Abstract
Similar to other malignancies, TCGA network efforts identified the detailed genomic picture of skin melanoma, laying down the basis of molecular classification. On the other hand, genome-wide association studies discovered the genetic background of the hereditary melanomas and the susceptibility genes. These genetic studies helped to fine-tune the differential diagnostics of malignant melanocytic lesions, using either FISH tests or the myPath gene expression signature. Although the original genomic studies on skin melanoma were mostly based on primary tumors, data started to accumulate on the genetic diversity of the progressing disease. The prognostication of skin melanoma is still based on staging but can be completed with gene expression analysis (DecisionDx). Meanwhile, this genetic knowledge base of skin melanoma did not turn to the expected wide array of target therapies, except the BRAF inhibitors. The major breakthrough of melanoma therapy was the introduction of immune checkpoint inhibitors, which showed outstanding efficacy in skin melanoma, probably due to their high immunogenicity. Unfortunately, beyond BRAF, KIT mutations and tumor mutation burden, no clinically validated predictive markers exist in melanoma, although several promising biomarkers have been described, such as the expression of immune-related genes or mutations in the IFN-signaling pathway. After the initial success of either target or immunotherapies, sooner or later, relapses occur in the majority of patients, due to various induced genetic alterations, the diagnosis of which could be developed to novel predictive genetic markers.
Keywords: skin melanoma, genomics, molecular pathology, prognostic and predictive markers
1. Introduction
Pathological diagnostics of cutaneous melanoma was established in the past decades and mostly based on histopathological characteristics completed with a relatively simple immunohistochemical marker set. However, this situation profoundly changed recently: the widespread dermatological melanoma screening programs detect premalignant lesions with a much higher frequency, which requires highly sensitive molecular tests to prove malignancy. On the other hand, the genetic background of hereditary melanoma became more and more complex, again defining the need for more complex genomic testing. Last but not least, since cutaneous melanoma is the most metastatic human cancer but detected at earlier stages, there is a clinical need of more precise prognostication, which can be based on newly developed genetic tests. Meanwhile, the identification of driver genes of skin melanoma helped to develop target therapies for what predictive genetic characterization became an everyday practice. However, the most effective therapy of skin melanoma is immunotherapy; unfortunately, its predictive markers have not yet entered clinical practice, although our knowledge of the immunogenomic characteristics of skin melanoma has increased enormously in the past years. In this review, we briefly summarize our basic knowledge on skin melanoma genomics, with the aim to show its clinicopathological relevance and highlight those areas where, although we have the required genetic knowledge, its introduction into clinical practice is urgently needed.
2. Molecular Epidemiology
Malignant melanoma is one of the most metastatic human cancers where a T1 sub-millimeter sized primary tumor of a ~106 cell population can have a significant metastatic potential, compared to most solid cancers, where a ten-times larger but similar T1 tumor of a population of 109 cells may not have it. Due to the novel lifestyles and global atmospheric changes, UV exposure of the skin has increased gradually, resulting in a paralleled increase in the incidence of melanoma [1]. In most European countries, melanoma can be found among the ten most frequent malignancies [1], and its prominent metastatic potential presents a significant burden for healthcare providers.
Malignant melanoma can develop from benign nevi or de novo. Considering the high incidence of benign nevi, the malignant transformation potential of these lesions is fortunately low. Meanwhile, nevi carry, at high frequency, the signature UV-induced mutation of BRAF (v-Raf murine sarcoma viral oncogene homolog B1) at exon 15, providing evidence of the etiological factor behind [2]. Malignant melanoma, however, can develop on non-UV-exposed skin, mucosal epithelium or uvea, and these melanoma types usually lack the characteristic BRAF mutation.
Both skin and uveal melanoma can have familial form, but their genetic background is different. Besides the loss of CDKN2A (cyclin-dependent kinase inhibitor 2A), germline mutations of CDK4 (cyclin-dependent kinase 4), MITF (microphtalmia-associated transcription factor) and BAP1 (BRCA1-associated protein 1) are the most significant contributors for hereditary melanoma [3]. However, the picture became more complex with the discovery of germline alterations of the pigmentation-related and DNA-repair-related genes in the development of melanoma. As far as the pigmentation-related genetic factors are concerned, besides MITF mutations, the alterations of MITF-regulated MC1R (melanocortin-1 receptor), SLC45A2 (solute carrier family 45 member 2) and OCA2 (oculocutaneous albinism type 2) genes, as well as those of the melanosomal TYR (tyrosinase) and TYRP1 (tyrosinase-related protein 1) and DNA repair gene defects of TERT (telomerase reverse transcriptase) and APEX1 (apurinic/apyrimidinic endodeoxyribonuclease 1), are also significant contributors, increasing the risk of melanoma development. Furthermore, novel germline alterations at the chromosomal region of 1q21.3 involving ARNT (aryl hydrocarbon receptor nuclear translocator) and SETDB1 (SET domain bifurcated histone lysine methyltransferase 1) were discovered lately as possible genetic risk factors for melanoma [3,4,5,6].
It is worth mentioning that, in the case of uveal melanoma, inherited homologous recombination or mismatch repair deficiencies due to PALB2 (partner and localizer of BRCA2) or MLH1 (MutL homolog 1) are the primary causes for heritability [7].
3. Molecular Classification
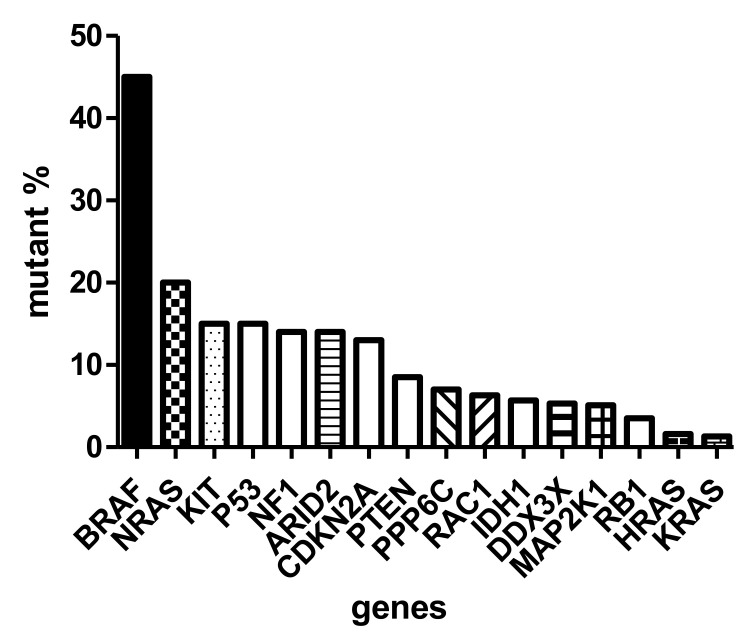
The sequencing of thousands of malignant melanomas worldwide defined the atlas of genomics of melanoma [8]. These analyses revealed that the most frequent gene defect of skin melanoma is the activating mutation of BRAF oncogene in exon 15/codon 600, characterizing almost half of these tumors. At a significantly lower frequency (~20%), the NRAS (neuroblastoma RAS viral (v-ras) oncogene homolog) oncogene is mutated in melanoma in exon 3/codon 61. Interestingly, almost with similar frequency (<15%) the KIT (KIT proto-oncogene, receptor tyrosine kinase) gene is also mutated in melanoma [9] (Figure 1). It has to be emphasized that the KIT receptor signaling pathway, containing NRAS and BRAF, is the dominating pathway in melanocytes (and evidently in melanoma), and it is responsible for activating the melanocyte-specific transcription factor MITF. As in other cancers, several oncosuppressor genes are mutated in melanoma, including TP53 (tumor protein p53), NF1 (neurofibromin 1), CDKN2A and PTEN (phosphatase and tensin homolog), at a similar relatively low frequency (~15%) (Figure 1). However, there are also genome-wide copy number alterations in melanoma: amplification affects the melanoma oncogenes, as well as CCND1 (cyclin D1) and MITF, while loss of heterozygosity (LOH) or complete loss may affect CDKN2A (p16) and PTEN [1]. Moreover, at much lower frequencies, chromosomal rearrangements affecting (beside PTEN) the kinase receptors ALK (anaplastic lymphoma kinase), RET (ret proto-oncogene) and NTRK (neurotrophic tyrosine receptor kinase) can also be detected [2].
Figure 1.
Mutation spectrum of driver genes (oncogenes and suppressor genes) of skin melanoma based on TCGA (The Cancer Genome Atlas).
Similar to the traditional histological subclassification of melanoma, today the molecular classification is also possible where there are four major categories, the BRAF-mutant, the RAS-mutant, the NF1-mutant and the so-called triple wild-type forms (Table 1) [8]. It is now evident that a cancer is characterized by a relatively well-described set of driver oncogenes. Accordingly, the BRAF-mutant melanoma is a p16-lost or negative tumor, where TP53 mutations are relatively rare, but this is the form where the MITF and PD-L1 (programmed death ligand 1) genes are amplified. RAS-mutant melanomas are different from BRAF-mutant ones because neither MITF nor PD-L1 are amplified, but TP53 is more frequently mutated. The NF1-mutant melanoma can be called a suppressor gene melanoma, since, besides NF1, CDKN2A, RB1 (retinoblastoma 1) and TP53 genes are all mutant. Last but not least, the so-called triple wild-type melanomas are TP53-wild-type but carry mutations of MDM2 (mouse double minute 2 homolog) and CCND1 [10]. All four molecular subtypes are characterized by IDH1 (isocitrate dehydrogenase 1) mutation involved in epigenetic regulation, while ARID2 (AT-rich interaction domain 2) is wild type only in the triple wild-type form but mutated in the other subclasses, resulting in disturbances in chromatin remodeling and transcriptional control. It is another difference that the AURKA (Aurora kinase A) inhibitor PPP6C (protein phosphatase 6 catalytic subunit) gene is mutated in BRAF- and RAS-mutant subclasses exclusively.
Table 1.
Molecular subtypes of melanoma [8].
| BRAF-Mutant | RAS-Mutant | NF1-Mutant | Triple Wild-Type | |
|---|---|---|---|---|
| MAPK Signaling | + | + | + | − |
| Cell cycle |
CDKN2Amut 60% CDK4mut rare |
CDKN2Amut 70% CDK4mut rare CCND1amp 10% |
CDKN2Amut 70% RB1mut 10% |
CDKN2Amut 40% CDK4amp 15% CCND1amp 10% |
| DDR | TP53mut 10% | TP53mut 20% | TP53mut 30% | MDM2amp 15% |
| Epigenetics |
ARID2mut 15% IDH1mut |
ARID2mut 15% IDH1mut |
ARID2mut 30% IDH1mut |
IDH1mut |
| Others |
PPP6Cmut 10% PD-L1amp MITFamp |
PPP6Cmut 15% |
amp, amplification; DDR, DNA damage response; mut, mutation.
The most frequent histological variant of skin melanoma is the superficial spreading melanoma (SSM) type. Other frequent variants are nodular melanoma (NM), acral lentiginous melanoma (ALM) and lentigo maligna melanoma (LMM). It is interesting that, in SSM or NM histological forms, the mutation order is BRAF > NRAS > KIT, while in the (acral-)lentiginous forms, the order of oncogene mutation frequency is KIT > BRAF > NRAS. Furthermore, ALM is characterized by chromosomal instability and a low mutational burden. There are rare histological variants of melanoma, with unique molecular signatures. The driver oncogene of deep penetrating melanoma is GRIN2A (N-methyl-D-aspartate receptor glutamate ionotropic receptor NMDA type subunit 2A), while the nevus-like melanoma is characterized by mutations of the lipid/AKT signaling pathway. A rare histological variant is the desmoplastic melanoma arising on chronic sun-damaged skin and is uniquely characterized by NFKBIE (NFKB inhibitor epsilon) promoter mutation, rare types of BRAF mutation and high tumor mutational burden (TMB) [11]. The blue nevus melanoma is a prototype of CDKN2A-lost tumor. As compared to these variants of (skin) melanoma, uveal melanoma is characterized by genetic alterations of the melanocortin receptor-1 signaling due to the mutations of GNAQ and GNA11 (guanine nucleotide-binding protein alpha subunit q and alpha subunit 11) genes [2].
4. Molecular Diagnostics
The identification of melanocytic lesions is based on specific markers of melanocytes which all associate with melanosomes not expressed by any other cell linages. Maturation of melanosomes is a four-step process, where lipid membranes of this organelle begin to contain melanosome-specific protein Pmel17/gp100, after which tyrosinase enzyme will be synthesized later, together with dopachrome tautomerase enzyme, and ultimately the organelle will contain MART-1/MelanA [12]. Based on this, the identification of melanocytic cells can be performed by immunohistochemistry detecting melanosomal proteins Pmel17/gp100, MART-1, or tyrosinase. Since the transcription of these genes is controlled by melanocytic MITF and SOX10 (Sry-related HMg-Box gene 10), the immunohistochemical detection of these transcription factors can also be used as a specific melanocytic marker. There is also a widely used, less specific protein marker of melanocytes, S100B (S100 calcium binding protein), which is expressed by neural cells as well (Table 2) [13].
Table 2.
Immunohistochemical markers of melanoma [13].
| Marker | Cellular Localization | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| S100B | cytoplasm | >93 | low |
| Pmel-17/gp100 | melanosome | >70 | >90 |
| MART-1/MelanA | melanosome | >85 | >95 |
| tyrosinase | melanosome | >80 | low |
| MITF | nuclear | >80 | low |
| SOX10 | nuclear | >95 | low |
Meanwhile, the diagnostic problem is frequently not the melanocytic origin of the lesion but the potential malignancy. Histopathology is the gold standard of differentiating these lesions, and the MPATHDx classification and its appropriate interpretation could help [14]. Immunohistochemical detection of the nuclear protein Ki67 is not suitable for this distinction since nevi, especially those mechanically damaged, may contain proliferating nevocytes. Until recently, morphological analysis of the melanocytic tumor cells served as the only diagnostic help, but today there are genetic techniques which could help in objectively defining the nature of the melanocytic lesions. One possibility is to use immunohistochemical markers of malignancy: two such markers have been evaluated and validated, p16 and PRAME. Loss of p16 protein alone may not be an optimal tool to differentiate benign or malignant lesions, but its combination with Ki67 and Pmel/gp100 may better suit the diagnostic need [14,15]. A new alternative to p16 is high PRAME protein expression, which has been validated relatively extensively [14]. Another possibility is to use a four-gene fluorescence in situ hybridization (FISH) probe applicable to formalin-fixed paraffin-embedded (FFPE) blocks. This probe set is composed of genes which characteristically suffer from copy number variations during malignant transformation of melanocytes: gene amplification generally occurs in RREB1 (rat responsive element binding protein 1) and CCND1 genes, while the loss of copies occurs in the case of CDKN2A and MYB (MYB proto-oncogene and transcription factor) genes (Table 3). A minimum of three copy number variations of these genes is required for malignancy definition [16]. Recently a gene expression signature was defined for melanoma, which could be applied to FFPE sections, as well, to discriminate melanomas from non-malignant melanocytic lesions. This molecular test (myPath; Myriad) is based on RNA evaluation of 14 genes, 7 of which are melanoma genes and 7 are tumor-microenvironment-associated ones (Table 3) [17].
Table 3.
| FISH [16] | myPath [17] | |
|---|---|---|
| TME | ||
| CCL5 | ||
| RREB1amp | Tumor | CXCL9/10 |
| CCND1amp | PRAME | CD38 |
| CDKN2A LOH | S100A7/8/9/12 | IRF1 |
| MYB LOH | PI3 | LCP2 |
| PTPRC | ||
| SEL1 | ||
amp, amplification; LOH, loss of heterozygosity; TME, tumor microenvironment.
5. Immunological Characteristics—The Tumor Immune Microenvironment
Skin melanoma is traditionally considered one of the most immunogenic tumor types, based in part on its long-known feature of frequently containing a characteristic lymphoid infiltrate; furthermore, it may be the only tumor type for which spontaneous regression can occur in the primary tumor; this regression is assumed to be the consequence of antitumor immune response [18]. More recent research and therapy results supported the unique immunological features of cutaneous melanoma from other aspects. It belongs to tumors with the highest tumor mutational burden (TMB), caused by high mutagen exposure (UV radiation) [19,20,21]. As a consequence of high mutation rate, the chance of production of mutant proteins that may function as neoantigens is increased, contributing to enhanced immunogenicity [19,22]. This presumably explains the outstanding efficiency of antitumor immunotherapies, including immune checkpoint inhibitors (ICIs), in melanoma patients. Studies applying different gene panels characterizing local immune activity, based on The Cancer Genome Atlas (TCGA), also indicate high immune activity in melanomas [23,24]. The above features, however, do not apply to the rarer melanoma types, such as acral types, mucosal types, or to uveal melanomas; in these, both the mutational burden and immune-associated gene expression generally show lower values, and, consequently, their immunotherapy sensitivity is lower [23,24,25,26].
Since cutaneous melanoma belongs to tumors with high mutation frequency, a further increase in TMB and the amount of neoantigens during progression is not expected, although an increase in defects of homologous recombination repair can be detected [19]. However, from the point of antigen presentation, genetic defects might abrogate the beneficial effect of high neoantigen burden. In melanoma, LOH or mutations in the chromosomal regions, where genes encoding human leukocyte antigen (HLA) class I heavy chains (chromosome 6) or beta2-microglobulin (B2M, chromosome 15) are located, are relatively frequent [19], and they may render such tumors immunoresistant because of the crucial role of HLA class I molecules in antigen presentation to CD8+ cytotoxic T lymphocytes. It is also important that the BRAF-mutant melanomas may also develop PD-L1 gene amplification [10], which may result in immunoresistance, although it could increase sensitivity to ICIs targeting the PD-1/PD-L1 axis.
6. The Molecular Background of Melanoma Progression
One of the outstanding questions in melanoma progression is how stable the oncogenic drivers are. Most of our data come from investigating primary tumors or locoregional metastases, while very few genomic data are available concerning visceral metastases. It is known that melanoma is also a clonally heterogeneous tumor where driver mutant and wild-type clones are present as a mixture in the primary. We have analyzed the driver gene presence and the ratio of the mutant clones in melanoma metastases as compared to the primary tumors. We did not observe a complete loss of the driver oncogenes (BRAF or NRAS) in visceral metastases. However, we have found an extreme heterogeneity concerning the relative clonal ratio of the driver clones, since we found genetic evidence for all the three possible scenarios: maintenance of the original ratio, significant decrease of the driver clones and significant increase of the driver clones in metastases [27]. Accordingly, based on the clonal dominance of a driver clone in the primary tumor (or their extreme subclonality), one cannot predict the situation in the metastases which can be important when indicating target therapies.
The natural genetic progression of melanoma without therapeutic pressure is an important process. The data indicate that there are several novel mutations which emerge in metastases, such as those of BRCA1 (breast cancer gene 1), EGFR4 (epidermal growth factor receptor 4) and NMDAR2 (N-methyl-D-aspartate receptor 2). Since BRCA1 mutation results in homologous recombination deficiency, it may open the way to explore the potential use of PARP inhibitors in those instances. Furthermore, copy number changes are also emerging, affecting MITF or MET (MET proto-oncogene and receptor tyrosine kinase) (amplifications), or the loss of the suppressor PTEN, increasing the genetic diversity of the metastases as compared to the primary tumor [2,10]. Furthermore, copy number variations developing in metastasis-associated genes, NEDD9 (neural precursor cell expressed, developmentally downregulated 9), TWIST1 (Twist family BHLH transcription factor 1), SNAI1 (Snail family transcriptional repressor 1) and TEAD (transcriptional enhanced associate domain) are also significant genetic contributors of progression [2,10]. A recent study focusing on the genetic analysis of visceral metastases of melanoma revealed organ-specific genetic alterations of progression. In the case of lung metastasis, copy number gains have been observed in several (19) immunogenic genes—most of them found to be expressed at protein levels (13)—termed as immunogenic mimicry, indicating a strong immunologic selection mechanism operational in this form of metastasis [28]. This observation may suggest that visceral metastases may not be equally sensitive to immunotherapy. In contrast to lung metastasis, in brain metastases of melanoma, besides TERT, amplifications of HGF (hepatocyte growth factor) and MET genes have been found, indicating the presence of a possible autocrine loop of signaling, offering a potential target therapy option for this type of metastasis. As compared to these organs, liver metastases did not contain many unique genetic alterations, except for amplifications of CDK6 (cyclin-dependent kinase 6) and MAPK (mitogen-activated protein kinase) genes; both can now be targeted by clinically tested drugs [28]. Collectively, these genetic data offer new possibilities for target therapies of progressing melanoma and hopefully would initiate new types of clinical trials.
7. Prognostic Markers: Gene Expression Pattern
Primary skin melanoma can be classified into three molecular categories based on gene expression signature: a proliferative one driven by the SOX10–MITF pathway, where CDKN2A is lost; an invasive one characterized by activity of epithelial–mesenchymal transition genes SNAI1, ZEB1 (zinc finger E-box binding homeobox 1) and TGFBR2 (transforming growth factor beta receptor 2); and a so-called immune-mediated one characterized by activation of the tumor microenvironment [8,10]. Other analyses mostly confirmed these genotypes, defining the MITF low/proliferative, the high-immune response, the MITF high/pigmentation and the normal subclasses [11] where the MITF low subclass has the poorest prognosis. Lately, the TCGA analysis of the melanoma gene expression signatures also found the MITF low group, the immune group and the keratin groups [11]. These genetic signatures also define the characteristic metabolic profiles of melanoma. The proliferative phenotype is characterized by a high expression of PGC1A (peroxisome proliferator γ coactivator α), which is responsible for the production of reactive oxygen species (ROS), the high level of which results in chemoresistance, as well as immunoresistance [29]. Furthermore, the TCGA melanoma database analysis identified a prognostic signature containing CAV1 (caveolin 1), CD36 and CPT1C (carnitine palmitoyltransferase 1C), members of which are responsible for fatty acid uptake and metabolism [30]. Unfortunately, none of these signatures has been analyzed in prospective clinical trials for their prognostic power. As single prognostic markers, EGFR4 overexpression was found to have a negative prognostic impact, while ALDH1 (aldehyde dehydrogenase 1) overexpression has a positive prognostic impact [31]. Furthermore, traditional serum biomarkers such as 5-S-cisteinyldopa or lactate dehydrogenase (LDH) may also serve as prognostic factors; a high expression of them characterizes poor prognosis [31]. LDH overexpression is the result of the increased glycolytic activity and is released from hypoxic and necrotic melanoma cells into the circulation. The continuous monitoring of LDH levels upon target therapy or immunotherapy can also be used to assess efficacy since responding patients are characterized by normalizing LDH levels [32].
A recent development is the application of circulating DNA tests as possible prognostic factors: several studies provided evidence for the prognostic power of BRAF and NRAS mutations detected in the peripheral blood as markers of poor prognosis, defining molecular residual disease [33]. However, the most extensively validated prognostic melanoma gene signature is a 31-gene expression panel constructed from meta-analyses of skin and uveal melanoma signatures, containing four inner control genes. This 31-gene panel was retrospectively and prospectively analyzed clinically and proved to have strong independent prognostic power (Table 4) [34].
Table 4.
DecisionDx prognosticator of melanoma: list of genes to be evaluated [34].
| Gene Symbol | Gene Name | Regulation |
|---|---|---|
| BAP1 | BRCA1-associated protein 1 | down |
| MGP | Matrix G1a protein | down |
| SPP1 | Osteopontin | up |
| CXCL14 | Chemokine ligand 14 | down |
| CLCA2 | Chloride channel accessory 2 | down |
| S100A8 | S100 Ca-binding protein A8 | down |
| S100A9 | S100 Ca-binding protein A9 | down |
| BTG1 | B-cell translocation gene 1 | down |
| SAP130 | Sin3A-associated protein | down |
| ARG1 | Arginase 1 | down |
| KRT6B | Keratin 6B | up |
| KRT14 | Keratin 14 | down |
| GJA1 | Gap junction protein A1 | down |
| ID2 | Inhibitor of DNA binding 2 | down |
| EIF1B | Eukaryotic translocation initiator 1B | up |
| CRABP1 | Cellular retinoic acid binding protein 1 | down |
| ROBO1 | Roundabout guidance receptor 1 | down |
| RBM23 | RNA binding protein 23 | down |
| TACSTD2 | Tumor-associated Ca-signal transducer 2 | down |
| DSC1 | Desmocollin 1 | down |
| SPRR1B | Small proline-rich protein 1B | down |
| TRIM29 | Tripartite motif 29 | down |
| AQP3 | Aquaporin 3 | down |
| TYRP1 | Tyrosinase-related protein 1 | down |
| PPL | Periplakin | down |
| LTA4H | Leukotriene A4 hydrolase | down |
| CST6 | Cystatin E/M | down |
8. Prognostic Markers: The Tumor Immune Microenvironment
Based on the favorable immunological features described above, one could expect that the density or composition of tumor-infiltrating immune cells will have a prognostic role as well. However, there is no clear association between the amount of tumor-infiltrating immune cells and TMB or neoantigen burden, and the intensity of infiltration by immune cells (e.g., CD8+ T cells, among others) in melanoma is not outstanding compared with other tumor types [19,35,36,37].
The prognostic value of immune cell infiltration in primary melanoma was analyzed in many studies. In the earliest investigations, the number of tumor-infiltrating lymphocytes (TILs) was determined based on hematoxylin–eosin staining; although a prominent lymphocyte infiltration (especially if it was determined in the vertical growth phase) proved to be a significant independent parameter of longer survival in several studies, in other cases, no such association was found, or the independent prognostic role was not confirmed [38,39]. In studies based on the immunohistochemical detection of immune cell type specific markers, controversial results were reported on the prognostic value of infiltration by T lymphocytes (including CD4+ and CD8+ subsets), or macrophages. On the other hand, a favorable prognostic effect of mature dendritic cells, as well as B cells, was described [38].
In studies evaluating immune cell infiltration in metastases of cutaneous melanoma (focusing mainly on lymph node, subcutaneous or cutaneous metastases), the amount of total TIL was found associated with patients’ survival [35]. Few studies have analyzed the prognostic impact of specific immune cell subsets. According to those comprising the largest patient cohorts [40,41], a high density of CD3+/CD8+ T lymphocytes, as well as that of CD20+ B cells, predicted favorable outcome.
In the past years, numerous transcriptomic analyses (performed on either primary or metastatic melanoma tissues) yielded the classification of samples based on the expression of immune-related genes, showing an association of “high immune” sample subsets or characteristic gene signatures with favorable outcome of the disease [8,23,42,43,44]. In contrast to the above observations on cutaneous melanomas, in uveal melanomas, a high expression of immune-cell-infiltration-associated genes or high immune scores were found to correlate with lower survival rate [45,46], in accordance with findings on the association of high density of T cells and macrophages with poor prognosis in this melanoma type [47,48].
9. Predictive Markers of Melanoma
The therapy of advanced/metastatic melanoma is based on its molecular classification since chemotherapies are more or less ineffective and irradiation has only a limited effectivity, mainly in the case of brain metastases. As was shown, there are three major driver oncogenes defining three major molecular forms of skin melanoma, BRAF-, NRAS- and KIT-mutant, and there is a fourth which is the so-called triple wild-type form. Accordingly, the molecular characterization of melanoma is necessary before making therapy decisions. However, target therapies are only approved in the case of exon 15/codon 600 BRAF-mutant melanoma, using a BRAF inhibitor with or without MEK inhibitors [49]. At the moment, there is no approved drug for NRAS-mutant melanoma, although MEK inhibitors are under clinical testing with some encouraging results [50]. In the case of KIT-mutant melanomas, it is important to define the KIT-inhibitor sensitive ones based on experiences with KIT inhibitor efficacy in gastrointestinal stromal tumor (GIST). In the case of the triple wild-type melanoma, the only recommended therapy option is immunotherapy in the form of anti-PD-1 (programmed cell death protein 1) and/or anti-CTLA-4 (cytotoxic T cell antigen 4) antibodies [49]. It is also important that immunotherapies can also be introduced in driver gene positive melanomas. It is a question what the rationale of the therapeutic decision would be in such cases. It was mentioned earlier that the clonal composition of the melanoma for a given oncogenic driver can be variable and can be objectively determined by assessing the variant allele frequency (VAF) of the mutation. In the case of subclonality (<20% VAF), the major part of the tumor is composed of tumor cells carrying the wild-type oncogene, where the efficacy of a target therapy is questionable, and early relapse and development of resistance is expectable. On the other hand, in the case of heterozygous mutation (~50% VAF), 100% of the tumor population carries the mutant oncogene, and the chance to control the disease is high.
Meanwhile, even with target or immunotherapies, a majority of melanomas progress due to genetic progression under special environmental pressure induced by the therapy. In the case of BRAF inhibitor/MEK inhibitor therapies, novel resistance mutations were reported, affecting BRAF outside V600, MEK1/2 and various members of the AKT signaling pathway (AKT1 (AKT serine/threonine kinase 1), PIK3CA (phosphoinositide-3-kinase catalytic subunit alpha) and PIK3R1/2 (phosphoinositide-3-kinase regulatory subunit 1/2)) [2,10,51]. Furthermore, gene amplifications of BRAF or MITF were also detected in target-therapy-resistant tumors. Last but not least, it seems that the loss of PTEN can also activate the AKT signaling pathway, resulting in BRAF inhibitor resistance [51].
The most widely used immunotherapies in patients with cutaneous melanoma are immune checkpoint inhibitors, monoclonal antibodies targeting PD-1 or CTLA-4. In the case of these agents, no clinically validated predictive markers exist in melanoma. PD-L1 expression of tumor cells and/or tumor-infiltrating immune cells is a prerequisite of anti-PD-1/PD-L1 treatment for several cancer types. However, there is no such requirement in the case of melanoma; although tumor-cell PD-L1 expression showed an association with therapeutic effect in several studies, responses can be observed in a significant proportion of PD-L1-negative cases, as well. Considering both the therapeutic benefit and the risk of side effects, in cases with positive tumor cell PD-L1 staining (>5%), anti-PD-1 monotherapy is advantageous, while, in negative cases, combination with anti-CTLA-4 could be more advantageous [52].
Besides PD-L1 expression, the most frequently analyzed tissue biomarker of immunotherapy efficacy is TMB, which showed a positive association with ICI therapy response [53,54]. Microsatellite instability of melanoma is rare; therefore, it cannot be efficiently used for selection for immunotherapy [2,10]. Moreover, several other potential predictive factors came to light, including clinical parameters as tumor burden or localization of metastases; blood or serum markers, e.g., absolute number or proportion of some blood cell types, or LDH level; the composition of the intestinal microbiome and microbial gene signatures; and tumor-infiltrating immune cells or expression of immune-associated genes in the tumor [39,54,55,56,57,58,59]. Loss of HLA class I expression could also contribute to both primary and acquired immunotherapy resistance through impeding antigen presentation to T cells [19,55,60,61,62]. In a small proportion of melanomas, loss of HLA class I expression is caused by mutation of the B2M gene [60,63]; however, epigenetic mechanisms or translational dysregulation are much more frequent mechanisms of HLA class I loss in cancers [64,65]. Loss of PTEN expression has been implicated as a mechanism of primary and acquired resistance to ICI therapy in melanoma [66,67]. Moreover, a cluster of cancer-germline antigens, located in chromosome Xq28, predicted resistance to CTLA-4 blockade (but not to PD-1 blockade) in melanoma patients [68]. Several studies have identified alterations of genes associated with interferon-γ (IFN-γ) signaling as mechanisms of immunotherapy resistance, such as mutations of JAK1/2 (Janus kinase 1/2) [63], loss of IFNGR1/2 (interferon gamma receptor 1/2) and gains of SOCS1 (suppressor of cytokine signaling 1) and PIAS4 (protein inhibitor of activated STAT 4) genes [69], as well as mutation of SERPINB3/4 (serpin family B members 3/4) genes [70], suggesting that IFN signaling plays a crucial role in these processes. Furthermore, an IFN-γ-related mRNA profile was found to be predictive of response to anti-PD-1 therapy in multiple tumors, including melanoma [71]. More recently, a type-I IFN resistance gene expression signature was identified in a human melanoma model, where a 17-gene component was found to be predictive for ICI therapy efficacy [72]. Collectively, there are several genetic alterations, inherent or acquired in melanoma progression, that have been defined to be associated with ICI therapy efficacy, which all wait for prospective clinical validation.
10. Concluding Remarks
Molecular pathology plays a critical role in the diagnostics and management of malignant melanoma. Extensive genetic analyses of melanoma patients not only resulted in the proper molecular classification of the tumors but also discovered several genetic conditions which are responsible for the hereditary forms or the increased risk of development. Since the diagnostics of melanoma can be difficult due to a wide range of premalignant melanocytic lesions, molecular markers and gene expression signatures can support diagnostics. Furthermore, the molecular pathology analyses revealed the genetic background of malignant progression and also identified clinically useful gene expression signatures with prognostic value and several potential targets for new therapies. However, the advent of target therapies and immunotherapies of melanoma present another array of selection pressure for the development of novel tumor cell clones characterized by selective genetic advantages for resistance toward those therapies. Accordingly, a continuous monitoring of the genetics of the progressing disease is necessary to optimize clinical management.
Abbreviations
AKT1, AKT serine/threonine kinase 1; ALDH1, aldehyde dehydrogenase 1; ALK, anaplastic lymphoma kinase; ALM, acral lentiginous melanoma; APEX1, apurinic/apyrimidinic endodeoxyribonuclease 1; ARID2, AT-rich interaction domain 2; ARNT, aryl hydrocarbon receptor nuclear translocator; AURKA, Aurora kinase A; B2M, beta2-microglobulin; BAP1, BRCA1-associated protein 1; BRAF, v-Raf murine sarcoma viral oncogene homolog B1; BRCA1, breast cancer gene 1; CAV1, caveolin 1; CCND1, cyclin D1; CDK4, cyclin-dependent kinase 4; CDK6, cyclin-dependent kinase 6; CDKN2A, cyclin-dependent kinase inhibitor 2A; CPT1C, carnitine palmitoyltransferase 1C; CTLA-4, cytotoxic T-cell antigen 4; DDR, DNA damage response; EGFR4, epidermal growth factor receptor 4; FFPE, formalin-fixed paraffin-embedded; FISH, fluorescence in situ hybridization; GIST, gastrointestinal stromal tumor; GNA11, guanine nucleotide-binding protein alpha subunit 11; GNAQ, guanine nucleotide-binding protein alpha subunit q; GRIN2A, N-methyl-D-aspartate receptor glutamate ionotropic receptor NMDA type subunit 2A; HGF, hepatocyte growth factor; HLA, human leukocyte antigen; ICI, immune checkpoint inhibitor; IDH1, isocitrate dehydrogenase 1; IFN, interferon; IFNGR1/2, interferon gamma receptor 1/2; JAK1/2, Janus kinase 1/2; KIT, KIT proto-oncogene, receptor tyrosine kinase; LDH, lactate dehydrogenase; LMM, lentigo maligna melanoma; LOH, loss of heterozygosity; MAPK, mitogen-activated protein kinase; MC1R, melanocortin-1 receptor; MDM2, mouse double minute 2 homolog; MET, MET proto-oncogene, receptor tyrosine kinase; MITF, microphthalmia-associated transcription factor; MLH1, MutL homolog 1; NF1, neurofibromin 1; MYB, MYB proto-oncogene, transcription factor; NEDD9, neural precursor cell expressed, developmentally downregulated 9; NFKBIE, NFKB inhibitor epsilon; NMDAR2, N-methyl-D-aspartate receptor 2; NM, nodular melanoma; NRAS, neuroblastoma RAS viral (v-ras) oncogene homolog; NTRK, neurotrophic tyrosine receptor kinase; OCA2, oculocutaneous albinism type 2; PALB2, partner and localizer of BRCA2; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; PIAS4, protein inhibitor of activated STAT 4; PIK3CA, phosphoinositide-3-kinase catalytic subunit alpha; PIK3R1/2, phosphoinositide-3-kinase regulatory subunit 1/2; PPP6C, protein phosphatase 6 catalytic subunit; PTEN, phosphatase and tensin homolog; RB1, retinoblastoma 1; RET, ret proto-oncogene; ROS, reactive oxygen species; RREB1, rat responsive element binding protein 1; S100B, S100 calcium binding protein; SERPINB3/4, serpin family B members 3/4; SETDB1, SET domain bifurcated histone lysine methyltransferase 1; SLC45A2, solute carrier family 45 member 2; SNAI1, Snail family transcriptional repressor 1; SOCS1, suppressor of cytokine signaling 1; SOX10, Sry-related HMg-Box gene 10; SSM, superficial spreading melanoma; TCGA, The Cancer Genome Atlas; TEAD, transcriptional enhanced associate domain; TERT, telomerase reverse transcriptase; TGFBR2, transforming growth factor beta receptor 2; TIL, tumor-infiltrating lymphocyte; TMB, tumor mutational burden; TME, tumor microenvironment; TP53, tumor protein p53; TWIST1, Twist family BHLH transcription factor 1; TYR, tyrosinase; TYRP1, tyrosinase-related protein 1; VAF, variant allele frequency; ZEB1, zinc finger E-box binding homeobox 1.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Research, Development and Innovation Office (Hungary) grants NKFI ANN-128524 (AL) and K-135540 (JT).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Karimkhani C., Green A.C., Nijsten T., Weinstock M.A., Dellavalle R.P., Naghavi M., Fitzmaurice C. The global burden of melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 2017;177:134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tímár J., Vízkeleti L., Doma V., Barbai T., Rásó E. Genetic progression of malignant melanoma. Cancer Metastasis Rev. 2016;35:93–107. doi: 10.1007/s10555-016-9613-5. [DOI] [PubMed] [Google Scholar]
- 3.Law M.H., MacGregor S., Hayward N.K. Melanoma genetics: Recent findings take us beyond well-travelled pathways. J. Investig. Dermatol. 2012;132:1763–1774. doi: 10.1038/jid.2012.75. [DOI] [PubMed] [Google Scholar]
- 4.Baxter A.J., Hughes M.C., Kvaskoff M., Siskind V., Shekar V.S., Aitken J.F., Green A.C., Duffy D.L., Hayward N.K., Martin N.G., et al. The Queensland Study of Melanoma: Environmental and genetic associations (Q-MEGA): Study design, baseline characteristics and repeatability of phenotypes and sun exposure measures. Twin Res. Hum. Genet. 2008;11:183–196. doi: 10.1375/twin.11.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amos C.I., Wang L.E., Lee J.E., Gershenwald J.E., Chen W.V., Fang S., Kosoy R., Zhang M., Qureshi A.A., Vattathil S., et al. Genome-wide association study identifies novel loci predisposing to cutaneous melanoma. Hum. Mol. Genet. 2011;20:5012–5023. doi: 10.1093/hmg/ddr415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett J.H., Iles M.M., Harland M., Taylor J.C., Aitken J.F., Andresen P.A., Akslen L.A., Armstrong B.K., Avril M.F., Azizi E., et al. Genome-wide association study identifies three new melanoma susceptibility loci. Nat. Genet. 2011;43:1108–1113. doi: 10.1038/ng.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jager M.J., Shields C.L., Cebulla C.M., Abdel-Rahman M.H., Grossniklaus H.S., Stern M.H., Carvajal R.D., Bedfort R.N., Jia R., Shields G.S., et al. Uveal melanoma. Nat. Rev. Dis. Primers. 2020;6:24. doi: 10.1038/s41572-020-0158-0. [DOI] [PubMed] [Google Scholar]
- 8.The Cancer Genome Atlas Network Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doma V., Barbai T., Beleaua M.A., Kovalszky I., Rásó E., Tímár J. KIT mutation incidence and pattern of melanoma in central-east Europe. Pathol. Oncol. Res. 2020;26:17–22. doi: 10.1007/s12253-019-00788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin L., Garraway L.A., Fisher D.E. Malignant melanoma: Genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- 11.Rabbie R., Ferguson P., Molina-Aguilar C., Adams D.J. Melanoma subtypes: Genomic profiles, prognostic molecular markers and therapeutic possibilities. J. Pathol. 2019;247:539–551. doi: 10.1002/path.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tímár J., Barbai T., Győrffy B., Rásó E. Chapter 2. Understanding Melanoma Progression by Gene Expression Signatures. In: Pfeffer U., editor. Cancer Genomics. Springer; Dordrecht, The Netherlands: 2013. pp. 47–79. [Google Scholar]
- 13.Ordonez N.G. Value of melanocytic-associated immunohistochemical markers in the diagnosis of malignant melanoma. Hum. Pathol. 2014;45:191–205. doi: 10.1016/j.humpath.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Deacon D.C., Smith E.A., Judson-Torres R.L. Molecular biomarkers for melanoma screening, diagnosis and prognosis: Current state and future directions. Front. Med. 2021;8:642380. doi: 10.3389/fmed.2021.642380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uguen A., Talagas M., Costa S., Duigou S., Bouvier S., De Braekeleer M., Marcorelles P. A p16-ki-67-HMB45 immunohistochemistry score system as an ancillary diagnostic tool in the diagnosis of melanoma. Diagn. Pathol. 2015;10:195. doi: 10.1186/s13000-015-0431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reimann J.D.R., Salim S., Velazquez E.F., Wang L., Williams K.M., Flejter W.L., Brooke L., Sunder S., Busam K.J. Comparison of melanoma gene expression score with histopathology, FISH and SNP array for the classification of melanocytic neoplasms. Mod. Pathol. 2018;31:1733–1743. doi: 10.1038/s41379-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke L.E., Flake D.D., Busam K., Cockerell C., Helm K., McNiff J., Reed J., Tschen J., Kim J., Barnhill K., et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617–628. doi: 10.1002/cncr.30385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aivazian K., Ahmed T., El Sharouni M.A., Stretch J.R., Saw R.P.M., Spillane A.J., Shannon K.F., Ch’ng S., Nieweg O.E., Thompson J.F., et al. Histological regression in melanoma: Impact on sentinel lymph node status and survival. Mod. Pathol. 2021;34:1999–2006. doi: 10.1038/s41379-021-00870-2. [DOI] [PubMed] [Google Scholar]
- 19.Ladányi A., Tímár J. Immunologic and immunogenomic aspects of tumor progression. Semin. Cancer Biol. 2020;60:249–261. doi: 10.1016/j.semcancer.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L., et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T., Dutton-Regester K., Brown K.M., Hayward N.K. The genomic landscape of cutaneous melanoma. Pigment Cell Melanoma Res. 2016;29:266–283. doi: 10.1111/pcmr.12459. [DOI] [PubMed] [Google Scholar]
- 22.Addeo A., Friedlander A., Banna G.L., Weiss G.J. TMB or not TMB as a biomarker: That is the question. Crit. Rev. Oncol. Hematol. 2021;163:103374. doi: 10.1016/j.critrevonc.2021.103374. [DOI] [PubMed] [Google Scholar]
- 23.Danaher P., Warren S., Lu R., Samayoa J., Sullivan A., Pekker I., Wallden B., Marincola F.M., Cesano A. Pan-cancer adaptive immune resistance as defined by the Tumor Inflammation Signature (TIS): Results from The Cancer Genome Atlas. J. Immunother. Cancer. 2018;6:63. doi: 10.1186/s40425-018-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roelands J., Hendrickx W., Zoppoli G., Mall R., Saad M., Halliwill K., Curigliano G., Rinchai D., Decock J., Delogu L.G., et al. Oncogenic states dictate the prognostic and predictive connotations of intratumoral immune response. J. Immunother. Cancer. 2020;8:e000617. doi: 10.1136/jitc-2020-000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarchoan M., Hopkins A., Jaffee E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D., Schilling B., Liu D., Sucker A., Livingstone E., Jerby-Amon L., Zimmer L., Gutzmer R., Satzger I., Loquai C., et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat. Med. 2019;25:1916–1927. doi: 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doma V., Kárpáti S., Rásó E., Barbai T., Tímár J. Dynamic and unpredictable changes in mutant allele fractions of BRAF and NRAS during visceral progression of cutaneous malignant melanoma. BMC Cancer. 2019;19:786. doi: 10.1186/s12885-019-5990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papp O., Doma V., Gil J., Markó-Varga G., Kárpáti S., Tímár J., Vízkeleti L. Organ specific copy number variations in visceral metastases of human melanoma. Cancers. 2021;13:5984. doi: 10.3390/cancers13235984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alkaraki A., McArthur G.A., Sheppard K.E., Smith L.K. Metabolic plasticity in melanoma progression and response to oncogene targeted therapies. Cancers. 2021;13:5810. doi: 10.3390/cancers13225810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nath A., Chan C. Genetic alterations in fatty acid transport and metabolism genes are associated with metastatic progression and poor prognosis of human cancers. Sci. Rep. 2016;6:18669. doi: 10.1038/srep18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safai B., Wu A.G., Hamby C.Y. Prognostic biomarkers in melanoma: Tailoring treatments to patient. J. Clin. Aenest. Dermatol. 2021;14:44–48. [PMC free article] [PubMed] [Google Scholar]
- 32.Valenti F., Falcone I., Ungania S., Desiderio F., Giacomini P., Bazzichetto C., Conciatori F., Gallo E., Cognetti F., Ciliberto G., et al. Precision medicine and melanoma: Multi-omics approaches to monitoring the immunotherapy response. Int. J. Mol. Sci. 2021;22:3837. doi: 10.3390/ijms22083837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanemaru H., Mizukami Y., Kaneko A., Kajihara I., Fukushima S. Promising blood-based biomarkers for melanoma: Recent progress of liquid biopsy and its future perspectives. Curr. Treat. Options Oncol. 2022;23:562–577. doi: 10.1007/s11864-022-00948-2. [DOI] [PubMed] [Google Scholar]
- 34.Hsueh E.C., DeBloom J.R., Lee J.H., Sussman J.J., Covington K.R., Caruso H.G., Quick A.P., Cook R.W., Slingluff C.L., McMaster K.M. Long-term outcomes in a multicenter prospective cohort evaluating the prognostic 31-gene expression profile for cutaneous melanoma. JCO Precis. Oncol. 2021;5:589–601. doi: 10.1200/PO.20.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spranger S., Luke J.J., Bao R., Zha Y., Hernandez K.M., Li Y., Gajewski A.P., Andrade J., Gajewski T.F. Density of immunogenic antigens does not explain the presence or absence of the T-cell–inflamed tumor microenvironment in melanoma. Proc. Natl. Acad. Sci. USA. 2016;113:E7759–E7768. doi: 10.1073/pnas.1609376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y.P., Zhang Y., Lv J.W., Li Y.Q., Wang Y.Q., He Q.M., Yang X.J., Sun Y., Mao Y.P., Yun J.P., et al. Genomic analysis of tumor microenvironment immune types across 14 solid cancer types: Immunotherapeutic implications. Theranostics. 2017;7:3585–3594. doi: 10.7150/thno.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varn F.S., Wang Y., Mullins D.W., Fiering S., Cheng C. Systematic pan-cancer analysis reveals immune cell interactions in the tumor microenvironment. Cancer Res. 2017;77:1271–1282. doi: 10.1158/0008-5472.CAN-16-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ladányi A. Prognostic and predictive significance of immune cell infiltrating cutaneous melanoma. Pigment Cell Melanoma Res. 2015;28:490–500. doi: 10.1111/pcmr.12371. [DOI] [PubMed] [Google Scholar]
- 39.Straker R.J., III, Krupp K., Sharon C.E., Thaler A.S., Kelly N.J., Chu E.Y., Elder D.E., Xu X., Miura J.T., Karakousis G.C. Prognostic significance of primary tumor-infiltrating lymphocytes in a contemporary melanoma cohort. Ann. Surg. Oncol. 2022 doi: 10.1245/s10434-022-11478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erdag G., Schaefer J.T., Smolkin M.E., Deacon D.H., Shea S.M., Dengel L.T., Patterson J.W., Slingluff C.L., Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabrita R., Lauss M., Sanna A., Donia M., Skaarup Larsen M., Mitra S., Johansson I., Phung B., Harbst K., Vallon-Christersson J., et al. Tertiary lymphoid structures improve immunotherapy in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 42.Sivendran S., Chang R., Pham L., Phelps R.G., Harcharik S.T., Hall L.D., Bernardo S.G., Moskalenko M.M., Sivendran E., Fu Y., et al. Dissection of immune gene networks in primary melanoma tumors critical for antitumor surveillance of patients with stage II–III resectable disease. J. Investig. Dermatol. 2014;134:2202–2211. doi: 10.1038/jid.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y., Schaafsma E., Gorlov I.P., Hernando E., Thomas N.E., Shen R., Turk M.J., Berwick M., Amos C.I., Cheng C. A leukocyte infiltration score defined by a gene signature predicts melanoma patient prognosis. Mol. Cancer Res. 2019;17:109–119. doi: 10.1158/1541-7786.MCR-18-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poźniak J., Nsengimana J., Laye J.P., O’Shea S.J., Diaz J.M.S., Droop A.P., Filia A., Harland M., Davies J.R., Mell T., et al. Genetic and environmental determinants of immune response to cutaneous melanoma. Cancer Res. 2019;79:2684–2696. doi: 10.1158/0008-5472.CAN-18-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Xu Y., Dai X., Lin X., Shan Y., Ye J. The prognostic landscape of adaptive immune resistance signatures and infiltrating immune cells in the tumor microenvironment of uveal melanoma. Exp. Eye Res. 2020;196:108069. doi: 10.1016/j.exer.2020.108069. [DOI] [PubMed] [Google Scholar]
- 46.Gu C., Gu X., Wang Y., Yao Z., Zhou C. Construction and validation of a novel immunosignature for overall survival in uveal melanoma. Front. Cell Dev. Biol. 2021;9:710558. doi: 10.3389/fcell.2021.710558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bronkhorst I.H.G., Jager M.J. Uveal melanoma: The inflammatory microenvironment. J. Innate Immun. 2012;4:454–462. doi: 10.1159/000334576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh L., Singh M.K., Kenney M.C., Jager M.J., Rizvi M.A., Meel R., Lomi N., Bakhshi S., Sen S., Kashyap S. Prognostic significance of PD-1/PD-L1 expression in uveal melanoma: Correlation with tumor-infiltrating lymphocytes and clinicopathological parameters. Cancer Immunol. Immunother. 2021;70:1291–1303. doi: 10.1007/s00262-020-02773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seth R., Messerschmith H., Kaur V., Kirkwood J.M., Kudchadkar R., McQude J.L., Provenzano A., Swami U., Weber J., Alluri K.C., et al. Systemic therapy of melanoma: ASCO guideline. J. Clin. Oncol. 2020;38:3947–3970. doi: 10.1200/JCO.20.00198. [DOI] [PubMed] [Google Scholar]
- 50.Rajkumar S., Berry D., Heney K.A., Strong C., Ramsay L., Lajoie M., Alkallas R., Nguyen T.T., Thomson C., Ahanfeshar-Adams M., et al. Melanomas with concurrent BRAF non-p600 and NF1 loss-of-function mutations are targetable by BRAF/MEK inhibitor combination therapy. Cell Rep. 2022;39:110634. doi: 10.1016/j.celrep.2022.110634. [DOI] [PubMed] [Google Scholar]
- 51.Tímár J., Hegedűs B., Rásó E. The role of lipid signaling in the progression of malignant melanoma. Cancer Metastasis Rev. 2018;37:245–255. doi: 10.1007/s10555-018-9729-x. [DOI] [PubMed] [Google Scholar]
- 52.Larkin J.L., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buder-Bakhaya K., Hassel J.C. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment—A review from the melanoma perspective. Front. Immunol. 2018;9:1474. doi: 10.3389/fimmu.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gide T.N., Wilmott J.S., Scolyer R.A., Long G.V. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin. Cancer Res. 2018;24:1260–1270. doi: 10.1158/1078-0432.CCR-17-2267. [DOI] [PubMed] [Google Scholar]
- 57.Balatoni T., Mohos A., Papp E., Sebestyén T., Liszkay G., Oláh J., Varga A., Lengyel Z., Emri G., Gaudi I., et al. Tumor-infiltrating immune cells as potential biomarkers predicting response to treatment and survival in patients with metastatic melanoma receiving ipilimumab therapy. Cancer Immunol. Immunother. 2018;67:141–151. doi: 10.1007/s00262-017-2072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balatoni T., Ladányi A., Fröhlich G., Czirbesz K., Kovács P., Pánczél G., Bence E., Plótár V., Liszkay G. Biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Pathol. Oncol. Res. 2020;26:317–325. doi: 10.1007/s12253-018-0466-9. [DOI] [PubMed] [Google Scholar]
- 59.McCulloch J.A., Davar D., Rodrigues R.R., Badger J.H., Fang J.R., Cole A.M., Balaji A.K., Vetizou M., Prescott S.M., Fernandes M.R., et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022;28:545–556. doi: 10.1038/s41591-022-01698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sade-Feldman M., Jiao Y.J., Chen J.H., Rooney M.S., Barzily-Rokni M., Eliane J.P., Bjorgaard S.L., Hammond M.R., Vitzthum H., Blackmon S.M., et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017;8:1136. doi: 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ladányi A., Papp E., Mohos A., Balatoni T., Liszkay G., Oláh J., Varga A., Lengyel Z., Emri G., Ferrone S. Role of the anatomic site in the association of HLA class I antigen expression level in metastases with clinical response to ipilimumab therapy in melanoma patients. J. Immunother. Cancer. 2020;8:e000209. doi: 10.1136/jitc-2019-000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladányi A., Hegyi B., Balatoni T., Liszkay G., Rohregger R., Waldnig C., Dudás J., Ferrone S. HLA class I downregulation in progressing metastases of melanoma patients treated with ipilimumab. Pathol. Oncol. Res. 2022;28:1610297. doi: 10.3389/pore.2022.1610297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torreyon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai L., Michelakos T., Yamada T., Fan S., Wang X., Schwab J.H., Ferrone C.R., Ferrone S. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol. Immunother. 2018;67:999–1009. doi: 10.1007/s00262-018-2131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maggs L., Sadagopan A., Moghaddam A.S., Ferrone S. HLA class I antigen processing machinery defects in antitumor immunity and immunotherapy. Trends Cancer. 2021;7:1089–1101. doi: 10.1016/j.trecan.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng W., Chen J.Q., Liu C., Malu S., Creasy C., Tetzlaff M.T., Xu C., McKenzie J.A., Zhang C., Liang X., et al. Loss of PTEN promotes resistance to T-cell-mediated immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kakavand H., Jackett L.A., Menzies A.M., Gide T.N., Carlino M.S., Saw R.P.M., Thompson J.F., Wilmott J.S., Long G.V., Scolyer R.A. Negative immune checkpoint regulation by VISTA: A mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod. Pathol. 2017;30:1666–1676. doi: 10.1038/modpathol.2017.89. [DOI] [PubMed] [Google Scholar]
- 68.Shukla S.A., Bachireddy P., Schilling B., Galonska C., Zhan Q., Bango C., Langer R., Lee P.C., Gusenleitner D., Keskin D.B., et al. Cancer-germline antigen expression discriminates clinical outcome to CTLA-4 blockade. Cell. 2018;173:624–633. doi: 10.1016/j.cell.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J., Shi L.Z., Zhao H., Chen J., Xiong L., He Q., Chen T., Roszik J., Bernatchez C., Woodman S.E., et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167:397–404. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riaz N., Havel J.J., Kendall S.M., Makarov V., Walsh L.A., Desrichard A., Weinhold N., Chan T.A. Recurrent SerpinB3 and SerpinB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat. Genet. 2016;48:1327–1329. doi: 10.1038/ng.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ayers M.A., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D.R., Albright A., Cheng J.D., Kang P., Shankaran V., et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ladányi A., Rásó E., Barbai T., Vízkeleti L., Puskás L.G., Kovács S., Győrffy B., Tímár J. Identification of a tumor cell associated type I IFN resistance gene expression signature of human melanoma, the components of which have a predictive potential for immunotherapy. Int. J. Mol. Sci. 2022;23:2704. doi: 10.3390/ijms23052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.