Abstract
Two groups’ research initiated to address two very different questions on problems of basic chemical research led, after much effort on catalyst development and studies of reaction mechanism, to the discovery and development of the coupling of amines with aryl halides in a practical fashion.
In the early 1990’s our groups at MIT and Yale were independently conducting experiments that would, ultimately, lead to the development of methods for the palladium-catalyzed formation of carbon-nitrogen bonds from aromatic and heteroaromatic electrophiles.[1–6] While these same connections had previously been forged by the venerable Ullmann and Goldberg coupling reactions,[7–8] a significant need existed for reactions that were more predictable and broader in scope, and that would reliably form products from complicated substrates under mild conditions. The latter need was particularly important for discovery groups in the pharmaceutical industry, given the complexity and oftentimes fragility of the molecules that chemists in this field study.
Both groups had encountered a seminal paper published in 1983 by Kosugi and Migita that described the use of a palladium catalyst, ligated by tri(ortho-tolyl) phosphine that enabled the conversion of diethylamino(tri-n-butyl stannane) and a limited number of aryl bromides to form the corresponding diethylanilines (an “amino-Stille” reaction).[9] While the results of this paper were intriguing, they led both groups to ask many questions about the mechanism and applicability of the results presented. To our both of their surprise, the paper had rarely been cited in the primary chemical literature during the ten years, up to 1993,[10–13] outside of the authors subsequent work (Although it had been mentioned in a small number of review articles).
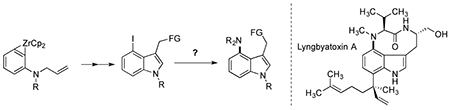
The MIT group had published a series of papers on the generation and use of zirconocene complexes of strained molecules, including benzynes, in carbon-carbon bond-forming reactions.[14] In one application, a suitably substituted substrate could be elaborated to a 3-iodomethyl-4-iodo indoline derivative, a precursor to highly substituted indoles, including Lyngbyatoxin[15] and the Teleocidins.[16] An amino-Stille reaction could install the amine at the 4-position directly from precursors available using their benzyne methodology. The facility of a synthesis of these complex molecules by the zirconocene chemistry, in combination with an amino-Stille reaction made clear the potential synthetic value of this undeveloped class of coupling reaction.
Contemporaneously, the Yale group was seeking to identify organometallic complexes that would form carbon-heteroatom bonds by unusual elementary reactions,[17] and studies of the mechanism of the transformation described in the Kosugi-Migita paper could reveal such reactions. They wondered whether the coupling reaction to form a carbon-nitrogen bond proceeded by a mechanistic pathway analogous to that followed by the better-studied palladium-catalyzed carbon-carbon bond-forming reactions (e.g., Stille, Suzuki-Miyaura or Negishi couplings).[18–23] If so, the product-forming step would involve the formation of an arylpalladium amido intermediate and an unknown reductive elimination from such a complex to form the carbon-nitrogen bond of an arylamine.[24–25] If this were the mechanism, why was the coupling reaction so rare? What factors controlled whether such a reductive elimination process occurred readily or under forcing conditions and what prevented a more common alternative pathway A→B involving β-hydrogen elimination to dominate?[26–27]

Neither group foresaw a day when palladium-catalyzed (or the Ni-catalyzed processes that both groups also studied in the 1990’s) couplings to form carbon-nitrogen bonds would become indispensable, everyday tools used by organic chemists.[28,29] Thus, this example, among countless others, shows how curiosity-driven basic research ultimately leads to practical science (in this case chemistry) that is widely employed throughout the world. At the same time, this example shows the importance of a sustained effort and financial support for a sustained effort. The process with catalysts that people now use did not come easily. It took many years, actually decades, of sequential improvements in catalysts and mechanistic studies to understand the effects of the catalyst structural features on reactivity to gain widespread use. The reaction and catalysts published by our groups initially would not form a majority of the products that people are currently making with this class of reaction. By expending the time and effort to understand the fundamental processes that form the basis of the catalytic systems that facilitated C–N coupling, we and others have been able to develop new and improved catalysts.[30–36] These catalysts, in turn, have greatly increased the range of substrates and applications that are suitable for the methodology that has been developed.
Over the past 25 years, both of our groups have used a combination of the elements of physical organic, structural and synthetic organic chemistry within the catalytic systems to learn about fundamental processes of interest to used. Given the original motivation for our studies on coupling reactions that form carbon-nitrogen bonds and the years of research required to make the reaction more predictable and reliable, we take this opportunity to emphasize our belief that the support of basic, curiosity-driven research is absolutely necessary for advancements in science and technology that drive economic progress. We implore those holding the purse strings to increase funding opportunities for research that addresses fundamental questions to which we currently do not have answers.
Acknowledgements
We thank our dedicated students and postdoctoral coworkers for their hard work, perseverance and creative input that led to the results for which we have received the Wolf Prize. We also acknowledge the National Science Foundation (initial funding to SLB), Department of Energy (initial funding to JFH for the fundamental organometallic chemistry), and the National Institutes of Health (long-term funding to SLB and JFH) for generous financial support of this work. SLB also thanks Dr. Christine Nguyen for help in preparing this manuscript.
References
- [1].Paul F, Patt J, Hartwig JF, J. Am. Chem. Soc 1994, 116, 5969–5970. [Google Scholar]
- [2].Louie J, Hartwig JF, Tetrahedron Lett. 1995, 36, 3609–3612. [Google Scholar]
- [3].Driver MS, Hartwig JF, J. Am. Chem. Soc 1996, 118, 7217–7218. [Google Scholar]
- [4].Guram AS, Buchwald SL, J. Am. Chem. Soc 1994, 116, 7901–7902. [Google Scholar]
- [5].Guram AS, Rennels RA, Buchwald SL, Angew. Chem. Int. Ed. Engl 1995, 34, 1348–1350. [Google Scholar]
- [6].Wolfe JP, Wagaw S, Buchwald SL, J. Am. Chem. Soc 1996, 118, 7215–7216. [Google Scholar]
- [7].Beletskaya IP, Cheprakov AV, Coord. Chem. Rev 2004, 248, 2337–2364. [Google Scholar]
- [8].Bhunia S, Pawar GG, Kumar SV, Jiang Y, Ma D, Angew. Chem. Int. Ed 2017, 56, 16136–16179; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 16352–16397. [Google Scholar]
- [9].Kosugi M, Kameyama M, Migita T, Chem. Lett 1983, 927–928. [Google Scholar]
- [10].Tunney SE, Stille JK. J. Org. Chem 1987, 52, 748–753. [Google Scholar]
- [11].Hatanaka Y, Hiyama T, J. Org. Chem 1988, 53, 918–920. [Google Scholar]
- [12].Bumagin NA, Gulevich YV, Beletskaya IP, Bull. Acad. Sci. USSR Div. Chem. Sci. (Engl. Transl.) 1984, 33, 879–879. [Google Scholar]
- [13].Scott WJ, Stille JK, J. Am. Chem. Soc 1986, 108, 3033–3040. [Google Scholar]
- [14].Broene R, Buchwald S, Science 1993, 261, 1696–1701. [DOI] [PubMed] [Google Scholar]
- [15].Fujiki H, Mori M, Nakayasu M, Terada M, Sugimura T, Moore RE. Proc. Natl. Acad Sci. USA 1981, 78, 3872–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakamura H, Yasui K, Kanda Y, Baran PS, J. Am. Chem. Soc 2019, 141, 1494–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hartwig JF, Nature 2008, 455, 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ozawa F, Ito T, Nakamura Y, Yamamoto A, Bull. Chem. Soc. Jpn 1981, 54, 1868–1880. [Google Scholar]
- [19].Ozawa F, Yamamoto A, Nippon Kagaka Kaishi 1987, 5, 773–784. [Google Scholar]
- [20].Ozawa F, Kurihara K, Fujimori M, Hidaka T, Toyoshima T, Yamamoto A, Organometallics 1989, 8, 180–188. [Google Scholar]
- [21].Gillie A, Stille JK, J. Am. Chem. Soc 1980, 102, 4933–4941. [Google Scholar]
- [22].Moraviskiy A, Stille JK. J. Am. Chem. Soc 1981, 103, 4182–4186. [Google Scholar]
- [23].Jutand A, J. Organomet. Chem 1999, 576, 254–278. [Google Scholar]
- [24].Driver MS, Hartwig JF. J. Am. Chem. Soc 1995, 117, 4708–4709. [Google Scholar]
- [25].Driver MS, Hartwig JF. J. Am. Chem. Soc 1997, 119, 8232–8245. [Google Scholar]
- [26].Hartwig JF, J. Am. Chem. Soc 1996, 118, 7010–7011. [Google Scholar]
- [27].Hartwig JF, Richards S, Barañano D, Paul F, J. Am. Chem. Soc 1996, 118, 3626–3633. [Google Scholar]
- [28].Brown DG, Boström J, J. Med. Chem 2016, 59, 4443–4458. [DOI] [PubMed] [Google Scholar]
- [29].Ruiz-Castillo P, Blackmond DG, Buchwald SL, J. Am. Chem. Soc 2015, 137, 3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ingoglia BT, Wagen CC, Buchwald SL, Tetrahedron 2019, 75, 4199–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gildner PG, Colacot TJ, Organometallics 2015, 34, 5497–5508. [Google Scholar]
- [32].Dorel R, Grugel CP, Haydl AM, Angew. Chem. Int. Ed 2019, 58, 17118–17129. [DOI] [PubMed] [Google Scholar]
- [33].Hartwig JF, Acc. Chem. Res 2008, 41, 1534–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grasa GA, Viciu MS, Huang JK, Nolan SP, J. Org. Chem 2001, 66, 7729. [DOI] [PubMed] [Google Scholar]
- [35].Valente C, Pompeo M, Sayah M, Organ MG, Org. Process Res Dev 2014, 18, 180–190. [Google Scholar]
- [36].Rataboul F, Zapf A, Jackstell R, Harkal S, Riermeier T, Monsees A, Dingerdissen U, Beller M, Chem. Eur. J 2004, 10, 2983. [DOI] [PubMed] [Google Scholar]


