Abstract
A diagnosis of perineural invasion (PNI) is widely accepted as an unfavorable prognostic factor in various solid malignancies. Although PNI has been described as a high-risk parameter in oral squamous cell carcinoma (OSCC), its role in the current staging manuals of the American Joint Committee on Cancer (AJCC) is rather subordinate. We analysed the prognostic value of PNI on survival and recurrence in a large, multicenter OSCC cohort and a population-based approach. A total of 493 OSCC patients with primary tumor resection to negative margins and concomitant neck dissection between 2010 and 2017 were enrolled. PNI was evaluated in relation to overall survival (OAS) and recurrence-free survival (RFS) using uni- and multi-variable Cox regression. The median follow-up time was 5.0 years and PNI was diagnosed in 48 patients (9.7%). A pathohistological verification of PNI correlated significantly with a deteriorated OAS in uni- (HR 2.312; 95% CI 2.312–3.493, p = 0.001) and multivariable Cox regression (HR 1.820; 95% CI 1.164–2.847, p = 0.009). Additionally, a diagnosis of PNI correlated with increased cumulative, as well as distant, metastasis 5-year-recurrence rates (p = 0.027 and p = 0.011, respectively). The application of adjuvant radiotherapy (RT) or radiochemotherapy (RCT) in patients with PNI did not alter OAS or RFS in survival analysis when compared to patients without PNI. The results underline the adverse impact of PNI on the survival and recurrence of surgically treated OSCC patients. Based on our findings, we highly recommend an emphasis on PNI in the TNM staging concept.
Keywords: oral squamous cell carcinoma, oral cancer, PNI, perineural invasion, survival, recurrence
1. Introduction
Despite the tremendous efforts of the recent decade aiming to ameliorate the outcome of patients suffering from oral squamous cell carcinoma (OSCC), survival rates are still regrettably low [1]. Certainly, the clinical application of immunotherapeutic approaches in the recent years has resulted in promising courses of disease—at least for a selected proportion of OSCC patients [2]. Among the many oncological hallmarks of solid malignancies, and particularly with regards to OSCC, metastasis and invasive growth are major challenges for surgical treatment [3]. Tumor size, anatomical site, and infiltration pattern determine the involvement and invasion of locoregional nerves due to a high anatomical density of these structures in the oral cavity and in the head and neck region, in general. For a tumor entity explicitly characterized as neurotropic [4,5], it is not surprising that perineural invasion (PNI) is commonly observed in OSCC [3]. When it comes to assessing outcomes in OSCC patients with a pathohistological verification of PNI, survival was fundamentally decreased in comparison to patients with an absence of PNI [6].
Since the first description of PNI by Cruveilhier in 1835 [7], numerous authors have characterized the phenomenon of tumor cells invading adjacent structures. The overall tenor of most studies explicitly considers tumors with pathohistological verified PNI as more aggressive, with a consecutively higher chance of adverse patient outcomes [8,9]. Although the exact molecular and cellular mechanisms of PNI are not entirely clear, recent studies have focused on the microenvironment closely located to nerval structures. In the classic concept, PNI was considered as a mainly cancer-driven nerve infiltration directly through the path of least resistance in the perineural space. This process includes a broad orchestra of different cell types and soluble, as well as non-soluble, factors [10,11,12]. However, more recent findings suggest that PNI requires reciprocal signaling interactions between tumor cells and nerve components, particularly Schwann cells. Specifically, OSCC can express neurotrophins and neurotrophin receptors that may contribute to cancer cell migration towards nerves, PNI, and neuritogenesis towards cancer. Schwann cells may play an important role in promoting PNI by migrating towards cancer cells, intercalating, and dispersing cancer, and facilitating cancer migration towards nerves [12]
Although PNI was correlated with various adverse cancer properties [4], its precise impact on prognosis in OSCC is not entirely clear [4]. Additionally, this topic is particularly relevant for the indication of adjuvant treatment modalities. We therefore evaluated the role of PNI in OSCC patients, focusing on therapeutic implications.
2. Materials and Methods
2.1. Patient Selection
This retrospective, multicenter cohort study comprises patients with newly diagnosed OSCC and surgical treatment between January 2010 and December 2017. All participants were residing in the region of Eastern Bavaria, which represents a German population of around 2.3 million people, and includes the districts of Upper Palatinate and Lower Bavaria. The population-based dataset was kindly provided by the Clinical Cancer Registry of the Tumor Center Regensburg. Diagnostic workup and treatment took place at three different centers: the Department of Cranio-Maxillofacial Surgery, as well as the Department of Otorhinolaryngology, both at the University Hospital Regensburg, and the Department of Otorhinolaryngology at the St. Elisabeth Hospital Straubing.
All included patients received resection of the primary lesion to negative margins based on clinical and radiologic examination. The standard surgical approach was transoral. In cases where this technique did not offer adequate exposure, temporary mandibulotomy and pull-through resection were applied. No robotic surgery was used in the three above-mentioned centers. In addition to the different types of oral defect reconstruction, a diligent elective/selective neck dissection was performed in each patient. Previous neck dissection, primary radio(-chemo)therapy of head and neck squamous cell carcinoma, or neoadjuvant treatment modalities were the exclusion criteria.
Consecutively, 493 patients were included in the present study. Staging was carried out in line with the “TNM classification of malignant tumors” by the Union Internationale Contre le Cancer (UICC) in its 7th edition [13]. Adjuvant treatment was based on the recommendation of the multidisciplinary tumor board, and radiotherapy or radio-chemotherapy was used accordingly. Patient-specific demographic, pathohistological, and clinical data were obtained from medical records, and included gender, age at diagnosis, positive anamnesis of nicotine and alcohol abuse, anatomical site, extranodal spread, grading, and application of adjuvant therapies. With consideration of comorbidities and age on survival of OSCC patients, the age-adjusted Charlson Comorbidity Index (ACCI) was calculated as previously described and without taking OSCC into account [14,15]. Recurrent disease was either diagnosed by radiologic evidence with clinical correlation or histologic confirmation by biopsy. Survival follow up data concerning recurrence-free survival (RFS) and overall survival (OAS) were gathered from medical records, death certificates, registration offices, and the Clinical Cancer Registry of the Tumor Center—Institute for Quality Management and Health Services Research, University of Regensburg. PNI was diagnosed if routine pathohistological examination of primary surgical specimen by an experienced pathologist showed a tumor in ultimate proximity to a nerve which is surrounded by the malignancy by at least one third circumference or penetrates at least one of three connective tissue layers of the nerve [16]. The cut-off date for this study was set at 31 March 2021.
2.2. Statistics
Continuous data are described as means, median, minimum, and maximum values. Categorical data are given as absolute frequencies and relative percentages. Patient characteristics were compared using the two-tailed Student’s t test for continuous data, in the case of normal distribution; otherwise, the Mann–Whitney U test was applied. Pearson’s Chi-square test was used for testing independence between categorical variables.
Recurrences were derived from clinical reports and were analyzed in detail, where local, locoregional relapse, as well as formation of distant metastases, were evaluated. Five -year-survival rates for OAS and RFS, as well as cumulative recurrence rates, were analyzed from the date of resection until the first event. Patients’ OAS and RFS were calculated with the Kaplan–Meier method. The follow-up period and survival times were right censored using 31 March 2021 as the cut-off date, rendering a median follow-up of 5.0 years (4.7 years for the PNI-group). Differences in survival outcomes were tested for statistical significance by the two-sided log rank test (Mantel–Cox), and the level of significance was set at 0.05. Besides univariable survival analysis, we additionally performed multivariable Cox regression to adjust for covariables.
Before setting up a multivariable model, multicollinearity among prognostic variables was checked by using the variance inflation factor (VIF), which measures the correlation between the predictor variables in a linear regression model. Besides the pathohistological diagnosis of PNI, we additionally adjusted for gender, ACCI, history of smoking and alcohol abuse, distinct tumor localizations, UICC stage, grading, lymph vessel invasion, and vein invasion. The variables were consecutively incorporated in multivariable analyses if p-values of univariable analysis were less than 0.100.
ACCI was categorized in three groups, reflecting increased age at diagnosis and comorbidities. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were estimated and considered statistically significant when the CI excluded 1.0, and a two-sided p-value was <0.05. All analyses were performed by using IBM SPSS Statistics, version 26.0 (IBM Corp., SPSS for Windows, Armonk, NY, USA).
3. Results
Here, we report on the results of a retrospective multicenter cohort study on 493 OSCC patients who had resection to negative margins combined with an elective neck dissection. The prevalence of PNI at the time of diagnosis was 9.7% (48 patients). Patient characteristics of the entire retrospective cohort, including demographic and clinical parameters, are provided in Table 1. Most patients were male (70.6%), and most patients had an age of between 50 and 69.9 years at the date of tumor resection (66.9%). Most patients displayed an ACCI of >0 (91.7%), with 240 patients (48.7%) presenting an ACCI of ≥3. The majority of patients additionally presented a history of alcohol abuse (67.7%), as well as nicotine abuse (73.8%). The predominant tumor localizations were the floor of mouth (36.7%), as well as the tongue (30.0%). The majority of tumors were staged as pT1 (38.7%) and pT2 (31.8); however, 190 patients (38.6%) had already presented cervical lymph node metastasis at the day of tumor resection (pN+). In summary, UICC stage IV could be diagnosed in 187 patients (37.9%). In 42.2% of patients, an adjuvant treatment was applied.
Table 1.
Clinicopathological characteristics of OSCC patients according to the diagnosis of PNI (n = 445).
| Category Group | Perineural Invasion | |||||||
|---|---|---|---|---|---|---|---|---|
| Pn0 | Pn1 | Total | χ2 | |||||
| N | (%) | N | (%) | N | (%) | p | ||
| Gender | Female | 133 | 29.9% | 12 | 25.0% | 145 | 29.4% | 0.480 |
| Male | 312 | 70.1% | 36 | 75.0% | 348 | 70.6% | ||
| Age at diagnosis | <50 | 48 | 10.8% | 5 | 10.4% | 53 | 10.8% | 0.983 |
| 50.0–59.9 | 156 | 35.1% | 17 | 35.4% | 173 | 35.1% | ||
| 60.0–69.9 | 143 | 32.1% | 14 | 29.2% | 157 | 31.8% | ||
| 70.0–79.9 | 77 | 17.3% | 9 | 18.8% | 86 | 17.4% | ||
| 80.0+ | 21 | 4.7% | 3 | 6.3% | 24 | 4.9% | ||
| Age-adjusted CCI | 0 | 36 | 8.1% | 5 | 10.4% | 41 | 8.3% | 0.724 |
| 1 and 2 | 190 | 42.7% | 22 | 45.8% | 212 | 43.0% | ||
| 3+ | 219 | 49.2% | 21 | 43.8% | 240 | 48.7% | ||
| Positive anmnesis smoking | No | 119 | 26.7% | 10 | 20.8% | 129 | 26.2% | 0.376 |
| Yes | 326 | 73.3% | 38 | 79.2% | 364 | 73.8% | ||
| Positive anmnesis alcohol | No | 146 | 32.8% | 13 | 27.1% | 159 | 32.3% | 0.420 |
| Yes | 299 | 67.2% | 35 | 72.9% | 334 | 67.7% | ||
| Anatomical tumor site | Buccal mucosa | 33 | 7.4% | 1 | 2.1% | 34 | 6.9% | 0.332 |
| Upper alveolus and gingiva | 19 | 4.3% | 1 | 2.1% | 20 | 4.1% | ||
| Lower alveolus and gingiva | 71 | 16.0% | 5 | 10.4% | 76 | 15.4% | ||
| Hard palate | 32 | 7.2% | 2 | 4.2% | 34 | 6.9% | ||
| Tongue | 132 | 29.7% | 16 | 33.3% | 148 | 30.0% | ||
| Floor of mouth | 158 | 35.5% | 23 | 47.9% | 181 | 36.7% | ||
| Tumor size | T1 | 183 | 41.1% | 8 | 16.7% | 191 | 38.7% | 0.002 |
| T2 | 141 | 31.7% | 16 | 33.3% | 157 | 31.8% | ||
| T3 | 37 | 8.3% | 9 | 18.8% | 46 | 9.3% | ||
| T4 | 84 | 18.9% | 15 | 31.3% | 99 | 20.1% | ||
| Cervical lymph node metastasis | N0 | 286 | 64.3% | 17 | 35.4% | 303 | 61.5% | <0.001 |
| N1 | 60 | 13.5% | 6 | 12.5% | 66 | 13.4% | ||
| N2/3 | 99 | 22.2% | 25 | 52.1% | 124 | 25.2% | ||
| Extranodal spread | No | 108 | 24.3% | 18 | 37.5% | 126 | 25.6% | <0.001 |
| Yes | 50 | 11.2% | 13 | 27.1% | 63 | 12.8% | ||
| Not applicable | 287 | 64.5% | 17 | 35.4% | 304 | 61.7% | ||
| Grading | G1 | 35 | 7.9% | 1 | 2.1% | 36 | 7.3% | 0.192 |
| G2 | 324 | 72.8% | 34 | 70.8% | 358 | 72.6% | ||
| G3/4 | 86 | 19.3% | 13 | 27.1% | 99 | 20.1% | ||
| Lymphatic invasion | L0 | 399 | 89.7% | 27 | 56.3% | 426 | 86.4% | <0.001 |
| L1 | 46 | 10.3% | 21 | 43.8% | 67 | 13.6% | ||
| Vascular invasion | V0 | 435 | 97.8% | 44 | 91.7% | 479 | 97.2% | 0.016 |
| V1 | 10 | 2.2% | 4 | 8.3% | 14 | 2.8% | ||
| UICC stage | I | 144 | 32.4% | 3 | 6.3% | 147 | 29.8% | <0.001 |
| II | 78 | 17.5% | 8 | 16.7% | 86 | 17.4% | ||
| III | 69 | 15.5% | 4 | 8.3% | 73 | 14.8% | ||
| IV | 154 | 34.6% | 33 | 68.8% | 187 | 37.9% | ||
| Adjuvant therapy | No | 277 | 62.2% | 8 | 16.7% | 285 | 57.8% | <0.001 |
| Radiotherapy | 109 | 24.5% | 24 | 50.0% | 133 | 27.0% | ||
| Radio-chemotherapy | 59 | 13.3% | 16 | 33.3% | 75 | 15.2% | ||
| Life status | Alive | 295 | 66.3% | 21 | 43.8% | 316 | 64.1% | 0.002 |
| Deceased | 150 | 33.7% | 27 | 56.3% | 177 | 35.9% | ||
| Death/recurrence | Alive without recurrence | 263 | 59.1% | 21 | 43.8% | 284 | 57.6% | |
| Death or recurrence | 182 | 40.9% | 27 | 56.3% | 209 | 42.4% | 0.041 | |
| Total | 445 | 100.0% | 48 | 100.0% | 493 | 100.0% | ||
Abbreviations: CCI: Charlson Comorbidity Index; UICC: Union Internationale Contre le Cancer.
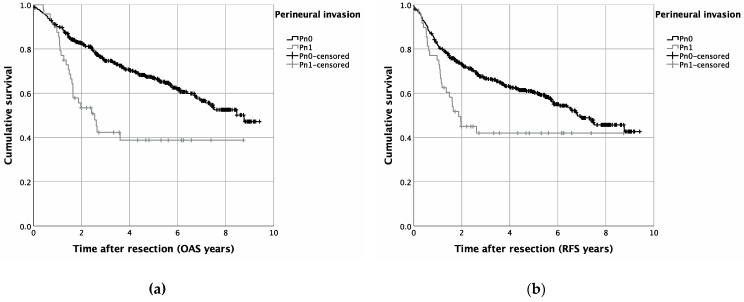
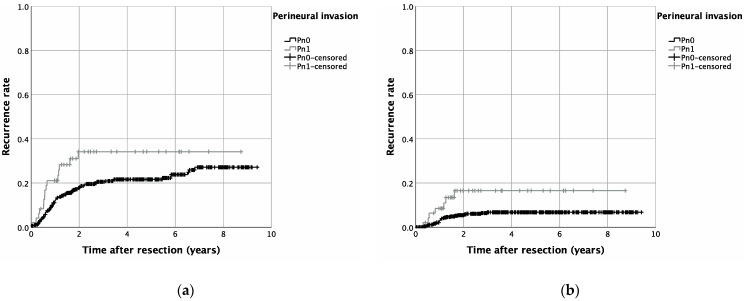
PNI was significantly associated with higher T-stages (p = 0.002), cervical lymph node involvement (p < 0.001), and pathohistological verification of extranodal spread (p < 0.001). Additionally, lymph vessel invasion (p < 0.001), as well as vascular invasion (p = 0.016), were more prevalent in patients with PNI (Pn1). Pearson’s chi-squared test identified increasing UICC stages to be significantly associated with PNI (p < 0.001) (Table 1). Univariable survival analysis revealed a significantly reduced OAS, as well as RFS, for patients with pathohistological verified PNI: hereby, a Kaplan–Meier analysis resulted in a five year OAS of 67.0% for patients without PNI (Pn0), whereas in case of PNI, five year survival was reduced to 38.8% (p < 0.001) (Figure 1). Similar results were seen for RFS, with a five year RFS of 60.6% (Pn1) and 42.0% (Pn1) (p = 0.004) (Figure 1). A cumulative five-year-recurrence rate of 21.6% (Pn0) and 34.2% (Pn1) was observed (p = 0.027) (Table 2, Figure 2). When focusing on recurrence in detail, a diagnosis of PNI did not significantly alter local (p = 0.134), as well as locoregional, recurrence rates (p = 0.303). A five-year distant metastasis recurrence rate, however, was significantly increased for patients diagnosed with PNI (16.6%) when compared to Pn0 patients (6.8%) (p = 0.011) (Table 2, Figure 2).
Figure 1.
Survival in OSCC patients with or without PNI (Pn1 and Pn0, respectively): Kaplan–Meier curves for OAS (a) (p = 0.001) and RFS (b) (p = 0.002).
Table 2.
Cumulative recurrence rates of as well as detailed forms of recurrence in OSCC patients, depending on PNI.
| Category | Group | N (Included Patients) |
N (Recurrent Disease) |
5-Year-Recurrence Rate | Log-Rank p |
|---|---|---|---|---|---|
| Cumulative recurrence rate |
Pn0 + Pn1 | 493 | 106 | 0.027 | |
| Pn0 | 445 | 91 | 21.6% | ||
| Pn1 | 48 | 15 | 34.2% | ||
| Local recurrence rate |
Pn0 + Pn1 | 493 | 67 | 0.134 | |
| Pn0 | 445 | 58 | 13.8% | ||
| Pn1 | 48 | 9 | 21.1% | ||
| Locoregional recurrence rate | Pn0 + Pn1 | 493 | 48 | 0.303 | |
| Pn0 | 445 | 42 | 10.5% | ||
| Pn1 | 48 | 6 | 17.1% | ||
| Distant metastasis recurrence rate | Pn0 + Pn1 | 493 | 33 | 0.011 | |
| Pn0 | 445 | 26 | 6.8% | ||
| Pn1 | 48 | 7 | 16.6% |
Figure 2.
Recurrence rates in OSCC patients with or without PNI (Pn1 and Pn0, respectively) for cumulative recurrence (a) (p = 0.001) as well as for recurrence by distant metastasis (b) (p = 0.002).
Multivariable survival analysis was additionally performed to adjust for covariables such as gender, ACCI, smoking and alcohol consumption, anatomical tumor site, UICC stage, grading, and lymphatic and vascular invasion. For OAS, a pathohistological verification of PNI significantly impaired the survival of OSCC patients in the present multicenter cohort study (HR 1.820; 95% CI 1.164–2.847, p = 0.009) (Table 3). Additionally, an ACCI of 3+ (HR 1.877; 95% CI 1.028–3.430, p = 0.040), as well as advanced tumor stages (HR 2.741; 95% CI 1.954–3.845, p < 0.001), were associated with diminished OAS (Table 4). For RFS, however, merely a trend in impaired multivariable survival was observed for PNI in OSCC patients (HR 1.461; 95% CI 0.934–2.284, p = 0.097) (Table 4). In line with the results of the multivariable analysis for OAS, UICC stages III and IV (HR 2.074; 95% CI 1.534–2.805, p < 0.001) and an elevated ACCI (p < 0.001) were significantly associated with impaired RFS (Table 4).
Table 3.
Effect of PNI and additional covariables on OAS using uni- as well as multivariable Cox regression.
| Overall Survival (OAS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Group | Univariable Cox Regression | Multivariable Cox Regression | ||||||
| p | HR | Lower 95%-CI |
Upper 95%-CI |
p | HR | Lower 95%-CI |
Upper 95%-CI |
||
| PNI | Pn0 | 1.000 | 1.000 | ||||||
| Pn1 | <0.001 | 2.312 | 1.530 | 3.493 | 0.009 | 1.820 | 1.164 | 2.847 | |
| Gender | Female | 1.000 | |||||||
| Male | 0.857 | 1.030 | 0.746 | 1.424 | |||||
| Age-adjusted CCI | 0 | <0.001 | 1.000 | <0.001 | 1.000 | ||||
| 1 and 2 | 0.778 | 0.915 | 0.491 | 1.704 | 0.774 | 0.912 | 0.488 | 1.707 | |
| 3+ | 0.043 | 1.854 | 1.020 | 3.369 | 0.040 | 1.877 | 1.028 | 3.430 | |
| Positive anamnesis smoking | No | 1.000 | |||||||
| Yes | 0.522 | 1.118 | 0.795 | 1.572 | |||||
| Positive anamnesis alcohol | No | 1.000 | |||||||
| Yes | 0.684 | 1.068 | 0.777 | 1.469 | |||||
| Anatomical tumor site | Upper alveolus, gingiva and hard palate | 0.23 | 1.000 | ||||||
| Tongue | 0.126 | 0.665 | 0.395 | 1.121 | |||||
| Lower alveolus, floor of mouth, buccal mucosa | 0.499 | 0.851 | 0.534 | 1.358 | |||||
| UICC stage | I/II | 1.000 | 1.000 | ||||||
| III/IV | <0.001 | 3.015 | 2.168 | 4.194 | <0.001 | 2.741 | 1.954 | 3.845 | |
| Grading | G1/2 | 1.000 | 1.000 | ||||||
| G3/4 | 0.068 | 1.395 | 0.976 | 1.993 | 0.124 | 1.328 | 0.925 | 1.906 | |
| Lymphatic invasion | L0 | 1.000 | 1.000 | ||||||
| L1 | 0.001 | 1.906 | 1.297 | 2.800 | 0.595 | 1.122 | 0.735 | 1.713 | |
| Vascular invasion | V0 | 1.000 | |||||||
| V1 | 0.036 | 2.055 | 1.049 | 4.027 | 0.185 | 1.583 | 0.803 | 3.119 | |
Abbreviations: CCI: Charlson Comorbidity Index; UICC: Union Internationale Contre le Cancer.
Table 4.
Effect of PNI and additional covariables on cumulative RFS using uni- as well as multivariable Cox regression.
| Recurrence-Free Survival (RFS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Group | Univariable Cox Regression | Multivariable Cox Regression | ||||||
| p | HR | Lower 95%-CI |
Upper 95%-CI |
p | HR | Lower 95%-CI |
Upper 95%-CI |
||
| PNI | Pn0 | 1.000 | 1.000 | ||||||
| Pn1 | 0.005 | 1.794 | 1.195 | 2.692 | 0.097 | 1.461 | 0.934 | 2.284 | |
| Gender | Female | 1.000 | |||||||
| Male | 0.894 | 1.020 | 0.758 | 1.373 | |||||
| Age-adjusted CCI | 0 | <0.001 | 1.000 | <0.001 | 1.000 | ||||
| 1 and 2 | 0.743 | 0.911 | 0.523 | 1.589 | 0.578 | 0.852 | 0.486 | 1.496 | |
| 3+ | 0.070 | 1.642 | 0.960 | 2.808 | 0.108 | 1.561 | 0.907 | 2.688 | |
| Positive anamnesis smoking | No | 1.000 | |||||||
| Yes | 0.684 | 1.066 | 0.782 | 1.454 | |||||
| Positive anamnesis alcohol | No | 1.000 | |||||||
| Yes | 0.638 | 1.072 | 0.801 | 1.436 | |||||
| Anatomical tumor site | Upper alveolus, gingiva and hard palate | 0.028 | 1.000 | 0.290 | |||||
| Tongue | 0.008 | 0.535 | 0.337 | 0.850 | 0.130 | 0.691 | 0.428 | 1.115 | |
| Lower alveolus, floor of mouth, buccal mucosa | 0.099 | 0.709 | 0.471 | 1.067 | 0.108 | 1.561 | 0.907 | 2.688 | |
| UICC stage | I/II | 1.000 | |||||||
| III/IV | <0.001 | 2.331 | 1.745 | 3.113 | <0.001 | 2.074 | 1.534 | 2.805 | |
| Grading | G1/2 | 1.000 | 1.000 | ||||||
| G3/4 | 0.058 | 1.375 | 0.989 | 1.910 | 0.136 | 1.289 | 0.923 | 1.800 | |
| Lymphatic invasion | L0 | 1.000 | 1.000 | ||||||
| L1 | 0.001 | 1.874 | 1.311 | 2.679 | 0.275 | 1.251 | 0.837 | 1.871 | |
| Vascular invasion | V0 | 1.000 | |||||||
| V1 | 0.161 | 1.614 | 0.826 | 3.152 | |||||
Abbreviations: CCI: Charlson Comorbidity Index; UICC: Union Internationale Contre le Cancer.
4. Discussion
PNI is a so-called optional descriptor in the established “TNM Classification of Malignant Tumours, 8th Edition” for OSCC, in addition to lymphatic and vascular invasion. Cancerous invasive growth in adjacent anatomical structures is characteristically linked to an increased risk of local, cervical lymphatic, and distant disease recurrence [17,18]. Nonetheless, it seems like PNI is relatively neglected in existing staging manuals [19]. The importance on OSCC patients’ survival after curative tumor resection is particularly of high clinical relevance.
Among the special features of our study are the large cohort, the population-based approach, the differentiation between types of recurrence, and the focus on patients’ comorbidities. With 9.7% of OSCC patients being diagnosed with PNI in the present study, a comparison with the prevalence of PNI in similar study designs might be of interest: a broad bandwidth (6.1–82%) for prevalence of PNI in OSCC can be found in the literature [20]. It is important to note that the diagnosis of PNI is closely related to different clinical, as well as pathohistological, parameters, including comorbidities, higher UICC stage, and heterogeneity of patient cohorts [21], especially blurring in the definition of PNI, as well as explicit pathohistological examination methods, to determine the evidence of PNI in clinical routine and scientific approaches.
A commonly applied definition of PNI entails the presence of malignant cells within any of the three layers of a nerval structure or tumor foci outside of the nerve with involvement of more than one third of the nerve’s circumference [16]. A less precise definition in this regard was given by Batsakis in 1985: PNI can be diagnosed if “tumor cell invasion in, around and through the nerve…” is present [22]. Undoubtedly, the definition of Batsakis leaves a scope for interpretation when it comes to precise definition of PNI. Therefore, objective, broadly accepted parameters for the diagnosis of PNI are urgently needed [23]. In addition, the establishment of standardized protocols would improve diagnoses of PNI. Certainly, immunohistochemical staining enhances the reliable detection of PNI based on neuronal markers such as S100 and several neurotrophins and their receptors [12,24]. In this regard, Shen et al. conducted a study on 116 previously resected OSCC patients, re-evaluating diagnoses of PNI by pathohistological stainings [25]. The initial PNI rate was increased by 17% through sheer review of hematoxylin and eosin-stained tissue biopsies by experienced pathologists. Additionally, IHC staining was applied using anti-S100 antibodies, which resulted in an overall PNI detection rate of 51% [25].
A major aim of the present study was to evaluate PNI as a prognostic oncological factor of surgically treated OSCC patients. Our results underline the findings of previous cohort studies on OSCC patients, indicating a significantly impaired outcome for Pn1 OSCC patients in uni- as well as multivariable survival analyses [6,26,27]. Moreover, with the present retrospective cohort study, we investigated whether PNI in OSCC patients affects different types of recurrence after tumor resection. Kaplan–Meier analysis clearly indicates a pronounced risk for recurrence, whether in terms of overall recurrence or recurrence by formation of distant metastasis. In this regard, we were able to attribute OSCC patients with PNI a 1.8-fold increased risk to develop distant metastasis, mainly in the lung or liver. This should be considered in follow-up care and assessment of OSCC patients.
However, the significance of PNI with regards to recurrence of malignancy is controversial in the current literature—notably, for the risk of developing distant metastasis, a diverging opinion exists. In this regard, Tarsitano et al. published results of not significantly increased distant metastasis recurrence rates for Pn1 patients compared to Pn0 OSCC cases [27]. In contrast to this, Rahima et al. attributed increased distant metastasis recurrence rates to oral, as well as oropharyngeal, squamous cell carcinoma patients with PNI; however, just 101 patients were included in this retrospective cohort study [28].
The results from our population-based, multicenter cohort study corroborate the findings of Rahima et. al. [28], and thereby assign PNI as a clearly detrimental prognostic impact on survival and recurrence in OSCC patients. In terms of predictive implications of PNI, and, chiefly, for adjuvant treatment modalities, we could not derive reliable implications of a potential outcome benefit after adjuvant RT or RCT due to small case numbers. Unsurprisingly, this question is of growing interest in the current literature. A main argument, potentially explaining a lack of effect for application of adjuvant treatment in Pn1 patients, might potentially be based on an already deteriorated outcome of Pn1 patients when compared to Pn0 cases. Particularly with regard to promising in vitro findings [11], the current inconsistent data situation regarding the role of adjuvant treatment of OSCC patients with PNI definitely demands further research [29].
5. Conclusions
PNI appears to be a relevant prognostic factor for the survival and recurrence of OSCC patients having received tumor resection to negative margins combined with a cervical lymphadenectomy. When focusing on recurrence in detail, the development of distant metastasis was significantly increased in Pn1 patients. Currently, an indication of adjuvant treatment for verified PNI in OSCC patients is under debate. So far, the present multicenter cohort study could not derive reliable implications of a potential outcome benefit after adjuvant RT or RCT. Additional in vivo, as well as in vitro, studies are necessary to unveil the molecular basis of PNI in OSCC and to develop targeted therapeutical approaches to address this special tropism of tumor spread. Based on our findings, we highly recommend emphasizing PNI in the TNM staging concept.
Acknowledgments
We thank Stefanie Wolf from St. Elisabeth Hospital Straubing for her excellent support. The present work was performed in fulfillment of the requirements for obtaining the degree of “Dr. med. dent.” by Stephanie Reil.
Author Contributions
Conceptualization, S.S. (Steffen Spoerl), S.S. (Silvia Spoerl), M.G. and G.S.; data curation, S.S. (Steffen Spoerl), M.G., S.R., R.F. and G.S.; formal analysis, S.S. (Steffen Spoerl), M.G. and G.S; investigation, J.T., R.F. and G.S.; methodology, S.S. (Silvia Spoerl), S.S. (Steffen Spoerl), S.R. and G.S.; project administration, S.S. (Silvia Spoerl), S.S. (Steffen Spoerl) and G.S.; resources, T.E. and T.E.R.; software, J.T. and N.L.; supervision, S.S. (Steffen Spoerl) and G.S.; validation, N.L., R.F., T.E. and T.E.R.; visualization, S.S. (Silvia Spoerl), J.T. and G.S.; writing—original draft, S.S. (Steffen Spoerl) and G.S.; writing—review and editing, S.S. (Silvia Spoerl), S.S. (Steffen Spoerl), N.L., J.T., R.F. and G.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Else Kröner-Fresenius-Stiftung, Germany.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Regensburg (GeschZ 12-101-0070, date of approval 11 November 2015).
Informed Consent Statement
Based on a retrospective analysis and a fully anonymized set of clinical data, and in agreement with the decision of the Ethics Committee, signing an informed consent form was not required.
Data Availability Statement
Data can be obtained by scientists that conducted the work independently from the industry on request. Data are not stored on publicly available servers.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonifazi M., Malvezzi M., Bertuccio P., Edefonti V., Garavello W., Levi F., La Vecchia C., Negri E. Age-period-cohort analysis of oral cancer mortality in Europe: The end of an epidemic? Oral Oncol. 2011;47:400–407. doi: 10.1016/j.oraloncology.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 3.Keerthi R., Dutta A., Agarwal S., Kani V., Khatua A. Perineural Invasion of Oral Squamous Cell Carcinoma: A New Hurdle for Surgeons. J. Maxillofac. Oral Surg. 2018;17:59–63. doi: 10.1007/s12663-016-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzistefanou I., Lubek J., Markou K., Ord R.A. The role of perineural invasion in treatment decisions for oral cancer patients: A review of the literature. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2017;45:821–825. doi: 10.1016/j.jcms.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz K.A., Hoffman H.T., Zimmerman M.B., Robinson R.A. Perineural and vascular invasion in oral cavity squamous carcinoma: Increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch. Pathol. Lab. Med. 2005;129:354–359. doi: 10.5858/2005-129-354-PAVIIO. [DOI] [PubMed] [Google Scholar]
- 6.Nair D., Mair M., Singhvi H., Mishra A., Nair S., Agrawal J., Chaturvedi P. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck. 2018;40:1780–1787. doi: 10.1002/hed.25170. [DOI] [PubMed] [Google Scholar]
- 7.Gaddikeri S. Perineural Invasion of Skin Cancers in the Head and Neck: An Uncommon Phenomenon Revisited. Otolaryngology. 2014;4:2. doi: 10.4172/2161-119X.1000169. [DOI] [Google Scholar]
- 8.Chen Y.W., Yu E.H., Wu T.H., Lo W.L., Li W.Y., Kao S.Y. Histopathological factors affecting nodal metastasis in tongue cancer: Analysis of 94 patients in Taiwan. Int. J. Oral Maxillofac. Surg. 2008;37:912–916. doi: 10.1016/j.ijom.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Woolgar J.A., Scott J. Prediction of cervical lymph node metastasis in squamous cell carcinoma of the tongue/floor of mouth. Head Neck. 1995;17:463–472. doi: 10.1002/hed.2880170603. [DOI] [PubMed] [Google Scholar]
- 10.Amit M., Na’ara S., Gil Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer. 2016;16:399–408. doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 11.Bakst R.L., Wong R.J. Mechanisms of Perineural Invasion. J. Neurol. Surg. Part B Skull Base. 2016;77:96–106. doi: 10.1055/s-0036-1571835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misztal C.I., Green C., Mei C., Bhatia R., Velez Torres J.M., Kamrava B., Moon S., Nicolli E., Weed D., Sargi Z., et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers. 2021;13:6011. doi: 10.3390/cancers13236011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobin L.H., Gospodarowicz M.K., Wittekind C., International Union against Cancer . In: TNM Classification of Malignant Tumours. 7th ed. Sobin L.H., Gospodarowicz M.K., Wittekind C., editors. Wiley-Blackwell; Chichester, UK: West Sussex, UK: Hoboken, NJ, USA: 2010. [Google Scholar]
- 14.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Spanier G., Böttcher J., Gerken M., Fischer R., Roth G., Lehn P., Klingelhöffer C., Meier J.K., Fraccaroli A., Tischer J., et al. Prognostic value of perioperative red blood cell transfusion and anemia on survival and recurrence in oral squamous cell carcinoma. Oral Oncol. 2020;107:104773. doi: 10.1016/j.oraloncology.2020.104773. [DOI] [PubMed] [Google Scholar]
- 16.Liebig C., Ayala G., Wilks J.A., Berger D.H., Albo D. Perineural invasion in cancer: A review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 17.Sahoo A., Panda S., Mohanty N., Jena D., Mishra N., Surabhi, Baisakh M.R. Perinerural, lymphovascular and depths of invasion in extrapolating nodal metastasis in oral cancer. Clin. Oral. Investig. 2020;24:747–755. doi: 10.1007/s00784-019-02921-0. [DOI] [PubMed] [Google Scholar]
- 18.Spoerl S., Gerken M., Fischer R., Mamilos A., Spoerl S., Wolf S., Pohl F., Klingelhoffer C., Ettl T., Reichert T.E., et al. Lymphatic and vascular invasion in oral squamous cell carcinoma: Implications for recurrence and survival in a population-based cohort study. Oral Oncol. 2020;111:105009. doi: 10.1016/j.oraloncology.2020.105009. [DOI] [PubMed] [Google Scholar]
- 19.Varsha B.K., Radhika M.B., Makarla S., Kuriakose M.A., Kiran G.S., Padmalatha G.V. Perineural invasion in oral squamous cell carcinoma: Case series and review of literature. J. Oral Maxillofac. Pathol. 2015;19:335–341. doi: 10.4103/0973-029X.174630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitd L.B., Scanlon C.S., D’Silva N.J. Perineural Invasion in Head and Neck Cancer. J. Dent. Res. 2018;97:742–750. doi: 10.1177/0022034518756297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita Y., Yanamoto S., Takahashi H., Yamada S., Naruse T., Sakamoto Y., Ikeda H., Shiraishi T., Fujita S., Ikeda T., et al. A clinicopathological study of perineural invasion and vascular invasion in oral tongue squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2015;44:543–548. doi: 10.1016/j.ijom.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Batsakis J.G. Nerves and neurotropic carcinomas. Pt 1Ann. Otol. Rhinol. Laryngol. 1985;94:426–427. [PubMed] [Google Scholar]
- 23.Chi A.C., Katabi N., Chen H.S., Cheng Y.L. Interobserver Variation Among Pathologists in Evaluating Perineural Invasion for Oral Squamous Cell Carcinoma. Head Neck Pathol. 2016;10:451–464. doi: 10.1007/s12105-016-0722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conte G.A., Qari O., Fasano G.A., Guinto R.K., Palo L., Parker G.S., Rangwala A.F., Minassian H., Greenberg P.J., Dewan A.A., et al. S100 Staining Adds to the Prognostic Significance of the Combination of Perineural Invasion and Lymphovascular Invasion in Colorectal Cancer. Appl. Immunohistochem. Mol. Morphol. AIMM. 2020;28:354–359. doi: 10.1097/PAI.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 25.Shen W.R., Wang Y.P., Chang J.Y., Yu S.Y., Chen H.M., Chiang C.P. Perineural invasion and expression of nerve growth factor can predict the progression and prognosis of oral tongue squamous cell carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2014;43:258–264. doi: 10.1111/jop.12133. [DOI] [PubMed] [Google Scholar]
- 26.Laske R.D., Scholz I., Ikenberg K., Meerwein C., Vital D.G., Studer G., Rössle M., Huber G.F. Perineural Invasion in Squamous Cell Carcinoma of the Oral Cavity: Histology, Tumor Stage, and Outcome. Laryngoscope Investig. Otolaryngol. 2016;1:13–18. doi: 10.1002/lio2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarsitano A., Tardio M.L., Marchetti C. Impact of perineural invasion as independent prognostic factor for local and regional failure in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015;119:221–228. doi: 10.1016/j.oooo.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Rahima B., Shingaki S., Nagata M., Saito C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004;97:423–431. doi: 10.1016/j.tripleo.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Blackburn T.K., Bakhtawar S., Brown J.S., Lowe D., Vaughan E.D., Rogers S.N. A questionnaire survey of current UK practice for adjuvant radiotherapy following surgery for oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2007;43:143–149. doi: 10.1016/j.oraloncology.2006.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained by scientists that conducted the work independently from the industry on request. Data are not stored on publicly available servers.