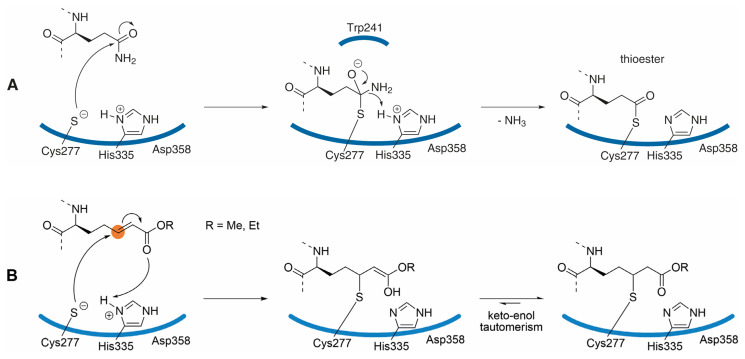
Figure 2.
(A) Transglutaminase reaction mechanism: the catalytic triad of human tissue transglutaminase is formed by the amino acids Cys277-His335-Asp358. The proposed thiolate-imidazolium ion pair is exceptionally nucleophilic enabling the attack of the otherwise inert carboxamide side-chain of protein bound glutamine to yield the thioester intermediate that itself is prone to react with the ε-amino group of lysine (not shown). The proposed tetrahedral oxyanion is stabilized by Trp241 and by the backbone nitrogen of Cys277; the driving force of the reaction is the release of ammonia. Hydrolysis of the reactive thioester is suppressed by the narrow hydrophobic tunnel excluding water from the catalytic site [48]. (B) The Michael acceptor warhead mimics the substrate glutamine side-chain, and when embedded in a suitable peptidic/peptidomimetic backbone the warhead addresses the catalytic center of tissue transglutaminase. The cysteinyl γ thiolate moiety of Cys277 attacks the complementary electrophilic β-carbon (marked in orange) of the α,β-unsaturated ester. The Michael addition to the alkene leads to the covalent, irreversible inhibition of TG2, following the mechanism previously described for cysteine proteases [51].