Abstract
Objective: The objective of this study is to create an overview of the possible aetiologies of windswept deformity and to emphasize the points of attention when presented with a case. Methods: A systematic search according to the PRISMA statement was conducted using PubMed, African Journals Online, Cochrane, Embase, Google Scholar, and Web of Science. Articles investigating the aetiology of windswept deformity at the knee in children, and articles with windswept deformity as an ancillary finding were included. The bibliographic search was limited to English-language articles only. The level of evidence and methodological appraisal were assessed. Results: Forty-five articles discussing the aetiology of windswept deformity were included. A variety of aetiologies can be brought forward. These can be divided into the following groups: ‘Rickets and other metabolic disorders’, ‘skeletal dysplasias and other genetic disorders’, ‘trauma’ and ‘descriptive articles without specific underlying disorder’. With rickets being the largest group. Interestingly, in the group without a specific underlying disorder, all patients were from African descent, being otherwise healthy and presented with windswept deformity between two and three years of age. Conclusion: We have presented an overview that may help identify the underlying disorder in children with windswept deformity. A step-by-step guide for clinicians who see a child with windswept deformity is provided. Even though, according to the Oxford level of evidence, most articles have a low level of evidence.
Keywords: windswept deformity, genu valgum, genu varum, children, rickets
1. Introduction
In 1975, Oyemade made mention of windswept deformity (WSD), under the term “varovalga”, characterised by the formation of a valgus deformity of one knee, and a varus deformity of the contralateral knee [1]. In 1976, the term windswept deformity was used by Fulford et al. to describe the general postural deformity acquired by children with cerebral palsy, in their first weeks of life [2]. Consequently, windswept deformity is used to describe the phenotypical presentation of a varus and valgus deformity, however, the location of this may vary, as well as the underlying pathology. In this article, we will focus on windswept deformity of the knee.
Although the long-term consequences of windswept deformity have not yet been described, it is presumable that it has a similar impact as other angular deformities of the knees. Untreated, it can lead to further deformity, gait abnormalities, limb shortening and osteoarthritis [3].
To date, the aetiology of windswept deformity remains unknown. Oyemade and Smyth described windswept deformity of the knee in previously healthy children, mostly of sub-Saharan descent, with normal developmental milestones [1,4]. The onset usually occurs in the second or third year of life, shortly after the onset of walking. According to Smyth, the valgus deformity develops first, rapidly followed by a varus deformity on the contralateral side [4]. Suggested hypotheses can be divided into the following categories: metabolic or dietary [5,6,7,8,9,10], mechanical pressure [1,11], reactive to unilateral disease [12], genetic/race [1,4,13] and traumatic [14]. Windswept deformity can be treated either surgically (corrective osteotomies, stapling) or conservatively (plaster casting) [11].
Apart from varovalgum at the knee, different forms of windswept deformity exist, including hip deformity in children with cerebral palsy [15]. The aim of this study is to perform a systematic search according to the PRISMA statement, in order to analyse which aetiologies of windswept deformity have been published, and assess their level and quality of evidence, as well as any bias. Our aim is to create an overview of the possible aetiologies for windswept deformity and emphasize the points of attention when presented with a case.
2. Materials and Methods
This systematic review was written according to the PRISMA statement for reporting systematic reviews and meta-analyses [16].
2.1. Eligibility Criteria
Types of studies: all articles investigating the aetiology of windswept deformity at the knee and all articles with windswept deformity as an ancillary finding. The language was restricted to articles written in English. There was no restriction on the date of publication.
Types of participants: all patients with a presentation of windswept deformity during childhood (0–18 years) were included. There were no restrictions on gender or race.
2.2. Information Sources
The following databases were searched on 16/10/2020: PubMed, African Journals Online, Cochrane, Embase, Google Scholar, and Web of Science. Articles were screened for eligibility, based on title, abstract and the full text. Additionally, the reference lists of the included articles were screened for further identification of any relevant articles and they were included where applicable.
2.3. Search Strategies
The following search terms were used. The limit “English” was added as mentioned in the eligibility criteria.
-
1.
(windswept deformity) OR (windswept);
-
2.
(((genu valgum) AND (genu varum)) OR ((genu valgum[MeSH Terms]) AND (genu varum[MeSH Terms]))) OR (combined valgus and varus knee) AND ((humans[Filter]) AND (allchild[Filter])) AND (English[Filter]);
-
3.
(((((varo-valgum) OR (varovalgum)) OR (genu varo-valgum)) OR (genu varovalgum)) OR (varo-valga)) OR (varovalga).
An overview of the search can be found in Appendix A.
2.4. Study Selection and Data Extraction
Studies were selected based on the eligibility criteria. First, duplicates were removed, followed by the selection based on title and abstract. The remaining articles were screened for their eligibility based on the full text. The included articles were screened upon a second survey and consensus amongst the authors was reached. The relevant data were extracted and reviewed by the authors.
2.5. Level of Evidence and Quality Assessment
The level of evidence was scored according to the Centre for Evidence-Based Medicine (CEBM) by three authors, followed by an assessment of methodological appraisal. The latter was performed according to the Joanna Briggs Institute (JBI) for case reports, case series, cohort studies, case-control studies, and cross-sectional studies [17]. Cut-off values were obtained using a scoring system, where a “yes” answer scored 2 points, “unclear” 1 point, “no” 0 points, and “not applicable” was subtracted from the maximum obtainable score. For each article, the obtained score was divided by the maximum obtainable score which led to a percentage. An article was considered to be of high quality when the score was 75% or higher, moderate between 50% and 75%, and low when the score was lower than 50%. Articles classified as “literature review” did not meet the criteria for level of evidence scoring or critical appraisal. An overview of the methodological appraisal can be found in Appendix B.
3. Results
An overview of the results and the number of records retrieved from the final search can be found in Figure 1.
Figure 1.
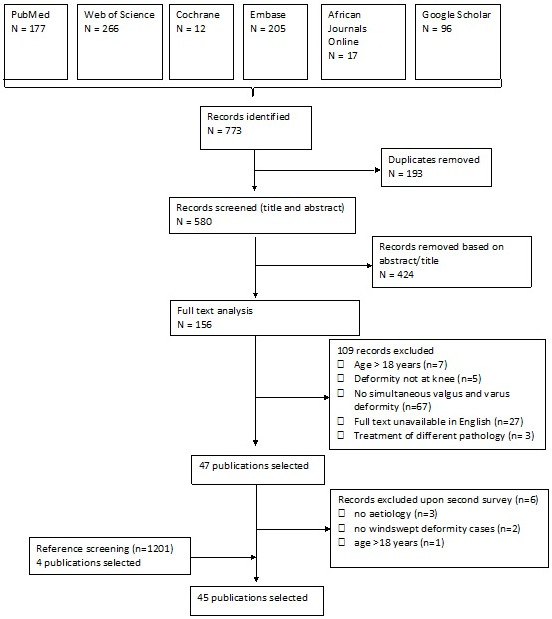
Flow diagram of studies screened and included in the review.
The three searches, performed in six databases, yielded a total of 773 records. After removing 193 duplicates, 580 records remained. Of these records, 424 were excluded based on title/abstract screening, leaving 156 records to be assessed based on the full text. From these 156 full-text records, 109 were excluded, as shown in Figure 1. This resulted in a total of 47 publications that were selected, of these, all the references (n = 1201) were screened from which four additional articles were selected. Upon second survey another six articles were excluded based on age > 18 years (n = 1), not being windswept deformity (n = 2) and no clear aetiology stated (n = 3). This resulted in a final number of 45 selected publications.
Although 45 articles is a substantial amount of included articles, only very few focus on the aetiology of windswept deformity, with most articles describing windswept deformity as an ancillary finding. The Oxford CEBM level of evidence regarding these publications is generally low, with most articles ranked as level IV. Additionally, the quality of the articles, assessed as described in the methods, varied greatly, ranging from a score of 50% to 100%.
In Table 1, general information can be found about the articles, including the country of study, study design, the aim of the study, and the main information related to windswept deformity, as well as the Oxford CEBM classification on the methodological quality score.
Table 1.
Overview of the demographic data of all included studies regarding windswept deformity at the knees.
| Article | Country of Study | Study Design | Aim of Study | Elaboration of WSD | WSD Aetiology | Level of Evidence (CEBM) and Methodological Quality |
|---|---|---|---|---|---|---|
| Akpede et al. [5] | Nigeria | Prospective cross-sectional | Determine the prevalence of clinical and biochemical rickets. | Two of ten patients who showed biochemical rickets, though not radiologically, did show WSD, suggesting a form of healed rickets. | Rickets, no radiographic evidence of active rickets | IV high |
| Al Kaissi et al. [18] | Austria | Case report | WSD in a patient with Schwartz-Jampel syndrome (SJS). | One patient with SJS, with WSD. | SJS | IV high |
| Al Kaissi et al. [19] | Austria | Case series | Record and discuss WSD in patients with X-linked hypophosphataemic rickets. | In seven patients with hypophosphataemic rickets, the most common angular deformity is WSD. | Hypophosphataemic rickets (from PHEX mutation) | IV moderate |
| Bar-On et al. [20] | Israel | Retrospective case series | Characterise deformities in patients with renal osteodystrophy (ROD). | One out of five patients showed WSD. | ROD | IV high |
| Bar-On et al. [14] | Israel | Retrospective case series | To investigate patients with insensitivity to pain. | One patient developed WSD as a consequence of growth disturbance due to untreated fractures of the growth plate. | Trauma | IV high |
| Bharani et al. [21] | India | Case report | To describe two siblings with sickle cell anaemia, presenting with bilateral lower limb deformities. | Two siblings, both male, 2 and 10 years old with progressive genu valgus on the right, genu varus on the left. | Distal renal tubular acidosis (dRTA) | IV High |
| Bhimma et al. [22] | Natal, South Africa | Case series | To determine the clinical spectrum of rickets among black children. | WSD was found in two patients with vitamin D deficiency and one patient with Ca deficiency in a total population of 37 patients. | Vitamin D and/or Ca deficiency rickets | IV high |
| Dudkiewicz et al. [23] | Israel | Case report | Describe the procedure of bone elongation in hypophosphataemic rickets. | One WSD with right genu valgum/left genu varum. | Hypophosphataemic rickets | IV moderate |
| Eralp et al. [24] | Turkey | Case report | Investigate the result of treatment with fixator-assisted intramedullary nailing in two cases with WSD. | Two patients with WSD, treated for vitamin-D-resistant rickets at younger age. | Vitamin-D-resistant rickets | IV high |
| Gigante et al. [25] | Italy | Case series | Evaluate temporary hemiepiphysiodesis in lower limb deformities in children with renal osteodystrophy (ROD). | One of the seven patients with ROD had WSD. Started as unilateral varus, developed valgus alignment in the contralateral knee. | ROD | IV high |
| Gupta et al. [26] | India | Literature review | Review the different types of nutritional vs. non-nutritional rickets. | Mention of WSD as a skeletal finding in nutritional rickets. It is not mentioned in non-nutritional rickets. | Nutritional rickets | n.a. * |
| Ikegawa [27] | Japan | Literature review | Review of the recent advances and current status of the genetic analysis of skeletal dysplasias. | Describes one WSD case, 17 years old with genu valgum on the left. | Skeletal dysplasia | n.a. * |
| Iyer and Diamond [28] | USA | Literature review | Review the effects of the resurgence of vitamin D deficiency and rickets. | Describes WSD as a possible clinical presentation of rickets. | Vitamin D deficiency rickets | n.a. * |
| Iyer and Diamond [29] | USA | Literature review | Review of the clinical, radiographic and biochemical manifestations of rickets. | Describes WSD as a possible clinical presentation of rickets. | Vitamin D deficiency rickets | n.a. * |
| Kenis et al. [30] | Austria | Case report | To describe the deformities in a patient with dysspondyloen-chondromatosis (DSC). | One patient with WSD with genu valgum of 30° on the right, genu varum of 10° on the left side. Age at start walking: 3 years. | DSC | IV high |
| Kim et al. [31] | Korea | Case series | Investigate the mutation frequency in individuals with multiple epiphyseal dysplasia (MED) and identify radiographic predictors. | Two of the fifty-five patients that identified with a previously reported mutation pathogenic for MED presented with WSD at the knee. One MATN3 and one COMP mutation. | MED | IV moderate |
| Lambert and Linglart [6] | France | Literature review | To describe the different causes and therapies of genetic and nutritional rickets. | WSD in walking children is a clinical manifestation of rickets. | Rickets | n.a. * |
| McKeand et al. [32] | USA | Case-control study | Describe the natural history of pseudoachondroplasia (PSACH). | WSD in 11/67 cases (16.4%), 8/11 cases (72.7%) needed a corrective operation for WSD. | PSACH | IIIb moderate |
| Muensterer et al. [33] | USA | Literature review | Describe pseudoachondroplasia, and its radiographic features. | In patients with pseudoachondroplasia, WSD typically develops around puberty, when genu varum transforms into WSD due to the progressive joint laxity. | PSACH | n.a.* |
| Nayak et al. [34] | India | Case report | Describe a case of epidermolytic hyperkeratosis (EHK) with rickets. | Epidermolytic hyperkeratosis (EHK) with rickets in a 6-year-old boy showed progressive WSD since age 3. | EHK | V high |
| Nishimura et al. [35] | Germany | Case series | Describe TRPV4 mutations in patients with spondylo-epiphyseal dysplasia (SED) and parastremmatic dysplasia. | A 7-year-old patient with reported low birth weight and length. Onset of walking at age 4. At 7 years she had a short stature (-4SD) and WSD. A TRPV4 mutation was found. | Parastremmatic dysplasia (TRPV4 mutation) | IV moderate |
| Nishimura et al. [36] | Switzerland | Literature review | To describe the different skeletal dysplasia’s related to TRPV4 mutations. | Patients with parastremmatic dysplasia have restricted joints and severe misalignment of the lower limbs (severe genu valgum, genu varum or WSD). | Parastremmatic dysplasia (TRPV4 mutation) | n.a. * |
| Oginni et al. [37] | Nigeria | Prospective case series | Response of oral calcium in Nigerian children with rickets. | Nine out of twenty-six children with underlying Ca-deficiency rickets presented with WSD, they were treated with calcium supplements, with good results. | Calcium-deficiency rickets | IV high |
| Oni and Keswani [38] | Nigeria | Case series | To describe the radiological findings of idiopathic or primary WSD. | Eight WSD patients were found, the onset of clinical and radiological alterations is abrupt, where the disease arises from a formerly normal epiphysis. The radiological features are similar to Blount, and therefore the etiological considerations that apply to Blount may also apply to primary WSD. | Hypotheses: similar to Blount, mechanical pressure, illness | IV moderate |
| Oni et al. [11] | Nigeria | Case series | To describe windswept deformity. | Eight patients with osteochondrosis with abrupt onset in previously healthy children, with formerly normal epiphyses. | Hypothesis: similar to Blount | IV moderate |
| Oyemade [13] | Nigeria | Case series | To describe the correction of primary knee deformities in children with and without rickets. | Rachitic patients WSD 12/47, Non-rachitic patients: WSD 15/67. | Rachitic or idiopathic (Blount) | IV moderate |
| Oyemade [1] | Nigeria | Case series | To clarify aetiological factors in primary deformities of the knee in children. | WSD: peak age male and female 2 years. Rachitic: WSD (12/47). Non-rachitic WSD (15/67). | Rachitic and non-rachitic Blount-like (weight-bearing) | IV moderate |
| Paruk et al. [39] | South Africa | Case report | Describe two cases of primary hyperparathyroidism (PHPT) in adolescence, mimicking rickets. | A 13-year-old male with progressive pain and WSD (right varus, left valgus). Caused by a parathyroid adenoma. | PHPT | IV high |
| Pavone et al. [40] | Italy | Literature review | Review hypophosphataemic rickets. | WSD described as a clinical feature of X-linked hypophosphataemic rickets. | X-linked hypophosphataemic rickets | n.a. * |
| Pettifor et al. [10] | South Africa | Literature review | Presentation of vitamin D deficiency and nutritional rickets in children. | In older children with vitamin D deficiency rickets, WSD may be present. | Vitamin D deficiency rickets and Calcium deficiency rickets | n.a. * |
| Pettifor et al. [41] | South Africa | Case series | Clinical, radiographic and biochemical findings in four children with severe bone deformities resembling rickets. | One out of four children had WSD. | Calcium-deficiency rickets | IV moderate |
| Prakash et al. [42] | India | Prospective cohort | To evaluate the behaviour of lower limb deformities due to rickets. | Five out of one-hundred and seventeen nutritional rickets patients had WSD. Varus deformity being the youngest, valgus and WSD being older. | Nutritional rickets | Iib high |
| Prentice et al. [43] | Gambia | Case-control study | Biochemical profile in Gambian children with rickets of unknown aetiology and normal 25OHD. | One out of thirty-seven patients had WSD. | Calcium-deficiency rickets | IIIb high |
| Shehzad and Shaheen [44] | Pakistan | Case report | Describe a case of epidermolytic hyperkeratosis (EHK) with rickets. | A 13-year old female with WSD, started around the age of 5. Scaling of the skin since birth. | Epidermolytic hyperkeratosis (EHK) with rickets | IV high |
| Simsek-Kiper et al. [45] | Turkey | Case series | Report on five patients from 2 unrelated families with SEMDFA (spondyloepimetaphyseal dysplasia Faden-Alkuraya type). | One patient presents with WSD (right genu varum, left genu valgum). | SEMDFA | IV High |
| Smyth [4] | Nigeria | Case report | Describe three cases of windswept deformity. | Three cases of WSD. Two cases with normal development, when suddenly WSD develops. In one case, there is a period of an acute febrile illness (possibly measles) preceding the development of WSD. | Period of epiphyseal instability + stress factor, geographical genetic dysplasia | IV moderate |
| Solagberu [12] | Nigeria | Prospective case series | Determine the varieties of angular deformities of the knee in children, in/around Ilorin, Nigeria. | Ten patients with WSD presented in one year. Age distribution between 2 and 5 years. | One bone diseased, while the other appears to be compensating | IV moderate |
| Teotia et al. [46] | India | Literature review | Report effects of endemic fluoride exposure on metabolic bone disease. | WSD as presentation of bony leg deformity due to high levels of fluoride exposure in drinking water. | Endemic chronic fluoride toxicity | n.a. * |
| Thacher et al. [47] | Nigeria/South Africa | Case report | Three cases of vitamin D-deficiency rickets associated with ichthyosis. | WSD in two patients with ichthyosis and rickets. | Ichthyosis with rickets | IV high |
| Thacher et al. [7] | Nigeria | Literature review | Describe the features of calcium-deficiency rickets. | Bowleg deformity is less specific for active rickets than knock-knee or WSD. | Calcium-deficiency rickets | n.a. * |
| Thacher et al. [9] | Nigeria | Case-control study | Determine whether low dietary calcium intake is associated with rickets in Nigerian children. | Of 123 Nigerian children with rickets: 16 (13%) had WSD. | Calcium-deficiency rickets | IIIb high |
| Thacher et al. [8] | Nigeria | Cohort study | Development of a clinical prediction model for active rickets. | The median age of onset of WSD was 24 months. WSD present in 39 of the 278 cases of active rickets (14%). Children presenting leg deformities over a span of 4 years, 95/736 had WSD (12.9%). |
Rickets | IIb moderate |
| Vatanavicharn et al. [48] | USA | Case report | Radiographic patellar finding in a patient with pseudoachondroplasia (COMP mutation). | At age 5, the patient developed WSD (right genu varum, left valgum). | Pseudoachondroplasia (following COMP mutation) | IV high |
| Weiner et al. [49] | USA | Case series | Characterise the typical orthopaedic findings in pseudoachondroplasia. | Angular deformity of knees: genu valgum n = 35 (22%), genu varum n = 89 (56%), WSD n = 35 (22%). Laxity in all patients. | Pseudoachondroplasia (following COMP mutation) | IV moderate |
| Yilmaz et al. [50] | Croatia | Cohort study | Temporary hemi epiphysiodesis for correction of angular deformities in children with skeletal dysplasia. | One patient with metaphyseal dysplasia had WSD and underwent correction for both varus and valgus deformity. | Metaphyseal dysplasia | Iib moderate |
* n.a.: not applicable, articles classified as “literature review” did not meet the criteria for level of evidence scoring or critical appraisal. Literature reviews did not provide the number of WSD patients included and therefore these numbers are not mentioned in the table for literature reviews.
From the selected articles, a variety of aetiologies for windswept deformity can be brought forward. These can be divided into the following groups:
Rickets and other metabolic disorders;
Skeletal dysplasias and other genetic disorders;
Trauma;
Descriptive articles without the specific underlying disorder.
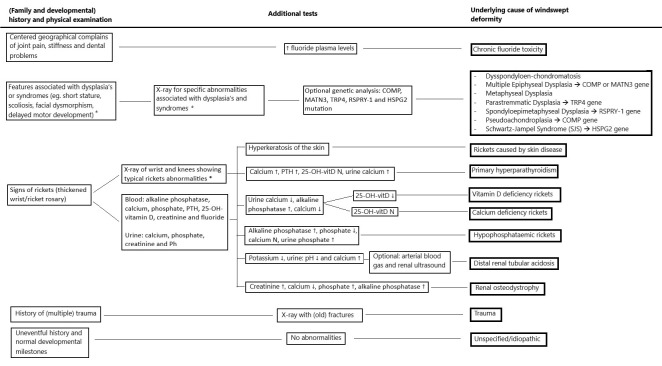
In Table 2, a quantitative summary was made on the demographic data of windswept deformity. A total of 184 patients with windswept deformity were included in Table 2, in 69 cases the gender was described: 45 (65.2%) males and 24 (34.8%) females. Of the 170 patients where the race is described, 108 (63.5%) are of African descent, 11 (6.5%) Asian and 50 (29.4%) Caucasian (37 of the Caucasian patients were retrieved from the same article about pseudoachondroplasia) [49]. One person of Bedouin descent was classified as Middle Eastern (0.6%). Based on the articles found in this study, an overview of the identifying features of each aetiology of windswept deformity is presented in Table 3. Figure 2 shows a flowchart that can be used to find the possible cause of windswept deformity in a child.
Table 2.
Quantitative overview of the demographic data from the studies describing the aetiology windswept deformity *.
| Author | Country of Study | WSD Cases | Male | Female | Suggested Aetiology or Hypothesis | Ethnicity | Onset Age (Months) | Onset Valgus | Onset Varus | Valgus Right | Valgus Left |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akpede et al. [5] | Nigeria | 2 | - | - | Biochemical rickets | African | - | - | - | - | - |
| Al Kaissi et al. [49] | Austria | 1 | 1 | 0 | Schwarz-Jampel Syndrome (SJS) | - | - | - | - | - | 1 |
| Al Kaissi et al. [19] | Austria | 7 | 7 | 0 | X-linked hypophosphataemic rickets | - | 14–18 | - | - | - | - |
| Bar-On et al. [20] | Israel | 1 | 1 | 0 | Renal osteodystrophy (ROD) | - | - | 1 | - | 1 | - |
| Bar-On et al. [14] | Israel | 1 | 0 | 1 | Congenital insensitivity to pain (trauma) | Middle-East | - | - | - | 1 | - |
| Bharani et al. [21] | India | 2 | 2 | 0 | Distal renal tubular acidosis (dRTA) | Asian | 36 | - | - | 2 | - |
| Bhimma et al. [22] | South Africa | 3 | - | - | Vitamin D and/or Ca deficiency rickets | African | - | - | - | - | - |
| Dudkiewicz et al. [23] | Israel | 1 | 1 | 0 | Hypophosphataemic rickets | - | - | - | - | 1 | - |
| Eralp et al. [24] | Turkey | 2 | 1 | 1 | Vitamin D-resistant rickets | - | - | - | - | 2 | - |
| Gigante et al. [25] | Italy | 1 | 1 | 0 | Renal osteodystrophy (ROD) | - | - | - | 1 | 1 | - |
| Ikegawa [27] | Japan | 1 | 1 | 0 | Pseudoachondroplasia | Asian | - | - | - | - | 1 |
| Kenis et al. [30] | Austria | 1 | 1 | 0 | Dysspondyloenchondromatosis (DSC) | Caucasian | - | - | - | 1 | - |
| Kim et al. [31] | Korea | 2 | 2 | 0 | Multiple epiphyseal dysplasia (MED) | Asian | - | - | - | - | - |
| McKeand et al. [32] | USA | 11 | - | - | Pseudoachondroplasia | Caucasian | - | - | - | - | - |
| Nayak et al. [34] | India | 1 | 1 | 0 | Rickets (epidermolytic hyperkeratosis) | - | 36 | - | - | - | - |
| Nishimura et al. [35] | Germany | 1 | 0 | 1 | Parastremmatic dysplasia (with TRPV4 mutation) | African | - | - | - | - | 1 |
| Oginni et al. [37] | Nigeria | 9 | - | - | Ca deficiency rickets | African | - | - | - | - | - |
| Oni and Keswani [38] | Nigeria | 8 | 3 | 5 | Similar to Blount mechanical pressure + illness | African | 6–24 | 1 | 1 | 2 | - |
| Oyemade [1] | Nigeria | 28 | 18 | 10 | Rickets (12) or non-rachitic (15) (3 hypotheses: weight-bearing, dietetic, genetic) | African | 12–108 | - | - | - | - |
| Paruk et al. [39] | South Africa | 1 | 1 | 0 | Primary hyperparathyroidism | African | 150 | - | - | - | 1 |
| Pettifor et al. [41] | South Africa | 1 | 0 | 1 | Ca deficiency rickets | African | - | - | - | 1 | - |
| Prakash et al. [42] | India | 5 | - | - | Nutritional rickets | Asian | 48–120 | - | - | - | - |
| Prentice et al. [43] | Gambia | 1 | - | - | Ca deficiency rickets | African | - | - | - | - | - |
| Shehzad and Shaheen [44] | Pakistan | 1 | 0 | 1 | Rickets (epidermolytic hyperkeratosis) | Asian | 60 | - | - | - | - |
| Simsek-Kiper et al. [45] | Turkey | 1 | 1 | 0 | Spondyloepimetaphyseal dysplasia Faden-Alkuraya type (SEMDFA) | Caucasian | 36–48 | - | - | - | 1 |
| Smyth [4] | Nigeria | 3 | 2 | 1 | Period of epiphyseal instability + stress factor, geographical genetics | African | 24–54 | 3 | - | 1 | 1 |
| Solagberu [12] | Nigeria | 10 | - | - | Compensation for 1 diseased bone | African | 24–60 | - | - | - | - |
| Thacher et al. [47] | Nigeria/South Africa | 2 | 1 | 1 | Ichthyosis with rickets | African | - | - | - | - | - |
| Thacher et al. [8] | Nigeria | 39 | - | - | Rickets | African | - | - | - | - | - |
| Vatanavicharn et al. [48] | USA | 1 | 0 | 1 | Pseudoachondroplasia | Caucasian | 60 | - | - | - | 1 |
| Weiner et al. [49] | USA | 35 | - | - | Pseudoachondroplasia | Caucasian | - | - | - | 1 | - |
| Yilmaz et al. [50] | Croatia | 1 | 0 | 1 | Metaphyseal dysplasia | Caucasian | - | - | - | - | 1 |
| Total | 184 | 45 | 24 | African n = 108 Asian n = 11 Caucasian n = 50 Middle-east n = 1 Missing ethnicity n = 14 |
5 | 2 | 14 | 8 |
* All literature reviews and other overlapping study populations were excluded from this quantitative overview.
Table 3.
An overview of the clinical presentation of each specific aetiology.
| Aetiology Group | Specific Aetiology | Family History | History | Physical Exam | Laboratory Findings | X-Ray | Articles |
|---|---|---|---|---|---|---|---|
| Rickets | Vitamin D deficiency rickets* | Generally uneventful | Decreased exposure to sunlight | Thickened wrists and ankles, rickety rosarymuscle weakness | Alkaline phosphatase ↑ Phosphate ↓/N/↑ Calcium ↓ 25-OH-vitD ↓ 1.25-di-OH-vit D ↓/N/↑ PTH ↑ Urine: Ca ↓ |
Widening of the growth plate and abnormal configuration of the metaphysis:
Anterior rib ends: rachitic rosary |
Bhimma et al. [22], Thacher [7], Iyer et al. [28,29], Gupta et al. [26], Prakash et al. [42] |
| Calcium deficiency rickets* | Generally uneventful | Low-calcium diet | Thickened wrists and ankles, rickety rosary | Alkaline phosphatase ↑ Phosphate ↓ Calcium ↓ 25-OH-vitD N 1.25-di-OH-vit D ↑ PTH ↑ Urine: Ca ↓ |
See above | Bhimma et al. [22], Oginni et al. [37], Prentice et al. [43], Pettifor et al. [41], Gupta et al. [26] | |
| Hypophosphataemic rickets | X-linked (PHEX mutation) or autosomal dominant (FGF23 mutation) transmission | Delayed walking, muscular weakness, bone pain, failure to thrive, tooth abscesses | Thickened wrists and ankles, rickety rosary, dental abnormalities | Alkaline phosphatase ↑ Phosphate ↓ Calcium N 25-OH-vitD N 1.25-di-OH-vitD N/↓ PTH N/↑ FGF23 ↑Urine: phosphate ↑ Genetic testing: PHEX mutation |
See above | Bhimma et al. [22], Al-Kaissi et al. [19], Dudkiewicz et al. [23], Pavone et al. [40], Eralp et al. [24], Gupta et al. [26], Prakash et al. [42] | |
| Skin disease (Epidermolytic hyperkeratosis or Ichthyosis) | Consanguinity and familial inheritance may occur | Bright red blisters after birth. Development of hyperkeratotic plaques | Generalised dry skin, hyperkeratotic and cobble-stone plaques. Rib beading, widening of wrists and ankles | Alkaline phosphatase ↑ Phosphate ↓ Calcium ↓ 25-OH-vitD ↓ PTH ↑ Skin biopsy: hyperkeratosis |
See above | Shehzad and Shaheen [44], Nayak et al. [34], Thacher [47] | |
| Other metabolic | Primary hyperparathyroidism | Generally uneventful | Progressive pain, normal developmental milestones | No abnormalities | Alkaline phosphatase ↑ Phosphate ↓ Calcium ↑ PTH ↑ 25-OH-vitD N 1.25-di-OH-vitD ↑ Urine: Ca ↑ |
See above and a sestamibi scan: increased focal uptake of the parathyroid glands | Paruk et al. [39] |
| Chronic fluoride toxicity | Affected family members, centred geographic distribution of fluoride levels | Mild: generalised bone and joint painmoderate: stiffness and rigiditySevere: flexion deformities at hips and knees | Stiff and rigid spine and joints, flexion deformity hips, knees and elbows, hypo-mineralisation of tooth enamel | Alkaline phosphatase ↑ Phosphate N Calcium N 25-OHD N1-25(OH)2D ↑ PTH ↑ Plasma fluoride ↑ |
Osteosclerosis, periosteal bone formation, calcifications of interosseus membrane, rickets-like metaphyses | Teotia et al. [46] | |
| Distal renal tubular acidosis | Familial inheritance | Sickle cell disease, failure to thrive, polyuria, polydipsia | Low weight/height, frontal bossing, wrist widening (signs of rickets) | ABG: Metabolic acidosis Potassium ↓ Chloride ↑ Urine: pH↓, calcium ↑ Renal ultrasound: nephrocalcinosis |
Osteopenia, angular deformities and signs of rickets | Bharani et al. [21] | |
| Renal Osteodystrophy | Familial inheritance may occur | Bone pain, muscle weakness | Significant growth retardation | Alkaline phosphatase ↑ Phosphate ↑ Calcium ↓ Creatinine ↑ PTH ↑ |
Widening and elongation of the growth plates and cupping of the metaphysis and signs of rickets | Bar-On et al. [20], Gigante et al. [25] | |
| Dysplasia’s and syndromes | Dysspondyloen-chondromatosis | Generally uneventful | Delays in motor development | Neonatal dwarfism, unequal limb length, flat midface with frontal prominence and progressive kyphoscoliosis | No abnormalities | Aniosospondyly and enchondroma-like lesions in the metaphyseal and diaphyseal portions of the long tubular bones | Kenis et al. [30] |
| Multiple Epiphyseal Dysplasia | Familial inheritance may occur | Joint pain, scoliosis, deformities hands, feet, knees and hips | Muscular hypotonia, ligamentous hyperlaxity, abnormal gait, angular deformities at hips and knees | Genetic testing: COMP or MATN3 mutations | COMP: small and round femoral head, MATN3: crescent-shaped femoral head | Kim et al. [31] | |
| Metaphyseal Dysplasia | Consanguinity and familial inheritance may occur | Mental, physical and height development are usually normal | Angular deformities of the knees, palpable widening of the distal femur and clavicles | no abnormalities | Erlenmeyer flask deformity | Yilmaz et al. [50] | |
| Parastremmatic Dysplasia | Generally uneventful | Normal mental milestones, motor development may be slightly delayed, short stature | Windswept and flexural deformity of the legs, scoliosis, platyspondyly | Genetic testing: TRP4 mutation | Flaky metaphyses with wide zones of radiolucencies and flocky calcifications, disorganised epiphyseal ossifications, severe platyspondyly | Nishimura et al. [35,36] | |
| Spondyloepimetaphyseal Dysplasia Faden-Alkuraya type | Parental consanguinity, autosomal recessive inheritance | Difficulty walking, short stature, delayed motor and mental development | Short stature, hypertelorism, brachycephaly, short nose with depressed nasal bridge, tented upper lip, proptosis | Genetic testing: RSPRY-1 mutation | Mild spondylar dysplasia, epi-metaphyseal dysplasia of long bones (flat and irregular epi- and metaphyseal flaring) | Siimsek-kiper et al. [45] | |
| Pseudoachondroplasia | Autosomal dominant inheritance | Normal birthweight and length, At around 2-4yr of age short stature and disproportionately short limbs appear | Short stature, disproportionately short limbs, short and stubby fingers, increased joint laxity, waddling gait | Genetic testing: COMP mutation | Irregular or fragmented epiphyses, flaring, widening or trumpeting of the metaphyses, anterior beaking of vertebrae | Muensterer et al. [33], Vatanavicharn et al. [27], Weiner et al. [49], McKeand et al. [32] | |
| Schwartz-Jampel Syndrome (SJS) | Parental consanguinity and familial inheritance | Normal gestation with severe muscle stiffness at birth | Dysmorphic facial features, trismus | Genetic: HSPG2 gene mutation | Kyphoscoliosis, platyspondyly with coronal clefts in vertebrae, inferior femoral and superior tibial epiphyses look enlarged and distorted | Al-Kaissi et al. [48] | |
| Trauma | Trauma | Generally uneventful | History of fractures | Abnormal gait, signs of bruises and evidence of (old) fractures | Non specific | Evidence of (old) fractures | Bar-On et al. [14] |
* Lab findings in vitamin D deficiency and hypocalcemia rickets depend on the phase of rickets. Phase 1: hypocalcemia causes PTH rise, leading to bone resorption and hyperphosphatemia and rise in alkaline phosphatase. This phase has a relative resistance to PTH. Phase 2: PTH rises further and overcomes the resistance. Calcium rises to normal or slightly lower than normal range and phosphate decreases further (renal excretion due to PTH). Phase 3: hypocalcemia returns worse because of depleted reserves, hypophosphatemia persists and further rise of alkaline phosphatase. In phase 3 X-ray abnormalities become visible. ↓ : lower compared to reference standard. ↑ : higher compared to reference standard.
Figure 2.
Flowchart to find the possible cause of windswept deformity in a child. * See Table 3 for more detailed descriptions.
3.1. Rickets and Other Metabolic Disorders
About half of the articles included reported rickets in patients with windswept deformity (n = 23). In five articles, accounting for a total of 68 patients, the rickets type was unspecified [1,5,6,8,13]. However, in three articles, for a total of eight patients, rickets was found to be (X-linked) hypophosphataemic, due to diminished reabsorption of phosphate in the kidneys [19,23,40]. Nutritional rickets due to vitamin D or calcium deficiency was found in another 12 articles, accounting for another 36 patients with windswept deformity [7,9,10,22,24,26,28,29,37,41,42,43]. In four patients, from three articles, rickets was caused by a skin disorder, namely epidermolytic ichthyosis, which is present at birth [34,44,47]. This adds up to 116 patients with windswept deformity, likely due to rickets of varying types.
A further four types of metabolic disorders were found with windswept deformity at presentation, accounting for an additional six cases. These include primary hyperparathyroidism (PHPT) (n = 1) [39], chronic fluoride toxicity (n = 1) [46], distal renal tubular acidosis (dRTA) (n = 1) [21] and renal osteodystrophy (ROD) (n = 3) [20,25].
3.2. Skeletal Dysplasia and Other Genetic Disorders
Six cases of windswept deformity were found in patients with different types of dysplasia’s: multiple epiphyseal dysplasia (MED) (n = 6) [31], dysspondyloenchondromatosis (DSC) (n = 1) [30], metaphyseal dysplasia (n = 1) [50], parastremmatic dysplasia (with TRPV4 mutation) (n = 1) [35,36] and spondyloepimetaphyseal dysplasia Faden-Alkuraya type (SEMDFA) (n = 1) [45]. These skeletal dysplasias are often characterised by a specific mutation. In MED cases, the mutations are either on the COMP or MATN3 gene, the former also being known to cause pseudoachondroplasia (PSACH), another disease in which windswept deformity can be found. We found 48 patients with PSACH presenting with windswept deformity, from five articles [27,32,33], 36 of which bring confirmed COMP mutations [48,49]. Schwartz-Jampel Syndrome (SJS) was another syndrome in which one patient with windswept deformity has been described [18]. Patients with skeletal dysplasia often present with scoliosis and consequently WSD towards the contralateral side.
3.3. Trauma
In one patient, trauma seemed to be the cause of the windswept deformity [14]. This patient was a young female with a history of fractures, often untreated due to congenital insensitivity to pain, presumably being the cause of the angular deformities of the legs.
3.4. Descriptive Articles without Specific Underlying Disorder
We grouped six articles together with a total of 36 patients, in which different hypotheses for the cause of windswept deformity were brought forward: a combination of mechanical pressure and a period of illness (similar cause as Blount disease) (n = 8) [11,38]; excessive or early weight-bearing, dietic or ethnicity (n = 15) [1,13]; a combination of the previously mentioned factors (n = 3) [4]; compensation, where only one side is diseased, while the other side compensates (n = 10) [12]. All of these hypotheses were (partly) based on mechanical loading or weight-bearing. The patients in these articles were all African and typically presented with windswept deformity between the age of 2 and 3 years, being otherwise healthy. None of these patients showed signs of (healed) rickets.
4. Discussion
This systematic review gives an overview of all previously published aetiology hypotheses for windswept deformity. Windswept deformity is generally limited to an individual with genu valgum on one side, and genu varum on the contralateral side. Further specific phenotypical descriptions vary depending on the aetiology.
Our results display that windswept deformity can be a manifestation of a broad variety of pathologies. However, in patients in whom no underlying illness was found, the deformity was deemed idiopathic, and the following hypotheses are brought forward: weight-bearing (due to excessive weight or early walking) [1,11,13,38], epiphyseal instability [4], stress factors (illness) [4] and geographical genetic factors [1,4,13]. Though these hypotheses are comparable between articles, they lack supporting evidence.
Rickets of different types was found to be the most common pathology manifesting windswept deformity. Although it appears to be the most frequent cause of windswept deformity, windswept deformity is far from the most common presentation of rickets. Thacher [7], presents different clinical features and their utility in predicting radiologically active rickets; only 14% of the children with radiologically active rickets have windswept deformity and the probability that a child with windswept deformity has radiologically active rickets is 41%. Unfortunately, no further research has been conducted on factors influencing the development of windswept deformity, genu valgum or genu varum in children with rickets. Bhimma et al. [22] concluded that mainly vitamin D deficiency is responsible for rickets, however, this may be aggravated by calcium deficiency. These two deficiencies may explain the geographical and ethnic distribution of windswept deformity. As seen in Table 2, most of the patients with windswept deformity were African, and about half of the included studies were performed in sub-Saharan African countries. The diet of rural African children is often low in milk and other dairy products, hence leading to a reduced calcium intake from the diet, despite the fortification of products with calcium [51]. The vitamin D deficiency may be explained by the increased sunlight exposure required for black children [22], or by cultural and/or religious factors that limit the exposure to sunlight, such as covering garments or veils. Hypophosphataemic rickets is most commonly found in its X-linked inheritance form and causes rickets due to its defects in renal handling of phosphorus [52]. Furthermore, epidermolytic ichthyosis is described in windswept patients caused by rickets due to marked hyperkeratosis of the skin [53]. Consequently, there is a decreased synthesis of vitamin D in the epidermis stimulating parathyroid hormone secretion, and a higher risk of rickets [54].
The cases of windswept deformity occurring in patients with other metabolic disorders are often comparable to the pathophysiology behind the different forms of rickets. In calcium deficiency rickets, patients present with secondary hyperparathyroidism as the low calcium levels stimulate the increased production of PTH. Similarly, primary hyperparathyroidism, distal renal tubular acidosis (dRTA) and renal osteodystrophy cause abnormalities in calcium and phosphorus levels. Exposure to high levels of fluoride in drinking water may lead to a decrease in strength by altering the structural integrity of the bone microarchitecture, which possibly leads to skeletal deformities, such as windswept deformity. Teotia et al. describe the difficulty in differentiating calcium-deficiency rickets from fluoride toxicity, and believe that every child presenting with bone disease in areas endemic to fluorosis is a case of skeletal fluorosis until proven otherwise [46].
A variety of skeletal dysplasias have been described in cases of windswept deformity. Often, these dysplasias are caused by a specific mutation, and a positive family history may therefore be present. Additionally, these patients often present additional symptoms, for example, brachydactyly and craniosynostosis for SEMDFA [45]. PSACH is caused by a mutation in the COMP gene and is usually inherited in an autosomal dominant manner. Although PSACH is not usually discovered until the age of 2-3 years, when disproportionate short stature, waddling gait and evidence of increased joint laxity starts to develop, these features should be used to distinguish PSACH as the cause of windswept deformity [49]. Most patients with a syndrome or dysplasia presenting with windswept deformity have other clinical features, such as malformations of the eyes and face, which can be used to identify the underlying syndrome.
Only a single article, by Bar-On et al. [14], describes trauma leading to windswept deformity. The precise location of the fractures is not described, and therefore, it is impossible to conclude whether this case of windswept deformity was due to compensation for one malformed leg, or if the trauma occurred in both legs, leading to the deformity. The child in this single case suffered from congenital insensitivity to pain, and therefore had a higher risk of multi-trauma leading to lower limb deformities.
The remaining articles, in which other hypotheses for windswept deformity were described, explained its development in patients where there is no underlying cause found. We found no explicit evidence of early walking or excessive weight. Although the beforementioned causes were poorly reported, all patients in these studies had comparable stories. All were of African descent, otherwise healthy, with no signs of healed rickets and the age of onset was between 2 and 3 years of age. There might be geographical or genetic factors that could explain the distribution of windswept deformity in the unspecified group.
When a child presents with windswept deformity, the number of possible underlying causes is extensive, and a complete overview is helpful to make the correct diagnosis. Table 3 shows an overview of the identifying features of each aetiology found in this review. To find a possible cause we advise extensive history taking, including family history (to exclude genetic dysplasias or syndromes) and developmental history; detailed physical examination, looking for clinical features suggestive of rickets (thickened wrists and ankles, and/or rickety rosary), or other identifying features associated with dysplasias or syndromes (facial dysmorphisms, ligamentous laxity, deformities at multiple joints, etc.); and additional testing, such as X-rays of the lower limb and wrist, looking for evidence of rickets or other abnormalities which might fit specific skeletal dysplasias, blood panel (alkaline phosphatase, calcium, phosphate, magnesium, PTH, 25-OH-vitamin D, albumin, creatinine and fluoride) and spot urine test (calcium, phosphate, creatinine and Ph) to exclude or identify rickets and/or other metabolic causes. If required, additional blood tests (e.g., chloride, potassium, 1.25-di-OH-vitamin D, arterial blood gas (ABG) and FGF23) can be performed. Genetic analysis may be indicated when a specific dysplasia or syndrome (COMP, MATN3, TRP4, RSPRY-1 and HSPG2 mutation), or hypophosphataemic rickets (PHEX mutation) is suspected. Additionally, a renal ultrasound can be performed in the case of distal renal tubular acidosis or a skin biopsy to confirm hyperkeratosis. However, when the cause of windswept deformity cannot be found it may be multifactorial, including mechanical loading or weight-bearing.
Despite the large number of articles included in this review, only very few focus on the aetiology of windswept deformity, and most articles have a different aim, describing the deformity as an ancillary finding. Additionally, most articles have old publishing dates. On the other hand, most articles occur in low and middle-income countries, which may result in an underestimation of the problem as less research is conducted in low and middle-income countries. Hence, the specific data available on patients with windswept deformity is often limited. Despite not being found in the literature as causes of windswept deformity, logically there are more underlying disorders that can cause windswept deformity, for instance, other skeletal dysplasias or metabolic disorders (e.g., hypomagnesemia or hypo-albuminemia).
5. Conclusions
Currently, the existing evidence on the aetiology of windswept deformity of the knee shows a broad spectrum of underlying causes that may lead to its development. However, when none of these specific causes can be identified, it appears that the aetiology is multifactorial, resting on the hypotheses of weight-bearing, epiphyseal instability, stress factors and geographical genetic factors. Presently, not enough evidence is available to confirm these hypotheses, and more research is necessary. Nevertheless, we have presented an overview, which helps guide clinicians presented with a case of windswept deformity. A thorough (family and developmental) history, followed by physical examination, and additional tests, such as X-rays, blood panels, urine tests, renal ultrasound and genetic analysis, may help identify the underlying disorder.
Acknowledgments
We would like to thank Prosper Moh, orthopaedic surgeon in St. John of God hospital in Duayaw Nkwanta (Ghana) for pitching the idea of a windswept deformity study.
Appendix A
Table A1.
Search terms.
| Database | Search 1 | Search 2 | Search 3 |
|---|---|---|---|
| Pubmed | (windswept deformity) OR (windswept) | (((genu valgum) AND (genu varum)) OR ((genu valgum[MeSH Terms]) AND (genu varum[MeSH Terms]))) OR (combined valgus and varus knee) AND ((humans[Filter]) AND (allchild[Filter])) AND (english[Filter]) | (((((varo-valgum) OR (varovalgum)) OR (genu varo-valgum)) OR (genu varovalgum)) OR (varo-valga)) OR (varovalga) |
| AJOL | (windswept deformity) OR (windswept) | ((genu valgum) AND (genu varum)) OR (combined valgus and varus knee) | (((((varo-valgum) OR (varo valgum)) OR (genu varo-valgum)) OR (genu varo valgum)) OR (varo-valgo)) OR (varovalga) |
| Cochrane | windswept deformity in Title Abstract Keyword OR windswept in Title Abstract Keyword | ((“genu valgum”):ti,ab,kw OR (“genu valgus”):ti,ab,kw) “AND” ((“genu varum”):ti,ab,kw OR (“genu varus”):ti,ab,kw) | (genu varovalgum) OR (genu varo-valgum) OR (varo-valgum) OR (varovalgum) OR (varovalga) OR (varo-valga) |
| Embase | windswept deformity.mp. OR windswept.mp. | valgus knee/AND varus knee/limit to (human and English language) | varo-valgum.mp. OR genu varo-valgum.mp. |
| G-scholar | knee or genu, “windswept deformity” or windswept, -cerebral, -palsy, -osteoarthritis | combined valgus and varus OR “simultaneous valgus and varus” OR “combined varus and valgus” OR “simultaneous varus and valgus” OR “simultaneous varus and valgus” | genu varovalgum |
| WoS | (windswept deformity OR windswept) | (TS = (genu valgum “and” genu varum) OR (TS = (combined valgus and varus knee “OR” simultaneous valgus and varus))) AND LANGUAGE: (English) |
genu varovalgum OR genu varo-valgum OR varovalgum OR varo-valgum OR varo-valga |
Appendix B
Table A2.
Overview Table of Methodological Appraisal and Scoring.
| Author | Study Design | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score | Maximum Score | Percentage | Low/Moderate/High |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akpede et al. [5] | cross-sectional | Y | Y | Y | Y | N | N | Y | Y | 12 | 16 | 75 | high | |||
| Al Kaissi et al. [18] | case report | Y | Y | Y | Y | Y | Y | Y | N | 14 | 16 | 87.5 | high | |||
| Al Kaissi et al. [19] | case series | N | Y | Y | UC | UC | Y | N | UC | Y | NA | 11 | 18 | 61.1 | moderate | |
| Bar-On et al. [20] | case series | Y | UC | UC | Y | UC | Y | Y | Y | Y | NA | 15 | 18 | 83.3 | high | |
| Bar-On et al. [14] | case series | Y | Y | Y | UC | UC | Y | Y | Y | Y | NA | 16 | 18 | 88.9 | high | |
| Bharani et al. [21] | case report | Y | Y | Y | Y | N | NA | N | N | 12 | 14 | 85.7 | high | |||
| Bhimma et al. [22] | case series | Y | Y | Y | UC | UC | Y | N | Y | Y | NA | 14 | 18 | 77.8 | high | |
| Dudkiewicz et al. [23] | case report | N | Y | N | N | N | Y | Y | Y | 8 | 16 | 50 | moderate | |||
| Eralp et al. [24] | case report | Y | N | Y | Y | Y | Y | N | Y | 12 | 16 | 75 | high | |||
| Gigante et al. [25] | case series | Y | Y | UC | Y | UC | N | Y | Y | Y | NA | 14 | 18 | 77,8 | high | |
| Kenis et al. [30] | case report | N | Y | Y | Y | Y | Y | N | Y | 12 | 16 | 75 | high | |||
| Kim et al. [31] | case series | Y | Y | Y | N | UC | Y | Y | NA | N | Y | 13 | 18 | 72.2 | moderate | |
| McKeand et al. [32] | case-control study | Y | UC | Y | Y | Y | N | N | Y | 11 | 16 | 68.75 | moderate | |||
| Nayak et al. [34] | case report | N | Y | Y | Y | Y | Y | N | Y | 12 | 16 | 75 | high | |||
| Nishimura et al. [35] | case series | Y | Y | Y | N | NA | N | N | Y | Y | NA | 10 | 16 | 62.5 | moderate | |
| Oginni et al. [37] | prospective case series | Y | Y | Y | Y | Y | N | N | Y | Y | Y | 16 | 20 | 80 | high | |
| Oni and Keswani [38] | case series | Y | UC | UC | UC | UC | N | Y | NA | Y | NA | 10 | 16 | 62.5 | moderate | |
| Oni et al. [11] | case series | Y | UC | UC | Y | UC | N | Y | NA | N | NA | 9 | 16 | 56.3 | moderate | |
| Oyemade [13] | case series | N | Y | Y | Y | UC | N | Y | Y | Y | NA | 13 | 18 | 72.2 | moderate | |
| Oyemade [1] | case series | Y | Y | Y | Y | UC | N | Y | N | Y | NA | 13 | 18 | 72.2 | moderate | |
| Paruk [39] | case report | Y | Y | Y | Y | Y | Y | Y | Y | 16 | 16 | 100 | high | |||
| Pettifor et al. [41] | case series | N | Y | Y | UC | UC | Y | Y | Y | N | NA | 12 | 18 | 66.7 | moderate | |
| Prakash [42] | prospective cohort | NA | NA | Y | NA | N | Y | Y | Y | Y | Y | Y | 14 | 16 | 87.5 | high |
| Prentice et al. [43] | case-control study | UC | UC | Y | Y | Y | N | Y | Y | 12 | 16 | 75 | high | |||
| Shehzad and Shaheen [44] | case report | Y | Y | Y | Y | Y | Y | N | N | 12 | 16 | 75 | high | |||
| Simsek-Kiper et al. [45] | case series | N | Y | Y | UC | UC | Y | Y | NA | Y | NA | 12 | 16 | 75 | high | |
| Smyth [4] | case report | Y | N | Y | Y | N | N | N | Y | 8 | 16 | 50 | moderate | |||
| Solagberu [12] | prospective case series | Y | UC | Y | Y | UC | N | Y | N | Y | Y | 14 | 20 | 70 | moderate | |
| Thacher et al. [47] | case report | Y | Y | Y | Y | Y | Y | N | Y | 14 | 16 | 87.5 | high | |||
| Thacher et al. [9] | case-control study | Y | Y | Y | Y | Y | N | N | Y | 12 | 16 | 75 | high | |||
| Thacher et al. [8] | cohort study | Y | Y | Y | N | N | N | Y | NA | NA | NA | Y | 10 | 16 | 62.5 | moderate |
| Vatanavicharn et al. [48] | case report | Y | Y | Y | Y | NA | NA | NA | Y | 10 | 10 | 100 | high | |||
| Weiner et al. [49] | case series | N | Y | UC | UC | UC | Y | Y | NA | Y | NA | 11 | 16 | 68.8 | moderate | |
| Yilmaz et al. [50] | cohort study | Y | Y | Y | N | N | Y | Y | Y | Y | UC | Y | 15 | 22 | 68.2 | moderate |
Author Contributions
Conceptualization, N.J.J., R.B.M.D., A.M.W., S.S. and H.M.S.; methodology, N.J.J., R.B.M.D. and H.M.S.; formal analysis, N.J.J. and R.B.M.D.; investigation, N.J.J. and R.B.M.D.; resources, H.M.S. and A.M.W.; writing—original draft preparation, N.J.J. and R.B.M.D.; writing—review and editing, A.M.W., S.S. and H.M.S.; supervision, A.M.W., S.S. and H.M.S.; funding acquisition, H.M.S. and A.M.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oyemade G. Aetiological Factors in Genu Valga, Vara and Varovalga in Nigerian Children. J. Trop. Pediatr. 1975;21:167–172. doi: 10.1093/tropej/21.4.167. [DOI] [PubMed] [Google Scholar]
- 2.Fulford F.E., Brown J.K. Position as a cause of deformity in children with cerebral palsy. Dev. Med. Child Neurol. 1976;18:305–314. doi: 10.1111/j.1469-8749.1976.tb03652.x. [DOI] [PubMed] [Google Scholar]
- 3.Sharma L., Song J., Felson D.T., Cahue S., Shamiyeh E., Dunlop D.D. The Role of Knee Alignment in Disease Progression and Functional Decline in Knee Osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 4.Smyth E.H. Windswept deformity. J. Bone Jt. Surg. Br. 1980;62:166–167. doi: 10.1302/0301-620X.62B2.7364828. [DOI] [PubMed] [Google Scholar]
- 5.Akpede G.O., Solomon E.A., Jalo I., Addy E.O., Banwo A.I., Omotara B.A. Nutritional rickets in young Nigerian children in the Sahel savanna. East Afr. Med. J. 2001;78:568–575. doi: 10.4314/eamj.v78i11.8945. [DOI] [PubMed] [Google Scholar]
- 6.Lambert A., Linglart A. Hypocalcaemic and hypophosphatemic rickets. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32:455–476. doi: 10.1016/j.beem.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Thacher T.D. Calcium-deficiency rickets. Endocr. Dev. 2003;6:105–125. doi: 10.1159/000072773. [DOI] [PubMed] [Google Scholar]
- 8.Thacher T.D., Fischer P.R., Pettifor J. The usefulness of clinical features to identify active rickets. Ann. Trop. Paediatr. 2002;22:229–237. doi: 10.1179/027249302125001525. [DOI] [PubMed] [Google Scholar]
- 9.Thacher T.D., Fischer P.R., Pettifor J.M., Lawson J.O., Isichei C.O., Chan G.M. Case-control study of factors associated with nutritional rickets in Nigerian children. J. Pediatr. 2000;137:367–373. doi: 10.1067/mpd.2000.107527. [DOI] [PubMed] [Google Scholar]
- 10.Pettifor J.M., Thandrayen K., Thacher T.D. Vitamin D. Academic Press; Cambridge, MA, USA: 2018. Vitamin D Deficiency and Nutritional Rickets in Children; pp. 179–201. [Google Scholar]
- 11.Oni O.O., Keswani H., Aganga M.O. Windswept deformity. Arch. Dis. Child. 1983;58:541–543. doi: 10.1136/adc.58.7.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solagberu B.A. Angular Deformities of the Knee in Children. Niger. J. Surg. Res. 2000;2:62–67. doi: 10.4314/njsr.v2i2.12187. [DOI] [Google Scholar]
- 13.Oyemade G.A. The correction of primary knee deformities in children. Int. Orthop. 1981;5:241–245. doi: 10.1007/BF00271077. [DOI] [PubMed] [Google Scholar]
- 14.Bar-On E., Weigl D., Parvari R., Katz K., Weitz R., Steinberg T. Congenital insensitivity to pain. Orthopaedic manifestations. J. Bone Jt. Surg. Br. 2002;84:252–257. doi: 10.1302/0301-620X.84B2.0840252. [DOI] [PubMed] [Google Scholar]
- 15.Persson-Bunke M., Hägglund G., Lauge-Pedersen H. Windswept hip deformity in children with cerebral palsy. J. Pediatr. Orthop. B. 2006;15:335–338. doi: 10.1097/01202412-200609000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Qureshi R., Mattis P., Lisy K., et al. Chapter 7: Systematic reviews of etiology and risk in: Aromataris E. In: Munn Z., editor. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute; Adelaide, Australia: 2017. [(accessed on 14 January 2022)]. Available online: https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- 18.Al Kaissi A., Ganger R., Klaushofer K., Grill F. Windswept deformity in a patient with Schwartz-Jampel syndrome. Swiss Med. Wkly. 2012;142:w13519. doi: 10.4414/smw.2012.13519. [DOI] [PubMed] [Google Scholar]
- 19.Al Kaissi A., Farr S., Ganger R., Klaushofer K., Grill F. Windswept lower limb deformities in patients with hypophosphataemic rickets. Swiss Med. Wkly. 2013;143:w13904. doi: 10.4414/smw.2013.13904. [DOI] [PubMed] [Google Scholar]
- 20.Bar-On E., Horesh Z., Katz K., Weigl D.M., Becker T., Cleper R., Krause I., Davidovits M. Correction of Lower Limb Deformities in Children With Renal Osteodystrophy by the Ilizarov Method. J. Pediatr. Orthop. 2008;28:747–751. doi: 10.1097/BPO.0b013e318186eb99. [DOI] [PubMed] [Google Scholar]
- 21.Bharani A., Manchanda R., Singh R.K., Prashant S. Distal renal tubular acidosis in sickle cell anemia. Saudi J. Kidney Dis. Transplant. 2018;29:1000–1004. doi: 10.4103/1319-2442.239637. [DOI] [PubMed] [Google Scholar]
- 22.Bhimma R., Pettifor J., Coovadia H.M., Moodley M., Adhikari M. Rickets in black children beyond infancy in Natal. S. Afr. Med. J. 1995;85:668–672. [PubMed] [Google Scholar]
- 23.Dudkiewicz I., Schindler A., Ganel A. Elongation of long bones for short stature in patients with hypophosphatemic rickets. Isr. Med. Assoc. J. 2003;5:66–67. [PubMed] [Google Scholar]
- 24.Eralp L., Kocaoglu M., Çakmak M., Ozden V.E. A correction of windswept deformity by fixator assisted nailing. J. Bone Jt. Surgery. Br. Vol. 2004;86:1065–1068. doi: 10.1302/0301-620X.86B7.14923. [DOI] [PubMed] [Google Scholar]
- 25.Gigante C., Borgo A., Corradin M. Correction of lower limb deformities in children with renal osteodystrophy by guided growth technique. J. Child. Orthop. 2017;11:79–84. doi: 10.1302/1863-2548-11-160172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta R., Sinha R., Banerjee S. Nutritional Verses Non Nutritional Rickets. Volume 13. The Institute of Child Health Calcutta; Kolkata, India: 2013. pp. 19–26. [Google Scholar]
- 27.Ikegawa S. Genetic analysis of skeletal dysplasia: Recent advances and perspectives in the post-genome-sequence era. J. Hum. Genet. 2006;51:581–586. doi: 10.1007/s10038-006-0401-x. [DOI] [PubMed] [Google Scholar]
- 28.Iyer P., Diamond F.B., Jr. Shedding Light on Hypovitaminosis D and Rickets. Adv. Pediatr. 2007;54:115–133. doi: 10.1016/j.yapd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Iyer P., Diamond F. Detecting Disorders of Vitamin D Deficiency in Children: An Update. Adv. Pediatr. 2013;60:89–106. doi: 10.1016/j.yapd.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Kenis V., Baindurashvili A., Melchenko E., Ganger R., Grill F., Al Kaissi A. Spinal and extraspinal deformities in a patient with dysspondyloenchondromatosis. Ger. Med. Sci. 2013;11:Doc06. doi: 10.3205/000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim O.H., Park H., Seong M.W., Cho T.J., Nishimura G., Superti-Furga A., Unger S., Ikegawa S., Choi I.H., Song H.R., et al. Revisit of multiple epiphyseal dysplasia: Ethnic difference in genotypes and comparison of radiographic features linked to the COMP and MATN3 genes. Am. J. Med. Genet. A. 2011;155:2669–2680. doi: 10.1002/ajmg.a.34246. [DOI] [PubMed] [Google Scholar]
- 32.McKeand J., Rotta J., Hecht J.T. Natural history study of pseudoachondroplasia. Am. J. Med. Genet. 1996;63:406–410. doi: 10.1002/(SICI)1096-8628(19960517)63:2<406::AID-AJMG16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.Muensterer O.J., Berdon W.E., Lachman R.S., Done S.L. Pseudoachondroplasia and the seven Ovitz siblings who survived Auschwitz. Pediatr. Radiol. 2012;42:475–480. doi: 10.1007/s00247-012-2364-8. [DOI] [PubMed] [Google Scholar]
- 34.Nayak S., Behera S.K., Acharjya B., Sahu A., Mishra D. Epidermolytic hyperkeratosis with rickets. Indian J. Dermatol. Venereol. Leprol. 2006;72:139–142. doi: 10.4103/0378-6323.25641. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura G., Dai J., Lausch E., Unger S., Megarbané A., Kitoh H., Kim O.H., Cho T.-J., Bedeschi F., Benedicenti F., et al. Spondylo-epiphyseal dysplasia, Maroteaux type (pseudo-Morquio syndrome type 2), and parastremmatic dysplasia are caused by TRPV4 mutations. Am. J. Med Genet. Part A. 2010;152:1443–1449. doi: 10.1002/ajmg.a.33414. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura G., Lausch E., Savarirayan R., Shiba M., Spranger J., Zabel B., Ikegawa S., Superti-Furga A., Unger S. TRPV4-associated skeletal dysplasias. Am. J. Med. Genet. Part C Semin. Med. Genet. 2012;160:190–204. doi: 10.1002/ajmg.c.31335. [DOI] [PubMed] [Google Scholar]
- 37.Oginni L.M., Sharp C.A., Badru O.S., Risteli J., Davie M.W.J., Worsfold M., Fischer P.R. Radiological and biochemical resolution of nutritional rickets with calcium. Arch. Dis. Child. 2003;88:812–817. doi: 10.1136/adc.88.9.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oni O.O., Keswani H. Idiopathic or primary windswept deformity: The etiological significance of the radiological findings. J. Pediatr. Orthop. 1984;4:293–296. doi: 10.1097/01241398-198405000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Paruk I.M., Pirie F.J., Motala A.A. Rickets mimicker: A report of two cases of primary hyperparathyroidism in adolescence. J. Endocrinol. Metab. Diabetes S. Afr. 2018;24:1–5. doi: 10.1080/16089677.2018.1546365. [DOI] [Google Scholar]
- 40.Pavone V., Testa G., Iachino S.G., Evola F.R., Avondo S., Sessa G. Hypophosphatemic rickets: Etiology, clinical features and treatment. Eur. J. Orthop. Surg. Traumatol. 2014;25:221–226. doi: 10.1007/s00590-014-1496-y. [DOI] [PubMed] [Google Scholar]
- 41.Pettifor J., Ross F., Travers R., Glorieux F., Deluca H. Dietary calcium deficiency: A syndrome associated with bone deformities and elevated serum 1,25-Dihyroxyvitamin D concentrations. Metab. Bone Dis. Relat. Res. 1981;2:301–305. doi: 10.1016/0221-8747(81)90013-8. [DOI] [Google Scholar]
- 42.Prakash J., Mehtani A., Sud A., Reddy B.K. Is surgery always indicated in rachitic coronal knee deformities? Our experience in 198 knees. J. Orthop. Surg. 2017;25:2309499017693532. doi: 10.1177/2309499017693532. [DOI] [PubMed] [Google Scholar]
- 43.Prentice A., Ceesay M., Nigdikar S., Allen S., Pettifor J. FGF23 is elevated in Gambian children with rickets. Bone. 2008;42:788–797. doi: 10.1016/j.bone.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Shehzad A., Shaheen S. Epidermolytic hyperkeratosis (bullous ichthyosiform erythroderma) with rickets: A case report. J. Pak. Assoc. Dermatol. 2008;18:182–186. [Google Scholar]
- 45.Simsek-Kiper P.O., Taskiran E.Z., Kosukcu C., Urel-Demir G., Akgun-Dogan O., Yilmaz G., Alikasifoglu M. Further delineation of spondyloepimetaphyseal dysplasia Faden-Alkuraya type: A RSPRY1-associated spondylo-epi-metaphyseal dysplasia with cono-brachydactyly and craniosynostosis. Am. J. Med. Genet. A. 2018;176:2009–2016. doi: 10.1002/ajmg.a.40427. [DOI] [PubMed] [Google Scholar]
- 46.Teotia M., Teotia S.P., Singh K.P. Endemic chronic fluoride toxicity and dietary calcium deficiency interaction syndromes of metabolic bone disease and deformities in India: Year 2000. Indian J. Pediatr. 1998;65:371–381. doi: 10.1007/BF02761130. [DOI] [PubMed] [Google Scholar]
- 47.Thacher T.D., Fischer P.R., Pettifor J.M., Darmstadt G.L. Nutritional Rickets in Ichthyosis and Response to Calcipotriene. Pediatrics. 2004;114:e119–e123. doi: 10.1542/peds.114.1.e119. [DOI] [PubMed] [Google Scholar]
- 48.Vatanavicharn N., Lachman R.S., Rimoin D.L. Multilayered patella: Similar radiographic findings in pseudoachondroplasia and recessive multiple epiphyseal dysplasia. Am. J. Med. Genet. A. 2008;146:1682–1686. doi: 10.1002/ajmg.a.32313. [DOI] [PubMed] [Google Scholar]
- 49.Weiner D.S., Guirguis J., Makowski M., Testa S., Shauver L., Morgan D. Orthopaedic manifestations of pseudoachondroplasia. J. Child. Orthop. 2019;13:409–416. doi: 10.1302/1863-2548.13.190066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yilmaz G., Oto M., Thabet A.M., Rogers K.J., Anticevic D., Thacker M.M., Mackenzie W.G. Correction of lower extremity angular deformities in skeletal dysplasia with hemiepiphysiodesis: A preliminary report. J. Pediatr. Orthop. 2014;34:336–345. doi: 10.1097/BPO.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 51.Pettifor J.M., Ross P., Moodley G., Shuenyane E. Calcium deficiency in rural black children in South Africa—A comparison between rural and urban communities. Am. J. Clin. Nutr. 1979;32:2477–2483. doi: 10.1093/ajcn/32.12.2477. [DOI] [PubMed] [Google Scholar]
- 52.Jagtap V.S., Sarathi V., Lila A.R., Bandgar T., Menon P., Shah N.S. Hypophosphatemic rickets. Indian J. Endocrinol. Metab. 2012;16:177–182. doi: 10.4103/2230-8210.93733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice A.S., Crane J.S. StatPearls. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2020. Epidermolytic Hyperkeratosis. [PubMed] [Google Scholar]
- 54.Milstone L.M., Ellison A.F., Insogna K.L. Serum Parathyroid Hormone Level Is Elevated in Some Patients With Disorders of Keratinization. Arch. Dermatol. 1992;128:926–930. doi: 10.1001/archderm.1992.01680170058005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.