Abstract
Gels are semisolid, homogeneous systems with continuous or discrete therapeutic molecules in a suitable lipophilic or hydrophilic three-dimensional network base. Innovative gel systems possess multipurpose applications in cosmetics, food, pharmaceuticals, biotechnology, and so forth. Formulating a gel-based delivery system is simple and the delivery system enables the release of loaded therapeutic molecules. Furthermore, it facilitates the delivery of molecules via various routes as these gel-based systems offer proximal surface contact between a loaded therapeutic molecule and an absorption site. In the past decade, researchers have potentially explored and established a significant understanding of gel-based delivery systems for drug delivery. Subsequently, they have enabled the prospects of developing novel gel-based systems that illicit drug release by specific biological or external stimuli, such as temperature, pH, enzymes, ultrasound, antigens, etc. These systems are considered smart gels for their broad applications. This review reflects the significant role of advanced gel-based delivery systems for various therapeutic benefits. This detailed discussion is focused on strategies for the formulation of different novel gel-based systems, as well as it highlights the current research trends of these systems and patented technologies.
Keywords: hydrogels, in situ gels, emulsion gels, microgels, nanogels, vesicular gels
1. Introduction
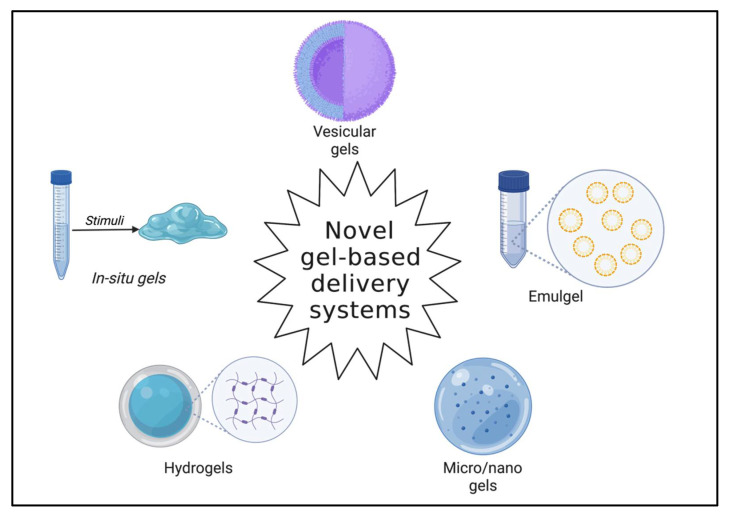
In recent years, novel drug delivery systems have proven very adept at delivering therapeutic molecules with site-specific and localized effects. Additionally, these systems facilitate drug release at desired rates and simultaneously lower the undesired effects [1]. Gels are three-dimensional, semi-solid systems consisting of polymeric matrices. These behave in the same way as solid systems; however, they consist of relatively higher liquid components than solid dispersions [2,3]. Gel systems comprise long, arbitrary chains, albeit with reversible links at precise points. These systems comprise minimum two components and are fundamentally coherent colloidal dispersion systems [4]. The system components, namely the dispersion medium and the dispersed constituent, are uniformly scattered throughout the system. Gels are usually transparent or translucent in appearance entailing higher amounts of solvent [5]. When a suitable solvent is employed, the gelling agents entangle to form a three-dimensional colloidal network that confines fluid movement by entrapment and achieves immobilization of solvent molecules [6]. The network governs the viscoelastic properties of the gel system by developing endurance against deformation. In other words, the thixotropic behavior is contributed by the matrix’s structure [7]. Gels are prepared mainly by fusion technique or by employing gelling agents. Gel-based systems can be alienated into two categories, organogels and hydrogels, based on the physical state of the gelling agent dispersion [8]. Dispersible colloids and water-soluble components constitute hydrogels, while lipophilic oleaginous components are employed in organogels [9]. The systems are further classified into xerogels or aqueous gels based on the nature of the solvents. Xerogels are solid gels with a minimum solvent concentration obtained mainly by solvent evaporation, thereby attaining a gel network [10,11]. However, the gel state can be reinstated by incorporating an imbibing agent that swells the matrix. Novel gels are capable of controlled and sustained release of loaded therapeutic molecules. Figure 1 portrays common novel gel-based delivery systems [12]. Smart gels can be developed which respond to biological and external stimuli, such as temperature, pH, chemical, enzymes, electrical, light, antigens, etc. These systems are highly instrumental in lowering undesired effects and are biodegradable and biocompatible [13]. High drug loading can be achieved. Their size (nanogels) expedites high drug accumulation at the tissue level and enables stealth systems by evading phagocytic cells [14,15,16]. Their distinctive surface properties enable passive and active targeting. This review underlines the advances in gel-based delivery systems, their developments, and a current update in the delivery of therapeutic molecules.
Figure 1.
Common novel gel-based delivery systems (Created with BioRender.com accessed on 28 April 2022).
2. Advances in Novel Gel-Based Delivery Systems
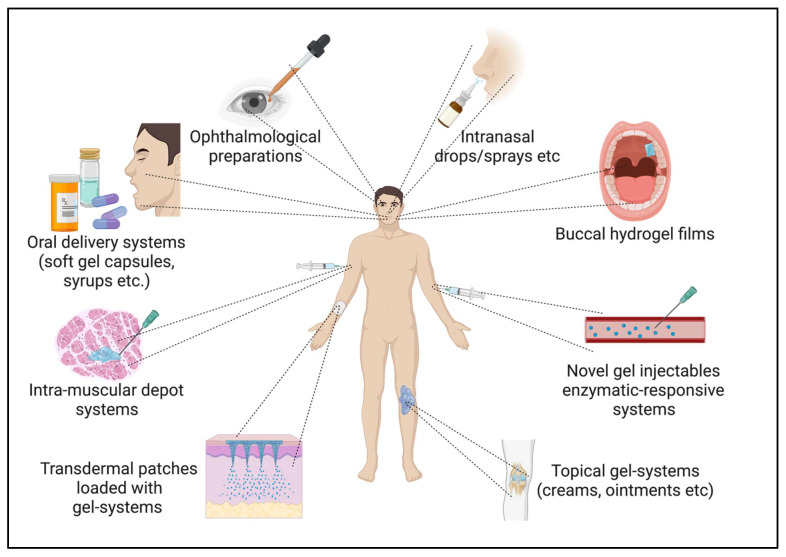
Novel gel-based drug delivery systems are classified by the nature of their structural network and by their response to stimuli [15]. The former is either a chemically aligned gel network or a physically aligned gel network system. At the same time, the latter category entails responsive, intelligent gel systems that imbibe solvents and swell on exposure to stimuli, such as temperature, pH, chemical, enzymes, electrical, light, antigens, etc. [17,18]. Novel gel systems are evaluated for rigorous characterizations to understand their efficacy as delivery systems. The most commonly employed evaluation parameters comprise swelling capacity, size and morphology, rheological properties, surface charge, etc. [19]. Further, they are scrutinized for physical appearance for compliance, physical state, homogeneity, and phase separation to understand their stability, extrudability, and spreading coefficient is significant for topical gels, as well as bioadhesive strength is a vital element for mucoadhesive gels [20]. Drug content, permeability, and release play a substantial role in any drug delivery system. The International Council on Harmonisation (ICH) (Geneva, Switzerland) dictates the stability guidelines. The gels are subjected to various stress conditions and later scrutinized for drug content, release, and entrapment efficiency to assess their compliance [21]. Figure 2 illustrates various potential delivery routes for novel gel-based delivery systems.
Figure 2.
Potential delivery routes for novel gel-based delivery system. (Created with BioRender.com accessed on 28 April 2022).
2.1. Intelligent Hydrogels
A three-dimensional network of hydrophilic polymers composes of hydrogels that inherently imbibe water and maintain the system’s integrity [22]. These systems are one of the most versatile delivery systems among novel gels. Researchers have comprehensively developed several intelligent hydrogels that precisely retort to numerous physical stimuli such as temperature, light, electric fields, pressure, sound, and magnetic fields in recent years. Furthermore, stimuli pertain to pH, ions, enzymes, etc. These systems are beneficial for formulating controlled delivery systems [23,24,25].
Temperature-responsive hydrogels are triggered by a precisely established temperature range. These hydrogels are formulated with polymers that are capable of temperature-triggered phase transitions [26]. Regular polymers exhibit higher solubility with an increase in temperature; however, the polymers employed in temperature-responsive systems, such as poly (N, N-diethylacrylamide), poly (tertramethyleneether glycol), poly(N-isopropylacrylamide), and others, possess lower critical solution temperatures. Polymers with lower critical solution temperatures tend to shrink with the increase in temperature; these hydrogels are known as negative temperature responsive [22]. Poly(acrylic acid) and polyacrylamide polymers inherently imbibe at higher temperatures and shrink at lower temperatures; hence, hydrogels prepared from these polymers are positive temperature responsive. However, tetronics and pluronics are applied to formulate thermally reversible gels [27].
Electrical signal-responsive hydrogels endure swelling and contracting when subjected to an electric field. The demerit of some systems is that due to the charge orientation, one side swells while the other contracts, thus, comprising the stability [28]. Polyelectrolytes, such as poly(2-acrylamido-2-methylpropane sulphonic acid-co-n-butlymethacrylate), are usually employed to formulate these systems [29,30].
pH-responsive hydrogels tend to release or accept protons depending on the pH of the site. Researchers have studied these systems extensively and reported encouraging results. Poly(N,N’-diethylaminoethylmethacrylate) is ionized at a low pH, unlike poly(acrylic acid), which ionizes at a higher pH. However, polycations tend to swell less at neutral pH [31,32].
In enzyme-responsive systems, a suitable enzyme is considered to trigger a release or deliver at a precisely targeted site where the enzyme is operational at a specific temperature or pH [33]. Enzyme-responsive hydrogels are usually prepared from cellulose and other suitable polymers that facilitate the macromolecular networks and function in a controlled environment [34]. The most explored enzymatic stimuli-responsive system consists of a triggerable agent (usually a polymer or a lipid) into which a therapeutic molecule is incorporated. Indeed, this active agent is sensitive to swelling or degradation when it reaches the target site. Some reported enzymes include protease- and glycosidase-based catalyzed enzymatic reactions [35].
Other intelligent systems include light-responsive hydrogels that are functional in ophthalmic delivery systems. These systems are responsive to light and other stimuli, including pressure, thrombin, antigen, and so forth [36,37,38].
2.2. In Situ Gels
Over the years, in situ gels have exhibited tremendous benefits in controlled drug delivery systems and emerged as a significant intelligent drug delivery technology [39]. These incredible systems remain in a liquid state at room temperature and achieve a sol-gel state when exposed to any biological environments, such as altered pH and temperature. In other words, in situ gelling is a spontaneous gelation process at a specific site post-administration [40].
This system accommodates numerous routes of administration, namely ocular, oral, intranasal, vaginal, rectal, depot system, etc. In situ systems have proven their benefits, including prolonged residence time at the site of application. As a result, there is a marked reduction in dosage regimen. Good thixotropic properties expedite the flexibility for formulation development. Rapid absorption and onset can be easily achieved. As a result, the therapeutic benefits can be achieved at lower doses with minimal side effects. Besides, they expedite systemic circulation, avoiding localized hepatic circulation, and targeting can be accomplished [41,42].
In situ gel systems principally work on stimuli such as temperature alteration (chitosan, poloxamer), ion activation (sodium alginate), pH changes (carbopol), solvent exchange, and environmental factors. The gels are ideally dependent on physical or chemical mechanisms. The physical mechanism constitutes of imbibing liquids, mainly water and diffusion, and absorbing water by gel polymers in site-specific locations. While diffusion entails the solvent penetration from the polymer solution to the neighboring tissues, the polymer solidifies. Temperature–responsive, pH-responsive, enzymatic cross-linking, and ionic cross-linking are effective mechanisms that govern the precipitation of solids in gel systems [43].
The pH-responsive systems include fewer polymers such as cellulose acetate phthalate, carbopol, pseudolatexes, polyethylene glycol, and polymethacrilicacid. The temperature-responsive systems primarily form gels with temperature variations and they include polymers such as pluronics, chitosan, xyloglucans etc. [44,45]. Enzymatic cross-linking is governed by natural biological enzymes. The rate of gel formation is proportional to the enzyme concentration. Insulin delivery was studied with a smart stimuli-responsive delivery system and exhibited positive outcomes, e.g., in a study reported by Podual et.al., where the glucose oxidase enzyme was employed to facilitate the release [46,47,48,49]. In ionic cross-linking, different ions dictate the phase transition of polymers. Gellan gum, carrageenan, and alginic acid are a few ion-responsive polymers. Several natural polymers are available in nature, such as carrageenan, which transform to a gel state on exposure to ions. Gellan gum is predominantly available as Gelrite (commercially available). It is an anionic polysaccharide that instinctively forms a gel in the presence of Mg++, Na+, K+, and Ca++. Electromagnetic radiation is applied to facilitate in situ gels in photo-polymerization techniques [50,51]. This is achieved by injecting reactive macromers or a solution with initiators and monomers into the desired tissue site. To initiate photo-polymerization, specific polymerizable functional groups and acrylate or similar macromers that undergo dissociation in the presence of a photo-initiator are subjected to radiation. In ultraviolet photo-polymerization, a ketone, such as 2,2 dimethoxy-2-phenyl acetophenone, is used as the initiator, while camphorquinone and ethyl eosin initiators are used in visible light systems [52,53,54,55].
2.3. Emulsion Gels
An emulgel is an amalgamation of gel technology and emulsions. This system offers controlled release, especially in topical formulations, as the therapeutic molecules are loaded in a dual delivery system emulsion and a gel core. The fusion of these dual delivery systems overcomes the demerits of these conventional systems, such as stability and drug loading [56]. Emulgels are prepared by incorporating gelling agents in the continuous (usually water) emulsion phase. Compared to other gels, these systems facilitate higher entrapment efficiency, desired thixotropic behavior, and better patient compliance. The formulation of emulgels is achieved by loading the drug-loaded emulsion into a pre-gel and applying a shear to achieve a homogenous gel system [57]. Gelling agents, emulsifiers, water, and penetration enhancers (primarily for topical formulations) are the fundamental components of emulgels. Polyethylene glycol, tweens, spans, and so forth are utilized as emulsifiers, while carbopols, including Hydroxypropyl methylcellulose (HPMC), are used as gelling agents. Menthol, oleic acid, etc., are mostly effective penetration enhancers. Emulgels are amphiphilic and enable the load of both hydrophilic and lipophilic moieties. Furthermore, the bioavailability of specific molecules was enhanced by lowering the globule size to a micron (µm). Microemulsions are isotropic, clearer, and stable systems. As a result, the increased effective surface facilitates a higher bioavailability [58]. These systems are proven better than emulgels due to the significant increase in the penetration of topical formulations and better compliance [59].
2.4. Microgels
Microgels are described as gels that have a size range in microns (µm). In contrast to typical gels, these systems have cross-linking structures in microns (µm) and are colloidal dispersions [60]. The molecular arrangements are different in these systems, in comparison to regular gels. Electric charge, polymer–water bonding, and cross-link density are a few factors that underlie the swelling capacity of these systems [61]. The internal forces impart steadiness to the microgels. Similar to microgels, stimuli-responsive methods yield novelty. The colloidal nature of microgels augments characteristics such as controlled delivery of therapeutic molecules, high responsiveness to stimuli, extrudability, mass transport, and high therapeutic efficacy with minimal adversity [62]. The microgels can be created by reducing the size of macrogels by applying a shear or by employing monomers or polymers. The basic underlying principles for formulating are emulsification, where a pregel is prepared in the oil phase, followed by polymerization, yielding microgels. In nucleation, the adjacent particles (in the solvent) initiate the nucleation process and deliver homogeneous microgels [63]. No external forces are required. In contrast, complexation, where complexes are formed in water, can be achieved by adding two mild polymers to water. Another peculiar method can be adopted for developing microgels by the mere addition of polyelectrolytes, which are oppositely charged and at diluted concentrations yield colloidal dispersion [64].
2.5. Nanogels
Nanogels are mostly hydrogel polymeric network dispersions with particles lying in the nanometer range (nm). Nanogels allow chemical modifications to facilitate ligand targeting and triggered release. This system is a blend of colloidal dispersions and cross-linking networks of polymers [65]. Nanogels are capable of intracellular delivery with enhanced cellular concentrations without significant cellular toxicity [66]. Other advantages include rapid swelling, sharp responsiveness to stimuli, high bioavailability, accommodating more therapeutic moieties, avoiding renal clearance, and remaining undetected by opsonins. Besides, nanogels have excellent stability and accommodate highly lipophilic moieties [67]. Distinctly, nanogels can be prepared by chemical cross-linking (emulsion polymerization), pulse radiolysis, and photopolymerization. In the first method, the cross-linking agent, monomers, surfactant, and water are added to an organic phase. Later, this organic phase is irradiated and purified. In pulse radiolysis, ionizing radiation is irradiated onto polymers in suitable solvents to promote internal structural reorientations of polymer radicles to yield nanogels. In the photopolymerization technique, monomers are exposed to UV radiation. Initiators, cross-linking agents, or surfactants are not required for this method; hence, pure nanogels are produced [68,69].
Similarly, other procedures include heterogenous controlled radical polymerization. Recently, developed techniques, including reversible addition–fragmentation chain transfer and atom transfer radical polymerization, have been instrumental in developing polymer-conjugate systems. Chemical cross-linking is more commonly employed to formulate varied nanogels and is further classified into Michael addition reactions, carbodiimide coupling, and free radical polymerization [70]. Hydrophilic monomers are polymerized in the presence of varied cross linkers to yield synthetic nanogels. Self-assembly of polymers includes an aggregation of the hydrophilic polymers by electrostatic interactions, hydrophobic bonds, or hydrogen bonding in an aqueous medium. These systems accommodate large molecules; thus, they are effective in incorporating macromolecules, such as proteins and peptides [71].
2.6. Vesicular Gels
Vesicular gels are composed of carrier systems deliberate with amphiphilic molecules comprising lipids, surfactants, and co-polymers [72]. The carrier system consists of a hydrophilic core within an amphiphilic bilayer [73]. Vesicular drug delivery has been on the rise in recent studies for its versatility and broad application in drug delivery, cosmetics, etc. Vesicular gels have exhibited significant results in topical drug delivery. A hydrogel matrix can be incorporated with preformed vesicles in a simple technique [74,75].
2.6.1. Liposomal Gels
Liposomes are a very effective and successful novel delivery system. One significant advantage of these systems is that they possess biosimilar structures with desirable properties [76]. Their amphiphilic properties enable the incorporation of both lipophilic and hydrophilic therapeutic molecules. These carriers are biodegradable, exhibit no toxicity, and support localization and site-specific release. They are capable of infiltrating several bio-obstacles otherwise difficult to any conventional systems [77,78]. However, often the topical applications are limited due to rheological constraints. Traditionally, liposomes can be formulated by the standard technique of lipid film hydration. Other techniques include the French pressure cell method and solvent injection methods. The addition of a gelling agent in the aqueous phase incorporates liposomes into the gel system. Carbopols, xanthan gum, poloxamers, gellan gum, polyvinyl alcohol, and others are explored as gelling agents [79].
2.6.2. Niosomal Gels
Niosomes are similar to the liposomal system; however, these are prepared from non-ionic surfactants. This vesicular system resolves the instability of traditional liposomes due to phospholipids [80]. The bilayer, depending on the preparation technique, yields unilamellar or multilamellar niosomes. The system’s integrity relies on the surfactant’s chemical composition and on the hydrophilic–lipophilic balance (HLB) value. Primarily, proniosomes, emulsion niosomes, and aspasomes are different types of niosomes. Proniosomes are preliminary niosomes which are devoid of any aqueous phase [81]. These are reconstituted with a suitable buffer to yield niosomes. Proniosomes are advantageous over niosomes regarding dose dumping and stability. Niosomal gels comprise a therapeutic moiety, surfactant, and cholesterol that yield vesicles, which are later loaded into a gel base. However, broad applications of these systems are restricted to achieving controlled release, stealth systemic circulations, and enhanced stability [82,83].
2.6.3. Transferosome Gels
Transferosomes are improved versions of liposomes. These systems overcome various shortcomings of other vesicular systems, including aggregation, dose dumping, and poor permeability [84]. Similar to liposomes, these systems also possess an aqueous core enclosed by a lipid layer. However, these bilayers are modified with edge activators, which enables flexibility. Usually span 80, tween 80, and sodium cholate are proven effective edge activators. Phospholipids, edge activators, alcohol, and hydrating media (buffers) constitute transferosomes [85]. Hydrocolloids are incorporated into buffers to yield transferosome gels. The delivery of proteins and peptides, such as insulin, has shown promising results when incorporated into the transferosome gel system [85,86,87].
3. Novel Gel-Based Delivery Approaches for Delivering Therapeutic Molecules—Recent Trends
3.1. Hydrogel Systems
Various nanocarrier systems have been utilized in drug delivery applications for different diseases. The number of cancer patients is increasing day by day worldwide. However, the therapy for cancer treatment is still adapted as a conventional method, i.e., surgery and radiotherapy followed by chemotherapy. There are several limitations of conventional chemotherapy, which produces long- or short-term side effects and adverse effects. Such chemotherapy delivery to cancer patients has low bioavailability due to poor solubility issues, resulting in adverse effects on the biological systems. Hence, researchers are finding a better way to deliver chemotherapeutics with enhanced bioavailability to resolve these problems safely. The hydrogel drug delivery system is one of the carrier systems that can deliver hydrophilic chemotherapeutics agents and hydrophobic drugs in a sustained and controlled manner. Generally, hydrogels are hydrated in nature and possess self-shrinking and self-swelling characteristics in different biological conditions [88]. Their 3D structural properties enable the efficient encapsulation of chemotherapeutic agents into their internal structure, which protects the drug from degradation either in storage or enzymatically during circulation in biological systems. The advantage of the hydrogel drug delivery system in cancer therapy is that nanogels can be modified according to the response to the cell or the tumor microenvironments [89].
Hydrogels can be functionalized by targeting ligands for enhanced, prolonged, and specific drug delivery, making it a safer carrier system. In general, hydrogels could achieve high drug delivery efficiency in cancer therapy with conformational changes and degradation under specific conditions, such as temperature, pH, redox, and ultrasound [90].
The second advantage is that hydrogel synthesis can be done as per the demand of the drug delivery to particular tumor microenvironments. Internal and external stimuli have been utilized to design hydrogels for the delivery of chemotherapeutic drugs to manage cancer. The thermo-sensitive stimuli hydrogels are the most common used hydrogels. These hydrogels are usually in a gel state at room temperature and are attributed to a low critical solution temperature. Once it is administered into the body, it will change its form into a solution due to the cellular temperature. Usually, poly(N-isopropylacrylamide) or elastin-like polypeptides have been used in thermo-sensitive hydrogels recently [91,92].
Moreover, thermo-sensitive hydrogel in situ sites avoid the accumulation of chemotherapeutic drugs in the liver or spleen, which overcomes the biosafety limitations of drugs [93]. The chemotherapeutic agent, 7-ethyl-10-hydroxycamptothecin or SN-38, has a lower solubility in drug delivery applications. Bai et al. developed a thermo-sensitive liposomal system, which showed a better antitumor effect and reduced systematic toxicities in in vivo models at the same dose of the pure drug [94]. Similarly, alginate-based thermo-sensitive gels enhanced cisplatin’s in vivo antitumoral effects through an in situ injection. They spurred the tumor growth up to 95% compared to the control group, along with an increased prolonged survival rate of the animals [95]. Furthermore, in another study, the interaction of ligand–receptor with the hydrogel enhances transcorneal permeability and precorneal retention of the drug activity. Upon topical instillation, dexamethasone and Arg-gly-asp supramolecular hydrogel increased the transcorneal permeability in rabbits’ eyes to treat ocular inflammation [96]. The development of a copolymer from a mono-functional polymer exhibits a good sol-gel transition phase, thereby enhancing water solubility to prolong the mucoadhesive system. The effect of silsesquioxane thermo-responsive hydrogel of FK506 improved drug solubility, biocompatibility, and prolonged retention time by enhancing drug efficacy in a murine dry eye model [97].
These hydrogels can be used for single-drug therapy and combinatorial therapy by co-loading with other chemotherapeutics drugs [98,99]. For instance, Doxorubicin (DOX), IL-2, and IFN-g were delivered by a poly (g-ethyl-L glutamate)-poly (ethylene glycol)-poly(g-ethyl-L-glutamate) (PELG-PEG-PELG) hydrogel. The prepared hydrogel system showed long-term sustained drug release behavior for more than three weeks. The combination therapy enhanced the antitumor effect against B16F10 melanoma cells by inducing cell apoptosis and cell cycle arrest in the G2/S phase. The nanocarriers have not shown any systematic side effects in the xenograft mice model, suggesting an effective and promising approach to drug delivery in melanoma therapies [98].
3.2. Thermosensitive Hydrogels
Likewise, the thermo-sensitive poly(3-caprolactone)-10R5-PCL hydrogels, co-loaded with tannic acid and oxaplatin to manage colorectal cancer, restricted the CT26 colon cancer growth in a mice model. They improved the survival time of the animals (11). Moreover, the co-delivery of gemcitabine and cisplatin through the PDLLA-PEG-PDLLA hydrogels synergistically improved the anti-cancer efficacy against pancreatic cancer with sustained drug release. The dual drug-loaded hydrogels exhibited superior antitumor effects in the xenograft model than in single-drug therapies [100]. Similarly, when the thermo-sensitive stimuli hydrogel was utilized to co-deliver paclitaxel (PTX) and temozolomide (TMZ), it produced a synergistic effect against glioblastoma cells. The in vivo studies suggested that the combination therapy potentially reduced tumor growth and sustained drug release in mice brains for one month with no apparent side effects [101].
Chitosan-based thermo-sensitive hydrogels, including several polyols, have gained a critical identity that transforms to a hydrogel form upon contact with body temperature from a solution state. A study indicated that a hydrogel formulation that employs a green synthesis approach with chitosan, genepin, and poloxamer 407 has proved to sustain drug release. Brinzolamide-loaded nanostructured lipid carrier was entrapped into a hydrogel matrix using a hot-melt emulsification and sonication method that showed a sustained drug release for a longer duration (24 h) than marketed eye drops (8 h) in the management of glaucoma [102]. Moxifloxacin hydrochloride thermosensitive gel was formulated using chitosan-β-glycerophosphate to advance the ocular delivery. The drug release profile of the formulation was shown to be delivered in a sustained pattern and at a slower rate with a release of 53% in 1 h and 83.3% in 8 h due to the hydrogel’s polymeric network, whereas a 75.6% release was identified from the drug solution. Hence, the formulated hydrogel was stated to be biodegradable, safe, and with a more significant drug loading to the administration site to manage bacterial infections [103]. The quaternized chitosan achieved a better swelling property as a therapeutic carrier. The thermo-sensitive transparent quaternized chitosan hydrogel was utilized to release timolol maleate in the management of glaucoma. Hemolysis and cytotoxicity profiles showed good biocompatibility. The in vitro release pattern of timolol maleate from the hydrogel showed a burst release initially and a linear release for one week, showing a sustained pattern. Hence, quaternized chitosan has a promising ability to sustain drug release in the anti-glaucoma model [104]. Similarly, chitosan has been an extensively used polysaccharide to prolong precorneal retention and corneal permeability, though it is poorly soluble in physiological solvents. Therefore, derivates of chitosan (glycol chitosan) have shown superior aqueous solubility in a broad pH range and are employed in ocular drug delivery systems, such as hydrogels, nanoparticles, and films. Hydrogel films for the topical ocular delivery of dexamethasone and levofloxacin were fabricated by utilizing many oxidation degrees of oxidized hyaluronic acid and glycol chitosan. The formulation showed potent activity in decreasing bacterial growth in different strains. Additionally, the formulation downregulated in vitro anti-inflammatory activities. Overall, the formulated hydrogel film would serve as a treatment for endophthalmitis with minimal corneal irritation and biocompatibility [105]. In another study, a combination of carbon dots and thermo-sensitive hydrogels was evaluated for their in vitro cellular toxicity after effectively delivering diclofenac sodium to an eye. The in vitro release showed characteristic biphasic release of the drug. Cellular toxicity studies revealed that the formulation has a better cytocompatibility with CD44 targeting and serves as a novel way for ocular delivery of drugs [106].
3.3. Light Stimuli Hydrogels
Furthermore, light stimuli hydrogels enhanced drug release from hydrogels. The mechanism of photosensitive hydrogels is that they can undergo structural and conformational changes under radiation, ultraviolet, and visible light sources and achieve sol-gel transition [107,108]. Photo stimuli hydrogels have been utilized in the past few years. For example, Fourniols and his co-worker developed a polyethylene glycol dimethacrylate-based photo-polymerized hydrogel for the local and sustained delivery of TMZ in the management of glioblastoma [109]. Similarly, azobenzene, α-cyclodextrin-functionalized hyaluronic acid, and gold nano-bipyramids-mesoporous silica nanoparticles-conjugated polymer-based in situ injectable hydrogels loaded with DOX and stimulated under near-infrared (NIR) radiation potentially improved the drug localization of drug into the nuclei of the tumor cells [110]. Another example of a NIR stimuli hydrogel was designed by Qui et al. in 2018. They synthesized DOX and black phosphorus-loaded agarose-based hydrogels. The drug release was enhanced after exposure to the light intensity of NIR, which therapeutically increased the anti-cancer efficacy for in vivo experiments [111].
3.4. pH Stimuli Hydrogels
Another most commonly used hydrogel for cancer therapy is the pH stimuli hydrogel. Due to the diverse microenvironments of tumor cells, the pH of the extracellular matrix of the tumor is in the range of 5.8–7.2 and the lysosomal or intracellular matrix pH is usually 5.5, which is acidic compared to normal cells (pH 7.4) [112,113]. Hence, both intracellular and extracellular acidic conditions stimulated the hydrogels for degradation and drug release at the tumor site. The pH-sensitivity could have been achieved by protonating the polymers’ ionizable moiety or acid-cleavable bond break [114].
Usually, the pH stimuli hydrogels act as prodrugs, inactive in normal biological pH (7.4), but once they reach the tumor site, upon the release of the drug, they change to their active chemotherapeutic form, producing its effect. The FER-8 peptide hydrogel loaded with PTX exhibited high drug encapsulation and self-assembly of the peptide at pH 7.4. The hydrogel was stimulated by the acidic conditions of the tumor microenvironment. The PTX-loaded hydrogel significantly accumulated the drug in the tumor site and showed sustained and prolonged retention of PTX through an intratumoral injection. This hydrogel showed enhanced tumor inhibition [115]. The polyacrylic acid-based pH stimuli hydrogel for DOX delivery triggered the specific site of drug delivery with a less acidic microenvironment. The hydrogels showed improved pharmacokinetics and drug accumulation in a mice xenograft model with significant tumor growth regression and lowered adverse and side effects [116]. Various hydrogels have received attention in the last few years due to their biocompatibility, biodegradability, and low toxicity. The hydrogel-based drug delivery of chemotherapeutic agents has been attended in recent years [27]. Most chemotherapeutic drugs are associated with lower solubility. Hence, efficient drug delivery and significant therapeutic effects cannot be achieved by a lower dose and the involvement of a higher dose in cancer management includes side effects. Therefore, the hydrogel system is the emerging nanocarrier for the delivery of chemotherapeutic agents. Some recent significant research related to novel gel-based systems is shown in Table 1.
Table 1.
Recent published research for novel gel-based delivery systems.
| Sno. | Types of Hydrogels | Composition | Drug Used | Disease | References |
|---|---|---|---|---|---|
| 1 | Thermo-stimuli Hydrogel | 7-ethyl-10-hydroxycamptothecin (SN-38) liposomal hydrogel | SN-38 | Hepatocellular carcinoma |
[94] |
| 2 | alginate nanogel co-loaded with cisplatin and gold nanoparticles | cisplatin | colorectal cancer | [95] | |
| 3 | poly(γ-ethyl-L-glutamate)-poly(ethylene glycol)-poly(γ-ethyl-L-glutamate) (PELG-PEG-PELG) hydrogel | DOX/IL-2/IFN-γ | melanoma | [98] | |
| 4 | PHB-b-PDMAEMA | paclitaxel and Temozolomide |
glioblastoma | [99] | |
| 5 | poly(3-caprolactone) (PCL)-10R5-PCL (PCLR) hydrogel | tannic acid/ oxaliplatin |
colorectal cancer | [117] | |
| 6 | Caprolactone-Polyethylene Glycol | Silibinin | melanoma | [118] | |
| 7 | α-Cyclodextrin co-polymeric PEGylated iron oxide-based hydrogels | PTX/DOX | breast cancer | [119] | |
| 8 | β- cyclodextrin complexed glycol chitosan hydrogel | PTX | Ovarian Cancer | [120] | |
| 9 | mPEG-b-PELG | CA4P and cisplatin | colorectal cancer | [121] | |
| 10 | Pluronic F127, Pluronic F68, and Hydroxy Propyl Methyl Cellulose. | Itraconazole | Fungal Keratitis | [122] | |
| 11 | Sulfobutylether-β-cyclodextrin (SBE-β-CD) | Ketoconazole | Fungal Keratitis | [123] | |
| 12 | Poloxamers (P407 and P188), Carbopol-93 | Dipivefrin hydrochloride |
Intraocular pressure | [124] | |
| 13 | Triacetin, Transcutol-P, Poloxamer 407, Poloxamer188 | Acyclovir | Ocular viral infections | [125] | |
| 14 | Poloxamer 407, disodium EDTA | Chlorhexidine digluconate | Acanthamoeba keratitis | [126] | |
| 15 | poloxamers, hyaluronic acid (HA), beta-lapachone (β Lap), | beta-lapachone (β Lap) | Restoring the synovial fluid | [127] | |
| 16 | Poloxamers, D—(+)-GlcN hydrochloride, papain, | Glucosamine (GlcN) | controlling inflammation and promoting cartilage re-generation | [128] | |
| 17 | Poloxamer, hyaluronic | Sulforaphane (SFN | SFN intra-articular release for OA treatment | [129] | |
| 18 | Photosensitive Hydrogels | azobenzene and α-cyclodextrin-functionalized hyaluronic acid with gold nanobipyramids | DOX | human epidermal keratinocyte | [110] |
| 19 | poly(N-isopropylacrylamide) hydrogel | Bortezomib and DOX | osteoblast | [130] | |
| 20 | Agarose based hydrogel | black phosphorus and DOX | breast cancer | [111] | |
| 21 | poly(N-phenylglycine)- poly (ethylene glycol) co-polymeric hydrogel | Cisplatin | breast cancer | [131] | |
| 23 | pH-stimuli Hydrogels | poly (acrylic acid) complexed with stabilized amorphous calcium carbonate | DOX | hepatocarcinoma | [116] |
| 24 | amphiphilic hyaluronan (HA)-and cystamin-pyrenyl | Organoiridium (III) | lung cancer | [132] | |
| 25 | Graphene oxide, L-arginine | 5-fluorouracil | breast cancer | [133] | |
| 26 | FER-8 peptide | PTX | hepatocarcinoma | [115] | |
| 27 | Dibenzaldehyde, poly (ethylene glycol) | DOX | hepatocarcinoma | [50] | |
| 28 | Redox- stimuli Hydrogels | dextrin nanogel | DOX | Breast Cancer | [134] |
| 29 | Polydopamine, poly (ethylene glycol) | DOX | breast cancer | [135] | |
| 30 | poly (ethylene glycol) monomethacrylate | Vorinostat and etoposide | cervical cancer | [136] | |
| 31 | polyglycerol nanogel | DOX | cervical cancer | [137] | |
| 33 | N-Isopropylacrylamide, Methacrylic acid, Benzalkonium chloride and poly (sulfobetaine methacrylate) | DOX and Indocyanine green | hepatocarcinoma | [138] | |
| 34 | Magnetism-Responsive Hydrogels | ferromagnetic vortex-domain iron oxide, chitosan and poly (ethylene glycol) | DOX | breast cancer | [139] |
| 35 | methacrylic acid, ethylene glycol dimethacrylate, 2,2′-azobisisobutyronitrile and glycidyl methacrylate | sunitinib | cervical cancer, breast cancer and Human Thyroid Tumor | [140] | |
| 36 | paramagnetic fullerene, DNA and Hyaluronic Acid | DOX | hepatocarcinoma | [141] | |
| 37 | Proniosomal gel | Surfactant, lecithin and cholesterol | Curcumin | Ocular Inflammation | [142] |
| 38 | Liposomal gel | Lecithin: cholesterol, Carbopol 934 | Travoprost | Glaucoma and ocular hypertension | [143] |
| 39 | Injectable hydrogel | horseradish peroxidase (HRP) and H2O, chitosan, hyaluronic acid (HA) | Dextrane Tyramine |
cartilage tissue regeneration | [144] |
| 40 | bone marrow mesenchymal stem cell (MSC) spheroids, short fibre fillers, Kartogenin (KGN) | Celecoxib | cartilage regeneration, and inflammation removal | [145] | |
| 41 | poly (ethylene glycol)-b-polythioketal-b-poly(ethylene glycol), micelles | dexamethasone acetate | preventing cartilage extracellular matrix degeneration | [146] | |
| 42 | Gelatin, ulbecco’s phosphate buffered saline (DPBS), methacrylic anhydride. | diclofenac sodium | preventing the development of degenerative changes in OA via the synergistical treatment of enhanced lubrication (COF reduction) and sustained drug release (inflammation down-regulation) | [147] | |
| 43 | Hexachlorocyclotriphosphazene, Poly (dichlorophosphazene, Methoxy poly (ethylene glycol), | Triamcinolone acetonide | Effective prevention and long-term anti-OA treatment | [148] | |
| 44 | Shear—sensitive hydrogels | Hyaluronic acid, aldehyde groups, amino Groups, HSPC lipid, |
Celecoxib | Minimizing shear-induced cartilage damage and inflammation | [149] |
4. Descriptive Patents Established for Novel Gel-Based Delivery Systems
Various publications illustrated the efficacy of novel gels over the last decades. The current section explains the established patents of novel gels. In patent 20210338211, aptamer and hydrogels cross-linked with DNAzyme are used for colorimetric identification of analytes in body fluids through an ocular device [150]. Patent 2021120395 tells that in situ hydrogels provide extended drug release by delivering to a tissue, usually agents with low water solubility [151]. Patent W/O/2021/113515 explains that hydrogels composed of Gelatin–hydroxyphenylpropionic acid (gelatin-HPA), hyaluronic acid–tyramine (HA-Tyr), catalyzer, cross-linker, or other combinations can treat ocular disorders [152]. Patent 20210069496 describes polymeric formulations, including hydrogels formed by a UV cross-linking method. Here, the hydrogels act as a nasal stimulator that stimulates the lacrimal glands to mimic the production of tears electronically and to manage dry eye syndromes [153]. Patent WO/2021/038279 tells of the invention related to ion-exchange polymeric hydrogels for ocular treatment [154].
Patent 202121042889 talks about etoricoxib, which is formulated as a nanosponge hydrogel for the management of arthritis by the method of emulsion solvent diffusion using a polymeric organic solvent, ethylcellulose eudragit, and an aqueous phase. The formulated nanosponges were evaluated for differential scanning calorimetry (DSC), Fourier-transform infrared spectroscopy (FTIR), polydispersity index (PDI), scanning electron microscopy (SEM), zeta potential, drug content, entrapment efficiency, viscosity, spreadability, in vitro diffusion, irritation test, and in vivo antiarthritic effect. The synthesized formulation proved to be effective as a novel way for managing arthritic pain [155]. In patent 202031000910, a cross-linked protein matrix hydrogel was prepared for topical application in skin regeneration and wound healing [156].
Conductive hydrogels with an adhesiveness method of preparation were invented and discussed in patent 112442194. The process involves dopamine modification of carbon nanotubes and grafting to saccharides, followed by acrylamide mixing and formation of hydrogels in the presence of an initiator and a cross-linking agent. Hydrogel structure, electrical conductivity, adhesiveness, and biocompatibility can be improved by dispersing the modified carbon nanotubes in an aqueous solution to form hydrogen bonds and cross-link with the supramolecules of the hydrogel. Hence, conductive hydrogels can be used in biomedical fields, as well as for human body monitoring and electronic skin, etc. [157].
Patent 20210023121 discloses thrombin-responsive hydrogels for prolonged heparin delivery for auto-anticoagulant regulation in a controlled feedback mechanism. The formulated microneedle, containing a patch, can activate the thrombin and release heparin to avoid blood coagulation. The insertion of a microneedle patch containing hydrogel regulates blood coagulation sustainably in response to thrombin without leakage [158].
Patent 20210393780 discloses the effectiveness of thermo-sensitive polymer–protein-based hydrogels in the field of cancer therapeutics. The invention is enriched by photosensitizers, dyes, photothermal agents, and drugs. Hence, the invention proved to be less expensive, highly effective, and thermosensitive, resulting in a sustained drug release for targeted delivery [159]. Patent WO/2021/174021 describes a degradable hydrogel system for immunotherapy with an extended-release pattern of an anti-cancer drug linked with a hydrogel matrix synergistically for cancer treatment [160]. Patent 9758/CHENP/2012 explains self-assembling peptides and their use in hydrogels for the adhesion, proliferation, differentiation of neural stem cells, and their auto-healing properties. They are reported to be non-toxic in central nervous systems, as well as to avoid bleeding and have faster nervous regeneration [161]. Patent 20140286865 explores di-block co-polypeptide synthetic hydrogels in the central nervous system [162].
5. Conclusions
In this review, the authors have focused on recent trends in novel gel-based drug delivery systems and their applications. In recent times, these novel systems have exhibited proficient delivery of multiple therapeutic moieties and expressed desired properties and functions, such as selective targeting. The systems offer abundant benefits compared to conventional drug delivery approaches including controlled drug release, high drug loading, biocompatibility and biodegradability, and enriching patient compliance and comfort. The responsive gel technology is significant in formulating intelligent delivery systems; these systems respond to stimuli such as pH, temperature, enzymes, and so forth. Hence, these systems are site-specific and facilitate the controlled release of therapeutic molecules. Although, fundamentally, these systems have proven capabilities for effective drug delivery, there is a scope to explore new polymers to fabricate novel gels; therefore, the currently employed components can be modified. Furthermore, recent studies revealed that employing plant extracts to develop novel delivery systems has enabled the development of various drug delivery systems with non-toxic procedures. The formulation of substances of natural origin has advantages in various magnitudes on the environment. Over the years, the evolution of green chemistry has provided more eco-friendly procedures resulting in minor harm to nature. Current findings suggest promising results for green synthesised delivery systems over conventional systems. Green technology does not require common harmful chemicals. Instead, this technology uses biological and biocompatible reagents. Besides, reports suggest that green technology delivery systems have better stability than traditional methods. Formulations developed with green technology employing plant extracts and biomaterials, such as proteins or peptides, yielded non-toxic and highly biocompatible systems; thus, they have resolved the most concerning issue with traditional delivery systems, i.e., toxicity. Green technology will play a significant role in formulating novel delivery systems. However, we require further understanding of the development of systems with green technology.
Acknowledgments
The author(s) express deep sense of gratitude towards JSS College of Pharmacy, JSS Academy of Higher Education & Research (JSS AHER), Mysuru for their constant support and motivation.
Author Contributions
Conceptualization, T.K.S., V.N.G.; Formal analysis, V.N.G.; Resources, T.K.S., S.C., S.M., H.K., P.C.; Data curation, T.K.S., S.C., S.M., H.K., P.C.; Writing original draft preparation, T.K.S., S.C., S.M., H.K., P.C.; Writing review and editing, V.J., B.V. and D.V.G.; Supervision, V.N.G., V.J., B.V. and D.V.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work received no external funding. The APC is supported by JSS Academy of Higher Education & Research, Mysuru.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iriventi P., Gupta N.V., Osmani R.A.M., Balamuralidhara V. Design & development of nanosponge loaded topical gel of curcumin and caffeine mixture for augmented treatment of psoriasis. DARU J. Pharm. Sci. 2020;28:489–506. doi: 10.1007/s40199-020-00352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uzunalli G., Guler M.O. Peptide gels for controlled release of proteins. Ther. Deliv. 2020;11:193–211. doi: 10.4155/tde-2020-0011. [DOI] [PubMed] [Google Scholar]
- 3.Nambiar M., Schneider J.P. Peptide hydrogels for affinity-controlled release of therapeutic cargo: Current and potential strategies. J. Pept. Sci. 2021;28:e3377. doi: 10.1002/psc.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S., Tiwari S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020;162:737–747. doi: 10.1016/j.ijbiomac.2020.06.110. [DOI] [PubMed] [Google Scholar]
- 5.Micale N., Citarella A., Molonia M.S., Speciale A., Cimino F., Saija A., Cristani M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules. 2020;25:3254. doi: 10.3390/molecules25143254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Op’t Veld R.C., Walboomers X.F., Jansen J.A., Wagener F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020;26:230–248. doi: 10.1089/ten.teb.2019.0281. [DOI] [PubMed] [Google Scholar]
- 7.Tavakoli S., Klar A.S. Advanced Hydrogels as Wound Dressings. Biomolecules. 2020;10:1169. doi: 10.3390/biom10081169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du W., Zong Q., Guo R., Ling G., Zhang P. Injectable Nanocomposite Hydrogels for Cancer Therapy. Macromol. Biosci. 2021;21:2100186. doi: 10.1002/mabi.202100186. [DOI] [PubMed] [Google Scholar]
- 9.Chen T., Hou K., Ren Q., Chen G., Wei P., Zhu M. Nanoparticle-Polymer Synergies in Nanocomposite Hydrogels: From Design to Application. Macromol. Rapid Commun. 2018;39:e1800337. doi: 10.1002/marc.201800337. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez G., Venkateswaran S., López-Ruiz E., Peran M., Pernagallo S., Díaz-Monchón J.J., Canadas R., Antich C., Oliveira J.M., Callanan A., et al. A soft 3D polyacrylate hydrogel recapitulates the cartilage niche and allows growth-factor free tissue engineering of human articular cartilage. Acta Biomater. 2019;90:146–156. doi: 10.1016/j.actbio.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Yu J.-K., Ren K., Zuo J., Ding J., Chen X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules. 2019;20:1478–1492. doi: 10.1021/acs.biomac.9b00043. [DOI] [PubMed] [Google Scholar]
- 12.Barouti G., Liow S.S., Dou Q., Ye H., Orione C., Guillaume S.M., Loh X.J. New Linear and Star-Shaped Thermogelling Poly([R]-3-hydroxybutyrate) Copolymers. Chem. A Eur. J. 2016;22:10501–10512. doi: 10.1002/chem.201601404. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y., Cheng Y., Tong J., Zhang L., Wei Y., Tian M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B. 2021;9:2979–2992. doi: 10.1039/D0TB02877K. [DOI] [PubMed] [Google Scholar]
- 14.Taghizadeh B., Taranejoo S., Monemian S.A., Moghaddam Z.S., Daliri K., Derakhshankhah H., Derakhshani Z. Classification of stimuli–responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015;22:145–155. doi: 10.3109/10717544.2014.887157. [DOI] [PubMed] [Google Scholar]
- 15.Marques A.C., Costa P.J., Velho S., Amaral M.H. Stimuli-responsive hydrogels for intratumoral drug delivery. Drug Discov. Today. 2021;26:2397–2405. doi: 10.1016/j.drudis.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Khan S., Akhtar N., Minhas M.U., Badshah S.F. pH/Thermo-Dual Responsive Tunable In Situ Cross-Linkable Depot Injectable Hydrogels Based on Poly(N-Isopropylacrylamide)/Carboxymethyl Chitosan with Potential of Controlled Localized and Systemic Drug Delivery. AAPS PharmSciTech. 2019;20:119. doi: 10.1208/s12249-019-1328-9. [DOI] [PubMed] [Google Scholar]
- 17.Oliva N., Conde J., Wang K., Artzi N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017;50:669–679. doi: 10.1021/acs.accounts.6b00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W., Zhang Y., Zhang Q., Zhang L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016;44:2049–2061. doi: 10.1007/s10439-016-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T., Chen L., Shen T., Wu D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016;93:775–782. doi: 10.1016/j.ijbiomac.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Raghuwanshi V.S., Garnier G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019;274:102044. doi: 10.1016/j.cis.2019.102044. [DOI] [PubMed] [Google Scholar]
- 21.Azeera M., Vaidevi S., Ruckmani K. Characterization Techniques of Hydrogel and Its Applications. Springer; Cham, Switzerland: 2018. pp. 1–24. [DOI] [Google Scholar]
- 22.Alexander A., Ajazuddin, Khan J., Saraf S., Saraf S. Polyethylene glycol (PEG)–Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014;88:575–585. doi: 10.1016/j.ejpb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Qu J., Zhao X., Ma P.X., Guo B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018;72:55–69. doi: 10.1016/j.actbio.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Malekmohammadi S., Aminabad N.S., Sabzi A., Zarebkohan A., Razavi M., Vosough M., Bodaghi M., Maleki H. Smart and Biomimetic 3D and 4D Printed Composite Hydrogels: Opportunities for Different Biomedical Applications. Biomedicines. 2021;9:1537. doi: 10.3390/biomedicines9111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasiński A., Zielińska-Pisklak M., Oledzka E., Sobczak M. Smart Hydrogels—Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020;15:4541–4572. doi: 10.2147/IJN.S248987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi J., Yu L., Ding J. PEG-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021;128:42–59. doi: 10.1016/j.actbio.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Sun Z., Song C., Wang C., Hu Y., Wu J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020;17:373–391. doi: 10.1021/acs.molpharmaceut.9b01020. [DOI] [PubMed] [Google Scholar]
- 28.Le T.M.D., Duong H.T.T., Thambi T., Giang Phan V.H., Jeong J.H., Lee D.S. Bioinspired pH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules. 2018;19:3536–3548. doi: 10.1021/acs.biomac.8b00819. [DOI] [PubMed] [Google Scholar]
- 29.Basu S., Pacelli S., Paul A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020;105:159–169. doi: 10.1016/j.actbio.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo F., Kehr N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2020;10:2001341. doi: 10.1002/adhm.202001341. [DOI] [PubMed] [Google Scholar]
- 31.Stayton P., El-Sayed M., Murthy N., Bulmuş V., Lackey C., Cheung C., Hoffman A. ‘Smart’ delivery systems for biomolecular therapeutics. Orthod. Craniofacial Res. 2005;8:219–225. doi: 10.1111/j.1601-6343.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y.J., Chen F. pH-Responsive Drug-Delivery Systems. Chem. Asian J. 2015;10:284–305. doi: 10.1002/asia.201402715. [DOI] [PubMed] [Google Scholar]
- 33.Chandrawati R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016;241:972–979. doi: 10.1177/1535370216647186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culver H.R., Clegg J.R., Peppas N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017;50:170–178. doi: 10.1021/acs.accounts.6b00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billah S.M.R., Mondal I.H., Somoal S.H., Pervez M.N., Haque O. Enzyme-Responsive Hydrogels. Springer; Cham, Switzerland: 2019. pp. 309–330. [DOI] [Google Scholar]
- 36.Rafael D., Melendres M.M.R., Andrade F., Montero S., Martinez-Trucharte F., Vilar-Hernandez M., Durán-Lara E.F., Schwartz S., Abasolo I. Thermo-responsive hydrogels for cancer local therapy: Challenges and state-of-art. Int. J. Pharm. 2021;606:120954. doi: 10.1016/j.ijpharm.2021.120954. [DOI] [PubMed] [Google Scholar]
- 37.Andrade F., Roca-Melendres M.M., Durán-Lara E.F., Rafael D., Schwartz S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers. 2021;13:1164. doi: 10.3390/cancers13051164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin D.R., Kasko A.M. Photoselective Delivery of Model Therapeutics from Hydrogels. ACS Macro Lett. 2012;1:1330–1334. doi: 10.1021/mz300366s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fakhari A., Subramony J.A. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Control. Release. 2015;220:465–475. doi: 10.1016/j.jconrel.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Fang G., Yang X., Wang Q., Zhang A., Tang B. Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater. Sci. Eng. C. 2021;127:112212. doi: 10.1016/j.msec.2021.112212. [DOI] [PubMed] [Google Scholar]
- 41.Choi B., Loh X.J., Tan A., Loh C.K., Ye E., Joo M.K., Jeong B. In-Situ Gelling Polymers. Springer; Singapore: 2015. Introduction to in situ forming hydrogels for biomedical applications; pp. 5–35. [DOI] [Google Scholar]
- 42.Loh X.J. In-Situ Gelling Polymers: For Biomedical Applications. Springer; Singapore: 2015. [Google Scholar]
- 43.Dimatteo R., Darling N.J., Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018;127:167–184. doi: 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shim W.S., Kim J.-H., Kim K., Kim Y.-S., Park R.-W., Kim I.-S., Kwon I.C., Lee D.S. pH- and temperature-sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel. Int. J. Pharm. 2007;331:11–18. doi: 10.1016/j.ijpharm.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 45.Kang J.H., Turabee H., Lee D.S., Kwon Y.J., Ko Y.T. Temperature and pH-responsive in situ hydrogels of gelatin derivatives to prevent the reoccurrence of brain tumor. Biomed. Pharmacother. 2021;143:112144. doi: 10.1016/j.biopha.2021.112144. [DOI] [PubMed] [Google Scholar]
- 46.Podual K., Doyle F.J., Peppas N.A. Dynamic behavior of glucose oxidase-containing microparticles of poly(ethylene glycol)-grafted cationic hydrogels in an environment of changing pH. Biomaterials. 2000;21:1439–1450. doi: 10.1016/S0142-9612(00)00020-X. [DOI] [PubMed] [Google Scholar]
- 47.Yu J., Zhang Y., Bomba H., Gu Z. Stimuli-responsive delivery of therapeutics for diabetes treatment. Bioeng. Transl. Med. 2016;1:323–337. doi: 10.1002/btm2.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X., Shang H., Zhang T., Shu P., Liu Y., Xie J., Zhang D., Tan H., Li J. A stimuli-responsive insulin delivery system based on reversible phenylboronate modified cyclodextrin with glucose triggered host-guest interaction. Int. J. Pharm. 2018;548:649–658. doi: 10.1016/j.ijpharm.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Wells C.M., Harris M., Choi L., Murali V.P., Guerra F.D., Jennings J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019;10:34. doi: 10.3390/jfb10030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qu J., Zhao X., Ma P.X., Guo B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017;58:168–180. doi: 10.1016/j.actbio.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Jalalvandi E., Shavandi A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci. Mater. Med. 2018;29:158. doi: 10.1007/s10856-018-6166-x. [DOI] [PubMed] [Google Scholar]
- 52.Prajapati V.D., Jani G.K., Moradiya N.G., Randeria N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013;92:1685–1699. doi: 10.1016/j.carbpol.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Bhardwaj T.R., Kanwar M., Lal R., Gupta A. Natural Gums and Modified Natural Gums as Sustained-Release Carriers. Drug Dev. Ind. Pharm. 2000;26:1025–1038. doi: 10.1081/DDC-100100266. [DOI] [PubMed] [Google Scholar]
- 54.Guo J.-H., Skinner G.W., Harcum W.W., Barnum P.E. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharm. Sci. Technol. Today. 1998;1:254–261. doi: 10.1016/S1461-5347(98)00072-8. [DOI] [Google Scholar]
- 55.Dodane V., Vilivalam V.D. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today. 1998;1:246–253. doi: 10.1016/S1461-5347(98)00059-5. [DOI] [Google Scholar]
- 56.Farjami T., Madadlou A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019;86:85–94. doi: 10.1016/j.tifs.2019.02.043. [DOI] [Google Scholar]
- 57.Singh L.P., Bhattacharyya S.K., Kumar R., Mishra G., Sharma U., Singh G., Ahalawat S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014;214:17–37. doi: 10.1016/j.cis.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Dickinson E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012;28:224–241. doi: 10.1016/j.foodhyd.2011.12.017. [DOI] [Google Scholar]
- 59.Zhao X., Chen B., Sun Z., Liu T., Cai Y., Huang L., Deng X., Zhao M., Zhao Q. A novel preparation strategy of emulsion gel solely stabilized by alkaline assisted steam-cooking treated insoluble soybean fiber. Food Hydrocoll. 2022;129:107646. doi: 10.1016/j.foodhyd.2022.107646. [DOI] [Google Scholar]
- 60.Smeets N.M.B., Hoare T. Designing responsive microgels for drug delivery applications. J. Polym. Sci. Part A Polym. Chem. 2013;51:3027–3043. doi: 10.1002/pola.26707. [DOI] [Google Scholar]
- 61.Imaz A., Forcada J. New Biocompatible Microgels. Macromol. Symp. 2009;281:85–88. doi: 10.1002/masy.200950711. [DOI] [Google Scholar]
- 62.Martín-Molina A., Quesada-Pérez M. A review of coarse-grained simulations of nanogel and microgel particles. J. Mol. Liq. 2019;280:374–381. doi: 10.1016/j.molliq.2019.02.030. [DOI] [Google Scholar]
- 63.Sung B., Kim C., Kim M.-H. Biodegradable colloidal microgels with tunable thermosensitive volume phase transitions for controllable drug delivery. J. Colloid Interface Sci. 2015;450:26–33. doi: 10.1016/j.jcis.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 64.Vinogradov S.V. Colloidal Microgels in Drug Delivery Applications. Curr. Pharm. Des. 2006;12:4703–4712. doi: 10.2174/138161206779026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kousalová J., Etrych T. Polymeric Nanogels as Drug Delivery Systems. Physiol. Res. 2018;67:S305–S317. doi: 10.33549/physiolres.933979. [DOI] [PubMed] [Google Scholar]
- 66.Varshosaz J., Taymouri S., Ghassami E. Supramolecular Self-Assembled Nanogels a New Platform for Anticancer Drug Delivery. Curr. Pharm. Des. 2018;23:5242–5260. doi: 10.2174/1381612823666170710121900. [DOI] [PubMed] [Google Scholar]
- 67.Ryu J.-H., Jiwpanich S., Chacko R., Bickerton S., Thayumanavan S. Surface-Functionalizable Polymer Nanogels with Facile Hydrophobic Guest Encapsulation Capabilities. J. Am. Chem. Soc. 2010;132:8246–8247. doi: 10.1021/ja102316a. [DOI] [PubMed] [Google Scholar]
- 68.Suhail M., Rosenholm J.M., Minhas M.U., Badshah S.F., Naeem A., Khan K.U., Fahad M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019;10:697–717. doi: 10.4155/tde-2019-0010. [DOI] [PubMed] [Google Scholar]
- 69.Müller R. Biopolymers Online. American Cancer Society; Atlanta, GA, USA: 2005. Biodegradability of Polymers: Regulations and Methods for Testing. [DOI] [Google Scholar]
- 70.Oh J.K., Drumright R., Siegwart D.J., Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008;33:448–477. doi: 10.1016/j.progpolymsci.2008.01.002. [DOI] [Google Scholar]
- 71.Ahmed S., Alhareth K., Mignet N. Advancement in nanogel formulations provides controlled drug release. Int. J. Pharm. 2020;584:119435. doi: 10.1016/j.ijpharm.2020.119435. [DOI] [PubMed] [Google Scholar]
- 72.Rajkumar J., Gv R., Sastri K T., Burada S. Recent update on proniosomal gel as topical drug delivery system. Asian J. Pharm. Clin. Res. 2019;12:54–61. doi: 10.22159/ajpcr.2019.v12i1.28558. [DOI] [Google Scholar]
- 73.Brandl M. Vesicular Phospholipid Gels: A Technology Platform. J. Liposome Res. 2007;17:15–26. doi: 10.1080/08982100601186490. [DOI] [PubMed] [Google Scholar]
- 74.Tian W., Schulze S., Brandl M., Winter G. Vesicular phospholipid gel-based depot formulations for pharmaceutical proteins: Development and in vitro evaluation. J. Control. Release. 2010;142:319–325. doi: 10.1016/j.jconrel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Breitsamer M., Winter G. Vesicular phospholipid gels as drug delivery systems for small molecular weight drugs, peptides and proteins: State of the art review. Int. J. Pharm. 2018;557:1–8. doi: 10.1016/j.ijpharm.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 76.Fetih G., Fathalla D., El-Badry M. Liposomal Gels for Site-Specific, Sustained Delivery of Celecoxib: In Vitro and In Vivo Evaluation. Drug Dev. Res. 2014;75:257–266. doi: 10.1002/ddr.21179. [DOI] [PubMed] [Google Scholar]
- 77.Pavelić Ž., Skalko-Basnet N., Schubert R. Liposomal gels for vaginal drug delivery. Int. J. Pharm. 2001;219:139–149. doi: 10.1016/S0378-5173(01)00637-8. [DOI] [PubMed] [Google Scholar]
- 78.Pavelić Z., Skalko-Basnet N., Jalsenjak I. Liposomal gel with chloramphenicol: Characterisation and in vitro release. Acta Pharm. 2004;54:319–330. [PubMed] [Google Scholar]
- 79.Singh S., Vardhan H., Kotla N.G., Maddiboyina B., Sharma D., Webster T.J. The role of surfactants in the formulation of elastic liposomal gels containing a synthetic opioid analgesic. Int. J. Nanomed. 2016;11:1475–1482. doi: 10.2147/IJN.S100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel K.K., Kumar P., Thakkar H.P. Formulation of Niosomal Gel for Enhanced Transdermal Lopinavir Delivery and Its Comparative Evaluation with Ethosomal Gel. AAPS PharmSciTech. 2012;13:1502–1510. doi: 10.1208/s12249-012-9871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muzzalupo R., Tavano L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Drug Deliv. 2015;4:23–33. doi: 10.2147/RRTD.S64773. [DOI] [Google Scholar]
- 82.Yoshida H., Lehr C.-M., Kok W., Junginger H.E., Verhoef J.C., Bouwstra J.A. Niosomes for oral delivery of peptide drugs. J. Control. Release. 1992;21:145–153. doi: 10.1016/0168-3659(92)90016-K. [DOI] [Google Scholar]
- 83.Bhardwaj P., Tripathi P., Gupta R., Pandey S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020;56:101581. doi: 10.1016/j.jddst.2020.101581. [DOI] [Google Scholar]
- 84.Das B., Sen S.O., Maji R., Nayak A.K., Sen K.K. Transferosomal gel for transdermal delivery of risperidone: Formulation optimization and ex vivo permeation. J. Drug Deliv. Sci. Technol. 2017;38:59–71. doi: 10.1016/j.jddst.2017.01.006. [DOI] [Google Scholar]
- 85.Cevc G., Blume G., Schätzlein A. Transfersomes-mediated transepidermal delivery improves the regio-specificity and biological activity of corticosteroids in vivo1Dedicated to the late Dr. Henri Ernest Bodde.1. J. Control. Release. 1997;45:211–226. doi: 10.1016/S0168-3659(96)01566-0. [DOI] [Google Scholar]
- 86.Malakar J., Sen S.O., Nayak A.K., Sen K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm. J. 2012;20:355–363. doi: 10.1016/j.jsps.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benson H.A.E. Transfersomes for transdermal drug delivery. Expert Opin. Drug Deliv. 2006;3:727–737. doi: 10.1517/17425247.3.6.727. [DOI] [PubMed] [Google Scholar]
- 88.Li Y., Maciel D., Rodrigues J., Shi X., Tomás H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015;115:8564–8608. doi: 10.1021/cr500131f. [DOI] [PubMed] [Google Scholar]
- 89.Sahiner N., Godbey W.T., McPherson G.L., John V.T. Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: Synthesis and characterization. Colloid Polym. Sci. 2006;284:1121–1129. doi: 10.1007/s00396-006-1489-4. [DOI] [Google Scholar]
- 90.Thambi T., Phan V.H.G., Lee D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016;37:1881–1896. doi: 10.1002/marc.201600371. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida R., Uchida K., Kaneko Y., Sakai K., Kikuchi A., Sakurai Y., Okano T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 1995;374:240–242. doi: 10.1038/374240a0. [DOI] [Google Scholar]
- 92.Meyer D.E., Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 93.Li Y., Lin T.-Y., Luo Y., Liu Q., Xiao W., Guo W., Lac D., Zhang H., Feng C., Wachsmann-Hogiu S., et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014;5:4712. doi: 10.1038/ncomms5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bai R., Deng X., Wu Q., Cao X., Ye T., Wang S. Liposome-loaded thermo-sensitive hydrogel for stabilization of SN-38 via intratumoral injection: Optimization, characterization, and antitumor activity. Pharm. Dev. Technol. 2018;23:106–115. doi: 10.1080/10837450.2017.1391287. [DOI] [PubMed] [Google Scholar]
- 95.Mirrahimi M., Abed Z., Beik J., Shiri I., Dezfuli A.S., Mahabadi V.P., Kamrava S.K., Ghaznavi H., Shakeri-Zadeh A. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019;143:178–185. doi: 10.1016/j.phrs.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Chen L., Deng J., Yu A., Hu Y., Jin B., Du P., Zhou J., Lei L., Wang Y., Vakal S., et al. Drug-peptide supramolecular hydrogel boosting transcorneal permeability and pharmacological activity via ligand-receptor interaction. Bioact. Mater. 2022;10:420–429. doi: 10.1016/j.bioactmat.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han Y., Jiang L., Shi H., Xu C., Liu M., Li Q., Zheng L., Chi H., Wang M., Liu Z., et al. Effectiveness of an ocular adhesive polyhedral oligomeric silsesquioxane hybrid thermo-responsive FK506 hydrogel in a murine model of dry eye. Bioact. Mater. 2022;9:77–91. doi: 10.1016/j.bioactmat.2021.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lv Q., He C., Quan F., Yu S., Chen X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018;3:118–128. doi: 10.1016/j.bioactmat.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao D., Song H., Zhou X., Chen Y., Liu Q., Gao X., Zhu X., Chen D. Novel facile thermosensitive hydrogel as sustained and controllable gene release vehicle for breast cancer treatment. Eur. J. Pharm. Sci. 2019;134:145–152. doi: 10.1016/j.ejps.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 100.Shi K., Xue B., Jia Y., Yuan L., Han R., Yang F., Peng J., Qian Z. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. 2019;12:1389–1399. doi: 10.1007/s12274-019-2342-7. [DOI] [Google Scholar]
- 101.Zhao M., Bozzato E., Joudiou N., Ghiassinejad S., Danhier F., Gallez B., Préat V. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J. Control. Release. 2019;309:72–81. doi: 10.1016/j.jconrel.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 102.Chakole C.M., Sahoo P.K., Pandey J., Chauhan M.K. A green chemistry approach towards synthesizing hydrogel for sustained ocular delivery of brinzolamide: In vitro and ex vivo evaluation. J. Indian Chem. Soc. 2021;99:100323. doi: 10.1016/j.jics.2021.100323. [DOI] [Google Scholar]
- 103.Asfour M.H., El-Alim S.H.A., Awad G.E.A., Kassem A.A. Chitosan/β-glycerophosphate in situ forming thermo-sensitive hydrogel for improved ocular delivery of moxifloxacin hydrochloride. Eur. J. Pharm. Sci. 2021;167:106041. doi: 10.1016/j.ejps.2021.106041. [DOI] [PubMed] [Google Scholar]
- 104.Pakzad Y., Fathi M., Omidi Y., Mozafari M., Zamanian A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: A transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020;159:117–128. doi: 10.1016/j.ijbiomac.2020.04.274. [DOI] [PubMed] [Google Scholar]
- 105.Bao Z., Yu A., Shi H., Hu Y., Jin B., Lin D., Dai M., Lei L., Li X., Wang Y. Glycol chitosan/oxidized hyaluronic acid hydrogel film for topical ocular delivery of dexamethasone and levofloxacin. Int. J. Biol. Macromol. 2021;167:659–666. doi: 10.1016/j.ijbiomac.2020.11.214. [DOI] [PubMed] [Google Scholar]
- 106.Wang L., Pan H., Gu D., Li P., Su Y., Pan W. A composite System Combining Self-Targeted Carbon Dots and Thermosensitive Hydrogels for Challenging Ocular Drug Delivery. J. Pharm. Sci. 2022;111:1391–1400. doi: 10.1016/j.xphs.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 107.Pereira R.F., Bártolo P.J. Hot Topics in Biomaterials. Future Science Ltd.; London, UK: 2014. Photopolymerizable hydrogels in regenerative medicine and drug delivery; pp. 6–28. [DOI] [Google Scholar]
- 108.Lim H.L., Hwang Y., Kar M., Varghese S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014;2:603–618. doi: 10.1039/C3BM60288E. [DOI] [PubMed] [Google Scholar]
- 109.Fourniols T., Randolph L.D., Staub A., Vanvarenberg K., Leprince J.G., Préat V., des Rieux A., Danhier F. Temozolomide-loaded photopolymerizable PEG-DMA-based hydrogel for the treatment of glioblastoma. J. Control. Release. 2015;210:95–104. doi: 10.1016/j.jconrel.2015.05.272. [DOI] [PubMed] [Google Scholar]
- 110.Chen X., Liu Z., Parker S.G., Zhang X., Gooding J.J., Ru Y., Liu Y., Zhou Y. Light-Induced Hydrogel Based on Tumor-Targeting Mesoporous Silica Nanoparticles as a Theranostic Platform for Sustained Cancer Treatment. ACS Appl. Mater. Interfaces. 2016;8:15857–15863. doi: 10.1021/acsami.6b02562. [DOI] [PubMed] [Google Scholar]
- 111.Qiu M., Wang D., Liang W., Liu L., Zhang Y., Chen X., Sang D.K., Xing C., Li Z., Dong B., et al. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA. 2018;115:501–506. doi: 10.1073/pnas.1714421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ojugo A.S.E., McSheehy P.M.J., McIntyre D.J.O., McCoy C., Stubbs M., Leach M.O., Judson I.R., Griffiths J.R. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: A comparison of exogenous19F and31P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(SICI)1099-1492(199912)12:8<495::AID-NBM594>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 113.van Dyke R.W. Acidification of Lysosomes and Endosomes. Springer; Boston, MA, USA: 1996. pp. 331–360. [DOI] [PubMed] [Google Scholar]
- 114.Hoffman A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013;65:10–16. doi: 10.1016/j.addr.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 115.Raza F., Zhu Y., Chen L., You X., Zhang J., Khan A., Khan M.W., Hasnat M., Zafar H., Wu J., et al. Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater. Sci. 2019;7:2023–2036. doi: 10.1039/C9BM00139E. [DOI] [PubMed] [Google Scholar]
- 116.Xu C., Yan Y., Tan J., Yang D., Jia X., Wang L., Xu Y., Cao S., Sun S. Biodegradable Nanoparticles of Polyacrylic Acid–Stabilized Amorphous CaCO3 for Tunable pH-Responsive Drug Delivery and Enhanced Tumor Inhibition. Adv. Funct. Mater. 2019;29:1808146. doi: 10.1002/adfm.201808146. [DOI] [Google Scholar]
- 117.Ren Y., Li X., Han B., Zhao N., Mu M., Wang C., Du Y., Wang Y., Tong A., Liu Y., et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2019;128:279–289. doi: 10.1016/j.ejps.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 118.Makhmalzadeh B.S., Molavi O., Vakili M.R., Zhang H.-F., Solimani A., Abyaneh H.S., Loebenberg R., Lai R., Lavasanifar A. Functionalized Caprolactone-Polyethylene Glycol Based Thermo-Responsive Hydrogels of Silibinin for the Treatment of Malignant Melanoma. J. Pharm. Pharm. Sci. 2018;21:143–159. doi: 10.18433/jpps29726. [DOI] [PubMed] [Google Scholar]
- 119.Wu H., Song L., Chen L., Zhang W., Chen Y., Zang F., Chen H., Ma M., Gu N., Zhang Y. Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents post-operative recurrence in a breast cancer model. Acta Biomater. 2018;74:302–311. doi: 10.1016/j.actbio.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 120.Hyun H., Park M.H., Jo G., Kim S.Y., Chun H.J., Yang D.H. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs. 2019;17:41. doi: 10.3390/md17010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu S., Wei S., Liu L., Qi D., Wang J., Chen G., He W., He C., Chen X., Gu Z. Enhanced local cancer therapy using a CA4P and CDDP co-loaded polypeptide gel depot. Biomater. Sci. 2019;7:860–866. doi: 10.1039/C8BM01442F. [DOI] [PubMed] [Google Scholar]
- 122.Permana A.D., Utami R.N., Layadi P., Himawan A., Juniarti N., Anjani Q.K., Utomo E., Mardikasari S.A., Arjuna A., Donnelly R.F. Thermosensitive and mucoadhesive in situ ocular gel for effective local delivery and antifungal activity of itraconazole nanocrystal in the treatment of fungal keratitis. Int. J. Pharm. 2021;602:120623. doi: 10.1016/j.ijpharm.2021.120623. [DOI] [PubMed] [Google Scholar]
- 123.Chaudhari P., Naik R., Mallela L.S., Roy S., Birangal S., Ghate V., Kunhanna S.B., Lewis S.A. A supramolecular thermosensitive gel of ketoconazole for ocular applications: In silico, in vitro, and ex vivo studies. Int. J. Pharm. 2022;613:121409. doi: 10.1016/j.ijpharm.2021.121409. [DOI] [PubMed] [Google Scholar]
- 124.Alkholief M., Kalam M.A., Almomen A., Alshememry A., Alshamsan A. Thermoresponsive sol-gel improves ocular bioavailability of Dipivefrin hydrochloride and potentially reduces the elevated intraocular pressure in vivo. Saudi Pharm. J. 2020;28:1019–1029. doi: 10.1016/j.jsps.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mahboobian M.M., Mohammadi M., Mansouri Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J. Drug Deliv. Sci. Technol. 2020;55:101400. doi: 10.1016/j.jddst.2019.101400. [DOI] [Google Scholar]
- 126.Cucina A., Filali S., Risler A., Febvay C., Salmon D., Pivot C., Pelandakis M., Pirot F. Dual 0.02% chlorhexidine digluconate—0.1% disodium EDTA loaded thermosensitive ocular gel for Acanthamoeba keratitis treatment. Int. J. Pharm. 2019;556:330–337. doi: 10.1016/j.ijpharm.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 127.Diaz-Rodriguez P., Mariño C., Vázquez J.A., Caeiro-Rey J.R., Landin M. Targeting joint inflammation for osteoarthritis management through stimulus-sensitive hyaluronic acid based intra-articular hydrogels. Mater. Sci. Eng. C. 2021;128:112254. doi: 10.1016/j.msec.2021.112254. [DOI] [PubMed] [Google Scholar]
- 128.Zhang T., Chen S., Dou H., Liu Q., Shu G., Lin J., Zhang W., Peng G., Zhong Z., Fu H. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater. Sci. Eng. C. 2021;118:111352. doi: 10.1016/j.msec.2020.111352. [DOI] [PubMed] [Google Scholar]
- 129.Nascimento M.H.M.D., Ambrosio F.N., Ferraraz D.C., Windisch-Neto H., Querobino S.M., Nascimento-Sales M., Alberto-Silva C., Christoffolete M.A., Franco M.K.K.D., Kent B., et al. Sulforaphane-loaded hyaluronic acid-poloxamer hybrid hydrogel enhances cartilage protection in osteoarthritis models. Mater. Sci. Eng. C. 2021;128:112345. doi: 10.1016/j.msec.2021.112345. [DOI] [PubMed] [Google Scholar]
- 130.GhavamiNejad A., Samarikhalaj M., Aguilar L.E., Park C.H., Kim C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016;6:33594. doi: 10.1038/srep33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ruan C., Liu C., Hu H., Guo X.-L., Jiang B.-P., Liang H., Shen X.-C. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy. Chem. Sci. 2019;10:4699–4706. doi: 10.1039/C9SC00375D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cai Z., Zhang H., Wei Y., Wei Y., Xie Y., Cong F. Reduction- and pH-Sensitive Hyaluronan Nanoparticles for Delivery of Iridium(III) Anticancer Drugs. Biomacromolecules. 2017;18:2102–2117. doi: 10.1021/acs.biomac.7b00445. [DOI] [PubMed] [Google Scholar]
- 133.Malekimusavi H., Ghaemi A., Masoudi G., Chogan F., Rashedi H., Yazdian F., Omidi M., Javadi S., Haghiralsadat B.F., Teimouri M., et al. Graphene oxide- l -arginine nanogel: A pH-sensitive fluorouracil nanocarrier. Biotechnol. Appl. Biochem. 2019;66:772–780. doi: 10.1002/bab.1768. [DOI] [PubMed] [Google Scholar]
- 134.Zhang F., Gong S., Wu J., Li H., Oupicky D., Sun M. CXCR4-Targeted and Redox Responsive Dextrin Nanogel for Metastatic Breast Cancer Therapy. Biomacromolecules. 2017;18:1793–1802. doi: 10.1021/acs.biomac.7b00208. [DOI] [PubMed] [Google Scholar]
- 135.Zhao J., Yang Y., Han X., Liang C., Liu J., Song X., Ge Z., Liu Z. Redox-Sensitive Nanoscale Coordination Polymers for Drug Delivery and Cancer Theranostics. ACS Appl. Mater. Interfaces. 2017;9:23555–23563. doi: 10.1021/acsami.7b07535. [DOI] [PubMed] [Google Scholar]
- 136.Kumar P., Wasim L., Chopra M., Chhikara A. Co-delivery of Vorinostat and Etoposide Via Disulfide Cross-Linked Biodegradable Polymeric Nanogels: Synthesis, Characterization, Biodegradation, and Anticancer Activity. AAPS PharmSciTech. 2018;19:634–647. doi: 10.1208/s12249-017-0863-5. [DOI] [PubMed] [Google Scholar]
- 137.Park H., Choi Y., Jeena M.T., Ahn E., Choi Y., Kang M.-G., Lee C.G., Kwon T.-H., Rhee H.-W., Ryu J.-H., et al. Reduction-Triggered Self-Cross-Linked Hyperbranched Polyglycerol Nanogels for Intracellular Delivery of Drugs and Proteins. Macromol. Biosci. 2018;18:1700356. doi: 10.1002/mabi.201700356. [DOI] [PubMed] [Google Scholar]
- 138.Li F., Yang H., Bie N., Xu Q., Yong T., Wang Q., Gan L., Yang X. Zwitterionic Temperature/Redox-Sensitive Nanogels for Near-Infrared Light-Triggered Synergistic Thermo-Chemotherapy. ACS Appl. Mater. Interfaces. 2017;9:23564–23573. doi: 10.1021/acsami.7b08047. [DOI] [PubMed] [Google Scholar]
- 139.Gao F., Xie W., Miao Y., Wang D., Guo Z., Ghosal A., Li Y., Wei Y., Feng S., Zhao L., et al. Magnetic Hydrogel with Optimally Adaptive Functions for Breast Cancer Recurrence Prevention. Adv. Healthc. Mater. 2019;8:1900203. doi: 10.1002/adhm.201900203. [DOI] [PubMed] [Google Scholar]
- 140.Parisi O.I., Morelli C., Scrivano L., Sinicropi M.S., Cesario M.G., Candamano S., Puoci F., Sisci D. Controlled release of sunitinib in targeted cancer therapy: Smart magnetically responsive hydrogels as restricted access materials. RSC Adv. 2015;5:65308–65315. doi: 10.1039/C5RA12229E. [DOI] [Google Scholar]
- 141.Wang L., Wang Y., Hao J., Dong S. Magnetic Fullerene-DNA/Hyaluronic Acid Nanovehicles with Magnetism/Reduction Dual-Responsive Triggered Release. Biomacromolecules. 2017;18:1029–1038. doi: 10.1021/acs.biomac.6b01939. [DOI] [PubMed] [Google Scholar]
- 142.Aboali F.A., Habib D.A., Elbedaiwy H.M., Farid R.M. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: In-vitro and in-vivo assessment. Int. J. Pharm. 2020;589:119835. doi: 10.1016/j.ijpharm.2020.119835. [DOI] [PubMed] [Google Scholar]
- 143.Shukr M.H., Ismail S., El-Hossary G.G., El-Shazly A.H. Design and evaluation of mucoadhesive in situ liposomal gel for sustained ocular delivery of travoprost using two steps factorial design. J. Drug Deliv. Sci. Technol. 2021;61:102333. doi: 10.1016/j.jddst.2021.102333. [DOI] [Google Scholar]
- 144.Jin R., Teixeira L.S.M., Dijkstra P.J., van Blitterswijk C.A., Karperien M., Feijen J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran–hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials. 2010;31:3103–3113. doi: 10.1016/j.biomaterials.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 145.Wei J., Ran P., Li Q., Lu J., Zhao L., Liu Y., Li X. Hierarchically structured injectable hydrogels with loaded cell spheroids for cartilage repairing and osteoarthritis treatment. Chem. Eng. J. 2022;430:132211. doi: 10.1016/j.cej.2021.132211. [DOI] [Google Scholar]
- 146.Zhou T., Xiong H., Wang S.Q., Zhang H.L., Zheng W.W., Gou Z.R., Fan C.Y., Gao C.Y. An injectable hydrogel dotted with dexamethasone acetate-encapsulated reactive oxygen species-scavenging micelles for combinatorial therapy of osteoarthritis. Mater. Today Nano. 2022;17:100164. doi: 10.1016/j.mtnano.2021.100164. [DOI] [Google Scholar]
- 147.Han Y., Yang J., Zhao W., Wang H., Sun Y., Chen Y., Luo J., Deng L., Xu X., Cui W., et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021;6:3596–3607. doi: 10.1016/j.bioactmat.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seo B.-B., Kwon Y., Kim J., Hong K.H., Kim S.-E., Song H.-R., Kim Y.-M., Song S.-C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022;7:14–25. doi: 10.1016/j.bioactmat.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lei Y., Wang X., Liao J., Shen J., Li Y., Cai Z., Hu N., Luo X., Cui W., Huang W. Shear-responsive boundary-lubricated hydrogels attenuate osteoarthritis. Bioact. Mater. 2022;16:472–484. doi: 10.1016/j.bioactmat.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ocular Inserts with Analyte Capture and Release Agents. 20,210,338,211. [(accessed on 30 April 2022)];U.S. Patent. 2021 May 3; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US340278458&_cid=P20-L2LQFZ-64472-1.
- 151.Drug Delivery from Hydrogels. 2,021,120,395. [(accessed on 30 April 2022)];JP Patent. 2021 May 11; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=JP334768825&_cid=P20-L2LQIS-64737-1.
- 152.Injectable Hydrogels for Cell Delivery to the Vitreous. 2,021,113,515. [(accessed on 30 April 2022)];WO Patent. 2020 June 10; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021113515&_cid=P20-L2LQN0-65347-1.
- 153.Polymer Formulations for Nasolacrimal Stimulation. 20,210,069,496. [(accessed on 30 April 2022)];U.S. Patent. 2020 September 11; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US319902595&_cid=P20-L2LQOA-65557-1.
- 154.A Method for Obtaining Ion-Exchange Polymeric Hydrogels for Eye Treatment and Hydrogel Lenses Thereof. 2,021,038,279. [(accessed on 30 April 2022)];WO Patent. 2021 March 4; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021038279&_cid=P20-L2LQQ0-65789-1.
- 155.An Etoricoxib Loaded Nanosponge Hydrogel for Arthritis & Process to Prepare Thereof. 202,121,042,889. [(accessed on 30 April 2022)];IN Patent. 2021 September 22; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=IN340501612&_cid=P20-L2LQRJ-65914-1.
- 156.Protein Hydrogel for Topical Application and Formulation for Skin Regeneration/Wound Healing. 202,031,000,910. [(accessed on 30 April 2022)];IN Patent. 2020 January 8; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=IN334769630&_cid=P20-L2LQSP-66090-1.
- 157.Preparation Method of Conductive Adhesive Hydrogel. 112,442,194. [(accessed on 30 April 2022)];CN Patent. 2019 September 4; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=CN320288668&_cid=P20-L2LQU0-66236-1.
- 158.Thrombin-Responsive Hydrogels and Devices for Auto-Anticoagulant Regulation. 20,210,023,121. [(accessed on 30 April 2022)];U.S. Patent. 2020 September 14; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US316414241&_cid=P20-L2LQV8-66462-1.
- 159.Thermosensitive Hydrogel for Cancer Therapeutics and Methods of Preparation Thereof. 20,210,393,780. [(accessed on 30 April 2022)];U.S. Patent. 2021 May 20; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US345561289&_cid=P20-L2LQWJ-66715-1.
- 160.Tunable Extended Release Hydrogels. 2,021,174,021. [(accessed on 30 April 2022)];WO Patent. 2021 September 2; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021174021&_cid=P20-L2LQXW-66853-1.
- 161.Novel Self-Assembling Peptides and Their Use in the Formaiion of Hydrogels. 9758/CHENP/2012. [(accessed on 30 April 2022)];IN Patent. 2012 November 19; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=IN211695000&_cid=P20-L2LQZK-67034-1.
- 162.Synthetic Diblock Copolypeptide Hydrogels for Use in the Central Nervous System. 20,140,286,865. [(accessed on 30 April 2022)];U.S. Patent. 2014 February 18; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US123273430&_cid=P20-L2LR0O-67168-1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.