Abstract
The Schinus molle tree is notoriously invasive in most parts of the world, and yet as a pseudospice, its berries potentially possess some significant health benefits which need to be explored. Therefore, polar metabolome of seed + husks (SH), husks (H), and de-hulled (DH) berries were profiled and quantified by untargeted metabolomics approach using UPLC-QTOF-MS. A total of 13 gallotannins, three phenolic acids, a phenolic acid glucoside, three phenolic acid esters, an organic acid, a gallotannin derivative, and nine flavonoids were detected and quantified. Phenolic acids ranged between 12.2–295.7; 4.9–77; and 89.7–1613.1 mg/kg in SH, DH seeds and H respectively. Flavonoids ranged between 1.8–267.5; 73.4–80.4; and 124–564.3 mg/kg in SH, DH seeds and H respectively. Gallotannins ranged between 1.1–146.6; 14.8–21.8; and 48.1–664.8 mg/kg in SH, DH seeds and H respectively. Feruloyltartaric A, quercetin 3-O-glucuronide, catechin digalloylshikimic acid B as well as digalloyl quinic acid were some of the dominant secondary metabolites revealed. These results indicate that S. molle berries are a rich source of secondary metabolites with elevated concentrations in the husks, while DH seeds possess lower concentrations to none. These findings open important insights into the potential of S. molle berries as a natural source of antioxidants for the food and pharmaceutical industries.
Keywords: flavonoids, phenolic acids, pseudo-spices, Schinus molle, tannins, underutilised indigenous foods
1. Introduction
Urbanisation and globalisation impacts including the proliferation of unhealthy foods, poverty and ultimately poor diets are the leading causes of NCDs especially in poor countries [1]. In fact, diets that are lacking in vegetables and fruits, seeds, nuts, omega-3 fatty acid rich foods and whole grains are the diet related driving forces [1]. Diets lacking these components are normally high in trans-fatty acids, sugar and sodium/salt which are detrimental to health [2]. The annual costs of treating and managing NCDs in South Africa are about US$34.2 billion and this is 10% of South Africa’s Gross Domestic Product [3]. A change in diet is recommended by the WHO [4]. This includes a massive reduction in salt intake, increasing diets that are high in antioxidants, and polyphenolic compounds as well as increased physical activity. The replacement of salt with spices could potentially reduce the salt intake while increasing the nutritional and polyphenolic compounds of the diet [5].
Non-communicable diseases (NCDs) including chronic respiratory diseases, cancer, diabetes, and cardiovascular diseases are the current leading cause of mortality worldwide [6]. These diseases account for 70% (40 million people) of global deaths [6]. In developing countries, these deaths account for about 50% of people under the age of 70 [7]. This data is disturbing because it shows the failure of developing countries to shake off the impact of NCDs which have previously been known as the developed countries’ curse. The World Health Organization (WHO) [4] estimated that by 2023, the NCD burden would have increased by 17% globally and 27% in Africa. The African continent is one of the poorest regions in the world. In South Africa, NCDs account for 51% of premature deaths as follows: cardiovascular diseases (19%), cancer (10%), diabetes (7%), chronic respiratory diseases (4%) and 11% for other NCDs [8].
Culinary herbs and spices have been used from time immemorial to enhance flavour in food and/or for their nutritional and health benefits [5]. However, indigenous/ pseudo-spices are generally neglected and underutilised and yet they have the potential to compliment the diet and improve its nutritional and health benefits. The effects of NCDs can potentially be ameliorated as well. If these pseudo-spices are neglected, their potential health benefits may never be realised. Schinus molle L. berries are a typical pseudo spice which needs to be investigated. S. molle is an Andean tree species from the Anacardiaceae family. This family comprises 83 genera and 860 known species [9]. The genus Schinus itself comprises about 30 known species [10]. This is native to South America and has several English names such as pink peppercorns, pepper tree, California pepper tree, peppercorn tree, pepperina, Peruvian pepper tree, and Peruvian mastic tree, among others [11]. This tree has now spread around the globe to the Mediterranean, tropical and subtropical regions including South Africa. Although this species is notoriously invasive in the semi-arid savanna of South Africa, some beneficial culinary uses of the berries have been reported especially in its native habitat. Besides substituting black pepper corns, some numerous uses have been reported, especially in the Andean region. According to García and Ormazabal [11], the whole berries are used to flavour drinks and syrups; in Mexico, an alcoholic fermented beverage known as ‘copalote’ or ‘copalocle’ is produced, in Californian they are cooked with vegetables or used as a garnish because of their mild, sweet taste that is also aromatic, citric, fruity, and floral. The dry whole berries are also mixed with butter and asparagus to prepare fish. A vinaigrette can also be derived while desserts and biscuits can be prepared using these berries. A drink known as ‘chicha’ or ‘molle’ in Peru is extracted from the whole berries for festivities [12]. These berries are generally not known in South Africa for these culinary benefits and therefore their potential nutritional and therapeutic role. The folkloric uses of the whole berries include uses as a mouth wash, sedative, purging agent, diuretic, disinfectant, analgesic, sciatica, antispasmodic, anti-septic, and hypotensor [13]. Furthermore, the berries reportedly possess anti-inflammatory properties and can cure rheumatic diseases, bronchitis, asthma, stomach and liver complaints, swelling of legs and arms as well as wound healing [13].
Numerous biological activities of the whole berries have also been reported against some microorganisms and pests [13,14,15]. The studies of [16,17,18,19] demonstrate the antioxidant, anti-inflammatory and antimicrobial activity of the berries. Perhaps these activities are strongly related to the polyphenolic compositions of the leaves [20,21,22,23,24,25], bark [26], as well as leaves and berries [17,19,27,28,29] of the S. molle tree. Fewer studies of the berries have been conducted. Specifically, refs. [16,30] reported the essential oil extract compounds while [31] and [32] reported these compounds in alcoholic extracts. Studies on the potential of S. molle berries as a spice and their associated pharmacological properties as well as phytochemistry and ethnobotany in South Africa are scarce. The full potential of this pseudo-spice needs to be explored and its full potential documented in South Africa. The cultivars that are actively growing also need to be investigated. The present study was therefore conducted to profile and quantify the secondary metabolites present in the ripe and mature berries of the species growing in the Western Cape province of South Africa.
2. Materials and Methods
2.1. Chemicals
All the solvents and chemicals that were used, including Tetrafluoro acetic acid and formic acid were of LC-MS/analytical grade. From Millipore water purification system, ultrapure water with 18.2 MΩ cm−1 at 25 °C resistivity was used. Catechin standards, trifluoroacetic acetic acid, formic acid, methanol, and sodium formate were purchased from Sigma Chemicals, Johannesburg, South Africa.
2.2. Plant Materials
Ripe and mature S. molle berries were collected from trees growing around Somerset West (34°05′ S 18°51′ E), Cape Town, South Africa in the month of April and May 2021 (Figure 1). The samples were put in sampling bags and immediately transported to the Cape Peninsula University of Technology laboratory for further processing. On arrival, the samples were separated into husks (H) and dehulled seeds (DH) while some were left in their original state, i.e., seed + husks (SH). The samples were dried at 50 °C in a dust free forced-draft oven to a constant weight. Samples were then finely ground and stored in glass vials in a refrigerator at 4 °C prior to further processing.
Figure 1.
(A)—S. molle tree; (B)—Ripe S. molle berries on the tree branches; (C)—Husks of S. molle berries; (D)—Dehulled S. molle berries.
2.3. Sample Methanolic Extraction
About 1 g of dry and ground S. molle samples was homogenised with 10 mL of methanol (80:20 (v/v)) containing 1% trifluoroacetic acid (TFA) using an E-UC13-HD-D Eins Sci (South Africa) ultrasonic bath. This was accomplished at 300 W and 35 °C for 30 min. Constant agitation on a shaker for 24 h then followed. To remove debris, the sample was vortexed and centrifuged (Hermle Z160M, Hermle LaborTechnik, Wehinger, Germany) at 5000× g/10 min. The supernatant was then filtered through a 0.22 μm Millipore filter and stored at −20 °C for UPLC-PDA-MS analysis and for the quantification of phytochemicals.
2.4. UPLC-QTOF-MS Profiling and Quantification
The experiment was conducted on a Waters Synapt G2 Quadrupole Time-of-Flight (QTOF) coupled to a Waters Acquity Ultra-Performance Liquid Chromatograph (UPLC) using the methods of [33,34]. Briefly, the column eluate was first detected by a Photodiode Array (PDA) prior to going through the MS, thus allowing for the simultaneous collection of MS and UV spectra. In negative mode, electrospray ionisation was used with a desolvation gas at 650 L/h and 275 °C respectively and a cone voltage of 15 V. The rest of the mass spectrometry settings were optimised for the best sensitivity and best resolution. In MSE or resolution mode, data were obtained by scanning from 150–1500 m/z. Two channels of MS data were obtained in MSE mode, i.e., at 4 V (low collision energy) and 40–100 V (collision energy ramp) to acquire fragmentation data as well. For accurate mass determination, Leucine enkaphalin was used as a reference mass/ lock mass while sodium formate was used to calibrate the instrument. To achieve separation, the Waters HSS T3 instrument was set at 2.1 × 100 mm, 1.7 μm column. The mobile phase comprised of 0.1% formic acid as solvent (B) as well as 0.1% formic acid containing acetonitrile as solvent A in a combined volume of 2 μL. In a linear way, the gradient commenced at 100% for a minute with solvent A and shifted to 28% for solvent B over 22 min. Consequently, the gradient progressed in solvent B to 40% over 50 s then a wash step of 1.5 min at 100% B, then re-equilibration to the original conditions was instituted for 4 min. Column temperature was kept constant at 55 °C while the flow rate was maintained at 0.3 mL/min. By injecting a range of catechin standards between 0.5 and 100 mg/L catechin, the metabolites were quantified in a relative manner against a calibration curve. The data were processed using MSDIAL and MSFINDER (RIKEN Center for Sustainable Resource Science: Metabolome Informatics Research Team, Kanagawa, Japan).
2.5. Chemometric Data Analysis
The UPLC-QTOF-MS data of S. molle SH, DH, and H berries were analysed by Principal Component Analysis (PCA) to identify potential discriminate variables. Using the Markerlynx v4.1., alignment and peak detection and raw data filtering were conducted. A mass range of 100–1000 Da, 5–21 min retention time as well as 50 mDa tolerance time were used as parameters. In addition, 0.4 min retention time tolerance, a 500-intensity threshold/ counts of collection parameters, and a noise elimination level of 1.00 were all set. SIMCA P+ (13.0) software (Umetrics, Umeå, Sweden) was used to determine m/z data pair and retention time for each peak. The data were then used to determine dataset relationships and various chemometric model constructions.
3. Results
3.1. Phenolic Acids and/or Derivatives
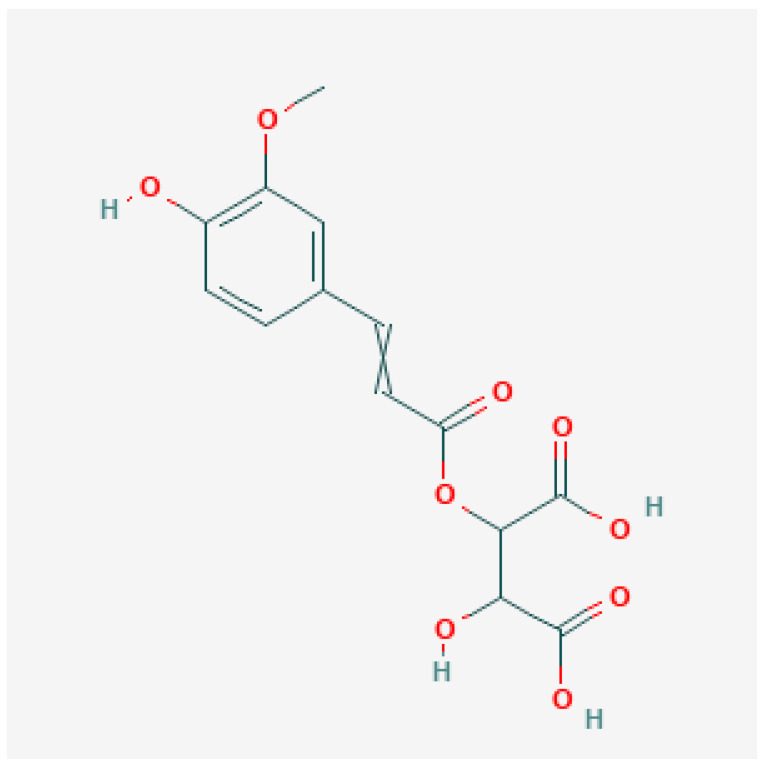
As shown in Table 1, MS analysis allowed for the detection and identification of seven phenolic acids and/ or their derivatives in the methanolic extracts of DH, SH, and H S. molle berries. A gallic acid and two gallic acid derivatives are phenolic acids that were detected and quantified in S. molle berries, while phenolic acid esters including 3-caffeoylquinic acid and feruloyltartaric A and B were also detected. In addition, gentisic acid 5-O-glucoside, a phenolic acid glucoside was also detected although this was undetectable in the dehulled seeds. The dominant phenolic acid was the ester feruloyltartaric A (Figure 2) which recorded 295.7 and 1613.1 mg/kg in SH and H samples respectively. These results show that the husks contained significantly higher phenolic acids in comparison with hull containing seeds and the dehulled seeds in the order H > SH > DH.
Table 1.
Secondary metabolites in Schinus molle berries (acidified methanolic extract) by LC-MS in negative ionization mode.
| RT | UV Max | m/z [M-H]− | Tentative Identification | Formula | Class | SH (mg/kg *) | DH (mg/kg *) | H (mg/kg *) |
|---|---|---|---|---|---|---|---|---|
| 5.602 | 229.6 | 331.06824 | β-Glucogallin A | C13H16O10 | Gallotannin | 76.0 | 14.8 | 443.6 |
| 5.787 | 271.6 | 169.01456 | Gallic acid | C7H6O5 | Phenolic acid | 62.7 | 4.9 | 252.3 |
| 6.04 | 273.6 | 331.06738 | β-Glucogallin | C13H16O10 | Gallotannin | 2.1 | nd | 147.8 |
| 6.646 | 268.6 | 343.06802 | Galloyl quinic acid A | C14H16O10 | Gallotannin | 30.8 | nd | 265.8 |
| 7.156 | 280.6 | 343.06808 | Galloyl quinic acid B | C14H16O10 | Gallotannin | 23.9 | nd | 251.9 |
| 7.444 | 255.6 | 411.02328 | Gallic acid derivative | C9H16O18 | Phenolic acid | 99.0 | 77.0 | 166.4 |
| 7.856 | 231.6 | 315.07346 | Gentisic acid 5-O-glucoside | C13H16O9 | Phenolic acid glucoside | 12.2 | nd | 130.3 |
| 8.437 | 261.6 | 325.05603 | Feruloyl tartaric A | C14H14O9 | Phenolic acid ester | 295.7 | 20.2 | 1613.1 |
| 8.804 | 267.6 | 325.05624 | Feruloyl tartaric B | C14H14O9 | Phenolic acid ester | 157.0 | 7.2 | 908.8 |
| 9.347 | 272.6 | 495.07687 | Digalloyl quinic acid | C35H28O22 | Gallotannin | 146.6 | 2.0 | 561.6 |
| 9.556 | 229.6 | 353.08759 | 3-caffeoylquinic acid | C16H18O9 | Phenolic acid ester | 95.1 | 36.4 | 543.9 |
| 10.781 | 274.6 | 577.12457 | Quinic acid derivative | C26H26O15 | organic acid | 9.0 | 20.5 | 31.0 |
| 11.11 | 275.6 | 469.06195 | Gallic acid derivative | C19H18O14 | Phenolic acid | nd | nd | 89.7 |
| 11.227 | 272.6 | 495.0744 | Digalloyl quinic acid | C35H28O22 | Gallotannin | 28.5 | 21.8 | 60.8 |
| 12.537 | 237.6 | 647.0879 | Pistafolin | C28H24O18 | Gallotannin | nd | nd | 147.9 |
| 13.094 | 237.6 | 635.0886 | Trigalloyl glucose | C27H24O18 | Gallotannin | nd | nd | 48.1 |
| 13.432 | 235.6 | 477.06815 | Digalloylshikimic acid A | C21H18O13 | Gallotannin | 65.8 | nd | 524.6 |
| 13.705 | 235.6 | 451.12579 | Galloylsalidroside | C21H24O11 | Gallotannin | nd | nd | 88.1 |
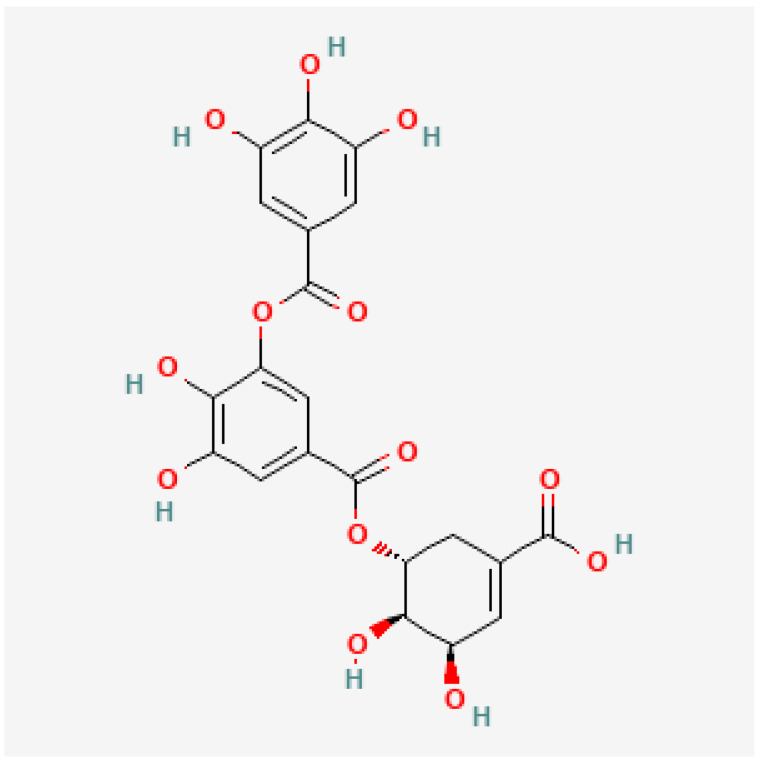
| 14.034 | 274.6 | 477.06717 | Digalloylshikimic acid B | C21H18O13 | Gallotannin | 102.4 | nd | 664.8 |
| 14.464 | 235.6 | 377.1821 | Unidentified | C17H30O9 | 121.4 | 103.0 | 155.4 | |
| 14.919 | 235.6 | 377.18033 | Unidentified | C17H30O9 | 233.3 | 240.7 | 378.0 | |
| 18.168 | 237.6 | 483.18625 | Gallic acid glycoside | C23H32O11 | Gallotannin | 108.1 | nd | 469.1 |
| 18.513 | 237.6 | 939.1104 | Pentagalloyl-β-D-glucose | C41H32O26 | Gallotannin | 1.1 | nd | 96.3 |
| 20.639 | 276.6 | 497.1662 | Eucaglobulin | C23H30O12 | Gallotannins derivative | nd | nd | 174.5 |
| 15.952 | 235.6 | 477.10321 | Isorhamnetin galactoside | C22H22O12 | Flavonol | 38.4 | nd | 430.1 |
| 16.299 | 235.6 | 477.10226 | Isorhamnetin glucoside | C22H22O12 | Flavonol | 27.4 | nd | 377.3 |
| 16.461 | 235.6 | 595.12994 | Quercetin 3-lathyroside | C26H28O16 | Flavonol | nd | nd | 124.0 |
| 16.978 | 237.6 | 615.09918 | Quercetin 3-(2-galloylglucoside) | C28H24O16 | Flavonol | 4.2 | nd | 224.1 |
| 17.67 | 235.6 | 303.04959 | Pentahydroxyflavanone | C15H12O7 | Flavonol | 16.5 | nd | 172.6 |
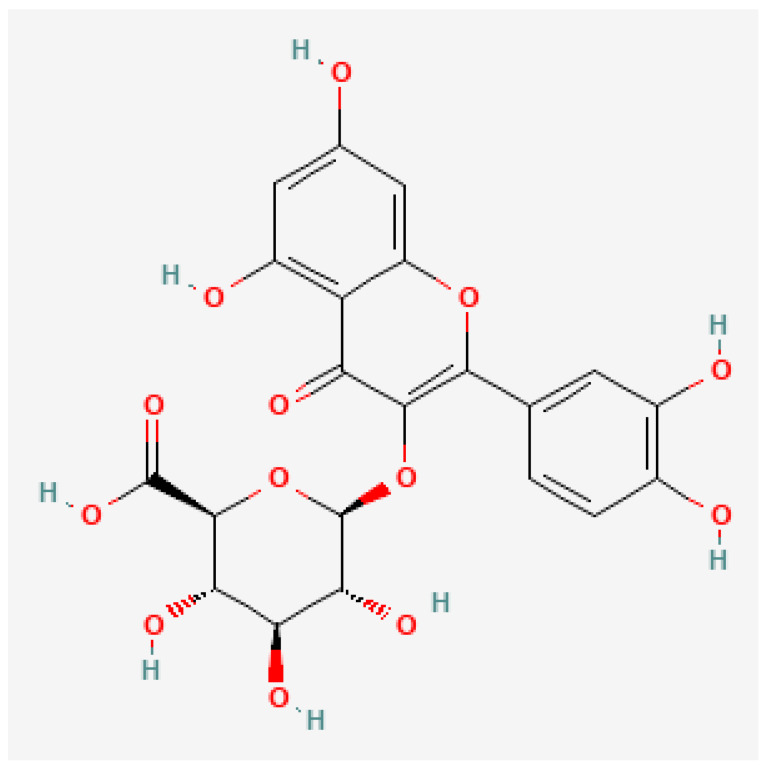
| 18.004 | 254.6 | 477.0668 | Quercetin 3-O-glucuronide | C21H18O13 | Flavonol | 267.5 | 73.4 | 564.3 |
| 19.198 | 237.6 | 447.08984 | Quercitrin | C21H20O11 | Flavonol | 1.8 | nd | 126.3 |
| 17.812 | 234.6 | 441.0809 | Catechin 3-O-gallate | C22H18O10 | Flavanol | 41.7 | nd | 326.8 |
| 11.651 | 229.6 | 289.07129 | Catechin | C15H14O6 | Flavanol | 122.0 | 80.4 | 551.9 |
Where: SH—Seed + Hulls; DH—Dehulled Seeds; H—Husks; nd—not detectable; * relative to catechin calibration curve; RT–retention Time; m/z—Mass-to-Charge Ratio; UV max—Maximum UV.
Figure 2.
Feruloyltartaric acid chemical structure [35].
3.2. Flavonoids
MS analysis allowed for the detection and identification of seven flavonols from the flavonoid class of secondary metabolites, in methanolic extracts of S. molle dehulled seeds, hulls and hull containing seeds (Table 1). Similar to phenolic acids, the results also indicate that more compounds were extracted from the hulls while the hull containing seeds and dehulled seeds possessed less compounds respectively. From the nine flavonols that were quantified in the husks, the compounds quantitatively decreased in the order quercetin 3-O-glucuronide > catechin > isorhamnetin galactoside > isorhamnetin glucoside > catechin 3-O-gallate > quercetin 3-(2-galloylglucoside) > pentahydroxyflavanone > quercitrin > quercetin 3-lathyroside. In the dehulled seeds, catechin and quercetin 3-O-glucuronide are the only flavonols that could be quantified. The compounds respectively ranged between 1.8 and 267.5/kg in quercitrin and quercetin 3-O-glucuronide in the seeds + husks. Quercetin 3-O-glucuronide (Figure 3) and catechin were conclusively the most dominant flavonoids in the present study.
Figure 3.
Quercetin 3-O-glucuronide chemical structure [36].
3.3. Tannins
As shown in Table 1, fourteen gallotannins, a subclass of hydrolisable tannins were detected and quantified in the husks of S. molle and decreased in the order: trigalloyl glucose > digalloyl quinic acid > galloylsalidroside > pentagalloyl-b-D-glucose > β-glucogallin > pistafolin > eucaglobulin > galloylquinic acid B > galloylquinic acid A > gallic acid glycoside > digalloylshikimic acid A > digalloyl quinic acid > digalloylshikimic acid B. In the seed + husks, the gallotannins ranged between 1.1 and 146.6 mg/kg in pentagalloyl-b-D-glucose and digalloyl quinic acid respectively. In the dehulled seeds, digalloyl quinic acid (21.8 mg/kg), β-glucogallin A (14.8 mg/kg), and digalloyl quinic acid (2 mg/kg) are the only gallotannins that were detected and quantified. Digalloylshikimic acid B (Figure 4) as well as digalloyl quinic acid were therefore the dominant tannins in the present study.
Figure 4.
Digalloylshikimic acid chemical structure [37].
Two compounds possessing the molecular formula C17H30O9 were also revealed although they could not be identified. Like phenolic acids, flavonols and gallotannins, the two compounds decreased in the order H > SH > DH. Also, an organic acid (quinic acid derivative) was detected and was found to be 9, 20 and 31 mg/kg in SH, DH, and H samples respectively. The dehulled samples of S. molle berries were surprisingly higher in quinic acid derivative than the seed + husk samples.
4. Discussion
4.1. Phenolic Acids and/or Derivatives
Acid and/or alkaline hydrolysis of tannins gives rise to gallic acids (GA), a trihydroxybenzoic compound of low molecular weight/ phenolic acid [38]. GA is naturally occurring and widely distributed in plants and can exist either as part of tannins or freely [38]. The diversity and antioxidant potential of GA and associated derivates are well known in the plant kingdom. Besides these antioxidant activities, GA derivatives have been reported to induce apoptosis in cancer cells, scavenge free radicals, signal pathway interference involving oxygen free radicals and Ca2+, as well as squalene epoxidase inhibition [39]. Also, some antitumoral, antiviral, antibacterial, antifungal, and anticancer activities have been linked with GA derivatives and esters, including activities in aging, neurodegenerative disorders, and cardiovascular diseases [38]. In addition, according to the previous authors, these activities are thought to be linked to the hydrophobic moiety of GA. Of particular interest is the earlier cytotoxicity study of [40], which showed that GA induced cell death in human promyelocytic leukaemia, mouse lymphoid neoplasm, human epithelial carcinoma, rat hepatoma, human hepatoma, and human epidermoid carcinoma cells, but with relatively high selectivity. Therefore, the presence of GA, its esters, and derivatives in S. molle berries offer insights into the potential of these berries in curing cancer and related diseases. However, besides the biological and pharmacological activities, esters of gallic acid prevent oxidation in foods while some cosmetic uses such as eye makeup, skin care, and hair shampoo among others have also been reported [41].
Some of the QA derivatives that were found in the present study are galloylquinic acid A and B (Gallotannins) as well as 3-caffeoylquinic acid (phenolic acid). Feruloyltartaric, a cinnamate ester obtained by formal condensation of the carboxy group of ferulic acid with one of the hydroxy groups of tartaric acid [35] is the most abundant compound in this study. The antidiabetic, antihypertensive, antioxidant, and anticancer qualities of ferulic acid are well known [42]. However, according to the previous authors, the bioavailability of this promising compound is low. But ferulic acid bioavailability is known to be improved by feruloyl esterases [42]. The presence of feruloyltartaric could therefore also conceivably help improve the bioavailability of ferulic acid and help improve the health promoting qualities of S. molle.
In comparison, the work of [43] was able to profile and quantify two phenolic acids including gallic acid and vanillic acid in two separate regions of Tunisia. Our work was able to profile and quantify three phenolic acids, 13 gallotannins, a phenolic acid glucoside and, three phenolic acid esters. Quantitatively, the Tunisian berries show more elevated concentrations. The previous authors were also able to show that S. molle berries quantitatively contained more phytochemical constituents in comparison with S. terebinthifolius, a close member of the species.
4.2. Flavonoids
The antioxidant, anti-inflammatory, and anti-carcinogenic role of flavonoids are well reported in literature. The results of the present study showed that flavonoid-3-O-glycosides dominated the flavonoid class and included isorhamnetin galactoside, isorhamnetin glucoside, quercetin 3-lathyroside, quercetin 3-(2-galloylglucoside), and quercitrin. The antioxidant, anti-inflammatory, antidiabetic, anticancer, anti-HIV activity, anti-obesity, anti-rotavirus activity, anti-influenza viral activity, anti-mayaro viral activity, anti-tumour, anti-coagulant, and anti-platelet activity of flavonoids have been widely reported [44,45,46,47,48]. The husks of S. molle berries were abundant in catechin, indicating the potential of the berries in human health.
In another study, the work of [43] showed only five flavonoids from S. molle and S. terebinthifolius berries. These authors reported catechin, epicatechin, rutin, luteolin, and kaempherol in their samples. Although our work reports nine flavonoids, only catechin was found in common between our works.
Other studies conducted so far show that S. molle essential oil extracts of the berries are different from the current methanolic extracts, for example the results of the studies of [16,19,27,29,30]. These authors reported different polyphenols from our findings. However, the alcoholic extracts of [31,32] compare well with the present results. These results further indicate that different extraction methods and instrumentation potentially yield different compounds. The essential oils in these previous studies were characterised by a high composition of α-pinene, the major monoterpene hydrocarbon class compound. The previous authors found α-pinene to dominate the essential oil compounds while phenolic acids dominated the methanolic extracts. Our findings showed that phenolic acids and their derivatives dominated the secondary metabolites while flavonoids were minimal.
Literature is awash with evidence that secondary metabolites are more concentrated in the peels/ or husks of fruits or berries [49,50]. It is therefore not surprising that the current results profiled and quantified more phenolic acids and/or their derivatives, tannins, and flavonoids in the husks in comparison to the dehulled seeds. As far as we know, the present study reports for the first time the secondary metabolites profile of S. molle berries grown in South Africa and the differences among the husks, dehulled seeds and seed containing husks in S. molle berries. A Korean study earlier quantified piperine, gallic acid, protocatechuic acid, epicatechin, and p-coumaric acid in the berries [32]. The Tunisian study of [31] also quantified these metabolites and many others in the berries of S. molle although these authors’ values are lower than our findings. We therefore conclude based on our results that the husks are very rich in both phenolic acids and/ or their derivatives, tannins, and flavonoids.
4.3. Tannins
The antioxidant, antitumor, and anticancer activities are among activities too many to mention that have been associated with catechin and its derivatives [51]. Tannins are particularly abundant in pulses, mostly red coloured beans, and are well known for their astringency [52,53]. Their molecular weight ranges from 500 to over 3000 and their ability to precipitate proteins, amino acids, and alkaloids have also been reported [52,53]. Anti-inflammatory studies indicate that tannins can control enteritis, bowel disorders, esophagitis, diarrhea, all forms of gastritis, kidneys, dysentery, fatigue, haemorrhaging, skin ulcers, and sore throats among others [54,55]. Furthermore, tannins have been shown to heal wounds and/or burns and stop bleeding simultaneously [55]. They heal wounds by forming a protective layer over the wound, and this consequently stops infections [55]. The anti-tumour, antibacterial, antiviral, and antiparasitic properties of tannins have also been reported [56]. S. molle berries are rich in gallotannins, a tannin subclass of hydrolysable tannins and as such potentially possess some of these health benefits. Heating tannins with hydrochloric or sulfuric acid may result in gallic or ellagic acids [57]. These changes from hydrolysable tannins to gallic or ellagic acid conceivably take place in the gut during digestion and thus some health benefits that are linked to these products of digestion may be realised. Gallic acid, a possible derivative of hydrolysable tannins is well known for inhibiting HIV replication [58]. S. molle is also rich in gallic or ellagic acids and derivatives as shown in Table 1 and as such could provide some health-related benefits.
Some of the tannins have not been widely studied and very little information exists on their role in pharmacology. For example, the study of [58] is the only study we found that reports on digalloylshikimic acid, while the studies of [59,60,61] report on pistafolin and showed some activity against ROS. The biological activities, mechanism of action, bioavailability among other pharmacological activities of these and other compounds need further investigation to reveal their full potential. These compounds are also abundant in S. molle berries as indicated in our results. However, phenolic acids in S. molle berries are promising especially in the husks. Table 1 further shows that most of the compounds that were detected are somewhat related, one being a product of hydrolysis of the other. Further studies are needed to further elucidate these relationships and possibly show how these interact in the human body as well as their bioavailability for nutrition and health.
Eucaglobulin, a gallotannin and one of the compounds that to the best of our knowledge is being reported for the first time in S. molle and possesses some anti-melanogenic and anti-inflammatory activity [62]. This compound has previously been reported in Eucalyptus globulus leaves and other Eucalyptus species [63].
Quinic acid (QA), a cyclohexanecarboxylic acid is an organic acid that is diverse in the plant kingdom [64]. According to the previous authors, although QA is naturally formed from dehydroquinic acid, which is an intermediate in the shikimic pathway, it has been synthetically hydrolysed from chlorogenic acid. This compound is dominant in the hulls of S. molle berries of the current study. In pharmaceuticals, QA is used as an astringent or a chiral substance and is an oseltamivir building block, an antiviral medicine that is used to treat infleunza A and B [65]. But biological activity studies have revealed the antioxidant, antidiabetic, radioprotective, and anti-neuroinflammatory activities of QA and its derivatives [66].
4.4. Multivariate Analysis
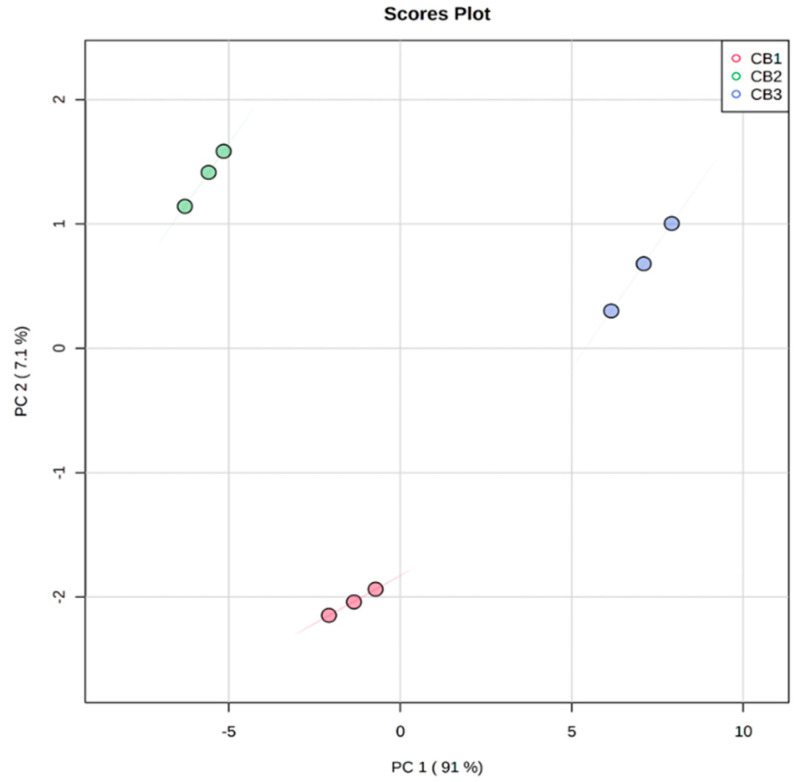
Using an untargeted metabolomics approach, phytochemical differences between SH, DH, and H of S. molle berries were broadly evaluated and characterised. The use of metabolomics as a tool for complex plant sample, feed product, product adulteration, cultivar variations and food quality elucidation studies are well documented [67]. In the present study, visualizing UPLC profiles of extracts from S. molle DH, SH, and H berries with varying phytochemical profiles and contents of individual metabolites with varying susceptibility led to the observation of variable but non complicated patterns. The metabolites shown in Table 1 were subjected to PCA using MetaboAnalyst 5.0 software for computing metabolomics data. Using this software, differences among DH, SH, and H of the berries were computed and profiled following the methods of [68] and following the workflow for untargeted metabolomics. The main principal component (PC); PC1 and PC2 accountable for 91 and 7.1% of the variance respectively as shown in Figure 5. The effectiveness of PCA in studies involving classification of species and when plotting exploratory analysis has been demonstrated [69]. PCA scores of the present study have unfolded a lot of data on polyphenolic compounds in S. molle SH, DH, and H berries. Three groups of populations have been separated and categorised by the two score plots shown in Figure 1. All three groups were placed separately with SH and H components towards the right and somewhat close to each other. DH seeds were placed far away from the H, showing the strong influence of the husks in determining the presence of secondary metabolites. The SH samples are close to either DH or H samples but not very close to either, showing that husks have an influence on the secondary metabolite profiles and quantities. Pigmented colours in fruits and vegetables are closely related to secondary metabolite profiles and quantities. The dehulled seeds of S. molle appear brownish in colour, the pink to reddish pigment is confined to the hulls. Therefore, removing the hulls conceivably reduces the secondary metabolites. It is also conceivable that the high oil contents of the seeds somewhat interfere with the presence of secondary metabolites. This is because the metabolites are drastically reduced when the seeds and husks are combined.
Figure 5.
Principal component analysis Score plot of PC1 Vs PC2 scores of S. molle Seeds + Husks (CB1), Dehulled Seeds (CB2) and Husks (CB3) analysed via UPLC-QTOF-MS.
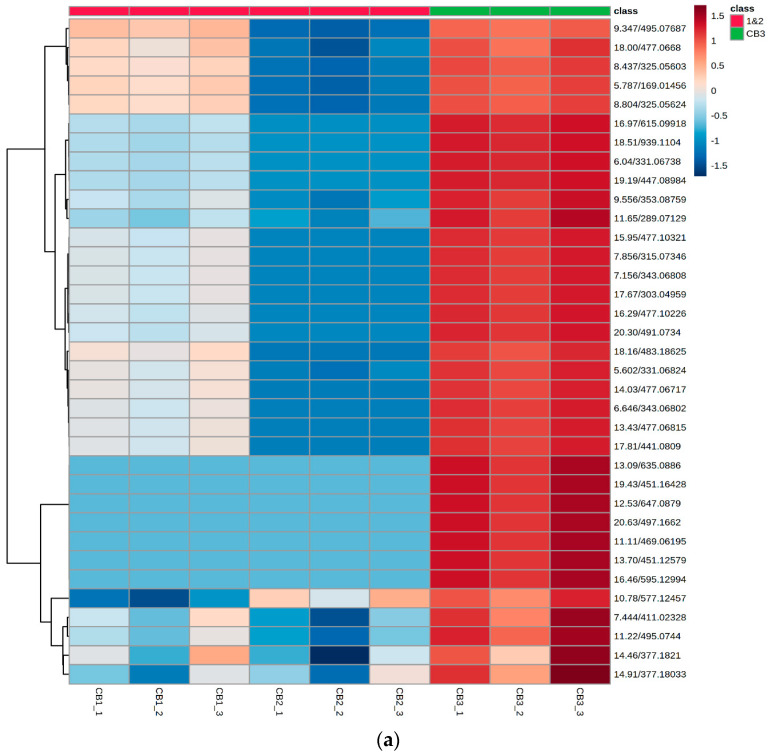
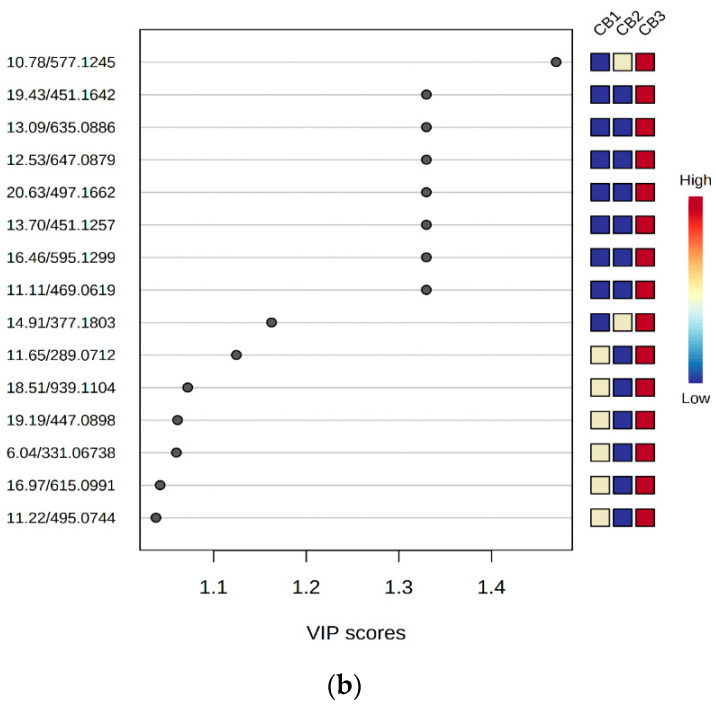
The intensity of the various untargeted polyphenolic compounds in S. molle SH, DH and H berries are shown in the heat map on Figure 6a. Included in this heat map are the clustergrams where the raw data represented the secondary metabolite compositions relating to whether the S. molle berries were dehulled, hulled or both. The colour block represented the intensity of the treatments of secondary metabolite concentrations. The lower concentrations were represented by the colour blue while the higher concentrations were represented by the colour red as shown in Figure 6b. Therefore, based on the heat map, husks had higher polyphenolic concentrations in comparison to lower concentrations in SH and DH samples respectively.
Figure 6.
(a): Heat map of S. molle Seeds + Husks (CB1), Dehulled Seeds (CB2), and Husks (CB3) secondary metabolites analysed via UPLC-QTOF-MS. The numbers on the right represent the RT and m/z [M-H]− shown on Table 1. (b): Heat map of S. molle Seeds + Husks (CB1), Dehulled Seeds (CB2), and Husks (CB3) secondary metabolites analysed via UPLC-QTOF-MS.
5. Conclusions
The results of the current study indicate that S. molle berries are a rich source of secondary metabolites. Thirteen gallotannins, three phenolic acids, a phenolic acid glucoside, three phenolic acid esters, an organic acid, a gallotannin derivative, and nine flavonoids were profiled and further quantified. The present study revealed that feruloyl tartaric A, a phenolic acid ester was the dominant phenolic acid while quercetin 3-O-glucuronide and catechin were the major flavonoids, and digalloylshikimic acid B as well as digalloyl quinic acid were the dominant tannins. The husks possessed more metabolites than the seeds + husks and dehulled seeds respectively. Although the use of the husks alone for culinary purposes may prove difficult, perhaps, the use of the whole berry would be ideal. However, to extract more secondary metabolites for pharmaceutical or food industry purposes, it would be ideal to extract from the hulls. Although S. molle is considered invasive in South Africa, both culinary and pharmaceutical applications can be derived from the ripe and mature berries, making this plant potentially an important nutraceutical plant. However, further studies need to be conducted to further elucidate the pharmacological properties and biological activities that can be linked to the reported polyphenolic compounds. The berries of these trees could also potentially provide sources of income in poor and marginalised communities if commercial linked applications can be harnessed. South African communities also need to learn about the existing culinary uses of these berries.
Acknowledgments
Support rendered by Ahmed Mohammed of the Department of Chemistry at the Cape Peninsula University of Technology is appreciated.
Author Contributions
Conceptualization, C.B. and L.K.; methodology, C.B. and L.K.; software, C.B. and L.K.; validation, C.B. and L.K.; formal analysis, C.B. and L.K.; investigation, C.B. and L.K.; resources, L.K.; data curation, C.B. and L.K.; writing—original draft preparation, C.B.; writing—review and editing, L.K.; supervision, L.K.; project administration, L.K.; funding acquisition, L.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Cape Peninsula University of Technology and the National Research Foundation of South Africa, Grant Number: IKS 160520165755.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang Y., Wang J. Modelling and prediction of global non-communicable diseases. BMC Public Health. 2020;20:822. doi: 10.1186/s12889-020-08890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bvenura C., Sivakumar D. The role of wild fruits and vegetables in delivering a healthy and balanced diet. Food Res. Int. 2017;99:15–30. doi: 10.1016/j.foodres.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 3.Davies J.I., Wagner R.G. Weighing up the Costs of Treating ‘Lifestyle’ Diseases in South Africa. 2019. [(accessed on 18 March 2021)]. Available online: https//www.wits.ac.za/news/latest-news/opinion/2019/2019-02/weighing-up-the-costs-of-treating-lifestyle-diseases-in-south africa.html#,~,text=Although%20HIV%20still%20causes%20the,are%20poor%2C%20black%20and%20male.
- 4.WHO . Global Action Plan for the Prevention and Control of Noncommunicable Diseases, 2013–2020. WHO Press; Geneva, Switzerland: 2013. [Google Scholar]
- 5.Asowata-Ayodele A.M., Afolayan A.J., Otunola G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. South Afr. J. Bot. 2016;104:69–75. doi: 10.1016/j.sajb.2016.01.001. [DOI] [Google Scholar]
- 6.UNSCN A World Free from Hunger and All Forms of Malnutrition Is Attainable in This Generation. 2021. [(accessed on 18 March 2021)]. Available online: https//www.unscn.org/en/topics/ncds.
- 7.Abegunde D.O., Mathers C.D., Adam T., Ortegon M., Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lance. 2008;370:1929–1938. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 8.WHO/NCD Country Profiles, South Africa. 2018. [(accessed on 18 March 2021)]. Available online: https//www.who.int/nmh/countries/zaf_en.pdf.
- 9.Christenhusz M.J.M., Byng J.W. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–217. doi: 10.11646/phytotaxa.261.3.1. [DOI] [Google Scholar]
- 10.Murray A.P., Murray M.G. Phytochemistry, traditional uses and bioactivity of the medicinal plant Schinus areira L. (Anacardiaceae), A review. Nat. Prod. J. 2017;7:97. doi: 10.2174/2210315507666170117145728. [DOI] [Google Scholar]
- 11.García N., Ormazabal C. Árboles Nativos de Chile. Enersis, S.A.; Santiago, Chile: 2008. p. 196. [Google Scholar]
- 12.Dellacassa E. Normalización de Productos Naturales Obtenidos de Especies de la Flora Aromática Latinoamericana, Proyecto CYTED IV.20. Edipucrs; Porto Alegre, Brasil: 2010. [Google Scholar]
- 13.Rebolledo V., Otero M.C., Delgado J.M., Torres F., Herrera M., Ríos M., Cabañas M., Martinez J.L., Rodríguez-Díaz M. Phytochemical profile and antioxidant activity of extracts of the peruvian peppertree Schinus areira L. from Chile. Saudi J. Biol. Sci. 2021;28:1052–1062. doi: 10.1016/j.sjbs.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guala M.S., Lapissonde M.O., Elder H.V., van Baren C.M., Bandoni A.L., Dellacassa E. Essential Oils in Food Preservation, Flavour and Safety. Academic Press; Cambridge, MA, USA: 2016. Chapter 78—Rose Pepper (Schinus molle L.) Oils; pp. 689–695. [Google Scholar]
- 15.Gundidza M. Antimicrobial activity of essential oil from Schinus molle Linn. Cent. Afr. J. Med. 1993;39:231–234. [PubMed] [Google Scholar]
- 16.Bendaoud H., Romdhane M., Souchard J.P., Cazaux S., Bouajila J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010;75:C466–C472. doi: 10.1111/j.1750-3841.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 17.Martins Mdo R., Arantes S., Candeias F., Tinoco M.T., Cruz-Morais J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014;151:485–492. doi: 10.1016/j.jep.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 18.Guala M., Elder H., Perez G., Chiesa A. Evaluation of the antioxidant power of fractions of Schinus molle L. essential oil obtained by vacuum distillation. Inf. Technol. 2009;20:83–88. [Google Scholar]
- 19.Silva-Junior E.F., Aquino P.G.V., Santos-Junior P.F.S., Nascimento I.J.S., Gomes E.A., Silva A.L.L., Verissimo R.C.S.S., Aquino T.M., Araújo-Junior J.X. Phytochemical compounds and pharmacological properties from Schinus molle Linnaeus and Schinus terebinthifolius Raddi (Anacardiaceae) J. Chem. Pharm. Res. 2015;7:389–393. [Google Scholar]
- 20.Belhamel K., Abderrahim A., Ludwig R. Chemical composition and antibacterial activity of the essential oil of Schinus molle L. grown in Algeria. Int. J. Essent. Oil Ther. 2008;2:175–177. [Google Scholar]
- 21.Doleski M.P.S., Ferreira C.C.H., Calil B.J., Palermo M.M. Chemical composition of the Schinus molle L. essential oil and their biological activities. Rev. Cuba. Farm. 2015;49:132–143. [Google Scholar]
- 22.El Hayouni A., Chraief I., Abedrabba M., Bouix M., Leveau J.Y., Mohammed H., Hamdi M. Tunisian Salvia officinalis L. and Schinus mole L. essential oils, Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008;125:242–251. doi: 10.1016/j.ijfoodmicro.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Marzouk M.S., Moharram F.A., Haggag E.G., Ibrahim M.T., Badary O.S. Antioxidant flavonol glycosides from Schinus molle. Phytother. Res. 2006;20:200–205. doi: 10.1002/ptr.1834. [DOI] [PubMed] [Google Scholar]
- 24.Prado A.C., Garces H.G., Bagagli E. Schinus molle essential oil as a potential source of bioactive compounds, Antifungal and antibacterial properties. J. Appl. Microbiol. 2018;126:516–522. doi: 10.1111/jam.14157. [DOI] [PubMed] [Google Scholar]
- 25.Turchetti G., Garzoli S., Laghezza Masci V., Sabia C., Iseppi R., Giacomello P., Tiezzi A., Ovidi E. Antimicrobial Testing of Schinus molle (L.) Leaf Extracts and Fractions Followed by GC-MS Investigation of Biological Active Fractions. Molecules. 2020;25:1977. doi: 10.3390/molecules25081977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed Z.M.S., Mohamed Z.Z., Hayssam M.A., Mamoun S.M.A. Chemical composition, antioxidant and antibacterial activities of extracts from Schinus molle wood branch growing in Egypt. J. Wood Sci. 2016;62:548–561. [Google Scholar]
- 27.Benzi B., Stefanazzi N., Ferrero A. Biological activity of essential oils from leaves and fruits of pepper tree (Schinus molle L.) to control rice weevil (Sitophilus oryzae L.) Chil. J. Agric. Res. 2009;69:154–159. doi: 10.4067/S0718-58392009000200004. [DOI] [Google Scholar]
- 28.Deveci O., Sukan A., Tuzun N., Kocabas E.E.H. Chemical composition, repellent and antimicrobial activity of Schinus molle L. J. Med. Plants Res. 2010;4:2211–2216. [Google Scholar]
- 29.Rocha P.M.D.M., Rodilla J.M., Díez D., Elder H. Synergistic antibacterial activity of the essential oil of Aguaribay (Schinus mole L.) Molecules. 2012;17:12023–12036. doi: 10.3390/molecules171012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eryigit T., Yildirim B., Ekici K., Çirka M. Chemical composition, antimicrobial and antioxidant properties of Schinus molle L. Essential oil from Turkey. J. Essent. Oil-Bear. Plants. 2017;20:570–577. doi: 10.1080/0972060X.2017.1304286. [DOI] [Google Scholar]
- 31.Feriani A., Tir M., Mufti A., Caravaca A., Contreras M., Taamalli A., Carretero A.S., Aldawood N., Nahdi S., Alwasel S., et al. HPLC-ESI-QTOF-MS/MS profiling and therapeutic effects of Schinus terebinthifolius and Schinus molle fruits, investigation of their antioxidant, antidiabetic, anti-inflammatory and antinociceptive properties. Inflammopharmacology. 2021;29:467–481. doi: 10.1007/s10787-021-00791-1. [DOI] [PubMed] [Google Scholar]
- 32.Kim M.J., Kim D.W., Kim J.G., Shin Y., Jung S.K., Kim Y.J. Analysis of the Chemical, Antioxidant, and Anti-Inflammatory Properties of Pink Pepper (Schinus molle L.) Antioxidants. 2021;10:1062. doi: 10.3390/antiox10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai Z., Tsugawa H., Wohlgemuth G., Mehta S., Mueller M., Zheng Y., Ogiwara A., Meissen J., Showalter M., Takeuchi K., et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods. 2018;15:53–56. doi: 10.1038/nmeth.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsugawa H., Cajka T., Kind T., Ma Y., Higgins B., Ikeda K., Kanazawa M., VanderGheynst J., Fiehn O., Arita M. MS-DIAL, data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods. 2015;12:523–526. doi: 10.1038/nmeth.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Biotechnology Information (NCBI) PubChem Compound Summary for CID 69501207, Feruloyltartaric acid. [(accessed on 20 March 2022)];2022 Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Feruloyltartaric-acid.
- 36.National Center for Biotechnology Information (NCBI) PubChem Compound Summary for CID 5274585, Quercetin 3-O-Glucuronide. [(accessed on 20 March 2022)];2022 Available online: https://pubchem.ncbi.nlm.nih.gov/compound/quercetin-3-O-glucuronide.
- 37.National Center for Biotechnology Information (NCBI) PubChem Compound Summary for CID 475266, 3-O-Digalloylshikimic acid. [(accessed on 20 March 2022)];2022 Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3-O-Digalloylshikimic-acid.
- 38.Al Zahrani N.A., El-Shishtawy R.M., Asiri A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry, A review. Eur. J. Med. Chem. 2020;204:112609. doi: 10.1016/j.ejmech.2020.112609. [DOI] [PubMed] [Google Scholar]
- 39.Pawlowska A., De Leo M., Braca A. Phenolics of Arbutus unedo L. (Ericaceae) Fruits, Identification of Anthocyanins and Gallic Acid Derivatives. J. Agric. Food Chem. 2006;54:10234–10238. doi: 10.1021/jf062230o. [DOI] [PubMed] [Google Scholar]
- 40.Inoue M., Suzuki R., Sakaguchi N., Li Z., Takeda T., Ogihara Y., Jiang B.Y., Chen Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995;18:1526–1530. doi: 10.1248/bpb.18.1526. [DOI] [PubMed] [Google Scholar]
- 41.Locatelli C., Filippin-Monteiro F.B., Creczynski-Pasa T.B. Alkyl esters of gallic acid as anticancer agents, a review. Eur. J. Med. Chem. 2013;60:233–239. doi: 10.1016/j.ejmech.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 42.Swamy S.K.P., Govindaswamy V. Therapeutical properties of ferulic acid and bioavailability enhancement through feruloyl esterase. J. Funct. Foods. 2015;17:657–666. doi: 10.1016/j.jff.2015.06.013. [DOI] [Google Scholar]
- 43.Tlili N., Yahia Y., Feriani A., Labidi A., Ghazouani L., Nasri N., Saadaoui E., Khaldi A. Schinus terebinthifolius vs Schinus molle: A comparative study of the effect of species and location on the phytochemical content of fruits. Ind. Crops Prod. 2018;122:559–565. doi: 10.1016/j.indcrop.2018.05.080. [DOI] [Google Scholar]
- 44.Andrae-Marobela K., Ghislain F.W., Okatch H., Majinda R. Polyphenols, A diverse class of multi-target anti-HIV-1 agents. Curr. Drug Metab. 2013;7:392–413. doi: 10.2174/13892002113149990095. [DOI] [PubMed] [Google Scholar]
- 45.Bae E.A., Han M.J., Lee M., Kim D.H. In vitro inhibitory effect of aome flavonoids on rotavirus infectivity. Biol. Pharm. Bull. 2000;23:1122–1124. doi: 10.1248/bpb.23.1122. [DOI] [PubMed] [Google Scholar]
- 46.Jin Y.R., Li A.X., Deng L.B. Progress in the research of inhibitory activities and structure-activity relationship of flavonoids on neuraminidases. Chin. J. New Drugs. 2012;21:2272–2278. [Google Scholar]
- 47.Morikawa T., Ninomiya K., Miyake S., Miki Y., Okamoto M., Yoshikawa M., Muraoka O. Flavonol glycosides with lipid accu mulation inhibitory activity and simultaneous quantitative analysis of 15 polyphenols and caffeine in the flower buds of Camellia sinensis from different regions by LCMS. Food Chem. 2013;140:353–360. doi: 10.1016/j.foodchem.2013.02.079. [DOI] [PubMed] [Google Scholar]
- 48.Xiao J. Dietary flavonoid aglycones and their glycosides, which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017;57:1874–1905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- 49.Bvenura C., Witbooi H., Kambizi L. Pigmented Potatoes, A Potential Panacea for Food and Nutrition Security and Health. Foods. 2022;11:175. doi: 10.3390/foods11020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh B., Singh J.P., Kaur A., Singh N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020;132:109114. doi: 10.1016/j.foodres.2020.109114. [DOI] [PubMed] [Google Scholar]
- 51.Fan F.Y., Sang L.X., Jiang M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules. 2017;22:484. doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izawa K., Amino Y., Kohmura M., Ueda Y., Kuroda M. 4.16—Human–Environment Interactions—Taste. Compr. Nat. Prod. II Chem. Biol. 2010;4:631–671. [Google Scholar]
- 53.Trugo L.C., von Baer D., Lupin E. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Cambridge, MA, USA: 2003. pp. 3623–3629. [Google Scholar]
- 54.Cheng H.Y., Lin C.C., Lin T.C. Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antivir. Res. 2002;55:447–455. doi: 10.1016/S0166-3542(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 55.Quideau S., Varadinova T., Karagiozova D., Jourdes M., Pardon P., Baudry C., Genova P., Diakov T., Petrova R. Main structural and stereochemical aspects of the antiherpetic activity of nonahydroxyterphenoyl-containing C-glycosidic ellagitannins. Chem. Biodivers. 2004;1:247–258. doi: 10.1002/cbdv.200490021. [DOI] [PubMed] [Google Scholar]
- 56.Ashok P.K., Upadhyaya K. Tannins are Astringent. J. Pharmacogn. Phytochem. 2012;1:45–50. [Google Scholar]
- 57.Milledge J.J., Nielsen B.V., Harvey P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2019;31:779–786. doi: 10.1007/s10811-018-1512-4. [DOI] [Google Scholar]
- 58.Nonaka G., Nishioka I., Nishizawa M., Yamagishi T., Kashiwada Y., Dutschman G.E., Bodner A.J., Kilkuskie R.E., Cheng Y.C., Lee K.H. Anti-AIDS agents, 2, Inhibitory effects of tannins on HIV reverse transcriptase and HIV replication in H9 lymphocyte cells. J. Nat. Prod. 1990;53:587–595. doi: 10.1021/np50069a008. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z., Guillen Quispe Y.N., Hwang S.H., Zuo G., Lim S.S. Pistafolin B is the major aldose reductase inhibitor of the pods of tara [Caesalpinia spinose (Molina) Kuntze] Ind. Crops Prod. 2018;122:709–715. doi: 10.1016/j.indcrop.2018.06.023. [DOI] [Google Scholar]
- 60.Zhao X., Sun H., Hou A., Zhao Q., Wei T., Xin W. Antioxidant properties of two gallotannins isolated from the leaves of Pistacia weinmannifolia. Biochim. Biophys. Acta. 2005;1725:103–110. doi: 10.1016/j.bbagen.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 61.Wei T., Sun H., Zhao X., Hou J., Hou A., Zhao Q., Xin W. Scavenging of reactive oxygen species and prevention of oxidative neuronal cell damage by a novel gallotannin, pistafolia A. Life Sci. 2002;70:1889–1899. doi: 10.1016/S0024-3205(02)01494-7. [DOI] [PubMed] [Google Scholar]
- 62.Hasegawa T., Takano F., Takata T., Niiyama M., Ohta T. Bioactive monoterpene glycosides conjugated with gallic acid from the leaves of Eucalyptus globulus. Phytochemistry. 2008;69:747–753. doi: 10.1016/j.phytochem.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 63.Ito H., Koreishi M., Tokuda H., Yoshida H., Nishino T. Cypellocarpin A–C, phenolic glycosides esterified with oleuropeic acid, from Eucalyptus cypellocarpa. J. Nat. Prod. 2000;63:1253–1257. doi: 10.1021/np0001981. [DOI] [PubMed] [Google Scholar]
- 64.Dhondge S.S., Shende P.H., Paliwal L.J., Deshmukh D.W. Volumetric and acoustic study of aqueous binary mixtures of quinine hydrochloride, guanidine hydrochloride and quinic acid at different temperatures. J. Chem. Thermodyn. 2015;81:34–43. doi: 10.1016/j.jct.2014.09.011. [DOI] [Google Scholar]
- 65.Pero R.W., Lund H., Leanderson T. Antioxidant metabolism induced by quinic acid increased urinary excretion of tryptophan and nicotinamide. Phytother. Res. 2009;23:335–346. doi: 10.1002/ptr.2628. [DOI] [PubMed] [Google Scholar]
- 66.Jang S.A., Park D.W., Kwon J.E., Song H.S., Park B., Jeon H., Sohn E.H., Koo H.J., Kang S.C. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed. Pharmacother. Biomed. Pharmacother. 2017;96:563–571. doi: 10.1016/j.biopha.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 67.Tang J.F., Li W.X., Zhang F., Li Y.H., Cao Y.J., Zhao Y., Li X.L., Ma Z.J. Discrimination of Radix Polygoni Multiflori from different geographical areas by UPLC-QTOF/MS combined with chemometrics. Chin. Med. 2017;12:34. doi: 10.1186/s13020-017-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pang Z., Chong J., Zhou G., de Lima Morais D.A., Chang L., Barrette M., Gauthier C., Jacques P.É., Li S., Xia J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farag M.A., Gad H.A., Heiss A.G., Wessjohann L.A. Metabolomics driven analysis of six Nigella species seeds via UPLC-qTOF-MS and GC-MS coupled to chemometrics. Food Chem. 2014;151:333–342. doi: 10.1016/j.foodchem.2013.11.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.