Abstract
Background: Alpha-fetoprotein-negative (<20 ng/mL) hepatocellular carcinoma (AFP-NHCC) cannot be easily diagnosed in clinical practice, which may affect early treatment and prognosis. Furthermore, there are no reliable tools for the prediction of AFP-NHCC early recurrence that have been developed currently. The objective of this study was to identify the independent risk factors for AFP-NHCC and construct an individual prediction nomogram of early recurrence of these patients who underwent curative resection. Methods: A retrospective study of 199 patients with AFP-NHCC who had undergone curative resection and another 231 patients with AFP-positive HCC were included in case-controlled analyses. All AFP-NHCC patients were randomly divided into training and validation datasets at a ratio of 7:3. The univariate and multivariate Cox proportional hazards regression analyses were applied to identify the risk factors, based on which the predictive nomogram of early recurrence was constructed in the training dataset. The area under the curve (AUC), calibration curve, and decision curve was used to evaluate the predictive performance and discriminative ability of the nomogram, and the results were validated in the validation dataset. Results: Compared to AFP-positive patients, the AFP-negative group with lower values of laboratory parameters, lower tumor aggressiveness, and less malignant magnetic resonance (MR) imaging features. AST (HR = 2.200, p = 0.009), tumor capsule (HR = 0.392, p = 0.017), rim enhancement (HR = 2.825, p = 0.002) and TTPVI (HR = 5.511, p < 0.001) were independent predictors for early recurrence of AFP-NHCC patients. The nomogram integrated these independent predictors and achieved better predictive performance with AUCs of 0.89 and 0.85 in the training and validation datasets, respectively. The calibration curve and decision curve analysis both demonstrated better predictive efficacy and discriminative ability of the nomogram. Conclusions: The nomogram based on the multivariable Cox proportional hazards regression analysis presented accurate individual prediction for early recurrence of AFP-NHCC patients after surgery. This nomogram could assist physicians in personalized treatment decision-making for patients with AFP-NHCC.
Keywords: hepatocellular carcinoma, alpha-fetoprotein-negative, early recurrence, nomogram
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common histological types of liver cancer, accounting for 90% of primary liver cancers with high morbidity and mortality [1,2]. Surgical resection, liver transplantation, and some locoregional treatment are the main curative treatments for HCC patients in the early stages [3]. Notably, immune checkpoint inhibitors (ICIs) have changed the treatment scenario of unresectable HCC in the last five years [4]. Nonetheless, the prognosis of HCC patients still remains grim due to inefficient diagnosis, especially in early or small HCC and higher rates of early recurrence [5,6].
Alpha-fetoprotein (AFP) is considered a reliable tumor biomarker that is widely used for screening, diagnosing, and monitoring tumor recurrence and metastasis of HCC in daily clinical practice [7,8]. Serum AFP concentration >400 ng/mL is the indication of HCC. A higher AFP concentration is closely correlated with a poorer prognosis, higher aggressiveness of the tumor, and lower response to therapies [9,10]. However, around one-third of HCC patients are defined as AFP-negative HCC (AFP-NHCC) with serum AFP levels < 20 ng/mL, which could affect early diagnosis and treatment [11,12]. Bai et al. [13] reported that patients with AFP-NHCC often had smaller tumor sizes, higher tumor differentiations, and better clinical outcomes. Thus, it is crucial to identify independent risk factors of AFP-negative HCC for the diagnostic and prognostic evaluation in this subgroup.
Several HCC staging systems including the Barcelona Clinic Liver Cancer (BCLC) staging system, Tumor-Node-Metastasis (TNM) staging system, and the Japan Society of Hepatology (JSH) staging system are widely accepted in prognostic evaluation, reasonable treatment option selection, and clinical researches [14]. However, there are varying degrees of defects with these systems in predicting prognosis and providing quantitative risk measures. Some studies have shown that the BCLC score is only suitable for the advanced stages of HCC. As TNM only pays close attention to tumor characteristics rather than liver function, its influence on prognosis remains controversial [15,16]. Therefore, these staging systems are not adequate in predicting early recurrence for AFP-NHCC patients. Novel methods for predicting prognosis and acquiring more precise prognostic information are urgently needed. Recently, the prediction of survival and recurrence of different types of cancers, for instance, urothelial carcinoma, lung cancer, and breast cancer, is convenient with the widespread use of nomograms [17,18,19]. The objective of our study was to identify the independent risk factors among the clinic-pathological factors and MR imaging features, then construct a nomogram for individual prediction of early recurrence in AFP-NHCC patients who underwent curative resection.
2. Materials and Methods
2.1. Patients
This retrospective study was approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital, and the written informed consent of each patient was waived. All HCC patients with preoperative AFP who underwent surgical resection at our institution between January 2015 and December 2018 were retrospectively analyzed. The inclusion criteria of patients were: (a) surgical pathology confirmed HCC with margin-negative; (b) clinicopathologic and follow-up information were complete. (c) the surgical resection was performed within 1 month after MRI; (d) the MR images with good image quality were available. The exclusion criteria included: (1) receipt of preoperative anti-HCC treatment; (2) distant metastasis or other malignant diseases. Overall, 430 patients (358 males and 72 females; median age, 59.00 (51.00–64.00) years) met the inclusion criteria and were included in this study. This study included 199 patients with AFP-negative HCC (AFP-NHCC) and 231 patients with AFP-positive HCC. These AFP-NHCC patients were divided randomly into a training dataset (n = 139; 121 males and 18 females; median age: 59.647 (52.384–68.910) years) and a validation dataset (n = 60; 52 males and 8 females; median age 58.617 (49.76–67.474) years) at a ratio 7:3.
2.2. Follow-Up
All patients were regularly followed up after discharge. Serum AFP levels, liver function tests, and various imaging examinations (ultrasound, contrast-enhanced CT, or MRI) were performed 1 month after surgery to monitor tumor recurrence, and every 3 or 6 months thereafter. Early recurrence was defined as intrahepatic and/or extrahepatic recurrence of HCC within 2 years after surgery. Recurrence-free survival (RFS) was defined as the interval between the date of surgery and the date of tumor recurrence.
2.3. Clinicopathologic Characteristics
Clinicopathologic characteristics were collected from electronic medical records (Table 1), including age, gender, underlying liver disease, Child-Pugh class, total bilirubin (TBIL), direct bilirubin (DBIL), albumin (ALB), aspartate transaminase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), carcinoembryonic antigen (CEA), platelet count (PLT), histologic differentiation, and the status of microvascular invasion, etc.
Table 1.
Baseline characteristics of alpha-fetoprotein (AFP)− and AFP+ patients.
| Characteristic | AFP− (<20 ug/mL) (n = 199) |
AFP+ (≥20 ug/mL) (n = 231) |
p-Value |
|---|---|---|---|
| Patient demographic | |||
| Age | 60.00 (54.00–66.00) | 56.00 (49.00–62.00) | <0.001 |
| Gender | 0.058 | ||
| Male | 173 | 185 | |
| Female | 26 | 46 | |
| Liver disease | 0.262 | ||
| HBV/HCV | 125 | 157 | |
| Other | 74 | 74 | |
| Liver cirrhosis | 0.018 | ||
| Present | 69 | 56 | |
| Absent | 130 | 175 | |
| Ascites | 0.309 | ||
| Present | 25 | 37 | |
| Absent | 174 | 194 | |
| Laboratory parameters | |||
| ALT (IU/L) | 32.00 (21.00–45.00) | 34.00 (24.00–53.00) | 0.114 |
| AST (IU/L) | 33.00 (25.00–48.00) | 38.00 (27.00–55.00) | 0.027 |
| ALB | 42.50 (40.10–45.20) | 42.20 (39.30–44.80) | 0.227 |
| TBIL | 16.40 (11.90–20.80) | 16.30 (13.20–20.80) | 0.459 |
| DBIL | 3.20 (2.30–4.10) | 3.30 (2.50–4.20) | 0.239 |
| CR | 65.00 (58.00–73.00) | 65.0 (57.00–75.00) | 0.708 |
| ALP (IU/L) | 98.00 (78.00–127.25) | 109.00 (89.00–133.00) | 0.002 |
| GGT (IU/L) | 49.00 (32.00–96.00) | 66.00 (39.00–120.00) | 0.002 |
| PLT | 165.00 (126.00–214.00) | 168.00 (122.00–217.00) | 0.966 |
| CA199 | 16.86 (8.70–29.34) | 20.91 (11.62–36.32) | 0.005 |
| CEA | 2.57 (1.58–3.74) | 2.67 (1.78–3.79) | 0.995 |
| Child-Pugh grade | 0.059 | ||
| A | 196 | 221 | |
| B | 3 | 11 | |
| MRI features | |||
| Multifocality | 0.100 | ||
| Solitary | 166 | 178 | |
| Multiple | 33 | 53 | |
| L-max | 0.007 | ||
| ≤5 cm | 118 | 110 | |
| >5 cm | 81 | 121 | |
| Tumor margin | 0.006 | ||
| Smooth | 45 | 29 | |
| Non-smooth | 154 | 202 | |
| Tumor-capsule | 0.183 | ||
| Present | 179 | 198 | |
| Absent | 20 | 33 | |
| Peritumoral enhancement | <0.001 | ||
| Present | 18 | 50 | |
| Absent | 181 | 181 | |
| Rim enhancement | <0.001 | ||
| Present | 35 | 79 | |
| Absent | 164 | 152 | |
| TTPVI | 0.001 | ||
| Present | 99 | 151 | |
| Absent | 100 | 80 | |
| Intra-hemorrhage | 0.152 | ||
| Present | 33 | 51 | |
| Absent | 166 | 180 | |
| Intra-necrosis | 0.401 | ||
| Present | 63 | 82 | |
| Absent | 136 | 149 | |
| Histologic characteristics | |||
| Histologic grade | <0.001 | ||
| Poor | 42 | 92 | |
| Mediate | 149 | 137 | |
| Well | 8 | 2 | |
| Satellite nodules | 0.002 | ||
| Present | 27 | 59 | |
| Absent | 172 | 172 | |
| MVI | <0.001 | ||
| Present | 79 | 131 | |
| Absent | 120 | 100 | |
| Early recurrence | <0.001 | ||
| Present | 75 | 131 | |
| Absent | 124 | 100 |
ALT = Alanine aminotransferase, AST = Aspartate aminotransferase, ALB = Serum albumin, TBIL = Total bilirubin, DBIL = Direct bilirubin, CR = Creatinine, ALP = Alkaline phosphatase, GGT = γ-glutamyl transpeptadase, PLT = Platelet count, CA199 = Carbohydrate antigen199, CEA = Carcinoembryonic antigen, L-max = Maximum tumor length, TTPVI = Two-trait predictor of venous invasion, MVI = Microvascular invasion.
2.4. MRI Analysis
All MR images were interpreted by 2 radiologists with 5 and 8 years of abdominal MRI experiences, respectively. Both radiologists were aware of HCC but blinded to other information. Discrepancies were resolved through discussion until consensus was reached. The radiologists assessed the following MRI features for each patient: (a) multifocality (0, solitary; 1, multiple); (b) maximum tumor length (0, L-max ≤ 5 cm; 1, L-max > 5 cm); (c) tumor margin (0, smooth margin; 1, non-smooth margin); (d) tumor capsule (0, well-defined tumor capsule; 1, ill-defined tumor capsule); (e) peritumoral enhancement (0, absent; 1, present); (f) rim enhancement (0, absent; 1, present); (g) intratumor necrosis (0, absent; 1, present); (h) intratumor hemorrhage (0, absent; 1, present); (i) Two-trait predictor of venous invasion, TTPVI, (0, TTPVI-absent; 1, TTPVI-present). These MRI features of the largest tumor were recorded when the lesions were multifocal.
2.5. Statistical Analysis
Statistical analysis was performed in this study with SPSS (version 26.0, Chicago, IL, USA) and R software (version 4.1.2 (November 2021); http://www.Rproject.org). The t-test and Mann-Whitney U test were performed to compare continuous variables between two independent groups; the Chi-square test was used in categorical variables. The selection of independent prognostic factors with the univariate and multivariate Cox proportional hazards regression analysis. The “survival” package, “rms” package, and the “survivalROC” package were used to construct a multivariate Cox proportional hazards model and plot nomogram, calibration curve, and ROC curve. The decision curve was drawn with a “ggDCA” package. A two-tailed p < 0.05 was considered to be statistically significant.
3. Results
3.1. Patient Characteristics
The baseline clinicopathological characteristics and MR imaging features of all patients in the study were shown in Table 1. Compared to the AFP-positive group, the patients in the AFP-negative group were older and had lower values of laboratory parameters, lower tumor aggressiveness, and less malignant MR imaging features. These differences in patient characteristics between the two groups may affect overall survival. RFS was longer for AFP-negative patients compared with AFP-positive patients, the early recurrence rates were 37.68% versus 56.70%, respectively (p < 0.0001, Figure 1).
Figure 1.
Early recurrence rate of surgically-resected hepatocellular carcinoma (HCC) patients with AFP− vs. AFP+.
3.2. Independent Prognostic Factors of AFP-NHCC Patients
A total of 199 HCC patients with AFP-negative were included in this study, these patients were divided randomly into a training dataset (n = 139, including 50 patients with early recurrence) and a validation dataset (n = 60, including 25 patients with early recurrence) at a ratio 7:3 (Table 2). In the training dataset, univariate Cox proportional hazards regression analysis revealed that AST, CA199, multifocality, tumor margin, tumor capsule, peritumoral enhancement, rim enhancement, and TTPVI were statistically significant predictors of early recurrence (Table 3). All these significant predictors were included in multivariate Cox proportional hazards regression analysis, and the results showed that AST (HR = 1.975, 95%CI: 1.045–3.732, p = 0.036), tumor capsule (HR = 0.422, 95%CI: 0.198–0.900, p = 0.026), rim enhancement (HR = 2.819, 95%CI: 1.267–6.273, p = 0.011), and TTPVI (HR = 11.665, 5%CI: 3.978–34.203, p < 0.001) were independent predictors of early recurrence for AFP-NHCC patients (Table 3).
Table 2.
Baseline characteristics in the training and validation datasets.
| Characteristic | Training Dataset | Validation Dataset | p-Value |
|---|---|---|---|
| n = 139 | n = 60 | ||
| Patient demographics | |||
| Age (y) | 59.647 (9.263) | 58.617 (8.857) | 0.466 |
| Gender | 1.000 | ||
| Female | 18 | 8 | |
| Male | 121 | 52 | |
| Liver disease | 1.000 | ||
| Hepatitis B/C virus | 87 | 38 | |
| Absent | 52 | 22 | |
| Liver cirrhosis | 0.821 | ||
| Present | 47 | 22 | |
| Absent | 92 | 38 | |
| Ascites | 0.654 | ||
| Present | 16 | 9 | |
| Absent | 123 | 51 | |
| Surgical treatment | |||
| Major hepatectomy | 33 | 20 | 0.160 |
| Minor hepatectomy | 106 | 40 | |
| Laboratory factors | |||
| ALB (g/L) > 40, ≤40 | 103, 36 | 49, 11 | 0.331 |
| ALT (IU/L) > 50, ≤50 | 30, 109 | 14, 46 | 0.930 |
| AST (IU/L) > 40, ≤40 | 49, 90 | 18, 42 | 0.578 |
| TBIL (μmol/L) > 19, ≤19 | 50, 89 | 16, 44 | 0.265 |
| DBIL (μmol/L) > 3.4, ≤3.4 | 64, 75 | 25, 35 | 0.678 |
| GGT (IU/L) > 40, ≤40 | 52, 87 | 32, 28 | 0.287 |
| ALP (IU/L) > 125, ≤125 | 38, 101 | 13, 47 | 0.507 |
| CR (μmol/L) > 110, ≤110 | 2, 137 | 0, 60 | 0.873 |
| CEA (ng/mL) > 3.4, ≤3.4 | 42, 97 | 14, 46 | 0.412 |
| CA199(U/mL) > 37, ≤37 | 22, 117 | 8, 52 | 0.14 |
| PT (s) > 13, ≤13 | 4, 135 | 2, 58 | 1.000 |
| PLT (109/L) > 300, ≤300 | 5, 134 | 2, 58 | 1.000 |
| Child-Pugh grade A, B | 137,2 | 59, 1 | 1.000 |
| MRI features | |||
| Multifocality | 0.851 | ||
| Solitary | 115 | 51 | |
| Multiple | 24 | 9 | |
| L-max > 5, ≤5 | 53, 86 | 28, 32 | 0.333 |
| Tumor margin | 1.000 | ||
| Smooth | 31 | 14 | |
| Non-smooth | 108 | 46 | |
| Tumor-capsule | 0.785 | ||
| Present | 124 | 55 | |
| Absent | 15 | 5 | |
| Peritumoral enhancement | 1.000 | ||
| Present | 13 | 5 | |
| Absent | 126 | 55 | |
| Rim enhancement | 0.700 | ||
| Present | 23 | 12 | |
| Absent | 116 | 48 | |
| Intra-hemorrhage | 25, 114 | 8, 52 | 0.547 |
| Intra-necrosis | 41, 98 | 22, 38 | 0.4055 |
| TTPVI | 1.000 | ||
| Present | 70 | 30 | |
| Absent | 69 | 30 | |
| Histologic features | |||
| Histologic grade | 0.758 | ||
| Poorly | 31 | 11 | |
| Moderately | 103 | 46 | |
| Well | 5 | 3 | |
| Satellite nodules | 0.693 | ||
| Present | 115 | 51 | |
| Absent | 24 | 9 | |
| MVI | 0.477 | ||
| Present | 132 | 51 | |
| Absent | 17 | 9 | |
| Early recurrence | 0.547 | ||
| Present | 50 | 25 | |
| Absent | 89 | 35 |
ALT = Alanine aminotransferase, AST = Aspartate aminotransferase, ALB = Serum albumin, TBIL = Total bilirubin, DBIL = Direct bilirubin, CR = Creatinine, ALP = Alkaline phosphatase, GGT = γ-glutamyl transpeptadase, PLT = Platelet count, CA199 = Carbohydrate antigen199, CEA = Carcinoembryonic antigen, L-max = Maximum tumor length, TTPVI = Two-trait predictor of venous invasion, MVI = Microvascular invasion.
Table 3.
Results of univariate and multivariate Cox analyses of the training dataset.
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Patient demographics | ||||
| Age (y) | 1.016 (0.986–1.047) | 0.286 | ||
| Gender | 1.940 (0.698–5.390) | 0.204 | ||
| Female | ||||
| Male | ||||
| Liver disease | 0.930 (0.525–1.646) | 0.803 | ||
| Hepatitis B/C virus | ||||
| Absent | ||||
| Liver cirrhosis | 1.275 (0.696–2.335) | 0.431 | ||
| Present | ||||
| Absent | ||||
| Ascites | 1.904 (0.925–3.919) | 0.081 | ||
| Present | ||||
| Absent | ||||
| Surgical treatment | 2.139 (1.190–3.843) | 0.011 | 1.047 (0.486–1.876) | 0.894 |
| Major hepatectomy | ||||
| Minor hepatectomy | ||||
| Laboratory factors | ||||
| ALB (g/L) > 40, ≤40 | 0.596 (0.329–1.080) | 0.088 | ||
| ALT (IU/L) > 50, ≤50 | 1.440 (0.765–2.711) | 0.258 | ||
| AST (IU/L) > 40, ≤40 | 2.324 (1.333–4.050) | 0.003 | 1.975 (1.045–3.732) | 0.036 |
| TBIL (μmol/L) > 19, ≤19 | 1.368 (0.780–2.399) | 0.275 | ||
| DBIL (μmol/L) > 3.4, ≤3.4 | 1.688 (0.965–2.952) | 0.066 | ||
| GGT (IU/L) > 40, ≤40 | 0.608 (0.348–1.060) | 0.079 | ||
| ALP (IU/L) > 125, ≤125 | 1.002 (1.000–1.005) | 0.069 | ||
| CR (μmol/L) > 110, ≤110 | 0.994 (0.974–1.014) | 0.529 | ||
| CEA (ng/mL) > 3.4, ≤3.4 | 1.209 (0.667–2.190) | 0.532 | ||
| CA199 (U/mL) > 37, ≤37 | 2.237 (1.168–4.284) | 0.015 | 1.023 (0.498–2.101) | 0.950 |
| PT (s) > 13, ≤13 | 1.433 (0.993–2.068) | 0.055 | ||
| PLT (109/L) | 0.998 (0.993–1.002) | 0.337 | ||
| Child-Pugh grade A, B | 2.089 (0.288–15.136) | 0.466 | ||
| MRI features | ||||
| Multifocality | 2.728 (1.503–4.949) | 0.001 | 1.424 (0.726–2.794) | 0.303 |
| Solitary | ||||
| Multiple | ||||
| L-max > 5, ≤5 | 1.228 (0.697–2.163) | 0.477 | ||
| Tumor margin | 3.056 (1.213–7.703) | 0.018 | 1.488 (0.563–3.929) | 0.422 |
| Smooth | ||||
| Non-smooth | ||||
| Tumor-capsule | 0.205 (0.105–0.398) | <0.001 | 0.422 (0.198–0.900) | 0.026 |
| Present | ||||
| Absent | ||||
| Peritumoral enhancement | 3.215 (1.604–6.448) | 0.001 | 1.183 (0.518–2.704) | 0.689 |
| Present | ||||
| Absent | ||||
| Rim enhancement | 6.173(3.438–11.084) | <0.001 | 2.819 (1.267–6.273) | 0.011 |
| Present | ||||
| Absent | ||||
| Intra-hemorrhage | 1.632 (0.853–3.124) | 0.139 | ||
| Intra-necrosis | 1.616 (0.907–2.880) | 0.104 | ||
| TTPVI | 18.061 (6.481–50.333) | <0.001 | 11.665 (3.978–34.203) | <0.001 |
| Present | ||||
| Absent | ||||
| Histologic features | ||||
| Histologic grade | 1.164 (0.756–1.791) | 0.491 | ||
| Poorly | ||||
| Moderately | ||||
| Well | ||||
| Satellite nodules | 1.067 (0.843–1.351) | 0.589 | ||
| Present | ||||
| Absent | ||||
| MVI | 1.139 (0.844–1.535) | 0.395 | ||
| Present | ||||
| Absent | ||||
ALT = Alanine aminotransferase, AST = Aspartate aminotransferase, ALB = Serum albumin, TBIL = Total bilirubin, DBIL = Direct bilirubin, CR = Creatinine, ALP = Alkaline phosphatase, GGT = γ-glutamyl transpeptadase, PLT = Platelet count, CA199 = Carbohydrate antigen199, CEA = Carcinoembryonic antigen, L-max = Maximum tumor length, TTPVI = Two-trait predictor of venous invasion, MVI = Microvascular invasion.
3.3. Construction and Evaluation of Nomogram
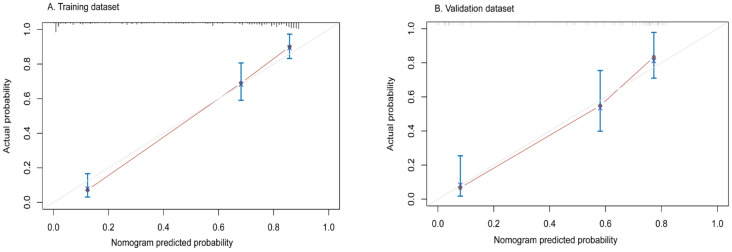
All independent predictors were integrated to develop the nomogram in the training dataset (Figure 2). The area under the curve (AUC) of the receiver operator characteristic (ROC) curve and calibration curve was used to evaluate the performance of the nomogram. The AUCs were 0.89 (95%CI: 0.83–0.94) and 0.85 (95%CI: 0.75–0.94) in the training and validation datasets, respectively (Figure 3). The calibration curve indicated that the predicted probabilities of the nomogram were similar to actual early recurrence probabilities in the datasets (Figure 4). The decision curve showed a higher net benefit for early recurrence in the reasonable threshold probability in the training and validation datasets (Figure 5).
Figure 2.
The recurrence-free survival (RFS) nomogram for HCC patients with AFP negative. The nomogram was scaled by the proportional regression coefficient of each predictor. (AST, Aspartate transaminase; Rim enhancement; Capsule, Tumor capsule; TTPVI, two-trait predictor of venous invasion.
Figure 3.
Receiver operating characteristics curve (ROC) for predicting early recurrence of AFP-NHCC patients in the training and validation datasets.
Figure 4.
Calibration curve in the training (A) and validation (B) datasets. The x-axis typifies the predicted probability of nomogram for early recurrence, y-axis shows the actual early recurrence probability in the patients.
Figure 5.
Decision curve for early recurrence in the training (A) and validation datasets (B). The y-axis is the net benefit; the x-axis measures the threshold probability. All patients with early recurrence (Green dotted line). No patients with early recurrence (Blue dotted line). The expected net benefit of each patient based on the nomogram (Red line).
4. Discussion
In this study, we included a cohort of patients with AFP-NHCC who underwent surgical resection in order to find out the independent risk factors of early recurrence. The results showed that a predictive model derived from AST, tumor capsule, rim enhancement, and TTPVI could be a helpful tool to estimate the probability of early recurrence in this subgroup. It might identify patients with a high risk of early recurrence using our predictive model, for whom liver transplantation, a wider extension of resection, and close follow-up should be considered [20,21]. The nomogram based on the multivariable Cox regression analysis displayed a better predictive performance, with AUC s of 0.89 and 0.85 in the training and validation datasets, respectively. The nomogram demonstrated a higher predicted precision and a better net benefit of early recurrence which were evaluated by the calibration curve and decision curve. The nomogram is a user-friendly graphical tool that can help physicians rapidly compute the probability of early recurrence and make personalized treatment options.
Our nomogram integrated four independent predictive factors for early recurrence, including rim enhancement, tumor capsule, TTPVI, and AST. Some studies have shown that rim enhancement is an important factor for poor prognosis in HCC patients [22,23]. The rim enhancement could be explained by the pathologic features which show the stromal fibrosis in the center of the lesion while the abundant tumoral cellularity in the periphery of the lesion [24]. The rim enhancement indicates infiltrative growth, poor differentiation, and worse prognosis, which may reflect the absence of a tumor capsule and microvascular invasion [25,26]. A tumor capsule is a layer of fibrous structure that limits the aggressiveness and spread of the tumor. Ng et al. reported that encapsulated tumors had a lower incidence of tumor microsatellites and direct liver invasion compared to non-encapsulated ones. Patients with encapsulated tumors tend to have a better prognosis [27]. A tumor capsule is a significant predictive factor of early recurrence in our study, which is in good agreement with the results of a previous study [28]. Segal et al. [29] first discovered that the TTPVI imaging feature might be used for predicting microvascular invasion of HCC. The study showed that TTPVI was associated with a specific HCC molecular profile, which was derived from a venous invasion gene profile related to angiogenesis, cellular proliferation, and matrix invasion. Renzulli et al. [30] confirmed that TTPVI had the same diagnostic accuracy in predicting microvascular invasion on CT and MR imaging. TTPVI can serve as a prognostic marker for HCC after hepatectomy [31]. In our study, TTPVI is also an important risk factor for early recurrence. The AST level is an indicator to activate inflammatory activity which reflects the etiopathogenetic mechanism of hepatocyte necrosis in patients with liver cirrhosis. Cirrhosis and chronic active hepatitis are risk factors for intrahepatic recurrence [32]. Therefore, AST is a significant predictive factor that reflects hepatic inflammation and affects long-term survival [33,34]. The AST level is remarkably associated with early recurrence in multivariate analysis in our study. All HCC patients in our cohort underwent hepatectomy with margin-negative resection confirmed by surgical pathology. Although initial surgical treatment (major or minor hepatectomy) was not an independent risk of early recurrence for AFP-NHCC patients in multivariate Cox analysis. We still think in theory that initial surgical treatment could have an effect on tumor recurrence and overall survival because it was a significant predictor in univariate analysis. It is still controversial for age to be a prognostic factor for HCC patients after hepatectomy [35,36]. In our research, age was significantly different between AFP-negative and AFP-positive groups, the patients in the AFP-negative group were older compared to the AFP-positive group. But the difference in age between recurrence and non-recurrence groups was small in this AFP-negative subgroup. In a word, the characteristics including rim enhancement, tumor capsule, TTPVI, and AST in AFP-NHCC patients should receive adequate attention. The nomogram including the above four predictive factors demonstrated superior discrimination ability of early recurrence in patients with AFP-NHCC. In addition, liver transplantation as an alternative treatment and a shorter interval time of follow-up should be considered for patients with a high risk of early recurrence predicted by the nomogram.
There were several limitations in our study although the nomogram had a better predictive performance. First, it was a retrospective study performed at a single institution, therefore, the selection bias of the predictive nomogram was unavoidable. The prospective studies are needed to further validate this result. Second, the number of patients with AFP-NHCC is limited, and the follow-up time is shorter in our study. Therefore, a larger number of AFP-NHCC patients with five-year follow-up and overall survival time data are required in future studies to verify our results. Third, all patients with AFP-NHCC underwent surgical resection in our study, whether the predictive nomogram would be suitable for patients who received other anti-tumor treatments remains uncertain. Fourth, since the follow-up time is short, it is impossible to evaluate the efficacy of different treatments in patients with tumor recurrence, and it is unknown whether these patients with hepatectomy had a higher overall survival than those who received other treatments, palliative or supportive care.
In conclusion, we used a novel method to construct and validate a nomogram based on the multivariable Cox proportional hazards regression analysis to predict early recurrence in patients with AFP-NHCC after curative resection. The nomogram including rim enhancement, tumor capsule, TTPVI, and AST independent predictive factors displayed a better predictive ability which could assist physicians in personalized treatment decision-making for patients with AFP-NHCC.
Abbreviations
AFP-NHCC: AFP-negative (<20 ng/mL) hepatocellular carcinoma; ER: early recurrence; MR: magnetic resonance; RFS: Recurrence-free survival; AUC: area under curve; ROC: receiver operator characteristic; DCA: decision curve analysis.
Author Contributions
Conceptualization and design: W.L., X.L. and Z.Y.; methodology, W.L., L.H. and B.X.; software and validation, W.L., L.H. and B.X.; formal analysis, investigation and resource W.L., L.H., B.X. and X.L.; writing—original draft preparation, W.L. and L.H.; writing—review & editing, W.L., L.H., B.X., X.L. and Z.Y.; project administration, X.L. and Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding by Breeding Project of National Science Foundation of China, Tianjin Medical University Cancer Institute and Hospital (grant number 210207).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Tianjin Medical University Cancer Institute and Hospital. Approval code: bc2020173.
Informed Consent Statement
Patient consent was waived due to this research was a retrospective study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gao T., Zhi J., Mu C., Gu S., Xiao J., Yang J., Wang Z., Xiang Y. One-step detection for two serological biomarker species to improve the diagnostic accuracy of hepatocellular carcinoma. Talanta. 2018;178:89–93. doi: 10.1016/j.talanta.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Guo S., Chen W., Luo Y., Ren F., Zhong T., Rong M., Dang Y., Feng Z., Chen G. Clinical implication of long non-coding RNA NEAT1 expression in hepatocellular carcinoma patients. Int. J. Clin. Exp. Pathol. 2015;8:5395–5402. [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet J.M., Kelley R.K., Augusto V., Singal A.G., Eli P., Sasan R., Riccardo L., Kazuhiko K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo A., Brandi G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021;27:100328. doi: 10.1016/j.ctarc.2021.100328. [DOI] [PubMed] [Google Scholar]
- 5.Chan A.W., Chan S.L., Wong G.L., Wong V.W., Chong C.C., Lai P., Chan H.L., To K.F. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann. Surg. Oncol. 2015;22:4138–4148. doi: 10.1245/s10434-015-4516-1. [DOI] [PubMed] [Google Scholar]
- 6.Tang A., Hallouch O., Chernyak V., Kamaya A., Sirlin C.B. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom. Radiol. 2018;43:13–25. doi: 10.1007/s00261-017-1209-1. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Galle P.R., Foerster F., Kudo M., Chan S.L., Llovet J.M., Qin S., Schelman W.R., Chintharlapalli S., Abada P.B., Sherman M., et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214–2229. doi: 10.1111/liv.14223. [DOI] [PubMed] [Google Scholar]
- 9.Cucchetti A., Piscaglia F., Grigioni A.D.E., Ravaioli M., Cescon M., Zanello M., Grazi G.L., Golfieri R., Grigioni W.F., Pinna A.D. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: A pilot study. J. Hepatol. 2010;52:880–888. doi: 10.1016/j.jhep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Chan A.W., Zhong J., Berhane S., Toyoda H., Cucchetti A., Shi K., Tada T., Chong C.C., Xiang B.D., Li L.Q., et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018;69:1284–1293. doi: 10.1016/j.jhep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 11.She S., Xiang Y., Yang M., Ding X., Liu X., Ma L., Liu Q., Liu B., Lu Z., Li S., et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int. J. Oncol. 2015;47:543–554. doi: 10.3892/ijo.2015.3042. [DOI] [PubMed] [Google Scholar]
- 12.Wang M., Devarajan K., Singal A.G., Marrero J.A., Dai J., Feng Z., Rinaudo J.A., Srivastava S., Evans A., Hann H.W., et al. The doylestown algorithm: A test to improve the performance of AFP in the detection of hepatocellular carcinoma. Cancer Prev. Res. 2016;9:172–179. doi: 10.1158/1940-6207.CAPR-15-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai D.S., Zhang C., Chen P., Jin S.J., Jiang G.Q. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci. Rep. 2017;7:12870. doi: 10.1038/s41598-017-12834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villanueva A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Rodríguez R., Romero-Gutiérrez M., Artaza-Varasa T., González-Frutos C., Ciampi-Dopazo J.J., de-la-Cruz-Pérez G., Sánchez-Ruano J.J. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev. Esp. Enferm. Dig. 2012;104:298–304. doi: 10.4321/S1130-01082012000600003. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Chen S.W., Liu L.L., Yang X., Cai S.H., Yun J.P. A model combining TNM stage and tumor size shows utility in predicting recurrence among patients with hepatocellular carcinoma after resection. Cancer Manag. Res. 2018;10:3707–3715. doi: 10.2147/CMAR.S175303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Necchi A., Sonpavde G., Vullo S.L., Giardiello D., Bamias A., Crabb S.J., Harshman L.C., Bellmunt J., De Giorgi U., Sternberg C.N., et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: Retrospective international study of invasive/advanced cancer of the urothelium (RISC) Eur. Urol. 2017;71:281–289. doi: 10.1016/j.eururo.2016.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desseroit M.C., Visvikis D., Tixier F., Majdoub M., Perdrisot R., Guillevin R., Cheze Le Rest C., Hatt M. Erratum to: Development of a nomogram combining clinical staging with 18F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1933. doi: 10.1007/s00259-016-3450-1. [DOI] [PubMed] [Google Scholar]
- 19.Su J., Miao L.F., Ye X.H., Cui M.S., He X.F. Development of prognostic signature and nomogram for patients with breast cancer. Medicine. 2019;98:e14617. doi: 10.1097/MD.0000000000014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrer-Fàbrega J., Forner A., Liccioni A., Miquel R., Molina V., Navasa M., Fondevila C., García-Valdecasas J.C., Bruix J., Fuster J. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology. 2016;63:839–849. doi: 10.1002/hep.28339. [DOI] [PubMed] [Google Scholar]
- 21.Tsilimigras D.I., Sahara K., Moris D., Hyer J., Paredes A.Z., Bagante F., Merath K., Farooq A.S., Ratti F., Marques H.P., et al. Effect of Surgical Margin Width on Patterns of Recurrence among Patients Undergoing R0 Hepatectomy for T1 Hepatocellular Carcinoma: An International Multi-Institutional Analysis. J. Gastrointest. Surg. 2020;24:1552–1560. doi: 10.1007/s11605-019-04275-0. [DOI] [PubMed] [Google Scholar]
- 22.Kang H.J., Kim H., Lee D.H., Hur B.Y., Hwang Y.J., Suh K.S., Han J.K. Gadoxetate-enhanced MRI Features of Proliferative Hepatocellular Carcinoma Are Prognostic after Surgery. Radiology. 2021;300:572–582. doi: 10.1148/radiol.2021204352. [DOI] [PubMed] [Google Scholar]
- 23.Cha D.I., Jang K.M., Kim S.H., Kim Y.K., Kim H., Ahn S.H. Preoperative Prediction for Early Recurrence Can Be as Accurate as Postoperative Assessment in Single Hepatocellular Carcinoma Patients. Korean J. Radiol. 2020;21:402–412. doi: 10.3348/kjr.2019.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S.H., Lim H.K., Lee W.J., Choi D., Park C.K. Scirrhous hepatocellular carcinoma: Comparison with usual hepatocellular carcinoma based on CT-pathologic features and long-term results after curative resection. Eur. J. Radiol. 2009;69:123–130. doi: 10.1016/j.ejrad.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura Y., Ikeda K., Seko Y., Hosaka T., Kobayashi M., Saitoh S., Kumada H. Heterogeneous type 4 enhancement of hepatocellular carcinoma on dynamic CT is associated with tumor recurrence after radiofrequency ablation. AJR Am. J. Roentgenol. 2011;197:W665–W673. doi: 10.2214/AJR.11.6843. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura Y., Ikeda K., Hirakawa M., Yatsuji H., Sezaki H., Hosaka T., Akuta N., Kobayashi M., Saitoh S., Suzuki F., et al. New classification of dynamic computed tomography images predictive of malignant characteristics of hepatocellular carcinoma. Hepatol. Res. 2010;40:1006–1014. doi: 10.1111/j.1872-034X.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- 27.Ng I.O., Lai E.C., Fan S.T., Ng M.M. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992;70:45–49. doi: 10.1002/1097-0142(19920701)70:1<45::AID-CNCR2820700108>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Lewis R.H., Glazer E.S., Bittenbinder D.M., O’Brien T., Deneve J.L., Shibata D., Behrman S.W., Vanatta J.M., Satapathy S.K., Dickson P.V. Outcomes following resection of hepatocellular carcinoma in the absence of cirrhosis. J. Gastrointest. Cancer. 2019;50:808–815. doi: 10.1007/s12029-018-0152-x. [DOI] [PubMed] [Google Scholar]
- 29.Segal E., Sirlin C.B., Ooi C., Adler A.S., Gollub J., Chen X., Chan B.K., Matcuk G.R., Barry C.T., Chang H.Y., et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 2007;25:675–680. doi: 10.1038/nbt1306. [DOI] [PubMed] [Google Scholar]
- 30.Renzulli M., Brocchi S., Cucchetti A., Mazzotti F., Mosconi C., Sportoletti C., Brandi G., Pinna A.D., Golfieri R. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology. 2016;279:432–442. doi: 10.1148/radiol.2015150998. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Zhang X., Li Z., Xie C., Qin S., Yan M., Ke Q., Jin X., Lin T., Zhou M., et al. Two-trait predictor of venous invasion on contrast-enhanced CT as a preoperative predictor of outcomes for early-stage hepatocellular carcinoma after hepatectomy. Front. Oncol. 2021;11:688087. doi: 10.3389/fonc.2021.688087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarao K., Takemiya S., Tamai S., Sugimasa Y., Ohkawa S., Akaike M., Tanabe H., Shimizu A., Yoshida M., Kakita A. Relationship between the recurrence of hepatocellular carcinoma (HCC) and serum alanine aminotransferase levels in hepatectomized patients with hepatitis C virus-associated cirrhosis and HCC. Cancer. 1997;79:688–694. doi: 10.1002/(SICI)1097-0142(19970215)79:4<688::AID-CNCR5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Obulhasim G., Yasen M., Kajino K., Mogushi K., Tanaka S., Mizushima H., Tanaka H., Arii S., Hino O. Up-regulation of dbpA mRNA in hepatocellular carcinoma associated with metabolic syndrome. Hepatol. Int. 2013;7:215–225. doi: 10.1007/s12072-012-9357-4. [DOI] [PubMed] [Google Scholar]
- 34.Li C.X., Zhang H., Wu X.F., Han S., Jiao C.Y., Wang D., Wang K., Li X.C. Clinical efficacy and prognostic factors analysis following curative hepatectomy for hepatocellular carcinoma patients with different China Liver Cancer Staging. Chin. J. Surg. 2021;59:134–143. doi: 10.3760/cma.j.cn112139-20200803-00605. [DOI] [PubMed] [Google Scholar]
- 35.Ng K.K., Cheng N.M., Huang J., Liao M., Chong C.C., Lee K.F., Wong J., Cheung S.Y., Lok H.T., Fung A.K., et al. Development and validation of a novel nomogram predicting 10-year actual survival after curative hepatectomy for hepatocellular carcinoma. Surgeon. 2021;19:329–337. doi: 10.1016/j.surge.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Tan J.T., Zhao C., Peng N.F., Yang Y., Zhong J.H., Yang T., Zheng M.H., Wang Y.Y., Gong W.F., Xiang B.D., et al. Association between age and overall survival of patients with hepatocellular carcinoma after hepatic resection. J. Surg. Oncol. 2016;114:966–970. doi: 10.1002/jso.24434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.