Abstract
Alasan, a high-molecular-weight bioemulsifier complex of an anionic polysaccharide and proteins that is produced by Acinetobacter radioresistens KA53 (S. Navon-Venezia, Z. Zosim, A. Gottlieb, R. Legmann, S. Carmeli, E. Z. Ron, and E. Rosenberg, Appl. Environ. Microbiol. 61:3240–3244, 1995), enhanced the aqueous solubility and biodegradation rates of polyaromatic hydrocarbons (PAHs). In the presence of 500 μg of alasan ml−1, the apparent aqueous solubilities of phenanthrene, fluoranthene, and pyrene were increased 6.6-, 25.7-, and 19.8-fold, respectively. Physicochemical characterization of the solubilization activity suggested that alasan solubilizes PAHs by a physical interaction, most likely of a hydrophobic nature, and that this interaction is slowly reversible. Moreover, the increase in apparent aqueous solubility of PAHs does not depend on the conformation of alasan and is not affected by the formation of multimolecular aggregates of alasan above its saturation concentration. The presence of alasan more than doubled the rate of [14C]fluoranthene mineralization and significantly increased the rate of [14C]phenanthrene mineralization by Sphingomonas paucimobilis EPA505. The results suggest that alasan-enhanced solubility of hydrophobic compounds has potential applications in bioremediation.
One of the main reasons for the prolonged persistence of hydrophobic hydrocarbons in contaminated environments is their low water solubility, which increases their sorption to soil particles and limits their availability to biodegrading microorganisms (4, 9, 20). Thus, approaches to enhancing biodegradation often attempt to increase the apparent solubility of hydrophobic hydrocarbons by treatments such as addition of synthetic surfactants (2, 13, 35) or biosurfactants (10, 19, 23, 29). Studies employing synthetic nonionic surfactants have contributed significantly to our understanding of the mechanisms that enhance apparent solubility and the interactions among degrading bacteria, the surfactant, and the hydrocarbons (14, 35, 36). However, the relative toxicity, low biodegradability, and limited efficiency at low concentrations reduce the potential for the applications of synthetic surfactants in contaminated sites (10). This purpose may be better served by biosurfactants whose primary function is to facilitate microbial life in environments dominated by hydrophilic-hydrophobic interfaces (12, 27, 29).
Biosurfactants are produced by numerous microorganisms and represent a wide diversity of chemicals and molecular structures (10). Several of them have found applications in environmental management (23) including the acceleration of the biodegradation of hydrophobic hydrocarbons in an oil-contaminated beach (15), soils (3, 33), and soil slurried in bioreactors (28). Most of these studies have employed small, well-characterized biosurfactants such as Pseudomonas aeruginosa rhamnolipids (3, 33), Torulopsis bombicola sophorose lipids (28), Rhodococcus erythropolis trehalose dimycolipids (5), and Bacillus sp. lichenysins (37). These are potent surfactants, as they dramatically reduce surface tension (from 72 to ≤30 dynes cm−1) and have low (micrograms per milliliter) critical micelle concentrations (CMC), which increase apparent solubilities of hydrophobic hydrocarbons by their solubilization into the hydrophobic core of micelles. Much less is known of how high-molecular-weight polymeric biosurfactants increase apparent solubilities of hydrophobic compounds and whether this increased solubility results in enhanced degradation rates of hydrophobic hydrocarbons. Polymeric biosurfactants, where hydrophobic groups are distributed over the entire molecule (27), as in emulsans from Acinetobacter calcoaceticus RAG-1 and BD-4 (29), are likely to form multimolecular structures, rather than micelles, in saturated aqueous solutions. Thus, they may enhance biodegradation of low-solubility hydrocarbons, by mechanisms other than micelle solubilization.
The goal of the work reported here was to test the effect of a polymeric biosurfactant, alasan, on the solubilization and biodegradation of polyaromatic hydrocarbons (PAHs). The bioemulsifier alasan is produced by Acinetobacter radioresistens KA53. It consists of a tightly bound complex of anionic polysaccharides and proteins and has an estimated molecular weight of 106 (26). Initial chemical characterization of the polysaccharide fraction showed the presence of N-acyl amino sugars, uronic acid, and a unique covalently bound alanine (26). The protein fraction consists of four major proteins of 45, 28, 21, and 31 kDa (24). Results show that alasan increases the apparent aqueous solubility of high-molecular-weight PAHs and enhances their biodegradation rate.
MATERIALS AND METHODS
Chemicals.
PAHs used in this study were phenanthrene (PHE; Aldrich Chemical Co., Milwaukee, Wis.), fluoranthene (FLA; Aldrich), and pyrene (PYR; Sigma, St. Louis, Mo.).
Preparation of alasan stock solutions.
Alasan was obtained from the cell-free extracellular fluid of A. radioresistens KA53 as described previously (26). Working stock solutions of alasan (a final concentration of 2 mg ml−1) were prepared fresh from lyophilized stocks by hydration in distilled water on ice followed by a 10-min heating in a boiling water bath. Stocks were then stored at 4°C.
Measurements of emulsifying activities.
Alasan preparations were routinely tested for their emulsifying activity by a standard assay (30). When small sample volumes were available, the assay volume was scaled down to 2 ml and the assay was performed in test tubes. Tubes were vortexed for 2 min, and emulsion formation was measured spectrophotometrically (Gilford Instruments; model 240) at 600 nm. The limit of detection of this assay was an A600 of 0.05. One unit of alasan emulsifying activity was defined as an increase in turbidity of 100 Klett units (in the standard assay [30]) or A600 of 1 (in test tube assays) per 1 mg of alasan.
PAH solubilization assays. (i) Test tube solubilization assay.
All solubilizations were performed in 20 mM Tris-HCl (pH 7.0). Solubilization in this buffer was slightly higher than in the standard buffer used for measuring emulsification (20 mM Tris–10 mM MgSO4 [26]), Bushnel-Haas (BH) growth medium (Difco Laboratories, Franklin Lakes, N.J.), 20 mM phosphate buffer (pH 7.0), or distilled water. Stock solutions of PAHs, prepared in acetone or hexane (Merck, Whitehouse Station, N.J.), were distributed into glass test tubes (10 by 170 mm) to yield 60 μg of PAH per tube. Tubes were left open inside an operating chemical fume hood to remove solvents, and 3 ml of assay buffer and other ingredients were added as required by the experimental design. All experiments were done in triplicate. Tubes were capped with plastic closures and incubated in a vertical position overnight at 30°C with shaking (150 rpm) in the dark. Samples were filtered through 1.2-μm-pore-size-grade GF/C glass microfiber filters (Whatman, Springfield Mill, United Kingdom), and 2 ml was removed to a clean tube to which 2 ml of hexane was added prior to extraction by vortexing for 2 min. A heavy emulsion that formed in tubes containing alasan necessitated a centrifugation step (12,000 × g for 10 min) to separate the aqueous and hexane phases. Concentrations of PAHs in the hexane extracts were measured spectrophotometrically at 252, 236, and 273 nm for PHE, FLA, and PYR, respectively, with calibration curves of PAHs in hexane. Assay buffers containing alasan at various concentrations but no PAHs were extracted with hexane as described above and served as blanks. Limits of detection by spectroscopy (rejecting absorbency readings of <0.05) were 0.17, 0.26, and 0.22 μg ml−1 for PHE, FLA, and PYR, respectively.
(ii) Solubilization in quartz cuvettes.
To determine the kinetics of solubilization, 100 μg of PHE was crystallized in the bottoms of 1-ml quartz cuvettes. The cuvettes were placed in a six-compartment holder of an Ultraspec 2000 spectrophotometer (Pharmacia, Uppsala, Sweden), and 1 ml of prefiltered (0.2-μm-pore-size polyethylsulfone membrane; Whatman) assay buffer containing various concentrations of alasan was added to each cuvette. Solubilization was carried out without shaking. A252 measurements were taken at room temperature every 30 s for 3.5 h with the parallel nonsynchronized mode of the kinetics software package provided by the manufacturer. Assay buffer (20 mM Tris, pH 7.0) was used as a control. The limit of detection of the assay was 0.9 μg of PHE ml−1 as determined by the A252 of a solution containing 500 μg of alasan ml−1. Absorbency readings were converted to concentrations of PHE by extrapolation from a calibration curve that was constructed by diluting a saturated PHE solution (1.6 μg ml−1) in assay buffer.
Effects of physicochemical factors on solubilization.
Assays were performed as described above for test tube solubilization assays except that, in experiments that tested the effect of temperature, incubations were for 2.5 h without shaking. A preliminary experiment showed that shaking had no effect on the extent of solubilization.
(i) Temperature effect.
Various incubation temperatures were achieved by placing the assay tubes in thermoblocks or in beakers containing water that were placed in temperature-controlled incubators.
(ii) pH effect.
Acetic acid–Na-acetate at 12.6:1, 1:2.4, and 1:9.7 (all at a final concentration of 20 mM) provided test systems at pH values of 3.8, 5.0, and 5.6, respectively. HCl was added to Tris buffer at the appropriate concentrations to obtain solutions of 20 mM Tris-HCl at pH values of 7.0, 8.0, and 9.0.
(iii) Salt concentration effect.
Stock solutions of 0.5 M NaCl (Sigma) and 0.5 M MgCl2 · 6H2O (Riedel-de Haën, Seelze-Hanover, Germany) were used to adjust the salt concentration of assay solutions to 0, 5, 10, 20, 30, 40, and 50 mM.
Dialysis experiments.
Saturated PHE solutions were prepared in the presence or absence of alasan (500 μg ml−1 filtered through a 0.2-μm-pore-size Super Acrodisc 32 filter [Gelman]). Flasks containing 6 mg of PHE and 60 ml of 20 mM Tris (pH 7.0) were shaken (150 rpm) overnight at 30°C in the dark. Solutions were filtered to remove remaining PHE crystals, samples were removed for time zero determinations, and the remaining volume was divided into 10-ml aliquots that were placed in dialysis tubing (Spectra/Por; molecular weight cutoff, 6,000 to 8,000; regenerated natural cellulose [Spectrum Medical Industries, Inc., Los Angeles, Calif.]). Samples were dialyzed against 5 liters of prechilled double-distilled water at 4°C, and one sample each of the alasan-treated and control solutions was removed every hour to determine remaining PHE concentrations and alasan emulsification activities as described above.
Mineralization of 14C-PAHs.
Mineralization assays were carried out in 250-ml Erlenmeyer flasks containing 50 ml of sterile BH medium, various concentrations of alasan (sterilized by filtration through 0.45-μm-pore-size nitrocellulose filters), and 1 mg of [3-14C]FLA (Sigma; specific activity, 0.1 μCi mg−1) or [9-14C]PHE (Sigma; specific activity, 0.21 μCi mg−1). The purity of both substrates was ≥98% as determined by high-pressure liquid chromatography with a Whatman ODS-2 column and detection of UV absorbance followed by radiochemical detection (Radiomatics detector [Packard, Groningen, The Netherlands]). The combined medium, PAHs, and alasan mixtures were shaken (100 rpm) overnight at 30°C to allow for solubilization of PAHs prior to inoculation. The inoculum, Sphingomonas paucimobilis EPA505 (22), was grown in glucose (10 g liter−1)-enriched Luria-Bertani medium at 30°C with shaking (150 rpm) to a cell density of 108 to 109 cells ml−1. Cells were centrifuged (6,000 × g, 10 min) and resuspended in 10 ml of BH medium. Cell concentration was determined spectroscopically with a predetermined relationship between A600 and cell counts, and the appropriate volume was added to each flask to give a cell density of approximately 2 × 108 cells ml−1. Flasks were immediately stoppered with silicon rubber stoppers from which CO2 traps consisting of glass vials with 0.5 ml of 1 N NaOH were suspended about 2 cm above the surface of the medium. Flasks were incubated as described above. The content of the traps was periodically exchanged with a fresh aliquot of the base solution and transferred to scintillation vials containing Ultima Gold (Packard). Samples were counted with a Beckman LS6500 scintillation counter (Fullerton, Calif.). Results are presented as percent 14C-PAH converted to 14CO2. Mineralization rates were calculated during time intervals when mineralization progressed linearly with time. Radioactivity that evolved during the interval was converted to micrograms of PAH by using specific activity values and divided by hours.
The toxicity of each PAH-alasan treatment was monitored with controls consisting of the same combinations of unlabelled PAHs, alasan, and EPA505 cells and 100 μg of [U-14C]glucose (Sigma; purity ≥98%, determined by high-pressure liquid chromatography with a Bio-Rad MNX HPX87C column and a radiochemical detector; specific activity of 9 nCi mg−1) ml−1. Glucose mineralization was monitored as described above for 14C-PAHs.
Determination of surface tension.
The method described by Miller and Zhang (21) was used to determine the surface tension of alasan in 20 mM Tris-HCl (pH 7.0) at 20°C with a Surface Tensiomat model 21 du Nouy tensiometer (Fisher Scientific, Pittsburgh, Pa.).
RESULTS
Solubilization of PAHs by alasan.
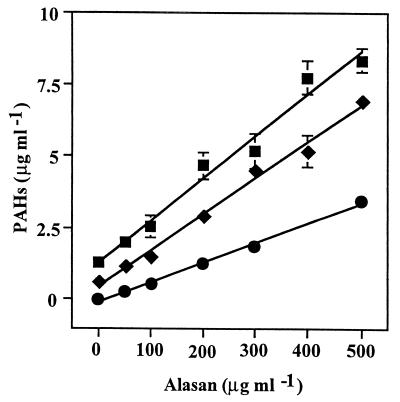
The effect of alasan on the apparent aqueous solubility of PHE, FLA, and PYR was determined by test tube solubilization assays in the presence of increasing concentrations of alasan (50 to 500 μg ml−1). The concentration of solubilized PAHs increased linearly with the addition of alasan (r2 ≥ 0.98). At 500 μg of alasan ml−1, the soluble PHE concentration was 8.34 ± 0.42 μg ml−1, 6.6 times higher than its solubility without alasan (1.26 μg ml−1); soluble FLA was measured at 6.94 μg ml−1, a 25.7-fold increase relative to the 0.27 μg ml−1 measured without alasan; and soluble PYR reached a concentration of 3.46 ± 0.05 μg ml−1, 19.8 times its previously reported (16) aqueous solubility of 0.175 μg ml−1. Solubilization with more than 500 μg of alasan ml−1 was not tested. The molar solubilization ratios (MSRs) for the test PAHs and alasan (approximate molecular weight of 106), calculated from the slopes of solubilization curves (Fig. 1), were 82.1 for PHE, 68.8 for FLA, and 28.9 for PYR. Normalized MSRs (in moles of PAH per mole of alasan per solubility value), obtained by dividing the MSR by the aqueous solubility of each compound, a measure of the enhancement of apparent aqueous solubilities by surfactants (19), showed that alasan affected FLA the most (normalized MSR of 52,000) followed by PYR (42,500) and PHE (11,400). Further work on the characterization of the solubilization activity was performed with PHE because its higher aqueous solubility allowed quantitative analysis of controls to which alasan was not added.
FIG. 1.
Relationship of alasan concentration to PAH solubilization. The figure shows the solubilization of PHE (■), FLA (⧫), and PYR (●) after an overnight incubation with the indicated alasan concentrations. Bars indicate the standard deviations of the means of three replicate samples. Standard deviations smaller than 0.4 μg ml−1 are hidden by the symbols.
Kinetics of solubilization.
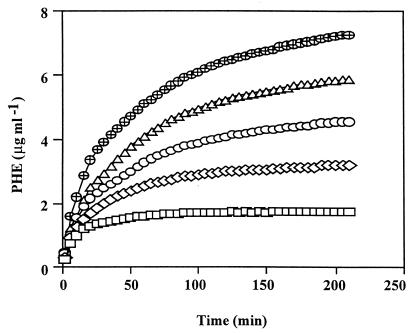
The increase in PHE concentration in solution was monitored to evaluate how alasan affected solubilization kinetics. Solubilization was rapid, reaching half its final magnitude within the first 30 min (Fig. 2). Both the rate (as indicated by the initial slopes of solubilization curves) and the final concentration of PHE were directly related to the amount of added alasan. The concentrations of PHE, 3.5 h after the initiation of solubilization, were 1.74, 3.19, 4.55, 5.83, and 7.23 μg ml−1 in the presence of 0, 100, 200, 300, and 400 μg of alasan ml−1, respectively, to give an MSR of 80.1 ± 3.9 (average ± standard deviation; n = 5).
FIG. 2.
Kinetics of PHE solubilization. Solubilization in the presence of 0 (□), 100 (◊), 200 (○), 300 (▵), and 400 (⊕) μg of alasan ml−1 was monitored photometrically (see Materials and Methods). Data obtained every 5 min are shown.
Effects of physicochemical factors on PHE solubilization.
The effect of factors that might alter alasan’s conformation, and thus interactions with ligands, on PHE solubilization was determined because these data could contribute to understanding of the solubilization mechanism. Temperature was the only factor tested that had an effect on solubilization, with alasan-induced solubilization reaching its optimum at 55°C (Table 1). Varying the assay pH (between 3.8 and 9.0) and the salt concentration of both mono- and divalent cations (NaCl and MgCl2) between 0 and 50 mM had no effect on solubilization (Table 1). These findings are in contrast to the pronounced effects of pH (optimum at 5.0) and ionic strength (stimulation by Mg2+) on the emulsification activity of alasan (26) and suggest that conformational changes of alasan that are induced by these factors, while modulating emulsification, do not affect its ability to solubilize PHE.
TABLE 1.
The effect of physicochemical factors on the solubilization of PHE
| Factor | Value or range tested | Amt of PHE (μg ml−1) solubilized by alasan concn (μg ml−1)a of:
|
|
|---|---|---|---|
| 250 | 0 | ||
| Temp (°C)b | 8 | 0.88 ± 0.07 | 0.29 ± 0.04 |
| 16 | 0.87 ± 0.13 | 0.49 ± 0.07 | |
| 25 | 2.28 ± 0.12 | 0.52 ± 0.02 | |
| 37 | 2.56 ± 0.02 | 1.45 ± 0.04 | |
| 42 | 3.80 ± 0.55 | 1.17 ± 0.35 | |
| 55 | 6.82 ± 0.05 | 3.35 ± 0.23 | |
| 69 | 3.03 ± 0.14 | 3.73 ± 0.47 | |
| pHc | 3.8–9.0 | 5.05 ± 0.53d | 1.17 ± 0.11d |
| Salt concn (mM)c | |||
| NaCl | 0–50 | 4.30 ± 0.68d | 1.02 ± 0.25d |
| MgCl2 | 0–50 | 3.30 ± 0.34d | 1.00 ± 0.08d |
Means ± standard deviations of triplicate samples.
Tested after 2.5 h of static incubation.
Tested at 30°C and with overnight incubation.
Means ± standard deviations of results of all assays performed at various pH values and salt concentrations.
Retardation of the dialysis of PHE by alasan.
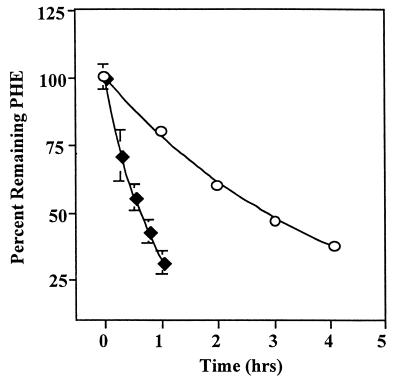
Dialysis experiments were conducted to test if binding of PHE by alasan occurs during solubilization. It was reasoned that if the high-molecular-weight alasan (about 106) bound PHE, the rate of PHE elimination from dialysis bags would be retarded relative to rates observed for solutions containing soluble PHE alone. Experimental results supported this binding hypothesis (Fig. 3). There was no loss of alasan during dialysis because emulsion activity of samples withdrawn from the bags remained stable at 16.0 ± 1.8 U ml−1. Quantitative interpretation of the results is complicated by the fact that the initial concentration of PHE in the alasan-treated sample was more than 10 times higher than its concentration in the sample without alasan (9.63 ± 0.33 versus 0.89 ± 0.08 μg ml−1). According to Fick’s law (31, 32), the rate of diffusion through a porous membrane is directly related to the concentration difference across the membrane. Thus, PHE diffusion from bags containing alasan should be approximately 10 times faster than its diffusion from bags without alasan. Flux calculations indicated that the former was only three times faster than the latter (1.9 compared to 0.6 μg of PHE ml−1 h−1, respectively), indicating that the diffusion of PHE was retarded by alasan.
FIG. 3.
Retardation of dialysis of PHE by alasan. Dialysis tubings containing an aqueous solution of PHE (⧫) or a PHE solution that was prepared in the presence of 500 μg of alasan ml−1 (○) were dialyzed against distilled water at 4°C. Samples were removed at the indicated intervals and analyzed for remaining PHE. Standard deviations smaller than 2% are hidden by the symbols.
The effect of alasan on PAH mineralization.
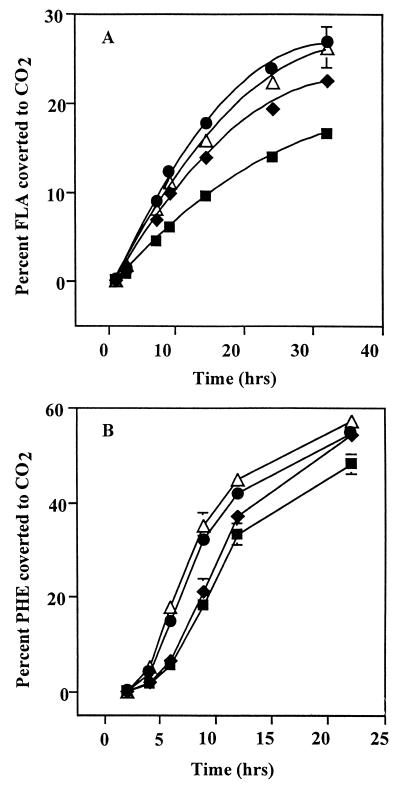
Mineralization of PHE and FLA by S. paucimobilis EPA505 was stimulated by the presence of alasan (Fig. 4), and the expected transition from linear to exponential growth kinetics (34) was observed in preliminary experiments that employed 400 μg of alasan ml−1 (data not shown). Stimulation of mineralization, however, was not directly correlated with the concentration of added alasan. Increasing the alasan concentration from 300 to 500 μg ml−1 did not result in a further increase in mineralization of either PHE or FLA. Thus, whereas mineralization rates increased from 7.9 to 13.4 and 16.6 μg of FLA h−1 by 0, 100, and 300 μg of alasan ml−1, respectively, only 14.2 μg of FLA h−1 was mineralized at 500 μg ml−1. Mineralization of PHE proceeded faster than that of FLA, was less affected by alasan (46.2 μg of PHE h−1 in the absence and 60.1 μg of PHE h−1 in the presence of 500 μg of alasan ml−1), and was completed after 22 h when 50 to 60% of the added substrate was converted to CO2. Controls showed that strain EPA505 could not grow on alasan (data not shown). The tested PAHs did not inhibit or stimulate [14C]glucose mineralization by EPA505 under the conditions employed in these experiments, indicating no toxicity by the increased solubility of PAHs nor effects of alasan on the metabolism of strain EPA505 (data not shown).
FIG. 4.
The effect of alasan on mineralization of 14C-PAHs. Mineralization of [14C]FLA (A) and [14C]PHE (B) was monitored in incubations containing 1 mg of the respective PAH and 0 (■), 100 (⧫), 300 (●), and 500 (▵) μg of alasan ml−1. Means ± standard deviations of triplicate incubations are presented. Standard deviations smaller than 2% are hidden by the symbols.
Effect of alasan on surface tension.
Increasing alasan concentrations from 0 to 200 μg ml−1 reduced the surface tension of 20 mM Tris from 69.1 ± 1.2 to 41.6 ± 0.5 dynes cm−1 (n = 3) (at 20°C), and no further decrease was noted when alasan concentrations were raised up to 500 μg ml−1. This observation suggests that at a concentration of 200 μg ml−1 alasan reached its saturation, forming multimolecular structures where hydrophobic moieties are located internally while hydrophilic moieties face the aqueous phase (21). Because it is unlikely that the high-molecular-weight alasan forms micelles, we refer to this concentration as the aggregation concentration for alasan rather than as the CMC.
DISCUSSION
In this paper, we present data indicating that the bioemulsifier alasan increases the apparent solubility of some PAHs, that this activity is likely due to a reversible binding of these compounds, and that it enhances the biodegradation of PAHs. Whereas there are a multitude of reports on increased apparent solubility and biodegradation of hydrophobic substances by low-molecular-weight biosurfactants, little attention has been given to polymeric ones (10, 27). As the mechanism of solubilization by high-molecular-weight polymers may be fundamentally different than that of small micelle-forming biosurfactants, research on the nature of this process might lead to the development of new approaches and tools for environmental management and industrial applications.
Results presented here (Fig. 1 and 2) indicate that a polymeric biosurfactant, alasan, increases apparent solubilities of PAHs and that the efficiency of this solubilization is similar to those reported for synthetic surfactants. Normalized MSRs for PHE and PYR (11,400 and 42,500, respectively) fell within ranges reported for a number of surfactants (5,000 to 22,000 for PHE and 59,000 to 103,000 for PYR; summarized by Miller [19]). One other group has demonstrated solubilization of PAHs by a bioemulsifier. Burd and Ward (8) demonstrated that a bioemulsifier consisting of protein and lipopolysaccharides produced by Pseudomonas marginalis PD-14B enhanced the dispersion of PAH crystals and growth on these PAHs. The isolated biosurfactant and the bacterium itself solubilized PHE (7). The nature of the solubilization mechanism was not addressed by these authors.
Data presented here suggest that interactions with hydrophobic regions in alasan are the most plausible explanation for the mechanism by which alasan solubilizes compounds with limited aqueous solubility. Mechanisms proposed for the enhancement of aqueous solubility of hydrophobic substances by surfactants include solubilization in the hydrophobic core of multimolecular surfactant structures formed at above-aggregation concentrations, such as micelles (11, 35) and liposomes (20); decreased surface tension of the solvent (38); and interaction with hydrophobic tails of surfactant monomers (1). Data presented in Fig. 1, showing a linear increase in apparent solubility of PAHs with increased alasan concentration (50 to 500 μg ml−1), and the observed aggregation concentration at 200 μg of alasan ml−1 rule out solubilization in multimolecular structures as the mechanism of solubilization by alasan. If alasan solubilized PAHs in the hydrophobic cores of such structures, no increase in solubility should be observed at concentrations below the CMC (11, 35). The possibility that reduced surface tension is the mechanism of solubilization is ruled out by the fact that enhanced solubilization of PAHs continued to increase at alasan concentrations (Fig. 1) that did not reduce surface tension at above the aggregation point (>200 μg ml−1). The possibility that alasan enhances apparent solubility by both mechanisms (solubilization in hydrophobic cores and reduction in surface tension at concentrations above and below aggregation concentration, respectively), as was suggested previously for the dispersion of octadecane by rhamnolipid (38), is unlikely because it would require that these two processes occurred with similar kinetics and stoichiometry (Fig. 2). This analysis, together with results of the dialysis experiment (Fig. 3) showing a physical association between alasan and PHE, strongly suggests interaction of low-aqueous-solubility compounds with hydrophobic regions of alasan as the mechanism of solubilization. Data presented indicate that this interaction is (i) reversible (Fig. 3), because 58.8 μg of PHE, the majority of which was initially associated with alasan, was removed from the bag after 4 h of dialysis; (ii) not affected by the formation of multimolecular structures of alasan at concentrations above the aggregation concentration (Fig. 1); and (iii) not affected by the conformation of alasan because varying pHs and salt concentrations did not affect the solubilization activity (Table 1). To the best of our knowledge, this is the first report to suggest a mechanism for the solubilization of solid hydrocarbons by polymeric biosurfactants.
The presence of alasan at concentrations of up to 300 μg ml−1 more than doubled the degradation rate of FLA and significantly increased the degradation rate of PHE (Fig. 4). The significance of this enhancement can be evaluated by comparison with the effect of synthetic and biologically produced surfactants on degradation of low-solubility substrates. Such comparisons are difficult because the degree of stimulation varies greatly with the test surfactant, the test substrate, growth conditions, and the degrading strains (36). Values reported in the literature show that the presence of surface-active compounds, e.g., rhamnolipids (38, 39) and synthetic surfactants (35), in growing cultures of hydrocarbon-degrading bacteria can stimulate degradation by factors of 2 to 8. We can more accurately compare the enhancement of FLA degradation by alasan with that by Tween 80 because a recent study using the same strain as used in this study, EPA505, and a similar experimental approach showed that degradation of FLA by 1.5 × 109 cells ml−1 (approximately an order of magnitude higher than that used here) was stimulated twofold by 0.48 mM Tween 80 (36). Thus, the degree of stimulation of PAH biodegradation by alasan was similar to that of other surface-active compounds, and further testing of alasan’s potential to enhance bioremediation of contaminated soils and sediments is well warranted. The attractiveness of alasan as a means in environmental remediation is its low toxicity, stability under various heat and alkaline conditions (26), and biodegradability (25) and the insensitivity of alasan-enhanced PAH solubilization to pH and salt concentration (Table 1). Alasan may be best suited for applications in wastewater and pump-and-treat efforts to clean up groundwater because the large size of alasan may restrict applications of the native polymer in soils where hydrocarbons persist in small pores (6).
Increasing the alasan concentration over 300 μg ml−1 had no further stimulatory effect on PAH mineralization (Fig. 4), although solubilization curves showed that the apparent solubility of these compounds continued to increase linearly with alasan additions at this concentration range (Fig. 1 and 2). This could be explained if PAHs that are associated with multimolecular structures of alasan, formed at concentrations above the CMC (about 200 μg ml−1), were not readily available for the degrading strain. Several studies (14, 35, 38, 40) showed that the availability of PAHs to degrading strains is limited by incorporation into micelles. Rather, PAHs released from micelles upon the degradation of aqueous-phase substrates stimulate growth in the presence of surfactants. This phenomenon together with the toxicity of some synthetic surfactants to some microbes (36) might explain why, in some instances, the addition of surfactants to contaminated soils inhibits, rather than stimulates, biodegradation (17, 18).
In summary, the demonstrated phenomenon of alasan-enhanced solubilization and biodegradation of PAHs has potential applications in the bioremediation of contaminated sites by accelerating the biodegradation rates of hydrophobic pollutants. Moreover, alasan, being a high-molecular-weight biopolymer, can serve as a useful model system for the study of the mode by which polymeric biosurfactants enhance the solubilization of solid compounds with low aqueous solubility.
ACKNOWLEDGMENTS
Thanks are due to Zohar Yerushalmi for her guidance during the early stages of this research; to Pia Willumsen for instruction with 14C-PAH mineralization assays and stimulating discussions; to Naomi Kayam for excellent technical assistance; to Valerie Walker for assistance with surface tension determinations; to Hap Pritchard for providing bacterial cultures and for stimulating discussions; and to Pia Willumsen, Hap Pritchard, and Joe Lepo for reviewing the manuscript.
This research was partially supported by contract RP8021/10 between the Electric Power Research Institute and Ramot of Tel Aviv University, by the Manja and Morris Leigh Chair for Biophysics and Biotechnology, and by the Ministry of Science, Israel.
REFERENCES
- 1.Almgren M, Grieser F, Powel J R, Thomas J K. A correlation between the solubility of aromatic hydrocarbons in water and micellar solutions, with their normal boiling points. J Chem Eng Data. 1979;24:285–287. [Google Scholar]
- 2.Aronstein B N, Alexander M. Effect of a non-ionic surfactant added to the soil surface on the biodegradation of aromatic hydrocarbons within the soil. Appl Microbiol Biotechnol. 1993;39:386–390. [Google Scholar]
- 3.Bai G, Brusseau M L, Miller R M. Biosurfactant-enhanced removal of residual hydrocarbon from soil. J Contam Hydrol. 1997;25:157–170. [Google Scholar]
- 4.Bartha R. Biotechnology of petroleum pollutant biodegradation. Microb Ecol. 1986;12:155–172. doi: 10.1007/BF02153231. [DOI] [PubMed] [Google Scholar]
- 5.Bruheim P, Bredholt H, Eimhjellen K. Bacterial degradation of emulsified crude oil and the effect of various surfactants. Can J Microbiol. 1997;43:17–22. doi: 10.1139/m97-003. [DOI] [PubMed] [Google Scholar]
- 6.Brusseau M L, Wang X, Hu Q. Enhanced transport of low-polarity organic compounds through soil by cyclodextrin. Environ Sci Technol. 1994;28:952–956. doi: 10.1021/es00054a030. [DOI] [PubMed] [Google Scholar]
- 7.Burd G, Ward O P. Bacterial degradation of polycyclic aromatic hydrocarbons on agar plates: the role of biosurfactants. Biotechnol Tech. 1996;10:371–374. [Google Scholar]
- 8.Burd G, Ward O P. Involvement of a surface-active high molecular weight factor in degradation of polycyclic aromatic hydrocarbons by Pseudomonas marginalis. Can J Microbiol. 1996;42:791–797. doi: 10.1139/m96-099. [DOI] [PubMed] [Google Scholar]
- 9.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol. 1993;4:331–338. [Google Scholar]
- 10.Desai J D, Banat I M. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards D A, Luthy R G, Liu Z. Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ Sci Technol. 1991;25:127–133. [Google Scholar]
- 12.Georgiou G, Lin S-C, Sharma M M. Surface-active compounds from microorganisms. Bio/Technology. 1992;10:60–65. doi: 10.1038/nbt0192-60. [DOI] [PubMed] [Google Scholar]
- 13.Grimberg S J, Nagel J, Aitken M D. Kinetics of phenanthrene dissolution into water in the presence of nonionic surfactants. Environ Sci Technol. 1995;29:1480–1487. doi: 10.1021/es00006a008. [DOI] [PubMed] [Google Scholar]
- 14.Guha S, Jaffé P R. Biodegradation kinetics of phenanthrene partitioned into the micellar phase of nonionic surfactants. Environ Sci Technol. 1996;30:605–611. [Google Scholar]
- 15.Harvey S, Elashvili I, Valdes J J, Kamely D, Chakrabarty A M. Enhanced removal of Exxon Valdez spilled oil from Alaskan gravel by a microbial surfactant. Bio/Technology. 1990;8:228–230. doi: 10.1038/nbt0390-228. [DOI] [PubMed] [Google Scholar]
- 16.Klevens H B. Solubilization of polycyclic hydrocarbons. J Phys Colloid Chem. 1950;54:283–298. doi: 10.1021/j150481a005. [DOI] [PubMed] [Google Scholar]
- 17.Laha S, Luthy R G. Inhibition of phenanthrene mineralization by nonionic surfactants in soil-water systems. Environ Sci Technol. 1991;25:1920–1930. [Google Scholar]
- 18.Laha S, Luthy R G. Effects of nonionic surfactants on the mineralization of phenanthrene in soil-water systems. Biotechnol Bioeng. 1992;40:1367–1380. doi: 10.1002/bit.260401111. [DOI] [PubMed] [Google Scholar]
- 19.Miller R M. Surfactant-enhanced bioavailability of slightly soluble organic compounds. In: Skipper H, Turco R, editors. Soil Science Society of America special publication 43. Bioremediation: science and applications. Madison, Wis: Soil Science Society of America; 1995. pp. 33–54. [Google Scholar]
- 20.Miller R M, Bartha R. Evidence from liposome encapsulation for transport-limited microbial metabolism of solid alkanes. Appl Environ Microbiol. 1989;55:269–274. doi: 10.1128/aem.55.2.269-274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller R M, Zhang Y. Measurement of biosurfactant-enhanced solubilization and biodegradation of hydrocarbons. Methods Biotechnol. 1997;2:59–66. [Google Scholar]
- 22.Mueller J G, Chapman P J, Blattmann B O, Pritchard P H. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl Environ Microbiol. 1990;56:1079–1086. doi: 10.1128/aem.56.4.1079-1086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller-Hurtig R F, Wagner F, Blaszcyk R, Kosaric N. Biosurfactants for environmental control. In: Kosaric N, editor. Biosurfactants: production, properties, applications. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 447–469. [Google Scholar]
- 24.Navon-Venezia, S. Unpublished data.
- 25.Navon-Venezia S, Banin E, Ron E Z, Rosenberg E. The bioemulsifier alasan: role of protein in maintaining structure and activity. Appl Microbiol Biotechnol. 1998;49:382–384. [Google Scholar]
- 26.Navon-Venezia S, Zosim Z, Gottlieb A, Legmann R, Carmeli S, Ron E Z, Rosenberg E. Alasan, a new bioemulsifier from Acinetobacter radioresistens. Appl Environ Microbiol. 1995;61:3240–3244. doi: 10.1128/aem.61.9.3240-3244.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neu T R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev. 1996;60:151–166. doi: 10.1128/mr.60.1.151-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberbremer A, Müller-Hurtig R, Wagner F. Effect of the addition of microbial surfactants on hydrocarbon degradation in a soil population in a stirred reactor. Appl Microbiol Biotechnol. 1990;32:485–489. doi: 10.1007/BF00903788. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg E. Microbial surfactants. Crit Rev Biotechnol. 1986;3:109–132. [Google Scholar]
- 30.Rosenberg E, Perry A, Gibson D T, Gutnick D L. Emulsifier of Arthrobacter RAG-1: specificity of hydrocarbon substrate. Appl Environ Microbiol. 1979;37:409–413. doi: 10.1128/aem.37.3.409-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz J S, Gerhardt P. Dialysis, culture of microorganisms: design, theory, and results. Bacteriol Rev. 1969;33:1–47. doi: 10.1128/br.33.1.1-47.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuwiner S B. Diffusion and membrane technology. New York, N.Y: Reinhold Publishing Corp.; 1962. [Google Scholar]
- 33.Van Dyke M I, Couture P, Brauer M, Lee H, Trevors T J. Pseudomonas aeruginosa UG2 rhamnolipid biosurfactants: structural characterization and their use in removing hydrophobic compounds from soil. Can J Microbiol. 1993;39:1071–1078. doi: 10.1139/m93-162. [DOI] [PubMed] [Google Scholar]
- 34.Volkering F, Breure A M, Sterkenburg A, Van Andel J G. Microbial degradation of polycyclic aromatic hydrocarbons: effect of substrate availability on bacterial growth kinetics. Appl Microbiol Biotechnol. 1992;36:548–552. [Google Scholar]
- 35.Volkering F, Breure A M, van Andel J G, Rulkens W H. Influence of nonionic surfactants on bioavailability and biodegradation of polycyclic aromatic hydrocarbons. Appl Environ Microbiol. 1995;61:1699–1705. doi: 10.1128/aem.61.5.1699-1705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willumsen P A, Karlson U, Pritchard P H. Response of fluoranthene-degrading bacteria to surfactant. Appl Microbiol Biotechnol. 1998;50:475–482. [Google Scholar]
- 37.Yakimov M M, Timmis K N, Wray V, Fredrickson H L. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl Environ Microbiol. 1995;61:1706–1713. doi: 10.1128/aem.61.5.1706-1713.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y M, Miller R M. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant) Appl Environ Microbiol. 1992;58:3276–3282. doi: 10.1128/aem.58.10.3276-3282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y M, Miller R M. Effect of rhamnolipid (biosurfactant) structure on solubilization and biodegradation of n-alkanes. Appl Environ Microbiol. 1995;61:2247–2251. doi: 10.1128/aem.61.6.2247-2251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y M, Maier W J, Miller R M. Effect of rhamnolipids on the dissolution, bioavailability and biodegradation of phenanthrene. Environ Sci Technol. 1996;31:2211–2217. [Google Scholar]