Abstract
The presence of Fe-oxidizing bacteria in the rhizosphere of four different species of wetland plants was investigated in a diverse wetland environment that had Fe(II) concentrations ranging from tens to hundreds of micromoles per liter and a pH range of 3.5 to 6.8. Enrichments for neutrophilic, putatively lithotrophic Fe-oxidizing bacteria were successful on roots from all four species; acidophilic Fe-oxidizing bacteria were enriched only on roots from plants whose root systems were exposed to soil solutions with a pH of <4. In Sagittaria australis there was a positive correlation (P < 0.01) between cell numbers and the total amount of Fe present; the same correlation was not found for Leersia oryzoides. These results present the first evidence for culturable Fe-oxidizing bacteria associated with Fe-plaque in the rhizosphere.
Wetland plants provide an important conduit for gas exchange between the atmosphere and saturated, anaerobic soils. For example, it is well known that much of the methane generated in anaerobic wetland soils is released into the atmosphere through vascular plants (24). Perhaps the most important influence plants have on gaseous fluxes into anaerobic soils is oxygen transport from stems to the roots and the subsequent release of O2 into the rhizosphere (1, 2, 5). Recently, microbial ecologists have become more cognizant of the potential for the rhizosphere of vascular wetland plants to provide an aerobic habitat in an otherwise anaerobic environment. For example, it has been shown that methane oxidizers inhabit the rhizosphere of wetland plants, where they consume a substantial portion of the methane produced (4, 6, 13, 14). Another recent discovery is that carbon monoxide-oxidizing prokaryotes can also live in the rhizosphere (19).
Wetland ecologists have long recognized that Fe oxidation occurs in the rhizosphere of many wetland plants based on the presence of Fe oxyhydroxide precipitates that often coat root surfaces (16). These Fe(III) deposits are commonly referred to as Fe-plaque. There have been few investigations into the potential role of microbes in the formation of Fe-plaque, and the results have been contradictory. Microscopic studies using scanning or transmission electron microscopes have shown the presence of bacterial cells in the iron matrix (20, 23). Trolldenier (23) took this work one step further and showed that reddish brown colonies formed when root plaque was inoculated into a semisolid iron sulfide agar medium; however, no further characterization was done to confirm that these were lithotrophic iron-oxidizing bacteria. There has been general speculation that most Fe oxidation in the rhizosphere results from chemical oxidation and that while bacteria may act as nucleation sites for the precipitation of Fe oxides, they do not actively catalyze the oxidation process (15, 16, 20). However, to our knowledge there have not been any specific investigations to discover whether lithotrophic, acidophilic, or neutrophilic Fe oxidizers could inhabit the rhizosphere of plants.
The ability of Fe-oxidizing bacteria to compete with the chemical oxidation of Fe(II) may depend on the rhizosphere pH. The kinetics of Fe oxidation are relatively slow at low pHs (<pH4) because Fe(II) is quite stable. At low pHs, acidophilic Fe-oxidizing bacteria, such as Thiobacillus ferrooxidans, may increase Fe oxidation kinetics (17) and thereby contribute to Fe-plaque formation. Recent findings suggest that Fe-oxidizing bacteria may also be favored in certain circumneutral, microaerophilic environments where low O2 concentrations could presumably limit abiotic Fe oxidation rates (10–12).
The purpose of this study was to determine the extent to which bacteria are associated with Fe-plaque and to establish whether acidophilic and neutrophilic Fe-oxidizing bacteria were present in the rhizosphere microbial community. The study site, Contrary Creek, is located in Northern Virginia and is a medium-sized creek with nearby pyrite mines that were abandoned more than 50 years ago. As a result of acidity generated by the mining spoils, the pH of the main creek ranged from around 3.5 to 5.5, and it contained large amounts of floc and streamers, typically associated with an acid mine drainage stream (17). The Fe(II) concentrations in the main creek ranged from tens to hundreds of micromoles per liter, as determined with ferrozine (21). Plants typical of wetland environments grew sparsely along the bank of Contrary Creek, where they were exposed to acidic waters. There was also a smaller circumneutral stream feeding into the main creek with a pH ranging between 6 and 7 and Fe(II) concentrations as high as 150 μM. In several places this stream was bordered by small wetlands supporting a variety of vascular wetland plant species. The sediment surfaces in both the stream and associated wetlands have large deposits of amorphous ferric hydroxides.
On three separate occasions we collected a total of four different plant species and their associated roots and rhizomes from both low pH and circumneutral pH sites (Table 1). The plants were collected primarily on the basis of their abundance at the particular site and secondly on the basis of their known ability to transport O2 to the rhizosphere. In all cases, a block of soil, approximately 15 by 15 by 25 cm, that contained the plant and its root system was removed. The soil blocks were returned to the laboratory, and the soil surrounding the roots of individual plants was removed aseptically with a metal spatula or spoon. The roots of each plant were cut into individual pieces, and loosely adhering soil was rinsed off with sterile deionized water. Visually, the majority of roots from these plants were rust colored to varying degrees, ranging from nearly white to a dark, rusty red; there was no noticeable pattern to the distribution of Fe-plaque on the roots.
TABLE 1.
Characteristics of plants and soil
| Site and date sampled | Plant species | Soil pH | Fe concn in:
|
Organic carbon content of soil (%)b | Soil composition
|
||||
|---|---|---|---|---|---|---|---|---|---|
| H2Oa (μM) | Plaque (μmol of Fe/g [dry wt]) | Bulk soil (μmol of Fe/g [dry wt]) | % Sandc | % Silt | % Clay | ||||
| Neutral tributary, July 1997 | S. australis | 6.25 | 65.9 | 6,256.6 | 2,544.0 ± 555.3 | 11.0 ± 0.3 | 70.3 ± 4.9 | 23.9 ± 7.1 | 5.85 ± 2.2 |
| Acidic stream | |||||||||
| October 1997 | J. effusus | 3.67 | 51.1 | 1,230.1 | 868.3 ± 122.0 | 6.7 ± 0.2 | 76.6 ± 3.9 | 14.4 ± 5.7 | 9.1 ± 1.8 |
| L. oryzoides | 4.45 | 22.4 | 771.8 | 2,418.1 ± 116.3 | 11.0 ± 0.1 | 75.9 ± 1.7 | 12.0 ± 4.0 | 12.1 ± 5.6 | |
| Unknown sedge | 4.57 | 57.6 | 4,027.7 | 941.7 ± 81.3 | 4.9 ± 0.3 | 81.7 ± 1.8 | 7.7 ± 1.9 | 10.6 ± 0.1 | |
| November 1998 | L. oryzoides | 3.49 | 288.2 | 302.1 | |||||
The total amount of iron on the root surface was extracted by the dithionate-citrate-bicarbonate (DCB) technique (22); the iron in the bulk soil was also extracted by the DCB technique, according to a slightly modified procedure (7). The DCB extractions efficiently reduce both noncrystalline and crystalline iron oxides and solubilize small amounts of exchangeable and organically bound Fe(II). Iron determinations were carried out with an Atomic Absorption Spectrometer (model 5100; Perkin-Elmer). All the plants had significant amounts of Fe-plaque associated with their roots (Table 1).
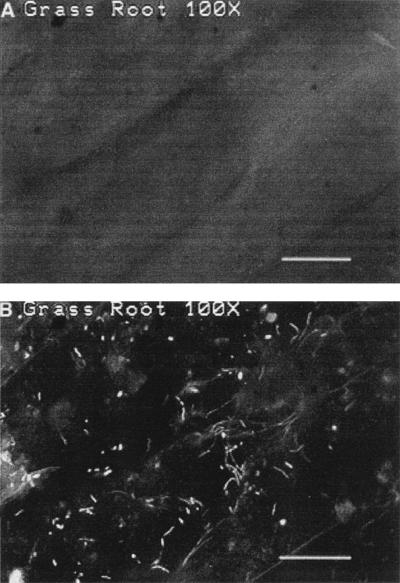
To determine the presence of cells in the Fe-plaque, subsections of root were placed in a dilute solution of acridine orange (0.001%) for 2 min, washed in deionized water, and inspected by epifluorescence light microscopy. Typical results are shown in Fig. 1. As with other observations of iron-oxidizing bacteria (10–12), light microscopy alone did not reveal the presence of any cells (Fig. 1A); however, when viewed by epifluorescence microscopy, a large number of cells were visible embedded in the iron oxide matrix (Fig. 1B). Morphologically these cell populations appeared quite diverse, with rods, cocci, filaments, and spiral-shaped forms present. The cells preferentially associated with the iron oxides. In regions of the plant root that did not have plaque, few visible cells adhered to the root surface.
FIG. 1.
Photomicrographs of a section of plant root from L. oryzoides encrusted with Fe-plaque that has been stained with acridine orange. (A) Light micrograph of a root section. (B) The same microscopic field viewed by epifluorescence. A large number of bacterial cells are visible in the Fe-plaque. Bar, 10 μm.
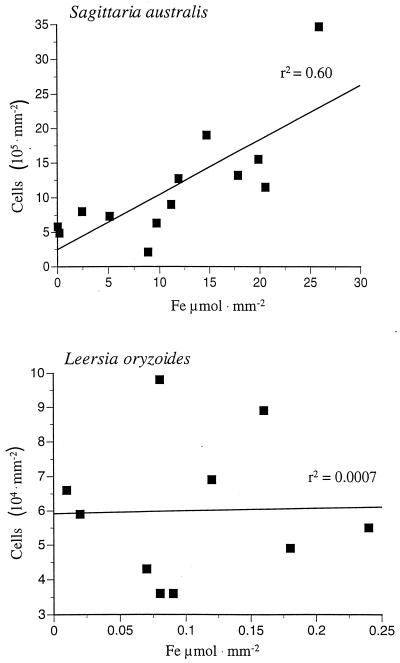
The total cell numbers and the quantity of total iron present on the roots were determined concurrently by a combined extraction procedure. For these analyses, plant roots containing visible Fe-plaque were cut into 1-cm sections, placed in 1.5 ml of deionized water in a microcentrifuge tube, vortexed for 30 s, and then centrifuged at 16,000 × g for 4 min to pellet any bacteria that were removed from the root surface. After centrifugation, the root, which remained upright in the supernatant, was removed, and the process was repeated two more times. The roots were treated with 1 ml of 0.5 M hydroxylamine–0.5 M HCl for 60 min. This procedure both dissolved the Fe-plaque on the root and released the cells that were embedded in the plaque matrix. After the reduction step, the sample was vortexed vigorously, an aliquot was removed for determination of Fe(II) by the ferrozine assay, and the remaining suspension was stained by adding 10 μl of a filter-sterilized solution of 0.1% (wt/vol) acridine orange per ml of sample. After the sample was allowed to stand for 4 min, it was filtered onto a 0.22-μm-pore-size Nucleopore filter. The filter was washed twice with 1 ml of sterile deionized water. The filters were counted by epifluorescence microscopy using the 100× objective lens on an Olympus BX 60 microscope. Twenty-five fields per filter were counted, and at least 10 filters were prepared for each plant species. The results of these studies are shown in Fig. 2. The Fe-plaque associated with two individual Sagittaria australis plants collected from the circumneutral wetland had as many as 2 × 106 cells/mm2 of root surface and a total reducible iron concentration of 25 μmol of Fe(II) · mm−2. There was a positive correlation (P < 0.01) between the number of cells and the concentration of iron associated with the root. In the rhizosphere of a grass, Leersia oryzoides, collected from an acidic site near the main channel of Contrary Creek, both the total number of cells and the iron concentration were at least 1 order of magnitude lower. In this case, there did not appear to be any correlation between the number of cells and the iron concentration. At present, it is not understood what factors may control the abundance of cells and iron in the rhizosphere of any given plant, but likely candidates include the amount of O2 transported into the roots, [Fe2+], the time of year, and physicochemical factors such as pH and redox potential (16).
FIG. 2.
Cell counts and Fe concentrations from the Fe-plaque on the roots of two different plants.
To demonstrate the presence of Fe-oxidizing bacteria in Fe-plaque, enrichments for lithotrophic Fe-oxidizing bacteria were established. The roots were washed in the manner described for the counting procedure. The washed roots were inoculated into gradient tubes that contained an Fe-sulfide plug overlaid with a 0.15% agarose semisolid gel made up from a mineral salts medium. The headspace contained ambient air. The medium was buffered to a pH of 6.2 to 6.3 with bicarbonate-CO2. This method has been described in detail previously (11). The principle of the gradient tubes is that opposing gradients of Fe(II) and O2 are established in the semisolid gel, allowing microaerophilic Fe-oxidizing bacteria to grow optimally on Fe(II) at the oxic-anoxic interface. For enrichment of acidophilic Fe oxidizers, root sections were inoculated into tubes of a liquid acidic medium, pH 3.0, typically used to cultivate T. ferrooxidans (18).
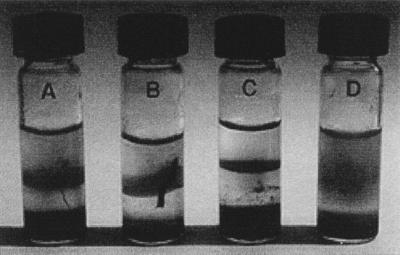
More than 80% of the circumneutral enrichment tubes from all the plant species produced successful enrichments of iron oxidizers. Figure 3 illustrates the growth of Fe-oxidizing bacteria in gradient tubes that have been inoculated either with plant roots or with a purified enrichment culture. Gradient tube enrichments were successful at circumneutral pHs from plant roots collected at both low and circumneutral pHs. One axenic culture of a putative lithotrophic Fe-oxidizing bacterium was obtained from Juncus effusus. This strain, CCJ, does not grow on heterotrophic medium either aerobically or microaerobically, and it does not utilize reduced S compounds (thiosulfate, sulfide, or tetrathionate), Mn(II), H2, or formate for growth. It is microaerophilic but cannot utilize NO3−. Liquid enrichments for acidophiles were successful for rhizosphere samples collected at acidic pHs but not circumneutral pHs. For the acidophiles, once growth was established, two consecutive end-point dilutions were carried out, one enrichment from L. oryzoides and the other from J. effusus. After two successive end-point dilutions, each culture contained small rod-shaped cells, similar in appearance to T. ferrooxidans; neither strain grew in a heterotrophic medium (18) designed for Acidiphilium spp.
FIG. 3.
Growth of Fe-oxidizing bacteria in gradient tubes. Tubes A and B were inoculated with sections of plant roots, tube C was inoculated with a purified enrichment culture of an Fe-oxidizing bacterium, and tube D is an uninoculated control. When the roots were inoculated into gradient tubes, the growth started as discrete foci on the root and continued to form a band of Fe oxides at the oxic-anoxic interface. In the control tube, Fe oxidation occurs throughout the overlaying semisolid gel.
This study showed that there are substantial numbers of bacteria associated with the root Fe-plaque of different wetland plants. Furthermore, these results provide the first proof of the existence of putatively lithotrophic, neutrophilic Fe-oxidizing bacteria associated with the Fe-plaque. The finding of both acidophilic and neutrophilic Fe oxidizers in acidic root systems indicates that the rhizosphere may be a dynamic environment with respect to pH. At present, we are undertaking a survey of different, primarily neutral pH wetlands in the Mid-Atlantic region. Results indicate that neutrophilic Fe oxidizers are present at all the sites (>6) we have investigated so far (25). We have obtained several more axenic cultures of Fe oxidizers and, like strain CCJ, all appear to be obligately microaerophilic Fe oxidizers. In at least one case it has been possible to show that there are as many as 105 Fe oxidizers per g of fresh root material, suggesting that microbial Fe oxidation could contribute substantially to the precipitation of Fe-plaque. We suspect that these microbes may also play a significant role in the biogeochemical cycles of other elements in the rhizosphere of wetland plants, such as P, Mn, and several other metals which coprecipitate with iron oxides.
Acknowledgments
We thank Ted Bradley of the Biology Department at GMU for help in plant identification and Tom Huff of the Shared Research Instrument Facility at GMU for assistance with the atomic absorption spectrometer.
This work was supported in part by a grant from the NSF (MCB-9723459 to D.E.) and a grant from the Jeffress Memorial Trust.
REFERENCES
- 1.Armstrong W, Strange M E, Cringle S, Beckett P M. Microelectrode and modelling study of oxygen distribution in roots. Ann Bot. 1994;74:287–299. [Google Scholar]
- 2.Armstrong W. Oxygen diffusion from the roots of some British bog plants. Nature. 1964;204:801–802. [Google Scholar]
- 3.Begg C B M, Kirk G J D, Mackenzie A F, Neue H-U. Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytol. 1994;128:469–477. doi: 10.1111/j.1469-8137.1994.tb02993.x. [DOI] [PubMed] [Google Scholar]
- 4.Bosse U, Frenzel P. Activity and distribution of methane-oxidizing bacteria in flooded rice soil microcosms and in rice plants (Oryza sativa) Appl Environ Microbiol. 1997;63:1199–1207. doi: 10.1128/aem.63.4.1199-1207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brix H. Macrophyte-mediated oxygen transfer in wetlands: transport mechanisms and rates. In: Moshiri G, editor. Constructed wetlands for water quality improvement. Boca Raton, Fla: CRC Press; 1993. pp. 391–398. [Google Scholar]
- 6.Calhoun A, King G M. Regulation of root-associated methanotrophy by oxygen availability in the rhizosphere of two aquatic macrophytes. Appl Environ Microbiol. 1997;63:3051–3058. doi: 10.1128/aem.63.8.3051-3058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darke A K, Walbridge M R. Estimating non-crystalline and crystalline aluminum and iron by selectable dissolution in a riparian forest soil. Commun Soil Sci Plant Anal. 1994;25:2089–2101. [Google Scholar]
- 8.Darke A K, Walbridge M R, Lockaby B G. Changes in Al and Fe crystallinity and P sorption capacity in a floodplain forest soil subjected to artificially manipulated flooding regimens in field mesocosms. Wetlands. 1997;4:235–244. [Google Scholar]
- 9.Day P R. Particle fractionation and particle size analysis. In: Black C A, editor. Methods of soil analysis. Madison, Wis: American Society of Agronomy; 1965. pp. 545–566. [Google Scholar]
- 10.Emerson D, Revsbech N P. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies. Appl Environ Microbiol. 1994;60:4022–4031. doi: 10.1128/aem.60.11.4022-4031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerson D, Moyer C L. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghiorse W C, Ehrlich H L. Microbial biomineralization of iron and manganese. In: Fitzpatrick R W, Skinner H C W, editors. Iron and manganese biomineralization processes in modern and ancient environments. Cremlingen-Destedt, Germany: Catena; 1993. [Google Scholar]
- 13.Gilbert B, Frenzel P. Rice roots and CH4 oxidation: the activity of bacteria, their distribution and the microenvironment. Soil Biol Biochem. 1998;10:1903–1916. [Google Scholar]
- 14.King G M. In situ analyses of methane oxidation associated with the roots and rhizomes of a bur reed, Sparganium eurycarpum, in a Maine wetland. Appl Environ Microbiol. 1996;62:4548–4555. doi: 10.1128/aem.62.12.4548-4555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laanbroek H J. Bacterial cycling of minerals that affect plant growth in waterlogged soils: a review. Aquat Bot. 1990;38:109–125. [Google Scholar]
- 16.Mendelssohn I A, Kleiss B A, Wakeley J S. Factors controlling the formation of oxidized root channels: a review. Wetlands. 1995;15:37–46. [Google Scholar]
- 17.Nordstrom D K, Southam G. Geomicrobiology of sulfide mineral oxidation. Rev Miner. 1997;35:361–390. [Google Scholar]
- 18.Pienta P, Tang J, Cote R, editors. ATCC bacteria and bacteriophages. 19th ed. Rockville, Md: American Type Culture Collection; 1996. [Google Scholar]
- 19.Rich J J, King G M. Carbon monoxide oxidation by bacteria associated with the roots of freshwater macrophytes. Appl Environ Microbiol. 1998;64:4939–4943. doi: 10.1128/aem.64.12.4939-4943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St-Cyr L, Fortin D, Campbell P G C. Microscopic observations of the iron plaque of a submerged aquatic plant (Vallisneria americana Michx) Aquat Bot. 1993;46:155–167. [Google Scholar]
- 21.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 22.Taylor G J, Crowder A A. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot. 1983;70:1254–1257. [Google Scholar]
- 23.Trolldenier G. Visualization of oxidizing power of rice roots and of possible participation of bacteria in iron deposition. Z Pflanzenernaehr Bodenkd. 1988;151:117–121. [Google Scholar]
- 24.Van Der Nat F-J W A, Middelburg J J. Effects of two common macrophytes on methane dynamics in freshwater sediments. Biogeochemistry. 1998;43:79–104. [Google Scholar]
- 25.Weiss, J. V. 1999. Unpublished data.