Abstract
Piriformospora indica (Hymenomycetes, Basidiomycota) is a newly described cultivable endophyte that colonizes roots. Inoculation with the fungus and application of fungal culture filtrate promotes plant growth and biomass production. Due to its ease of culture, this fungus provides a model organism for the study of beneficial plant-microbe interactions and a new tool for improving plant production systems.
Fungi interact with plants as pathogens or benefactors and may influence yields in agroforestry and floriculture. Knowledge concerning plant-growth-promoting cultivable root endophytes is low (7), and most studies have been conducted with mycorrhizal fungi. These mutualists improve the growth of crops on poor soils with lower inputs of chemical fertilizers and pesticides (2, 9). Arbuscular mycorrhizal (AM) fungi, forming the order Glomales of the Zygomycota (15), occur on the roots of 80% of vascular flowering plant species (19), but they are obligate biotrophs and cannot be cultured without the plant. In contrast, many ericoid and ectomycorrhizal fungi can be grown in pure culture, but their host spectrum is restricted to the Ericaceae or to woody plants (14).
Piriformospora indica Verma, Varma, Kost, Rexer & Franken, a plant root-interacting fungus, can be easily grown on various complex and minimal substrates, where it asexually forms chlamydospores containing 8 to 25 nuclei (28). Stages of a sexual life cycle have not been observed. Analysis for taxonomic position by molecular methods based on 18S rRNA sequences and by electron microscopy suggests that this fungus is related to the Hymenomycetes of the Basidiomycota. Our objectives in this study were to determine the pattern of root colonization by this fungus and to assess its effect on the growth of six plant species.
P. indica is deposited at the Deutsche Sammlung für Mikroorganismen und Zellkulturen, Braunschweig, Germany (DSM 11827). The fungus was grown on plates or in liquid culture containing a complete medium (21).
Seeds of maize (Zea mays L.), tobacco (Nicotiana tabaccum L.), and parsley (Petroselinum crispum L. ‘Hamburger Schnitt’) were surface sterilized for 2 min in ethanol followed by 10 min in a NaClO solution (0.75% Cl) and washed six times with sterile water. Seeds were germinated on water-agar plates (0.8% Bacto Agar; Difco, Detroit, Mich.) at 25°C in the dark. Plantlets of tobacco, Artemisia annua L., and Bacopa monnieri L. were grown from calli on a standard medium for micropropagation as described by Varma (27). H. Rennenberg (University of Freiburg) kindly provided micropropagated poplar (Populus tremula L.) plantlets. After germination or micropropagation, plantlets of maize (1 week after germination), tobacco and parsley (2 weeks after germination), and A. annua, B. monnieri, and poplar (4 weeks of micropropagation) were placed in pots (9-cm height by 10-cm diameter) containing expanded clay (2 to 4 mm in diameter). One gram of wet fungal mycelium was mixed with 100 ml of substrate; in control pots, autoclaved mycelium was added. Plants were grown in a growth chamber under the following constant conditions: 75% relative humidity, 25°C, 16-h day (300 μmol m−2 s−1), and weekly fertilization with Long Ashton solution (12). Roots of maize and tobacco were harvested 4, 7, and 15 days after inoculation and stained with chlorazol black E (4).
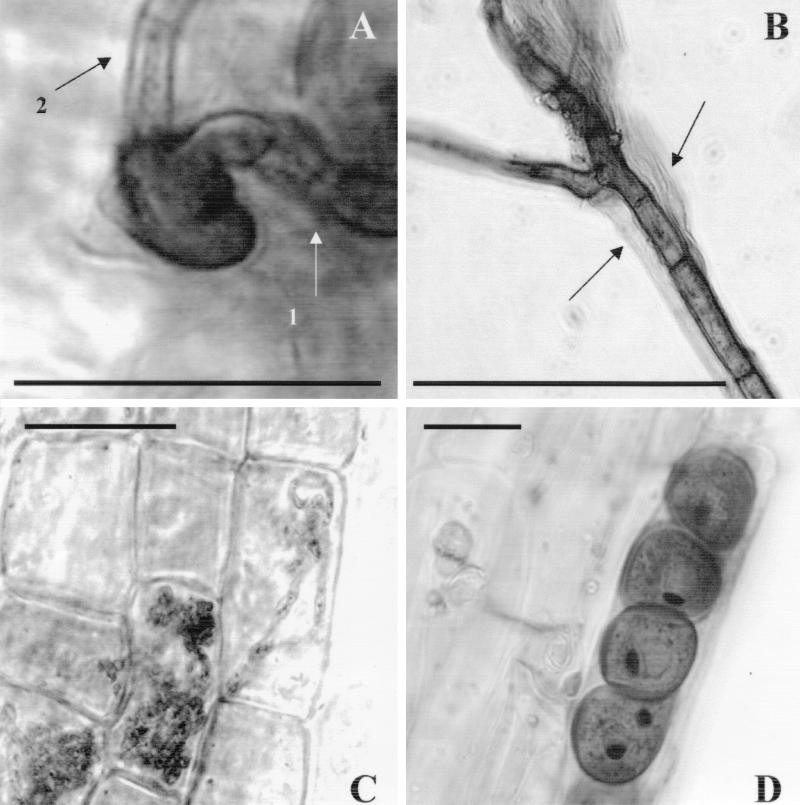
Colonization was observed under a light microscope. Control plants contained no fungal structures. When hyphae of P. indica contacted roots, they developed appressoria (Fig. 1A) and roots were colonized intercellularly (Fig. 1B). The fungus also colonized cortex cells intracellularly, where it formed coils and branches (Fig. 1C) or round bodies (Fig. 1D), but a typical arbuscule such as that developed by the Glomales (3, 10) was not observed. The round bodies, however, might function as storage organs, as has been considered for the intraradical vesicles of the Glomineae (29). P. indica never invaded the stelar tissue nor traversed upwards into the shoot. We did not determine whether P. indica causes invagination of the host plasma membrane during colonization, as do many other biotrophic organisms, or if it interacts with plant roots as a necrotroph or a saprophyte.
FIG. 1.
Plant colonization by P. indica. Maize plants were inoculated with P. indica. Roots were harvested 4 (A), 7 (B), and 16 (C and D) days after inoculation, stained, and observed by light microscopy. Bars, 10 μm. (A) A fungal appressorium attached to the root surface. Arrow 1 indicates a hypha leading to the appressorium; arrow 2 indicates a hypha leading away from the appressorium into the plant. (B) A root segment showing intercellular hyphae. Arrows indicate plant cell walls. (C) Cortical cells with coiled and branched intracellular hyphae. (D) Cortical cells showing round bodies.
Four weeks after inoculation, whole plants (all species) were harvested and divided into roots and shoots, and fresh weights of each were determined. The root and shoot biomasses for the P. indica treatment were about twice those of the controls (Table 1). Expanded clay was used as a substrate for the experiment whose results are shown in Table 1; however, similar growth responses were also obtained when plants were cultivated on other substrates, e.g., vermiculite and commercially available garden soils (data not shown). Germinated tobacco seeds also were directly inoculated on water agar by placing disks (1-cm diameter) from complete medium with and without the fungus close to the roots. Seedlings were harvested after 3 weeks of further incubation in a growth chamber. Root and shoot weights from these seedlings were similar to those grown in pot cultures (Table 1).
TABLE 1.
Growth-promoting effect of P. indica
| Plant | Treatment | Fresh wt (g)
|
|
|---|---|---|---|
| Shoot | Root | ||
| Maizea | Control | 45 ± 3.1 | 48 ± 2.5 |
| P. indica | 90 ± 6.7 | 75 ± 4.8 | |
| Tobaccoa | Control | 4.3 ± 0.3 | 3.4 ± 0.2 |
| P. indica | 7.8 ± 0.5 | 6.2 ± 0.4 | |
| Bacopaa | Control | 2.7 ± 0.1 | 2.0 ± 0.2 |
| P. indica | 7.8 ± 0.4 | 6.9 ± 0.5 | |
| Artemisiaa | Control | 8.6 ± 0.5 | 4.0 ± 0.3 |
| P. indica | 15 ± 1.2 | 9.9 ± 0.8 | |
| Parsleya | Control | 2.6 ± 0.2 | 2.1 ± 0.1 |
| P. indica | 5.9 ± 0.3 | 5.1 ± 0.3 | |
| Poplara | Control | 4.6 ± 0.2 | 2.4 ± 0.2 |
| P. indica | 8.7 ± 0.5 | 4.6 ± 0.3 | |
| Tobacco seedlingsb | Control | 1.2 ± 0.2 | 0.9 ± 0.2 |
| P. indica | 4.3 ± 0.3 | 1.4 ± 0.3 | |
| Tobacco plantletsc | Control | 3.9 ± 0.1 | |
| P. indica | 5.9 ± 0.1 | ||
| Maizea | Control | 45 ± 3.8 | 51 ± 3.4 |
| Culture filtrate | 97 ± 7.5 | 48 ± 3.2 | |
Mean values and standard deviations from 10 different plants grown in pots for 4 weeks.
Mean values and standard deviations from 10 different seedlings grown on agar plates for 3 weeks.
Mean values and standard deviations from seven different plantlets grown from calli on agar plates for 4 weeks.
Calli of tobacco plants were placed on MS medium without any supplements (18) close to agar disks with the fungus. For control treatments, the agar disks were empty, and the medium was supplemented with benzyl amino purine (2 mg/liter) and naphthaleneacetic acid (0.1 mg/liter). Seven replications were carried out, and culture bottles were incubated under the following controlled conditions: 60% relative humidity, 24°C, 16-h day (2,000 lx). Calli of fungus-treated tobacco rooted earlier (within 7 to 10 days) than those of controls supplemented with growth regulators (30 days and later). Biomass production by fungus-treated calli increased by approximately 50% after 4 weeks (Table 1). Six weeks after inoculation, three plantlets per replication (control and inoculated) were transferred to plastic pots (10-cm height by 6-cm diameter) filled with a mixture of sterile soil and sand (3:1; garden soil from the Jawaharlal Nehru University campus and acid-washed river bed sand). Pots were placed in a mist chamber (90% relative humidity, 24°C, 16-h day [1,000 lx]). After 4 weeks, microscopical analysis of chlorazol black E-stained roots showed that 87% ± 10% (mean ± standard deviation) of the cortex was colonized by the fungus. The survival rate of P. indica-inoculated plantlets was 95%, but only 57% of untreated plantlets survived the transfer to pots.
We analyzed culture filtrates to determine whether P. indica produces compounds that influence plant development. P. indica was grown for 2 weeks at 30°C under constant shaking (130 rpm) in 1 1 Erlenmeyer flasks with 500 ml of liquid minimal medium (21). Flask contents were filtered through a normal filter paper (595½; Schleicher & Schuell, Dassel, Germany), and the resulting filtrate was then pressed through a 0.2-μm-pore-size filter (Millipore). Maize plants were grown on expanded clay and fertilized three times per week with 1 ml of culture filtrate per 100 ml of substrate. Control plants were treated in parallel with minimal medium. Plants were harvested after 4 weeks, and fresh weight was measured (Table 1). The effect of culture filtrate alone on shoot development was similar to that of inoculation with the fungus. Root growth, however, was not affected by the culture filtrate. Production of substances which influence plant development has been observed by various plant-interacting fungi, including plant pathogens (5), ectomycorrhizal fungi (23), and AM fungi (1). AM fungi, however, improve not only plant development but also their nutrition (17) by bidirectional transfer of nutrients (24) and their health by protection against pathogens (13). Whether P. indica, which can regulate development, is also able to act as a biofertilizer or as a bioprotector remains unknown.
P. indica has growth-promoting effects on a broad range of plants, as do the AM fungi, but has the added trait of being able to be grown in axenic cultures. Most studies concerned with root endophytes beyond mycorrhiza were up to now carried out on fungi in plants of alpine and subalpine regions (22, 26) which were later also detected in other ecosystems (20). A positive influence on plant growth could be shown but seemed to be species specific (11). In some instances, other fungi were also studied, but those reports concerned only the effect on one plant and/or the enhancement of plant growth was relatively low (6, 8, 16, 25). We suggest that P. indica is a good candidate to improve commercial plant production and might be especially useful in agroforestry and flori-horticulture applications. Research on the cellular and molecular basis of the interaction should contribute to the understanding of the beneficial associations between plants and fungi.
Acknowledgments
We thank H. Rennenberg (Freiburg) for providing us with micropropagated poplar plants, as well as P. Bonfante (Torino) and H. Steele (Marburg) for critically reading the manuscript.
A. Varma, Sudha, and N. Sahay are thankful to the government of India (Department of Biotechnology and University Grant Commission) for partial funding.
REFERENCES
- 1.Barea J M, Azcon-Aguilar C. Production of plant growth-regulating substances by the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Appl Environ Microbiol. 1982;43:810–813. doi: 10.1128/aem.43.4.810-813.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethlenfalvay G J. Mycorrhizae and crop productivity. In: Bethlenfalvay G J, Linderman R G, editors. Mycorrhizae in sustainable agriculture. Madison, Wis: American Society of Agronomy; 1992. pp. 1–27. [Google Scholar]
- 3.Bonfante P, Perotto S. Strategies of arbuscular mycorrhizal fungi when infecting host plants. New Phytol. 1995;130:3–21. [Google Scholar]
- 4.Brundrett M C, Piche Y, Peterson R L. A new method for observing the morphology of vesicular-arbuscular mycorrhizae. Can J Bot. 1984;62:2128–2134. [Google Scholar]
- 5.Candau R, Avalos J, Cerdá-Olmedo E. Gibberellins and carotenoids in the wild type and mutants of Gibberella fujikuroi. Appl Environ Microbiol. 1991;57:3378–3382. doi: 10.1128/aem.57.11.3378-3382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewan M M, Sivasithamparan K. A plant growth promoting sterile fungus from wheat and rye grass with potential for suppressing take-all. Trans Br Mycol Soc. 1988;91:687–717. [Google Scholar]
- 7.Dix N J, Webster J. Fungal ecology. New York, N.Y: Chapman & Hall; 1995. [Google Scholar]
- 8.Gasoni L, Stegman de Gurfinkel B. The endophyte Cladorrhinum forcundissimum in cotton roots: phosphorus uptake and host growth. Mycol Res. 1997;101:867–870. [Google Scholar]
- 9.Gianinazzi S, Trouvelot A, Lovato P, Van Tuinen D, Franken P, Gianinazzi-Pearson V. Arbuscular mycorrhizal fungi in plant production of temperate agroecosystems. Crit Rev Biotechnol. 1995;15:305–311. [Google Scholar]
- 10.Gianinazzi-Pearson V, Gianinazzi S. Cellular and genetical aspects of the interactions between host and fungal symbionts in mycorrhizae. Genome. 1989;31:366–341. [Google Scholar]
- 11.Haselwandter K, Read D J. The significance of a root-fungus association in two Carex species of high-alpine plant communities. Oecologia. 1982;53:352–354. doi: 10.1007/BF00389012. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt E J. Sand and water culture methods. Technical communication no. 22 (2nd revised). London, United Kingdom: Commonwealth Agricultural Bureau; 1966. [Google Scholar]
- 13.Linderman R G. Role of VAM fungi in biocontrol. In: Pfleger F L, Linderman R G, editors. Mycorrhizae and plant health. St. Paul, Minn: APS Press; 1994. pp. 1–26. [Google Scholar]
- 14.Molina R, Massicotte H, Trappe J M. Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen M F, editor. Mycorrhizal functioning: an integrative plant-fungal process. New York, N.Y: Chapman & Hall; 1992. pp. 357–423. [Google Scholar]
- 15.Morton J B, Benny G L. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon. 1990;37:471–491. [Google Scholar]
- 16.Mucciarelli M, Scannerini S, Bricarello M, Varese C, Sacco T, Maffei M. In vitro growth promoting effects of a fungus (WSF) on the yield of Mentha X piperita. A chance for sustainable agriculture. G Bot Ital. 1995;129:121. [Google Scholar]
- 17.Mulongoy K, Gianinazzi S, Roger P A, Dommergues Y. Biofertilizers: agronomic and environmental impacts, and economics. In: Da Silva E, Sasson A, Ratledge C, editors. Microbial technology: economic and social aspects. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 59–69. [Google Scholar]
- 18.Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:431–487. [Google Scholar]
- 19.Newman E I, Reddel P. The distribution of mycorrhizas among families of vascular plants. New Phytol. 1987;106:745–751. doi: 10.1111/j.1469-8137.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 20.O’Dell T E, Trappe J M. Root endophytes of lupine and some other legumes in Northwestern USA. New Phytol. 1992;122:479–485. doi: 10.1111/j.1469-8137.1992.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 21.Pontecorvo G, Roper J A, Hemmons L M, MacDonald K D, Bufton A W J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- 22.Read D J, Haselwandter K. Observations on the mycorrhizal status of some alpine plant communities. New Phytol. 1981;88:341–352. [Google Scholar]
- 23.Scagel C F, Linderman R G. Relationships between differential in vitro indole-acetic acid or ethylene production capacity by ectomycorrhizal fungi and conifer seedling responses in symbiosis. Symbiosis. 1998;24:13–34. [Google Scholar]
- 24.Smith S E, Gianinazzi-Pearson V, Koide R, Cairney J W G. Nutrient transport in mycorrhizas: structure, physiology and consequences for efficiency of the symbiosis. Plant Soil. 1994;159:103–113. [Google Scholar]
- 25.Sneh B, Zeidan M, Ichielevich-Auster M, Barash I, Koltin Y. Increased growth responses induced by a nonpathogenic Rhizoctonia solani. Can J Bot. 1986;64:2372–2378. [Google Scholar]
- 26.Stoyke G, Currah R S. Endophytic fungi from the mycorrhizae of alpine ericoid plants. Can J Bot. 1990;69:347–352. [Google Scholar]
- 27.Varma A. Mycorrhizae: their application in micropropagated plantlets. Crit Rev Biotechnol. 1995;15:179–199. [Google Scholar]
- 28.Verma S, Varma A, Rexer K-H, Hassel A, Kost G, Sarbhoy A, Bisen P, Bütehorn B, Franken P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia. 1998;90:898–905. [Google Scholar]
- 29.Walker C. AM or VAM: what’s in a word? In: Varma A, Hock B, editors. Mycorrhizas: structure, function, molecular biology and biotechnology. Heidelberg, Germany: Springer Verlag; 1995. pp. 25–26. [Google Scholar]