Abstract
The nor mutant of Aspergillus flavus has a defective norsolorinic acid reductase, and thus the aflatoxin biosynthetic pathway is blocked, resulting in the accumulation of norsolorinic acid, a bright red-orange pigment. We developed a visual agar plate assay to monitor yeast strains for their ability to inhibit aflatoxin production by visually scoring the accumulation of this pigment of the nor mutant. We identified yeast strains that reduced the red-orange pigment accumulation in the nor mutant. These yeasts also reduced aflatoxin accumulation by a toxigenic strain of A. flavus. These yeasts may be useful for reducing aflatoxin contamination of food commodities.
Aflatoxins constitute a group of toxic carcinogenic metabolites produced by Aspergillus flavus Link: Fr., A. parasiticus Spear, and A. nomius Kurtzman (5, 8, 21). Aflatoxin B1 (AFB1), the most toxic of this group, is the most potent carcinogen known. Contamination of tree nuts, peanuts, corn, and cottonseed by aflatoxins is a serious food safety hazard to both humans and animals. A. flavus is the most common aflatoxin-producing fungus on corn, cotton, tree nuts, and peanuts (6, 7, 9, 10, 18, 23). This fungus is a wound-invading pathogen that infects plants damaged by insects, animals, early splits, and mechanical harvesting. Under conditions extremely favorable for infection, this fungus can colonize corn kernels directly (10, 18, 22). Any established infection of A. flavus will result in rapid accumulation of aflatoxins in the harvested commodity under conditions of warm temperatures and high humidity. The domestic market of food for human consumption presently allows a maximum level of 20 ppb for AFB1. Even very low levels of infection by A. flavus can result in aflatoxin levels exceeding this standard. In a worst-case scenario, the commodity may be banned from domestic and export markets. The presence of aflatoxins in a food commodity reduces the economic return to both the growers and processors.
Saprophytic yeasts are common on the surface of plant leaves and fruits. Some yeasts effectively compete with postharvest fungal pathogens, such as Penicillium expansum and Botrytis cinerea (11, 14, 19, 24). The potential of saprophytic yeasts to be used in a variety of application for reducing aflatoxin contamination in food has not been hitherto extensively explored.
More than 25 genes are involved in the biosynthesis of aflatoxins via the polyketide pathway. A regulatory gene, aflR, is required for the expression of several identified genes in the pathway (2, 3, 15, 16, 25). At least 16 enzyme-catalyzed steps are required to complete the synthesis of AFB1 from norsolorinic acid, the first stable intermediate. We used A. flavus Papa 827, a nor mutant (17), as an indicator strain to visually monitor the interactions of yeasts with A. flavus. The nor mutant of A. flavus has a mutation in the gene coding for norsolorinic acid reductase, and aflatoxin biosynthesis is blocked at this step. This mutant strain accumulates norsolorinic acid, a bright red-orange pigment, that can be easily seen (1, 12). The rationale for using the nor mutant as an indicator strain is that accumulation of the red-orange pigment during growth implies that the aflatoxin biosynthetic pathway is operating up to the formation of norsolorinic acid. Thus, if no visible pigment is produced, it implies that aflatoxin biosynthesis is inhibited at an earlier step in the polyketide pathway and that this inhibition also may occur in field strains of aflatoxigenic A. flavus.
Aspergillus flavus Papa 827 is a white-spored mutant that accumulates norsolorinic acid, a red-orange pigment. The toxigenic strain A. flavus 42-12 (NRRL-25347), isolated from a pistachio nut, was used to examine inhibition of aflatoxin production by yeasts. This strain produces AFB1 and traces of AFB2. The fungal strains were maintained on potato-dextrose agar (PDA) (Difco Laboratories, Detroit, Mich.). Fungal spores were resuspended in 0.05% Tween 80, and the number of spores in the suspension was determined microscopically with a hemacytometer.
We used the following bioassay protocol: (i) the yeast isolate was grown in a flask containing potato dextrose broth (Difco) for 24 h on a shaker at 28°C, and the fresh culture was used for inoculation; (ii) spore suspensions of the nor mutant were prepared (104 spores/ml); (iii) two streaks of 20-μl yeast cultures were applied 15 mm from the center line of a petri dish containing PDA; (iv) 4 h later, 20 μl of the A. flavus spore suspension was inoculated along the center line of the PDA agar; (v) the petri dish was sealed with Parafilm M (American National Can, Neenah, Wis.) and incubated at 28°C for 10 days.
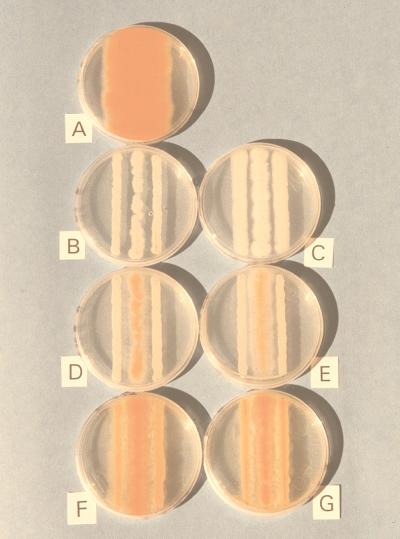
We determined the effects of yeast isolates on A. flavus by visually estimating the amount of red-orange color formed by the nor mutant (Fig. 1). A score of ++++ was assigned to the bright orange pigment level of control (Fig. 1A), and a score of − was assigned to an almost imperceptible level of pigment (Fig. 1B and C). The growth of the nor mutant was restricted to and reduced within the boundary of the two streaks of the yeast zone in the bioassay (Fig. 1). As shown in Fig. 1A, the fungal colony expanded toward the edges of the PDA in the petri dish in the absence of the yeasts.
FIG. 1.
A visual system for monitoring interactions of yeasts and A. flavus. nor mutant A. flavus Papa 827 is used as an indicator strain. Details of the experiment are given in the text. (A) nor mutant in the absence of yeasts; (B) WRL-076; (C) WRL-038; (D) WRL-084; (E) WRL-015; (F) WRL-024; (G) WRL-053.
The amount of norsolorinic acid produced was quantified spectrophotometrically to verify the reliability of the visual bioassay. The bright red-orange pigment in the mycelium was extracted by alkaline methanol (methanol–1 N NaOH [90:10, vol/vol]). Five agar discs (7-mm diameter) of fungal cultures were cored and transferred to a centrifuge tube containing 10 ml of solvent. Norsolorinic acid concentration in the supernatant was determined spectrophotometrically at a wavelength of 560 nm in a Beckman DU 640 spectrophotometer. Purified norsolorinic acid was dissolved in alkaline methanol and used as a standard. The visual scores correlate well with the concentration of norsolorinic acid (Table 1).
TABLE 1.
Effects of saprophytic yeasts on toxigenic strain 42-12a
| Yeast | External pH | Visual scoreb | Norsolo-rinic acid (μg/5 discs) | Biomass (mg/5 discs) | AFB1 (ng/4 discs) |
|---|---|---|---|---|---|
| WRL-015 | 4.7 ± 0.06 | + | 16 ± 2.1 | 7.2 ± 0.7 | 770 ± 74 |
| WRL-024 | 4.8 ± 0.07 | +++ | 66 ± 8.3 | 6.8 ± 0.7 | 1,900 ± 170 |
| WRL-038 | 5.2 ± 0.09 | − | 3 ± 0.4 | 5.5 ± 0.5 | 180 ± 20 |
| WRL-053 | 4.9 ± 0.09 | +++ | 60 ± 7.2 | 7.0 ± 0.6 | 360 ± 35 |
| WRL-076 | 4.4 ± 0.05 | − | 4 ± 0.8 | 3.8 ± 0.4 | 60 ± 9 |
| WRL-084 | 5.6 ± 0.16 | + | 21 ± 3.5 | 4.5 ± 0.4 | 410 ± 40 |
| Control | 4.8 ± 0.12 | ++++ | 112 ± 12 | 8.8 ± 0.7 | 4,800 ± 370 |
Values are means ± standard deviations (n = 6) of two sets of triplicate samples from two experiments
Visual score of red-orange pigment in fungal agar cultures shown in Fig. 1; ++++ was assigned to the bright red-orange pigment level of control, +++ was assigned to a pigment level not as high as the control, + was assigned to a low pigment level but visible, and − was assigned to an imperceptible level of pigment.
Saprophytic yeasts were isolated from fruits of almond, pistachio, and walnut trees. The six selected isolates, Candida guilliermondii WRL-015, Cryptococcus laurentii WRL-024, Candida krusei WRL-038, Rhodotorula mucilaginosa WRL-053, Pichia anomala WRL-076, and Candida oleophila WRL-084, exhibited a wide range of activity inhibitory toward norsolorinic acid biosynthesis and accumulation (Fig. 1 and Table 1). Yeasts WRL-076 and WRL-038 were the most inhibitory to aflatoxin biosynthesis; the mycelium of the nor mutant in the center of the PDA inhibitor plate was completely white (Fig. 1B and C, respectively). Yeasts WRL-084 and WRL-015 were less inhibitory, and red-orange color was observed in the fungal mycelium (Fig. 1D and E, respectively). Yeasts WRL-024 and WRL-053 were not very inhibitory, and large amounts of norsolorinic acid accumulated in the mycelium (Fig. 1F and G, respectively).
The norsolorinic acid synthesized and accumulated by the nor mutant is probably localized on the membrane inside the fungal hypha (12, 16). It is unlikely that the yeast streaks on the agar can degrade this compound, since it does not diffuse out from the nor mutant. We also determined the pH of the agar near the yeast streaks. To do this, five agar discs near the streak of yeast were cored, transferred to a microcentrifuge tube, and mixed with 0.5 ml of distilled H2O. After centrifugation at 14,000 rpm in an Eppendorf centrifuge for 10 min at 4°C, the pH of the supernatant was measured in a pH meter (Table 1). The pH readings near the most effective yeasts, WRL-038 and WRL-076, were 5.2 and 4.4, respectively. Acidic pH favors aflatoxin synthesis in toxigenic strains of Aspergillus spp. and norsolorinic accumulation in the nor mutant (4, 13). The nor mutant of A. flavus Papa 827 grown between the streaks of these two yeasts was expected to produce and accumulate larger quantities of norsolorinic acid so that more red-orange pigment would be seen in the agar. However, the opposite was observed. The fungal cultures had very little visible red-orange color (Fig. 1B and C). Therefore, the pH of the agar surrounding the nor mutant was not a determining factor for inhibiting the production of norsolorinic acid. Instead, we think that diffusible metabolites produced by the yeasts mediate the inhibitory effect.
To measure growth inhibition of the nor mutant, we determined the dry weight of the fungal mass in the agar between the two streaks of yeast. Five agar discs (7-mm diameter) of fungal mycelium were cored and transferred to boiling water. The floating mycelial mat was washed once in boiling water to remove agar traces and air dried at room temperature for 16 h. The dry weight of the fungal mass depended upon the yeast strain in the petri dish (Table 1). Growth and norsolorinic acid were not correlated (r = 0.43 and p = 0.18 based on the Kendall correlation), so the yeasts must affect norsolorinic acid accumulation in some manner other than simply reducing the amount of biomass available to accumulate the compound.
We selected the toxigenic strain A. flavus 42-12 to test the effectiveness of the six yeasts for inhibiting aflatoxin biosynthesis in the bioassay. Four agar discs (7-mm diameter) of fungal cultures were cored in the center of a petri dish and transferred to a glass tube containing methanol. Aflatoxin was extracted and analyzed by high performance liquid chromatography (20). When grown on PDA, strain 42-12 produced 4,800 ng of aflatoxin per four discs. Aflatoxin production was drastically reduced by yeasts WRL-076 and WRL-038 and moderately reduced by WRL-015, WRL-053, and WRL-084 (Table 1). The findings are consistent with that of the visual bioassay with the exception of WRL-053 in that the norsolorinic acid accumulated to a fairly high level in the nor mutant but aflatoxin production of 42-12 was much lower (Fig. 1 and Table 1).
The visual bioassay described here provides a simple and useful monitoring system for screening large numbers of yeast strains for their effectiveness in blocking early steps of the polyketide pathway in the biosynthesis of aflatoxin by A. flavus. The nor mutant also is safer for use in routine experiments than is a wild-type strain of A. flavus, since the mutant produces little if any aflatoxin during routine laboratory culture. This report is the first to demonstrate that yeasts can interact with A. flavus to reduce aflatoxin levels. Applications of these yeasts for reducing aflatoxin contamination of food commodities warrants further research.
ACKNOWLEDGMENTS
We thank B. C. Campbell, T. A. McKeon, J. Roitman, and S. Schwimmer for helpful comments on the manuscript; N. Keller (Texas A&M University) for providing the nor mutant and valuable discussion; D. Bhatnagar for providing norsolorinic acid; M. T. Smith (Centraalbureau voor Schimmelcultures, Yeast Division, Delft, The Netherlands) for identifying yeasts; and L. Whitehand for performing statistical analysis.
REFERENCES
- 1.Bennett J W. Aflatoxin and anthraquinones from diploids of Aspergillus parasiticus. J Gen Microbiol. 1979;113:127–136. doi: 10.1099/00221287-113-1-127. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J W, Bhatnagar D, Chang P K. The molecular genetics of aflatoxin biosynthesis. In: Powell K A, Renwick A, Peberdy J F, editors. The genus Aspergillus. New York, N.Y: Plenum Press; 1994. pp. 51–58. [Google Scholar]
- 3.Bhatnagar D, Cary J W, Chang P-K, Chu F S, Cleveland T E, Keller N P, Linz J E, Payne G A, Woloshuk C P. Consolidated information on aflatoxin pathway genetics. In: Robens J F, Cleveland T E, editors. Proceedings of the Aflatoxin Elimination Workshop. 1996. pp. 72–75. [Google Scholar]
- 4.Cotty P J. Aflatoxin and sclerotia production by Aspergillus flavus: influence of pH. Phytopathology. 1988;78:1250–1253. [Google Scholar]
- 5.Cotty P J, Bayman P, Egel D S, Elias K S. Agriculture, aflatoxins and Aspergillus. In: Powell K A, Renwick A, Peberdy J F, editors. The genus Aspergillus. New York, N.Y: Plenum Press; 1994. pp. 1–27. [Google Scholar]
- 6.Diener U L, Cole R J, Sanders T H, Payne G A, Lee L S, Klich M A. Epidemiology of aflatoxin formation by Aspergillus flavus. Annu Rev Phytopathol. 1987;25:249–270. [Google Scholar]
- 7.Doster M A, Michailides T J. Aspergillus molds and aflatoxins in pistachio nut in California. Phytopathology. 1994;84:583–590. [Google Scholar]
- 8.Ellis W O, Smith J P, Simpson B K. Aflatoxins in food: occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit Rev Food Sci Nutr. 1991;30:403–439. doi: 10.1080/10408399109527551. [DOI] [PubMed] [Google Scholar]
- 9.Gradziel T M, Wang D. Susceptibility of California almond cultivars to aflatoxigenic Aspergillus flavus. Hortscience. 1994;29:33–35. [Google Scholar]
- 10.Horn B W, Dorner J W, Green R L, Blankenship P D, Cole R. Effect of Aspergillus parasiticus soil inoculum on invasion of peanut seeds. Mycopathologia. 1994;125:179–191. doi: 10.1007/BF01146524. [DOI] [PubMed] [Google Scholar]
- 11.Katz H, Berkovitz A, Chalutz E, Droby S, Hofstein R, Keren-Tzoor M. Compatibility of Ecogen’s biofungicide ASPIR™, a yeast based preparation, with other fungicides commonly used for the control of postharvest decay of citrus. Phytopathology. 1995;85:1123. [Google Scholar]
- 12.Keller N P, Butchko R A E, Sarr B, Phillipp T D. A visual pattern of mycotoxin production in maize kernels by Aspergillus spp. Phytopathology. 1994;84:483–488. [Google Scholar]
- 13.Keller N P, Nesbitt C, Sarr B, Phillips T D, Burow G B. pH regulation of sterigamatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology. 1997;87:643–648. doi: 10.1094/PHYTO.1997.87.6.643. [DOI] [PubMed] [Google Scholar]
- 14.Leibinger W, Breuker B, Hahn M, Mendgen K. Control of postharvest pathogens and colonization of apple surface by antagonistic microorganisms in the field. Phytopathology. 1997;87:1103–1110. doi: 10.1094/PHYTO.1997.87.11.1103. [DOI] [PubMed] [Google Scholar]
- 15.Liu B-H, Chu F S. Regulation of aflR and its product, AflR, associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1998;64:3718–3723. doi: 10.1128/aem.64.10.3718-3723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyers D M, Obrian G, Du W L, Bhatnagar D, Payne G A. Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl Environ Microbiol. 1998;64:3713–3717. doi: 10.1128/aem.64.10.3713-3717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papa K E. Genetics of Aspergillus flavus: linkage of aflatoxin. Can J Microbiol. 1984;30:68–73. doi: 10.1139/m84-012. [DOI] [PubMed] [Google Scholar]
- 18.Payne G A, Thompson D L, Lillehoj E B, Zuber M S, Adkins C R. Effect of temperature on the preharvest infection of maize kernels by Aspergillus flavus. Phytopathology. 1988;78:1376–1380. [Google Scholar]
- 19.Roberts R G. Post harvest biological control of gray mold of apple by Cryptococcus laurentii. Phytopathology. 1990;80:526–530. [Google Scholar]
- 20.Rodriguez S, Mahoney N E. Inhibition of aflatoxin production by surfactants. Appl Environ Microbiol. 1994;60:106–110. doi: 10.1128/aem.60.1.106-110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scudamore K A. Aspergillus toxins in food and animal feeding stuffs. In: Powell K A, Renwick A, Peberdy J F, editors. The genus Aspergillus. New York, N.Y: Plenum Press; 1994. pp. 59–71. [Google Scholar]
- 22.Smart M G, Wicklow D T, Caldwell R W. Pathogenesis in Aspergillus ear rot of maize: light microscopy of fungal spread from wounds. Phytopathology. 1990;80:1287–1294. [Google Scholar]
- 23.Sommer N F, Buchanan J R, Fortlage R J. Relation of early splitting and tattering of pistachio nuts to aflatoxin in the orchard. Phytopathology. 1986;76:692–694. [Google Scholar]
- 24.Wilson C L, Wisniewski M E. Biological control of postharvest diseases of fruits and vegetables: an emerging technology. Annu Rev Phytopathol. 1989;27:425–451. [Google Scholar]
- 25.Woloshuk C P, Foutz K R, Brewer J F, Bhatnagar D, Cleveland T E, Payne G A. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl Environ Microbiol. 1994;60:2408–2414. doi: 10.1128/aem.60.7.2408-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]