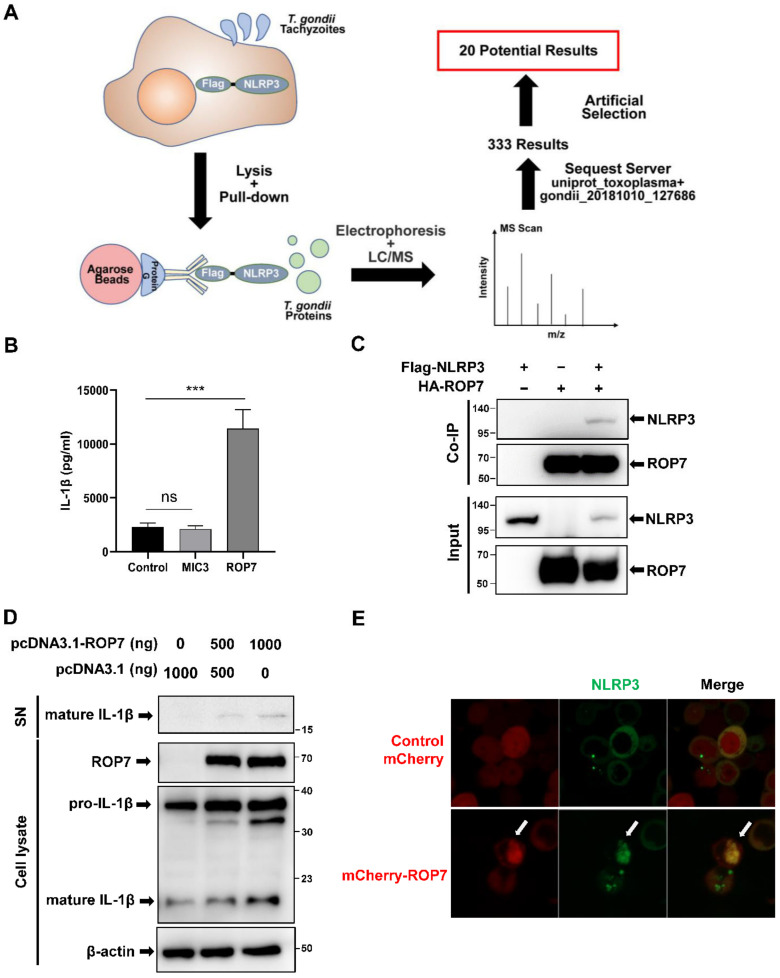
Figure 1.
ROP7 is associated with NLRP3 and promotes the maturation of IL-1β in 293T cells. (A) Schematic for the identification of potential proteins interacting with NLRP3. Protein G binding with anti-Flag-precipitated parasite proteins from 293T cell lysates following parasite invasion. Immunoprecipitated proteins were separated by SDS-PAGE and further analyzed by LC/MS after elution. Parasite proteins were identified using a T. gondii database from Uniprot. (B) The 293T cells were co-transfected with inflammasome-component plasmids (pro-IL-1β, pro-caspase-1, NLRP3, and ASC) and ROP7, MIC3, or an empty vector. The IL-1β secretion in cell-free supernatant was measured after 36 h. Data are expressed as mean ± SEM values. ns: non-significant, *** p < 0.001, as compared with the empty vector groups. (C) The 293T cells were transfected with pcDNA3-HA-ROP7 and pcDNA3.1-Flag-NLRP3. The cell lysates were subjected to immunoprecipitation using anti-HA antibody and further analyzed by immunoblotting with an anti-Flag antibody (NLRP3) and an anti-HA antibody (ROP7). (D) The 293T cells were co-transfected with inflammasome-component plasmids and different concentrations of ROP7 or an empty vector. At 48 h post-transfection, the cells and cell-free medium were harvested respectively, and the cleavage of pro-IL-1β was detected by Western blot using an anti-Flag antibody. (E) The 293T cells were transfected with pcDNA3-NLRP3-EGFP and pmCherry or pmCherry-ROP7. At 48 h post-infection, ROP7 and NLRP3 co-localization was captured via confocal microscopy.