Abstract
Cottage cheese whey is a cheese industry by-product still rich in proteins and lactose. Its recycling is seldom cost-effective. In this work we show that the lactose-utilizing yeast Kluyveromyces lactis, engineered for production of recombinant human lysozyme, can be grown in cottage cheese whey, resulting in high-level production of the heterologous protein (125 μg/ml).
The food industry produces a considerable amount of by-products that are still rich in organic substances. Although they are a potential source both for the extraction of valuable compounds and for the production of edible biomass, similar by-products are often discarded for economic reasons (6). This is also the case for cheese whey (CW) and cottage cheese whey (CCW), which are the major by-products of the cheese-making industry. CW, which is still rich in proteins, lactose, vitamins, and minerals (16), has been used in a variety of valorization processes such as drying and constituent extraction (19) and production of biomass (3, 14, 21), butanol (13), ethanol (18), and glycerol (10). However, owing to the high collection costs and the seasonal fluctuations in production, it is often discarded in sewage or in the environment, causing water and soil pollution (5, 6). CCW, which is still rich in lactose and minerals but contains smaller amounts of proteins, remains largely unused apart from a few experimental applications (4, 8, 12).
In this work we have used CW and CCW as culture media for growing Kluyveromyces lactis strains, engineered to produce recombinant human lysozyme (h-Lys). Luxuriant growth was obtained in both media, with the secretion of large amounts of the heterologous protein and the production of microbial biomass, suggesting that CW and CCW could be profitably recycled for production of valuable heterologous proteins by engineered microorganisms. A simple protocol for purification of the recombinant h-Lys from the whey culture supernatant is also described.
The K. lactis strains used in this work, K6 and K7, have been previously described (20). Briefly, they are K. lactis WM37 derivatives stably transformed with an integrative expression vector that directs production of recombinant h-Lys as a soluble protein secreted into the culture medium. In this vector, expression of the heterologous cDNA is under the control of the galactose-inducible K. lactis GAL7 promoter. The two strains differ in the copy number and integration pattern of the expression cassettes and produce different amounts of h-Lys in defined culture media (20).
Yeast strains were grown in synthetic defined (SD) medium, CW, or CCW. SD medium was made of Yeast Nitrogen Base (Difco Laboratories, Detroit, Mich.), 6.7 g/liter, supplemented with glucose (0.2 g/liter) (SD-GLU), galactose (45 g/liter) (SD-GAL), or lactose (45 g/liter) (SD-LAC) as the carbon source. CW and CCW were from ewe’s milk. They were collected at the time of production, stored at 4°C, and used within 2 days. Under these conditions no significant decrease in the lactose concentration caused by the contaminant microbial flora was observed. The reducing sugar concentration was determined by the method of Miller (15), with lactose as a standard. The total protein concentration was determined by the method of Bradford (2), with bovine serum albumin as a standard. The total microbial count present in CW and CCW was determined by an agar-inclusion assay using plate count agar medium. Compared with CW, CCW contained smaller amounts of proteins and lipids and also had a lower microbial count owing to the temperature and pH conditions used for preparation of the cottage cheese (heating up to 90°C for 10 min at pH 4.5). Sterilized CW and CCW were prepared by autoclaving at 121°C for 15 min, followed by removal of any insoluble material by centrifugation at 10,000 × g for 15 min at room temperature.
Biomass and recombinant protein production by K. lactis K6 and K7 grown in CW and CCW.
Starter yeast cultures of each strain were prepared by growing cells in SD-GLU overnight at 28°C in an orbital shaker (New Brunswick Scientific, Edison, N.J.) at 150 rpm. Each starter culture was used to inoculate CW, CCW, SD-GAL, and SD-LAC. The inoculation was carried out at a ratio of 1:8 (100 ml of starter culture in 700 ml of fresh medium), which was suitable for preventing the outgrowth of the contaminating bacteria present in unsterilized whey. The cultures were grown at 28°C for 42 h at 150 rpm, and several parameters, including biomass yield and the concentrations of lysozyme, reducing sugars, and total protein in the culture supernatant, were monitored at various time intervals. The biomass yield was determined as wet weight after centrifugation at 10,000 × g. Lysozyme activity was assayed by the lysoplate method using Micrococcus luteus AH-47 as the substrate (17). Briefly, M. luteus cells were suspended in saline, autoclaved at 121°C for 15 min, washed twice in the same manner, and added to tryptose phosphate agar medium to obtain a final optical density (A590) of 1. The molten medium was poured into petri dishes to obtain a layer of 6 mm. Samples were inoculated into 4-mm-diameter wells cut into the solidified medium. The amount of enzyme was indicated by the diameter of the halo of micrococcal lysis formed around the wells after incubation for 24 h at 37°C. Purified human lysozyme (Sigma Chemical Co., St. Louis, Mo.) was used to prepare a calibration curve in the concentration range 0.1 to 100 μg/ml. The yeast cell count at the end of each experiment was determined on Sabouraud dextrose agar (Difco), and the degree of contamination by other microorganisms was estimated from the difference between total microbial counts and yeast cell counts.
The amounts of h-Lys obtained by growing K. lactis K6 in unsterilized CW or CCW were lower (by about 75%) than those obtained with K7, and for this reason the former strain was not investigated further (data not shown).
The results obtained by growing K. lactis K7 on unsterilized CCW are shown in Table 1. The maximum biomass amount (≅24 g/liter) was obtained after 36 h and remained constant thereafter. Sugar was rapidly utilized, becoming undetectable at 30 h, while the total protein concentration was reduced to approximately 50% of the initial value by the same time. No basal bacteriolytic activity was detectable in the medium. h-Lys was detectable in the culture supernatant after 14 to 18 h, and its concentration reached a peak of up to about 130 μg/ml after 30 h. Subsequently, the h-Lys concentration in the supernatant of unsterilized CCW showed a decrease, being reduced to less than 70% of the peak value after 36 h. This phenomenon could be due to some proteolytic degradation occurring in the late stages of the culture; it did not occur in sterilized CCW or in other sterilized media. At the end of fermentation, the total microbial count-to-yeast count ratio was always <1.5. No significant differences were observed when CW was used instead of CCW (Table 2). Only unsterilized CCW showed a ratio higher than 1. When K. lactis K7 was grown on either SD-GAL or SD-LAC, the maximum amount of h-Lys produced was lower than that obtained in whey.
TABLE 1.
Biomass yield and h-Lys, reducing sugar, and protein concentration in unsterilized CCW used to grow engineered K. lactis
| Time (h) of fermentation | Scorea for the following parameter:
|
|||
|---|---|---|---|---|
| Lactose (g/liter) | Total protein (g/liter) | Biomass (g [wet wt]/liter) | h-Lys (mg/liter) | |
| 0 | 46 | 6.2 | <1 | ND |
| 6 | 42 | 5.9 | <1 | ND |
| 12 | 31 | 5.4 | 2 | ND |
| 18 | 18 | 4.4 | 4 | 5 |
| 24 | 7 | 3.5 | 6 | 14 |
| 30 | <1 | 3.1 | 11 | 127 |
| 36 | <1 | 3.0 | 24 | 85 |
ND, not detectable.
TABLE 2.
Differential production of h-Lys and biomass by K. lactis K7 grown on CW, CCW, or synthetic media
| Medium | Maximum amt detected in the fermentation process
|
Ratio of total microbial count/yeast count | ||
|---|---|---|---|---|
| Lysozyme (mg/liter) | Biomass (g [wet wt]/liter) | Yeast cell count | ||
| Sterilized CW | ∼100 | 28 | 1.3 × 1011 | 1.0 |
| Sterilized CCW | ∼125 | 29 | 1.0 × 1011 | 1.0 |
| Unsterilized CCW | ∼130 | 24 | 2.9 × 1010 | 1.3 |
| Synthetic medium (galactose) | ∼50 | 7 | 1.8 × 1010 | 1.0 |
| Synthetic medium (lactose) | ∼50 | 15 | 1.5 × 1010 | 1.0 |
Purification and characterization of h-Lys from whey cultures.
The h-Lys produced by K. lactis K7 grown in CCW was purified as follows. The culture supernatant was centrifuged at 6,000 × g for 15 min at 4°C. The supernatant was adjusted to 40% ammonium sulfate saturation, and the precipitate was removed by centrifugation at 10,000 × g for 20 min at 4°C. The ammonium sulfate saturation in the supernatant was then adjusted to 80%, and the precipitate was collected as described above. The pellet of the second precipitation, which contained the bacteriolytic activity, was resolubilized in 20 mM phosphate buffer (PB), pH 7.0 (1/20 of the original volume), dialyzed against 50 volumes of the same buffer for 14 h at 4°C, and loaded onto a carboxymethyl–Sephadex G-50 (Pharmacia Biotech, Uppsala, Sweden) column (90 by 3 cm) equilibrated with 50 mM PB, pH 7.0. The column was washed with 50 mM PB, pH 8.0, and eluted with a 0.1 to 1 M NaCl gradient in the same buffer. Most of the contaminating proteins cofractionated with h-Lys following the ammonium sulfate precipitation, did not bind to the carboxymethyl-Sephadex matrix, or were eluted at low NaCl concentrations. Fractions containing the bacteriolytic activity were eluted in a single peak at 0.5 M NaCl (data not shown). These fractions were pooled, dialyzed against 20 mM PB, pH 7.0, and concentrated by ultrafiltration with an Amicon concentrator (model 8400; Amicon Inc., Beverly, Mass.) equipped with a 3,000-Da-cutoff membrane. The yield of the purification process, evaluated by comparison of the bacteriolytic activity in the CCW supernatant with that of the purified preparation, was reproducibly around 15%.
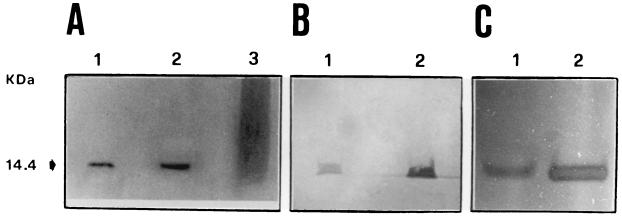
In sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the purified h-Lys preparation appeared as a single band with an Mr of ≅14,000 and was estimated to be >95% pure (Fig. 1A). Immunoblot analysis of the purified h-Lys preparation confirmed its reactivity with the anti-h-Lys antiserum (Fig. 1B). Zymogram analysis of the purified h-Lys preparation after renaturing SDS-PAGE confirmed the bacteriolytic activity of the 14-kDa band (Fig. 1C). SDS-PAGE was performed according to the method of Laemmli (11) with an acrylamide concentration of 5% (wt/vol) in the stacking gel and of 15% in the separating gel. Immunoblotting was performed according to the method of Towbin et al. (22) and Tsang et al. (23) with a semi-dry transfer apparatus (Bio-Rad Laboratories, Richmond, Calif.), nylon membranes, and a goat anti-h-Lys antiserum (Calbiochem, San Diego, Calif.). The antibody was revealed by use of an alkaline phosphatase-protein G conjugate (Calbiochem) and 5-bromo-4-chloro-3-indolyl phosphate–nitroblue tetrazolium as a substrate (Boehringer, Mannheim, Germany). Zymography after renaturing SDS-PAGE was performed as previously described (1).
FIG. 1.
Detection of purified h-Lys produced by engineered K. lactis. (A) Coomassie blue-stained PAGE. Lane 2, h-Lys highly purified by the column chromatography protocol employed; lane 1, commercial human lysozyme; lane 3, unpurified lysozyme. (B) Western blot of purified h-Lys. Lane 1, h-Lys from K. lactis; lane 2, commercial human lysozyme. (C) Zymogram of purified h-Lys. In this picture the enzyme appears as a halo of bacteriolytic activity on the M. luteus-containing regenerating polyacrylamide gel. Lane 1, K. lactis purified h-Lys; lane 2: commercial lysozyme.
Concluding remarks.
Recycling of whey for biomass production with lactose-utilizing microorganisms is a well-established process (3–5, 7, 8, 10, 14, 18). However, little information is available concerning the use of CW and CCW for growth of engineered microorganisms to produce valuable heterologous proteins, which could greatly improve the cost-effectiveness of whey recycling. Results of this work indicated that both CW and CCW can be used successfully for a similar application in combination with a K. lactis strain genetically engineered for heterologous gene expression. In fact, under the small-scale laboratory conditions evaluated in this work the process yielded considerable amounts of the heterologous protein, which were significantly higher than those obtained by growing the same strain in defined laboratory media (reference 20 and present results). Since large-scale fermentation technology for K. lactis in whey is well established (5, 9), it is possible that, under optimized fermentation conditions, even higher protein yields could be obtained. In our experiments, even unsterilized CCW was found to be suitable as a fermentation medium provided that it was kept refrigerated and used reasonably soon after collection. However, it should be considered that, in this case, the heterologous protein was an enzyme with antibacterial activity, while production of recombinant products lacking antibiotic activity could require a whey sterilization step or the use of more inoculum, with an eventual impact on the economy of the process.
With a similar expression system, in which the recombinant product is secreted into the medium during yeast growth, the generated biomass does not need to be processed for protein extraction and can be used as an animal food ingredient (9), resulting in an optimal productivity of the recycling process.
Acknowledgments
We are grateful to the Podda Company (Cagliari) for kindly supplying the CW and CCW used to perform this study. Thanks are due to Cesira Galeotti (Chiron-Biocine, Siena, Italy) for critical discussions and advice. The technical assistance of R. Murru is also acknowledged.
REFERENCES
- 1.Berlutti F, Thaller M C, Rossolini G M, Pantanella F, Schippa S, Pezzi R. Production of bacteriolytic enzymes as a tool for characterizing enterococci. J Appl Bacteriol. 1996;80:447–452. doi: 10.1111/j.1365-2672.1996.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Champagne C P, Goulet J, Lachance R A. Production of baker’s yeast in cheese whey ultrafiltrate. Appl Environ Microbiol. 1990;56:425–430. doi: 10.1128/aem.56.2.425-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawel J, Kosikowski F V. Improving alcohol fermentation in concentrated ultrafiltration permeates of cottage cheese whey. J Food Sci. 1978;43:1717–1719. [Google Scholar]
- 5.Ghaly A E, Singh R K. Pollution potential reduction of cheese whey through yeast fermentation. Appl Biochem Biotechnol. 1989;22:181–203. doi: 10.1007/BF02921744. [DOI] [PubMed] [Google Scholar]
- 6.Glazer A N, Nikaido H. Disposal of whey. A case study. In: Glazer A N, Nikaido H, editors. Microbial biotechnology. Fundamentals of applied microbiology. New York, N.Y: W. H. Freeman and Company; 1995. pp. 44–46. [Google Scholar]
- 7.Guimaraes W V, Dudey G L, Ingram L O. Fermentation of sweet whey by ethanologenic Escherichia coli. Biotechnol Bioeng. 1992;40:41–45. doi: 10.1002/bit.260400107. [DOI] [PubMed] [Google Scholar]
- 8.Holsinger V H. Fortification of soft drinks with protein from cottage cheese whey. Adv Exp Med Biol. 1978;105:735–747. doi: 10.1007/978-1-4684-3366-1_35. [DOI] [PubMed] [Google Scholar]
- 9.Irvine D M, Hill R M. Cheese technology. In: Moo Young M, editor. Comprehensive biotechnology. Vol. 3. Oxford, United Kingdom: Pergamon Press; 1985. pp. 523–565. [Google Scholar]
- 10.Jenq W, Speckman R A, Crang R A, Steinberg M P. Enhanced conversion of lactose to glycerol by Kluyveromyces fragilis utilizing whey permeate as a substrate. Appl Environ Microbiol. 1989;55:573–578. doi: 10.1128/aem.55.3.573-578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Lindenfelser L A, Ciegler A. Penicillic acid production in submerged culture. Appl Environ Microbiol. 1977;34:553–556. doi: 10.1128/aem.34.5.553-556.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddox I S. Production of n-butanol from whey filtrate using Clostridium acetobutylicum N.C.I.B. 2951. Biotechnol Lett. 1980;2:493–498. [Google Scholar]
- 14.Michel A, Jacob F, Perrier J, Poncet S. Yeast production from crude sweet whey. Biotechnol Bioeng. 1987;30:780–783. doi: 10.1002/bit.260300611. [DOI] [PubMed] [Google Scholar]
- 15.Miller G L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;13:420–428. [Google Scholar]
- 16.Moulin G, Galzy P. Whey, a potential substrate for biotechnology. Biotechnol Genet Eng Rev. 1984;1:347–373. [Google Scholar]
- 17.Pompei R, Caredda E, Piras V, Serra C, Pintus L. Production of bacteriolytic activity in the oral cavity by nutritionally variant streptococci. J Clin Microbiol. 1990;28:1623–1627. doi: 10.1128/jcm.28.7.1623-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porro D, Martegani E, Ranzi B M, Alberghina L. Lactose/whey utilization and ethanol production by transformed Saccharomyces cerevisiae cells. Biotechnol Bioeng. 1992;39:799–805. doi: 10.1002/bit.260390802. [DOI] [PubMed] [Google Scholar]
- 19.Rosner G. Opération Rhône-Alpes de valorisation des sous-produits de l’agroalimentaire par l’alimentation animale, 2ème rapport. Lyons, France: Etablissement Publique Régional Rhône-Alpes; 1982. [Google Scholar]
- 20.Rossolini G M, Riccio M L, Gallo E, Galeotti C L. Kluyveromyces lactis rDNA as a target for multiple integration by homologous recombination. Gene. 1992;119:75–81. doi: 10.1016/0378-1119(92)90068-z. [DOI] [PubMed] [Google Scholar]
- 21.Tahoun M K, El-Merheb Z, Salam A, Youssef A. Biomass and lipids from lactose or whey by Trichosporon beigelii. Biotechnol Bioeng. 1987;29:358–360. doi: 10.1002/bit.260290311. [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang V C, Peralta J M, Simons A R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]