Abstract
Water is a vital resource that is required for social and economic development. A rapid increase in industrialization and numerous anthropogenic activities have resulted in severe water contamination. In particular, the contamination caused by heavy metal discharge has a negative impact on human health and the aquatic environment due to the non-biodegradability, toxicity, and carcinogenic effects of heavy metals. Thus, there is an immediate need to recycle wastewater before releasing heavy metals into water bodies. Hydrogels, as potent adsorbent materials, are a good contenders for treating toxic heavy metals in wastewater. Hydrogels are a soft matter formed via the cross-linking of natural or synthetic polymers to develop a three-dimensional mesh structure. The inherent properties of hydrogels, such as biodegradability, swell-ability, and functionalization, have made them superior applications for heavy metal removal. In this review, we have emphasized the recent development in the synthesis of hydrogel-based adsorbent materials. The review starts with a discussion on the methods used for recycling wastewater. The discussion then shifts to properties, classification based on various criteria, and surface functionality. In addition, the synthesis and adsorption mechanisms are explained in detail with the understanding of the regeneration, recovery, and reuse of hydrogel-based adsorbent materials. Therefore, the cost-effective, facile, easy to modify and biodegradable hydrogel may provide a long-term solution for heavy metal removal.
Keywords: hydrogels, heavy metals removal, wastewater
1. Introduction
1.1. Problem Statement
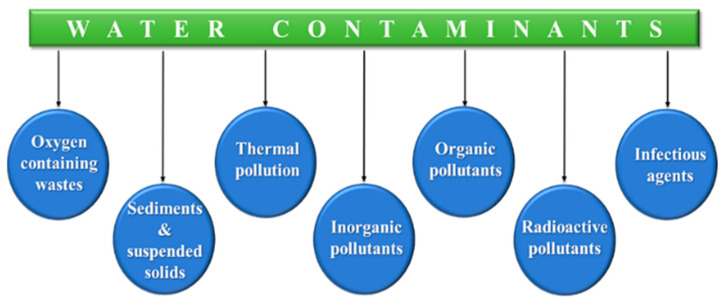
Water is essential for all living organisms on the planet. Although, it occupies 71% of the total surface area of the earth, only 3% of water is available as freshwater and less than 1% is potable. The remaining percentage of water is inaccessible in different forms such as ice, glaciers, and snow on the south and north poles [1]. Water plays a significant part in the hydrological cycle, food-processing industries, chemical weathering, domestic usage, agricultural irrigation, and so on. Therefore, there is an increasing need for freshwater, but the availability is limited. Freshwater is contaminated by discarding waste in various water bodies in the form of marine dumping, oil leakage, industrial waste, sewage waste, etc. Different pollutants present in wastewater are summarized in Figure 1. Among them, heavy metals are found to be the most common pollutant in contaminated water, which deteriorates the sustainable environment. Water contamination by heavy metals has harmed human health all around the world due to the fast development in industries, economics, and population [2].
Figure 1.
Different pollutants in contaminated water.
1.2. Heavy Metals and Their Hazardous Effect
Heavy metals are referred to as metals with a density of 5 gm/cm3 and are poisonous, toxic, and hazardous even at very low concentrations. The sources of heavy metal contamination into water are categorized in two ways: (1) natural ways like soil erosion, rainfall, dissolution of soluble salts, etc., and (2) artificial ways like industrial waste, and urban wastewater [3]. Heavy metals include mercury (Hg), zinc (Zn), arsenic (As), cadmium (Cd), silver (Ag), iron (Fe), lead (Pb), tin (Sn), and the platinum group of metals. Heavy metals are non-biodegradable elements [4] that cause detrimental effects on the natural ecosystem and human health when their concentration goes beyond permissible limits. For instance, persistent intake of inorganic arsenic causes lung, bladder, skin, and kidney cancer in humans via consumption of drinking water [5]. Mercury accumulation in the food chain shows a negative impact on human health such as kidney and pulmonary function impairment, chest pain, and damage to the central nervous system [6]. Some other examples are listed in Table 1.
Table 1.
| Heavy Metals | Leading Source | Path of Entry | Toxic Effects on Human Health | Environmental Hazards | MCL (mg/L) |
|---|---|---|---|---|---|
| Lead (Pb) | Mining, automobile emissions, smoking, pesticide, paint, burning of coal | Ingestion and inhalation | Damages the central nervous system, fetal brain, kidney, reproductive system, liver, basic cellular processes, and causes diseases, namely, anemia, nephrite syndrome, hepatitis, etc. | Soil and water pollution | 0.015 |
| Cadmium (Cd) | Pesticide fertilizer, electroplating, Cd-Ni batteries, welding | Ingestion and inhalation | Irritation of respiratory system, damages liver, kidney, and lungs | Soil and water pollution | 0.005 |
| Nickel (Ni) | Electrochemical industries | Inhalation | Causes lung, kidney, and gastronomical pain, renal edema, pulmonary fibrosis, and skin dermatitis | Soil and water pollution | 0.1 |
| Zinc (Zn) | Plumbing, refineries, metal plating, brass manufacture | Ingestion, inhalation, and through skin | Vomiting, pain in the stomach, skin irritation, nausea, and anemia | Soil and water pollution | 0.8 |
Heavy metals are not degraded by natural mechanisms and hence persist in the environment for a long duration of time. They may be converted into insoluble compounds or other forms. Water, air, and soil are the three key environmental compartments that get affected by heavy metal contamination (Table 1). Runoffs from cities, villages, towns, and factories transport the heavy metals that accumulate in a flowing stream. Even if a low concentration is transferred to water streams, it is extremely harmful to humans and the natural ecosystem [7]. Air pollution is caused by dust and particulate matters such as PM2.5 and PM10 which are discharged by various natural and anthropogenic processes. Soil erosion, dust storms, rock weathering, and volcanic eruptions are examples of natural processes that release particulate matter in the air, whereas anthropogenic activities are mainly transport-related and industrial. These particulate matters cause corrosion, haze, and eutropication, and lead to the formation of acid rains [8]. Heavy metals pollute soil by damping wastes like animal manures, pesticides, fertilizers, sewage sludge, spillage of petroleum distillates, etc. The use of this untreated waste has resulted in a high concentration of heavy metals in agricultural fields, which affects the entire biosphere. They are directly absorbed by plants, causing a risk to the plant and the food chain that consumes it. They affect soil qualities such as color, pH, and porosity and also pollute water [9]. Therefore, it is urgent and necessary to remove toxic heavy metals from contaminated wastewater. A variety of wastewater recycling techniques have been developed, which are further discussed in this review article.
2. Methods Used for Recycling Wastewater
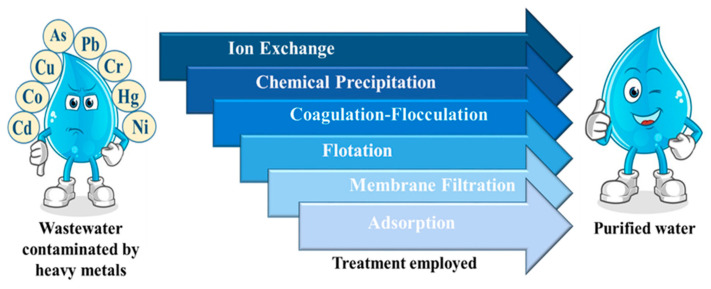
Over the years, numerous methods have been developed to remediate heavy metal-contaminated wastewater before discharging it into the environment. Heavy metals can be removed from wastewater by using a variety of methods, including ion exchange, coagulation-flocculation, flotation, membrane filtration, chemical precipitation, and adsorption (Figure 2). However, each method has its own set of advantages and disadvantages (Summarized in Table 2).
Figure 2.
Wastewater recycling methods.
Table 2.
Advantages and disadvantages of various methods used for recycling wastewater.
| Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Ion exchange | Does not produce a large amount of sludge, easy regeneration of resins | High operational cost, selective towards certain metal ions | [25] |
| Chemical precipitation | Low capital cost, simple process | Produces a large amount of sludge, ineffective in treating low concentration of heavy metal ions | [26] |
| Coagulation-flocculation | Easy to employ, inexpensive, low energy consumption | Complete removal of heavy metals is difficult, generation of a large quantity of sludge | [27] |
| Flotation | Economically efficient | Low elimination efficiency, | [28,29] |
| Membrane filtration | Small space requirement, high efficiency, high separation selectivity | complex process, high operational expense due to membrane fouling | [30] |
| Adsorption | Technologically feasible, effective, low-cost adsorbent, no waste generation, easy operation conditions | Low selectivity | [3,13] |
Among the presented methods in Figure 2, adsorption is considered to be one of the most efficient, low-cost, and simple-to-operate methods to remove heavy metals from contaminated water when compared with other methods [12]. Moreover, the adsorption process is technologically feasible and attractive, as the adsorbent material can be reused and regenerated. In this process, no secondary waste is generated during the removal of heavy metals [13]. The process also has the advantage of removing low concentrations of heavy metals from the solution with low energy consumption [14,15]. Adsorption is a mass transfer surface phenomenon that leads to the binding of molecules from liquid bulk (adsorbate) onto the solid surface (adsorbent) [16]. This binding occurs due to the presence of residual imbalance forces that attracts and retains molecules on the surface of the solid or liquid phase [17]. The adsorbent adsorbs the adsorbate via bonding interactions like a covalent bond or Van der Waals forces [18].
Over the years, researchers have developed adsorbent materials for the removal of toxic heavy metals from wastewater like rice husk bio-char, sugar beet pulp, TiO2, activated carbon, clay, etc. [19,20,21,22,23]. However, these adsorbent materials suffer from certain disadvantages such as their difficulty separating from the water after the decontamination process, higher production cost, economic unsustainability for large-scale applications, and many other reasons [24]. This calls for the immediate development of an adsorbent material that is cost-effective, easy to handle, biodegradable, and biocompatible adsorbent material to purify contaminated water. In recent years, hydrogels have gained tremendous attention as the potential adsorbent material owing to their excellent water affinity, controllable swelling behavior, high porosity, better mechanical properties, and easy handling; these are the main factors for the reuse of adsorbent material. Hydrogel-based material has shown substantial attention for applications in different fields, such as biomedicine, agriculture, food additives, drug delivery, wound dressing, regenerative medicine, and cosmetics. Even though hydrogel-based adsorbents have a long history in a few of the above-mentioned fields, their application for contaminant removal from wastewater has been only reported over the last decade. Our emphasis will be on the application of hydrogel-based material for the removal of toxic and hazardous heavy metals from wastewater.
3. Hydrogels for Removal of Heavy Metals
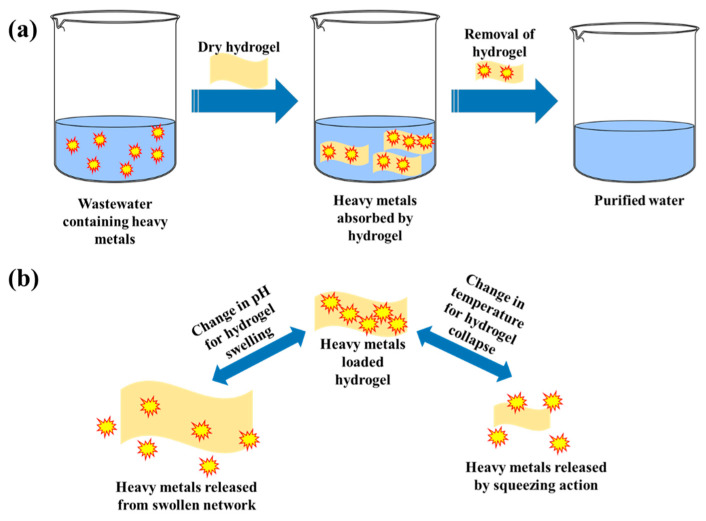
In 1894, the term “hydrogel” was first coined by Bemmelen to explain colloidal gels [31]. DuPont scientists reported the first synthetic hydrogel, poly (2-hydroxyethyl methacrylate) (PHEMA), in 1936 [32]. Witcher and Lim were the first to report the use of PHEMA for the application of contact lenses in 1960 [33]. Since then, hydrogels have been an intriguing topic for researchers, and they now represent a developing and active research field aimed at providing better solutions for various needs in many applicative fields. The use of hydrogels for the extraction of heavy metals from wastewater is becoming more popular, as they can capture and store different heavy metals found in wastewater within their network structure. Hydrogels are regarded as hydrophilic gels, which consists of chemically reactive functional groups and physically distinct three-dimensional (3D) network [34]. The porous three-dimensional network of hydrogel allows absorption and retention of a large volume of water without dissolving [35]. The hydrophilic groups in the polymeric network enable the formation of a flexible structure, which allows easy diffusion of solute into the three-dimensional framework of gels and forms a stable complex with the functional group present on a long polymeric chain [36]. Due to distinct properties like hydrophilicity, biocompatibility, biodegradability, viscoelasticity, and superabsorbancy, hydrogel adsorbents can play a prime role in the capture of heavy metals from contaminated water (Figure 3a) and can discharge these hazardous pollutants upon changes in the external environment (change in pH, temperature, etc.) (Figure 3b) [34,37].
Figure 3.
(a) Schematic illustration for removal of heavy metals from wastewater by using hydrogel-based adsorbent material. (b) Change in structure on applying external stimuli like pH and temperature.
In recent years, hydrogel adsorbents have shown a high potential for the effective elimination of heavy metals. Hydrogel absorbs heavy metals in a three-dimensional interstitial structure ensuring more sites per unit volume [38]. Unlike other adsorbents, hydrogels adsorb heavy metals in a three dimensional, highly porous network that leads to high adsorption efficiency [37]. Adsorption or desorption of heavy metals is mainly due to the surface chemistry and presence of hydrophilic functional groups (−COOH, −NH2, −OH, −SO3H, etc.) that act as a complexing agent for heavy metal removal from aqueous media [36,37]. Moreover, hydrogels can be modified by the addition of new functional groups or the preparation of composites with natural or synthetic sources to enhance heavy metal absorption capacities [39]. The swelling behavior of hydrogels is associated to an extent with hydrophilic functional groups present in the polymer backbone, the degree of cross-linking, the elasticity of the network, and the porosity of the polymer [40]. The hydrophilic polymers in hydrogel can swell up to several times their original volume in aqueous media and hold large content of water about 400 times its original weight [41]. Hydrogels are insoluble in water which leads to easy regeneration because of the presence of chemically cross-linked polymers, which enhance their mechanical strength as well as decreases the swelling ratio. Therefore, it is necessary to balance the amount of crosslinking and swelling ratio to obtain a stronger hydrogel [42]. Some other advantages of hydrogels are that they can be synthesized with the desired charges, controllable sizes, and functional groups [43]. The three most important parameters on which the capacity of cross-linked hydrogel synthesized depend are: (a) polymer volume fraction of hydrogel in swollen shape, which determines the quantity of fluid absorbed into hydrogel network, (b) the average molecular weight between two cross-links, which determines the degree of cross-linking for the prepared hydrogel, and (c) the network mesh size, which determines the degradability, mechanical strength, and diffusivity of releasing components into hydrogel structure [44].
An ideal hydrogel should have the following characteristics to be widely applied for the effective removal of heavy metals from polluted wastewater [24].
Cost-effective
High adsorption capacity to absorb heavy metals from wastewater
High adsorption rate (determined by porosity and particle size)
Biodegradable
Easy to modify
The low content of the unreacted residual monomer
High stability and durability during swelling and storage
Non-toxic, colorless, and odorless
pH neutrality after swelling in aqueous media
Deswelling capabilities and re-watering (able to release back the stored water)
4. Properties of Hydrogel
An ideal hydrogel has distinctive characteristic properties such as swelling or deswelling in the presence of external stimuli, responsiveness to the change in temperature, pH, light, etc., and biodegradability.
4.1. Swelling or Deswelling of Hydrogels
Hydrogels are the cross-linked polymer that swells and imbibes water when immersed in the aqueous media. These three-dimensional structures can swell up to several times their dry weight [45]. Hydrogels can respond to external stimuli (temperature, pH, light, salt, magnetic fields, biomolecules, and ionic strength) by shrinking, swelling, and discoloration [46]. Factors affecting swelling kinetics and equilibrium are the chemical structure of the polymers, cross-linking ratio, synthesis state, and ionic media. Chemical structure affects the swelling of hydrogel; hydrophilic groups swell more in comparison to hydrophobic groups. Cross-linking also has a significant effect on swelling behavior, as a highly cross-linked polymer network will show less swelling and vice versa. The swelling behavior of hydrogels is also affected by temperature and pH [47]. Temperature-sensitive hydrogels undergo swelling or de-swelling (change in volume) with the change in the temperature. These hydrogels swell below the low critical solution temperature and shrink above the low critical solution temperature [48]. pH-sensitive hydrogels undergo swelling or deswelling by varying pH levels. These hydrogels consist of ionizable acidic and basic groups connected to the polymer backbone that can add or release protons by varying pH levels. At the high pH value, the acidic groups on polymer chains deprotonate whereas at low pH values, the basic groups get protonated [49].
Swelling in hydrogels takes place in three steps:—(a) the diffusion of water into the three-dimensional network of hydrogel, (b) loosening of the polymeric chains, and (c) expansion of hydrogel structure. A hydrogel in swollen form is referred to as the rubbery state and the dry form as the glassy state. When dry hydrogel comes in contact with the solvent, the free space in a polymer network permits the solvent to enter the hydrogel matrix easily. This transforms the dry or glassy state into a swollen or rubbery state. The de-swelling of hydrogel occurs when water is removed from a hydrogel matrix [47]. Experimentally, the swelling ratio can be calculated by the following formula [50].
where, Ws and Wd are the weight of the swollen and dry hydrogel, respectively.
4.2. Stimuli-Responsive Hydrogels
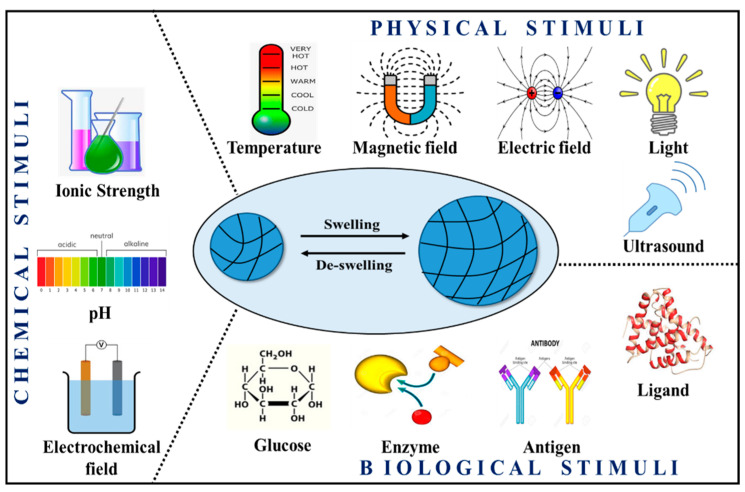
Gels that respond to changes in the external environment (temperature, pH, magnetic field, electric field, etc.) are termed stimuli-responsive hydrogels. These hydrogels have characteristics to transform their shape (from solution to gel) based on the application [51]. Furthermore, stimuli-responsive hydrogels are categorized into three classes: chemical, physical, and biological. pH, solvency, ionic strength, and electrochemical field are typical chemical stimuli. Physical stimuli include temperature, magnetic field, electric field, light, mechanical force, and ultrasound. Biological stimuli include enzymes, glucose, antigen, ligands, etc. (Figure 4) [52]. Multi-responsive hydrogels are the kind of hydrogels that respond to two or more stimuli [53].
Figure 4.
Response of hydrogel to different stimuli.
4.3. Biodegradable
Biodegradability refers to the ability of the hydrogel to break down into harmless and non-toxic end products by bacteria or other organisms. Hydrogels’ biodegradability is determined by the functional groups present in the system as well as the method of synthesis. The degradation process involves solubilization and hydrolysis of biological entities of hydrogel into safer end products. Biodegradable polymers include a wide range of hydrophilic synthetic and natural polymers. Due to diffusion, these polymers absorb an ample amount of water and expand to a large extent. The breakdown of these polymers is influenced by various parameters such as molecular weight, hydrophilicity, and the interaction of the polymer with water. Other environmental conditions like temperature and pH also influence the breakdown of polymers via solubilization.
Chemical hydrolysis can be used to degrade a variety of polymers that cannot be destroyed by simple hydrolysis. These polymers do not produce hydrogel; rather they mix with hydrogel to form a hydrophilic monomer, which is then combined to form a biodegradable hydrogel. The formed hydrogel undergoes degradation via chemical hydrolysis via ester bonds. Furthermore, hydrogels can be degraded by enzyme hydrolysis and this category of hydrogels involves polymers such as proteins, polysaccharides, and synthetic polypeptides. Enzyme hydrolysis takes place by a set of hydrolases that catalyze the hydrolysis of C-N, C-O, and C-C bonds. Peptidases and proteinases are hydrolases that degrade polypeptide and protein hydrogels, respectively. Moreover, glycosidase is the sole enzyme that degrades polysaccharide hydrogels [47].
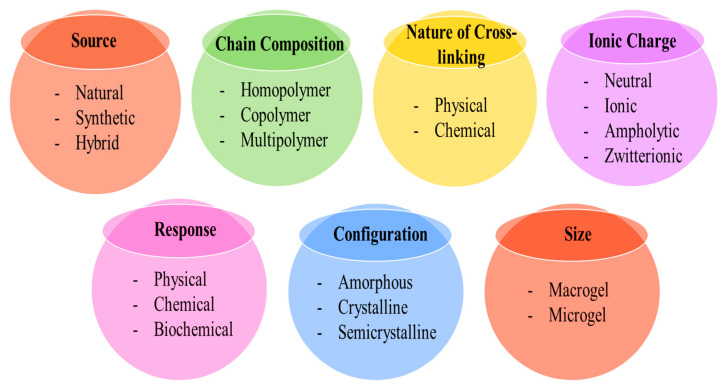
5. Classification of Hydrogel
Hydrogels are classified depending on their source, nature of cross-linking, chain composition, ionic charge, response to external stimuli, configuration, and size. The most important parameters for the classification of hydrogels are depicted in Figure 5.
Figure 5.
Classification of hydrogels.
5.1. Based on Source
Hydrogels can be classified as natural, synthetic, and hybrid.
-
(a)
Natural hydrogels are synthesized by using natural sources including chitosan, agar-agar, cellulose, lignin, gelatin, alginate, dextran, collagen, and many other materials [54].
-
(b)
Synthetic hydrogels are prepared by using synthetic polymers namely hydroxy methyl methacrylate (HEMA), acrylic acid (AA), vinyl acetate (VAc), ethylene glycol (EG), ethyleneglycoldimethacrylate (EGDMA), methacrylic acid, N-vinyl 2-pyrrolidone (NVP), and many other materials [54].
-
(c)
Hybrid hydrogels are made by combining natural and synthetic sources [55]. Zhang et al. prepared chitosan-g-poly (acrylic acid)/attapulgite/sodium humate hydrogel for effective removal of Pb2+ [56].
5.2. Based on the Nature of Chain Composition
Hydrogels can be classified into three principal classes: homo-polymeric hydrogels, co-polymeric hydrogels, and multipolymer hydrogels.
-
(a)
Homo-polymeric hydrogels are cross-linked polymer-network originating from a single type of monomer unit [57]. The structural unit of these hydrogels depends on the type of monomer, cross-linker, and polymerization technique [58].
-
(b)
Co-polymeric hydrogels are composed of two or more types of monomer units with at least one hydrophilic monomer, arranged in a random, block, and irregular structure along the backbone of the polymer network [59]. These hydrogels are prepared by cross-linking or polymerization between both the monomers by using a cross-linker and initiator. An example of such hydrogels is chitosan, k-carrageenan, carboxymethyl cellulose composite hydrogel which is used to remove metal ions.
-
(c)
Multipolymer hydrogels are cross-linked polymer-network prepared by three or more monomer units via cross-linking and polymerization reactions. For example, Kim et al. synthesized chitosan-based multicomponent functional gel comprising multiwall carbon nanotubes, polyaniline, poly (acrylic acid), and poly (4-amino diphenyl amine) [60].
-
(d)
Interpenetrating polymeric hydrogels are comprised of two independent, intertwined polymer networks, having natural and/or synthetic polymer components. In a semi-interpenetrating polymer hydrogel, one polymer has a linear network that diffuses into another cross-linked network. There is no chemical bonding between the polymers [54].
5.3. Based on the Nature of Cross-Linking
Hydrogels can be classified into two categories: physically cross-linked hydrogels and chemically cross-linked hydrogels.
-
(a)
Physically cross-linked hydrogels have a transient junction that arises due to physical interaction such as hydrogen bond, ionic interaction, and hydrophobic interaction.
-
(b)
Chemically cross-linked hydrogels have permanent junctions that arise due to covalent bonds [50].
5.4. Based on the Reaction of Hydrogel with the External Stimulus
Hydrogels can be classified into two distinct categories: traditional hydrogels and environmentally sensitive hydrogels.
-
(a)
Traditional hydrogel is not reactive to environmental changes
-
(b)
An environment-sensitive hydrogel can detect changes caused by chemical (pH, concentration), biochemical (antigen, enzyme, ligand), and physical (temperature, pressure, light) factors [50].
5.5. Based on the Configuration
On the basis of the physical structure and chemical composition, hydrogels can be classified as amorphous, semi-crystalline (mixture of crystalline and amorphous phases), and crystalline [41].
5.6. Based on the Size
Hydrogels can be classified into two classes: macrogel and microgel. The macrogel is further classified as a porous sponge, columnar, membranous, fibrous, and spherical according to its morphology. The prepared microgel also can be classified into nanometer and micron [50].
5.7. Based on Ionic Charge
Hydrogels can be classified into four categories based on the electric charge placed on the cross-linked network: neutral, ionic, ampholytic, and zwitterionic.
-
(a)
Neutral hydrogels are also known as non-ionic hydrogels. These hydrogels contain no charge on side groups or polymer backbone.
-
(b)
Ionic hydrogels are further classified as anionic and cationic. Anionic hydrogels carry negatively charged functional groups like sulfonyl, carboxyl, etc. and at high pH values show an increase in swelling behavior. Cationic hydrogels carry positively charged functional groups like amines, thiol, etc., and at low pH values exhibit an increase in swelling behavior.
-
(c)
Ampholytic or amphoteric hydrogels contain acidic as well as basic groups.
-
(d)
Zwitterionic hydrogels contain cationic and anionic groups in their structure [61,62].
6. Surface Functional Groups of Hydrogel
A group of atoms in a compound responsible for chemical reactions is known as the functional group. Functional groups play a significant role in determining the chemical reactivity of the molecule as well as the type and strength of intermolecular forces. The paramount functional groups incorporated in a three-dimensional network of hydrogels for metal adsorption are classified into three groups:—(a) nitrogen-containing functional groups, (b) oxygen-containing functional groups, and (c) sulfur-containing functional groups [63]. Table 3 summarizes hydrogels containing different functional groups and removed heavy metals.
Table 3.
Different hydrogel-based functionalized adsorbent materials for the removal of heavy metals.
| Hydrogel | Active Functional Group | Heavy Metals Removed | References |
|---|---|---|---|
| Graphene oxide-chitosan-poly(acrylic acid) (GO-CS-AA) hydrogel nanocomposite | R-COOH | Pb2+ | [84] |
| Hydrous ferric oxide-Poly(trans-aconitic acid/2-hydroxyethyl acrylate (HFO-P(TAA/HEA)) hydrogel | R-OH | Cu2+, Cd2+, Pb2+ and Ni2+ | [85] |
| Chitosan-sodium lignosulfonate-acrylic acid (CS-SLS-AA) hydrogel | R-NH2 | Co2+ and Cu2+ | [86] |
| Poly(3-acrylamidopropyl) trimethyl ammonium chloride/ɤ-Fe2O3 | R-N+(CH3)3 | Cr4+ | [87] |
| Sulfathiazole-based novel UV-curved hydrogel | R-SH | Hg2+, Cd2+ and Zn2+ | [88] |
| Magnetic anionic hydrogel (nFeMAH) | R-SO3Na | Cu2+ and Ni2+ | [64] |
| Poly(2-acrylamido-2-methyl-1-propane sulfonic acid) magnetic hydrogel | R-SO3H | Cd2+, Co2+, Fe2+, Pb2+,Cu2+, Cr2+ and Ni2+ | [39] |
| Acrylamide/crotonic acid (AAm/CA) hydrogel | R-COOH, and R-CONH2 | Hg2+ | [89] |
| Glucan/chitosan hydrogel | R-OH and R-NH2 | Co2+, Cu2+, Cd2+, Ni2+ and Pb2+ | [90] |
| Malic acid enhanced chitosan hydrogel beads (mCHBs) | R-COOH and R-NH2 | Cu2+ | [91] |
| Carboxymethyl cellulose/polyacrylamide (CMC/PAM) composite hydrogel | R-OH, R-COOH and R-NH2 | Cd2+, Pb2+ and Cu2+ | [92] |
| Chitosan poly(acrylic acid) supermacroporous hydrogel | R-OH, R-COOH and R-NH2 | Cu2+ and Pb2+ | [93] |
| Lignosulfonate-modified graphene hydrogel | R-C=O, R-OH and R-COOH | Pb2+ | [94] |
| Polyacrylonitrile-chitosan-graphene oxide (PCG) hydrogel composite | R-C(NH2)=N-OH | U6+ | [73] |
6.1. Nitrogen-Containing Functional Groups
6.1.1. Amine Group
The amine group contains a nitrogen atom that has a lone pair of electrons, that readily attach to cationic metal ions. The methods used to functionalize the amine group on the hydrogel surface are atom-transfer radical polymerization, formaldehyde treatment, and gamma ray-induced polymerization [63].
6.1.2. Amide Group
The general formula for the amide group is –CONH. In general, amine groups are more often functionalized on the hydrogel surface than amide groups. Moreover, monomers like 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt have an amide group in the polymer backbone, that complexes with heavy metals Cu(II) and Ni (II) [64].
6.1.3. Quaternary Ammonium Groups
Quaternary ammonium groups [R-N+(CH3)3] show strong attraction toward metal oxyanions (Cr2O72−, HCrO4−, AsO43− and CrO42−) [65]. Quaternary ammonium groups are highly stable and unaffected by pH change. Hence, they can captivate oxyanions of metals irrespective of the pH of the medium. Monomers, namely, (vinylbenzyl)trimethyl ammonium chloride, (3-acrylamidopropyl)trimethyl ammonium chloride and 2,3-epoxypropyltrimethylammonium chloride contains quaternary ammonium group as an active functional group in hydrogel preparation [66,67]. By ion exchange, hydrogels that consist of these monomers led to the exchange of the chloride (Cl¯) ions with oxyanions of metals.
6.2. Oxygen-Containing Functional Groups
6.2.1. Hydroxyl Group
A hydroxyl (R-OH) group is composed of one oxygen atom bonded to one hydrogen atom. According to the International Union of Pure and Applied Chemistry (IUPAC), the word “hydroxyl” refers to a hydroxyl radical. A hydroxyl group can easily remove the proton to attract metal cations.
6.2.2. Carboxyl Group
A carboxyl (R-COOH) group is composed of an electronegative oxygen atom that is double-bonded to the carbon atom and singly bonded to the –OH group. According to literature, the carboxyl group is found to be the most prominently used group to adsorb heavy metals onto the hydrogel surface. A carboxyl group gets ionized by giving an H+ ion from its R-OH group at alkaline pH, forming RCOO− ion that readily attracts the divalent metal cations. To functionalize the carboxyl group onto the hydrogel surface, different methods used in post-treatment are surface grafting, etherification, and 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO)-mediated oxidation, whichconverts a primary hydroxyl group into a carboxyl group [63].
6.3. Sulfur-Containing Groups
6.3.1. Thiol Group
The thiol (R-SH) group plays an important role in functionalizing the hydrogel surface for heavy metal adsorption [68]. The thiol group acts as a Lewis base and interacts with the heavy metal (Lewis acid) by forming a coordinate bond [69]. From the literature, it is demonstrated that the thiol group shows strong bonding with mercury (Hg). The bonding can be well explained by Hard-Soft-Acid-Base (HSAB) theory, where mercury acts as a soft acid and prefers to bind with the thiol group (soft base) [70]. In an article by Kumar et al. the thiol group prefers to form a stable complex with highly polarizable soft heavy metals like mercury (Hg), gold (Au), and silver (Ag); and to a lesser extent with cadmium (Cd) and zinc (Zn), failing to form a coordinate bond with lighter metals like sodium (Na), calcium (Ca), and magnesium (Mg) [71].
6.3.2. Sulfonic Acid Group
A sulfonic acid (R-SO3H) group is a sulfur-containing functional group that contains an electronegative sulfur atom that is double bonded to two oxygen atoms and single bonded to the –OH group. Sulfonic acid turns into sulfonate group (R-SO3−) on disassociation of hydrogen atom. Functionalizing a sulfonate group onto the surface of hydrogel makes the surface negatively charged, irrespective of the pH of the medium. A monomer 2-acrylamido-2-methyl propane sulfonic acid (AMPS) has been used to synthesize hydrogel via 60cobalt gamma-ray irradiation for the adsorption of Co2+, Mn2+, Cu2+,and Fe3+ [72].
6.4. Other Functional Groups
6.4.1. Amidoxime Group
The general formula for the amidoxime group is (R-C(NH2)=N-OH). The amidoxime group forms stable complexes with many heavy metals such as Co2+, Cu2+, Ni2+, and Pb2+ but shows a strong affinity towards uranium. Therefore, hydrogel-based adsorbent material has been functionalized with the amidoxime group for the adsorption of uranium [73,74]. Guibal and coworkers synthesized amidoxime grafted chitosan magnetic hydrogel for sorption of uranium (U6+) and europium (Eu3+) [75]. Zeng et al. prepared amidoxime-modified hydrogel via graft copolymerization for adsorption of Cu2+. The maximum adsorption capacity of 40.7 mg/g at pH 5 was achieved over the contact time of 25 h [76].
6.4.2. Phosphate-Containing Functional Group
Phosphate-containing functional groups, namely phosphine, phosphate, and phosphoramide are used to functionalize hydrogel surface. However, phosphate-based functional groups are more popular for functionalizing hydrogel for biomedical areas rather than metal adsorption. There are very few papers reported for metal adsorption. Liao et al. prepared phosphate functionalized graphene hydrogel for electrosorption of U6+. The maximum electrosorption capacity of 545.7 mg/g at 1.2 V and pH 5 was obtained [77].
6.4.3. Chelating Group
The chelating agent such as aminopolycarboxylic acids (APCAs) helps in enhancing the adsorption affinity of the hydrogel by chelation [78]. Because there are many nitrogen- and oxygen-containing functional groups present in the aminopolycarboxylic acid structure. Especially, the nitrogen-containing functional group shows strong bonding interaction with divalent metal cations [79,80]. The four common aminopolycarboxylic acids for metal adsorption are ethylenediaminetetra acetic acid (EDTA), iminodiacetic acid (IDA), diethylenetriaminepentaacetic acid (DTPA), and nitrilotriacetic acid (NTA). Many biosorbents have been functionalized with EDTA because of their chemical stability, chelating ability, and low price [81]. IDA is a tridentate ligand forming a metal complex by chelation [82]. No studies have been reported for hydrogel surface modification by an NTA chelating agent. DTPA possesses five carboxylate groups, which show a high binding affinity for heavy metals after grafting on the hydrogel surface. For example, Huang et al. prepared DTPA-modified chitosan/alginate hydrogels for removal of Cu2+ from electroplating wastewater [83].
7. Synthesis of Hydrogel
A plethora of issues arising from the overuse of non-biodegradable materials and fossil resources have shifted researchers’ focus to renewable and environmentally friendly materials. In the present time, polymers are extensively used in different areas, namely agriculture, biomedical applications, wastewater treatment, and food packaging [95,96,97,98]. Similarly, for the removal of toxic heavy metals from wastewater by adsorption, polymeric hydrogels are the most promising adsorbent material due to their increased surface area, good solubility in organic solvents, improved functionality, low-priced, biodegradability, recyclability, enhanced adsorption capacity, and ease of fabrication. In addition, the excellent hydrophilic character makes these hydrogels suitable for wastewater treatment [35,99]. However, the effectiveness of the adsorbent material is highly dependent on the physicochemical properties of the adsorbent [100]. As a result, the first and most important step in developing an effective adsorption process is to synthesize a suitable hydrogel-based adsorbent material with high absorptivity of heavy metals present in wastewater.
The essential chemicals required for the synthesis of the hydrogel are a monomer, an initiator, and a cross-linker. Acryl amide (AAm), polyvinyl alcohol (PVA), polyvinyl pyrrolidone, acrylic acid (AA), 2-dimethylamino ethyl methacrylate (DMAEM), polyethylene glycol methyl ether methacrylate (PEGMEM), (3-Acrylamidopropyl) trimethylammonium chloride (APTMACI), N-isopropylacrylamide, 2-acrylamido-2-methyl-1-propan-sulfonic acid (AMPS), and 4-vinyl pyridine, 2-hydroxyethylmetacrylate are the examples of some monomers used in hydrogel synthesis [38,39,101,102,103,104,105,106]. Distinct monomers have different properties in terms of adsorption capacity, physical strength, and so on. In the synthesis of hydrogels, researchers were able to develop a solution to overcome the limitation of specific monomers. For example, to reduce the physical weakness of biopolymer chitosan, Sun et al. [107] and Liu et al. [108] used cellulose as the blending polymer in the synthesis of chitosan-based hydrogel for heavy metal adsorption.
Cross-linkers or cross-linking agents play a crucial role in the synthesis of polymeric hydrogels because they help to build up the polymeric three-dimensional network by stabilizing the binding sites amid the functional monomer and adsorption target molecule. Therefore, cross-linkers influence the polymers’ hydrophilic or hydrophobic properties, selectivity, mechanical stability, and morphology [109]. A cross-linker of organic or inorganic nature can be used in the synthesis process. Moreover, inorganic cross-linkers are mainly used to synthesize hydrogel adsorbents as organic cross-linkers that have certain disadvantages in terms of lower mechanical strength and thus cannot withstand stressed conditions; additionally, they also have a lower swelling capacity [41,110]. Furthermore, the characteristic properties of a hydrogel can differ depending on whether the cross-linking between the chains is covalent or non-covalent. Permanently cross-linked junctions exist in hydrogels that have been cross-linked with covalent bonds. Hydrogels cross-linked with non-covalent bonds (ionic interaction, hydrophobic interaction, or hydrogen bonding), on the other hand, have transient junctions [41,50].
For polymerization reaction, a cross-linker must have more than one active functional group to help linear polymer chains to join with other chains to form a stable three-dimensional structure. A low degree of cross-linking, in particular, corresponds to a small quantity of cross-linker, resulting in poor mechanical strength of polymeric material. As a result, the three-dimensional structure of hydrogel distorts during the application, and adsorption sites are disrupted, giving rise to a high number of non-specific perforations. When the degree of cross-linking is high, a densely packed three-dimensional mesh structure is generated, having excellent mechanical strength and an unexpectedly high mass transfer number. Resulting in the reduction of adsorption sites, as well as the degree of swelling of the hydrogel, causing heavy metals to barely penetrate the hydrogel surface. Therefore, it is optimal to maintain the quantity of the cross-linker in the specified range. Polymers having a cross-link ratio greater than 80% are generally used [109,111,112].
In the synthesis of hydrogels, an initiator is a chemical that helps to initiate the polymerization process. Table 4 summarizes various hydrogel-based adsorbents and the monomers, initiators, and cross-linkers used in their synthesis process.
Table 4.
Different hydrogel adsorbents and associated monomers, cross-linker, and initiators in the synthesis process.
| Hydrogel | Monomer | Cross-Linker | Initiator/Accelerator | References |
|---|---|---|---|---|
| Poly(2-acrylamido-2-methyl-1-propansulfonic acid-co- vinylimidazole) hydrogel | 2-acrylamido-2-methyl-1-propansulfonicacid (AMPS), N- vinyl imidazole | N,N′ methylenebisacrylamide (MBA) | 2,2′-azobis(2-methyl propionamide) (MPA) dihydrochloride | [37] |
| Cationic hydrogel | (3-acrylamidopropyl) trimethylammonium chloride (APTMCI) | N,N′ methylenebisacrylamide (MBA) | Ammoniumpersulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) | [38] |
| Hydrogel biochar composite | Acrylamide (AAm) | N,N′ methylenebisacrylamide (MBA) | Ammonium persulfate (APS) | [101] |
| Fe2O3 nanoparticles functionalized polyvinyl alcohol/chitosan magnetic composite hydrogel | Polyvinyl alcohol (PVA) | Glutaraldehyde vapor | Glacial acetic acid | [102] |
| Methacrylate-based hydrogel | Polyethylene glycol methyl ether methacrylate (PEGMEM), 2-dimethylamino ethyl methacrylate | N,N′ methylenebisacrylamide (MBA) | Ammonium persulfate (APS) | [104] |
| (p-4-VP-co-HEMA) composite hydrogel | 4-vinyl pyridine (4-VP), 2- hydroxyethylmetacrylate (HEMA) | N,N′ methylenebisacrylamide (MBA) | Ammonium persulfate (APS), N,N,N′,N′-tetramethylenediamine (TEMED) | [106] |
| Chitosan-cellulose hydrogel | Chitosan | Cellulose | - | [107] |
| Superabsorbent polymer hydrogels | Acrylic acid (AA), acrylamide (AAm) | N,N′ methylenebisacrylamide (MBA) | Ammoniumpersulfate (APS) | [113] |
| Poly(N-hydroxymethylacrylamide) hydrogel | N-hydroxymethylacrylamide | Polyethylene glycol (400) diacrylate | Ammonium persulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) | [114] |
| EDTA Functionalized Chitosan/Polyacrylamide double network hydrogel | Chitosan, acrylamide | N,N′ methylenebisacrylamide (MBA) | Potassium persulfate (KPS) | [115] |
| N-vinyl-2-pyrrolidone/Itaconic acid hydrogel | Itaconic acid (IA), N-vinyl-2-pyrrolidone | N,N′ methylenebisacrylamide (MBA) | Ammoniumpersulfate (APS/N,N,N′,N′- tetramethylenediamine (TEMED) | [116] |
| Polyampholyte hydrogel | Methyl methacrylate (MMA), acrylic acid (AA) | N,Nʹ methylenebisacrylamide (MBA) | Ammonium persulfate (APS)/N,N,N′,N′- tetramethylenediamine (TEMED) | [117] |
| Poly(acrylic acid) hydrogel adsorbent | Acrylic acid (AA) | Calcium hydroxide (Ca(OH)2) nano-spherulites (CNS) | Ammonium persulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) | [118] |
| Magnetic chitosan hydrogel beads | Chitosan | Glutaraldehyde | - | [119] |
| Hydrogel-based on novel cross-linker | Chitosan, acrylic acid, glucose | Allyl pentaerythritol(AP)15/allyl mannitol (AP)14/allyl sorbitol | Potassium persulfate (KPS) | [120] |
Hydrogels are synthesized via two routes: Chemical and physical.
7.1. Synthesis via the Chemical Route
Polymer chains in chemically cross-linked hydrogels are formed by covalent bonds. The subsequent sections describe various methods for the synthesis of the hydrogel by chemical modification.
7.1.1. Chemical Route of Cross-Linking via Free Radical Polymerization
Free radical polymerization is one of the well-studied approaches for the synthesis of hydrogel in the presence of cross-linking agent N, N′ methylene bisacrylamide (MBA). The method involves three steps namely, initiation, polymeric chain propagation, and termination. In this process, the first step involves the generation of free radicals by using an initiator such as ammonium persulfate (APS), potassium persulfate (KPS), etc. in the vicinity of temperature, light, redox reaction, or ultraviolet or gamma radiation [121,122]. After that, in the second step, the free radical will react with the monomer to produce a radical monomer, which then reacts with the other monomers present in the solution to form polymeric chains. The cross-linker is added during the propagation of the polymeric chain, resulting in the formation of a three-dimensional structure of hydrogel. In the last step, the polymeric chain is terminated via disproportionation or combination reaction. The combination reaction connects two growing chains into one long polymeric chain. However, in the case of disproportionation reaction, a hydrogen atom is abstracted from the end of one growing chain and added to the other growing chain. As a result, a polymer with the unsaturated end group and a saturated end group is obtained. To speed up the process, an accelerating agent such as N,N,N′,N-tetramethylene diamine is added to the reaction mixture [123]. For instance, Shah et al. prepared superabsorbent polymer hydrogels containing acrylamide and acrylic acid as monomers via one-step free-radical polymerization. In this work, they aimed to remove multi-metals (Ni2+, Cd2+, Co2+, and Cu2+) from an aqueous medium [113].
7.1.2. Chemical Route of Cross-Linking via High Energy Irradiation
The hydrogel synthesis via ultraviolet light radiation, electron beams, and ɤ-radiation is carried out at ambient or sub-ambient temperatures without the requirement of initiators, catalysts, or cross-linkers [124]. This synthetic route outperforms chemically initiated processes in regards to one-step hydrogel formation with no waste generation as a byproduct [125]. In this method, the density of cross-linking is estimated by duration and dose of irradiation. Polyethylene glycol (PEG), polyvinyl alcohol (PVA), alginate, chitosan, gelatin, hyaluronic acid (HA), carboxymethyl cellulose (CMC) are among the natural and synthetic polymers proposed for the hydrogel synthesis using this method [126,127]. This cross-linking method is similar to free radical polymerization in terms of three-step hydrogel formation: initiator, propagation of polymeric chain, and termination. When the mixture of the reaction solution is irradiated, a hydroxyl free radical is generated, resulting in the formation of a free-radical monomer. The hydrogel is synthesized when the network has reached the critical stage of gelling [128]. Maziad et al. prepared polyacrylic acid/polyvinyl alcohol based hydrogel to treat water decontamination via gamma radiation. They found that the hydrogel swelled 273%and had removal capacity of 150 mg/g, 155mg/g, and 193 mg/g for Ni2+, Co2+, and Cu2+ ions, respectively, at acidic pH 5 and after 24 h [129].
7.1.3. Chemical Route of Cross-Linking via Grafting Reactions
In this method, the hydrophilic functional group like carboxyl (−COOH), sulfonic (−SO3H), amino (−NH2), and acylamino (−CONH2) are grafted on the surface of hydrogel [92,130]. Grafting the functional groups helps in improving the adsorption or desorption efficiency, as well as selectivity for specific heavy metals. As a result of this, there is an increase in surface polarity, hydrophilicity, and enhancement in the number of active sorption sites [131]. For example, Qi et al. prepared a new salecan polysaccharide-based hydrogel via graft copolymerization of sodium vinyl sulfonate and acrylamide onto the salecan for the effective decontamination of Pb2+ from wastewater [132].
7.1.4. Chemical Route of Cross-Linking via Reaction of Functional Groups
The reaction involves the bond formation between the cross-linker and the functional moieties present in the polymer molecule. Hydrophilic groups such as amine (–NH2 in chitosan and proteins) and hydroxyl (–OH in cellulose and its derivatives) are bonded with cross-linking agents (such as glutaraldehyde) having an aldehyde functional group resulting in aldol product via covalent interaction. Hydrogel synthesis involving the polymers having hydroxyl groups needs certain specific conditions like methanol as a quencher, high temperature, and low pH. However, in the case of protein-based hydrogel no specific conditions are required [112,133,134]. Polymers with ester functional groups, on the other hand, undergo chemical cross-linking through the condensation process in the presence of a cross-linking agent, resulting in the formation of Schiff bases [130].
7.2. Synthesis via Physical Route
The physical route of cross-linking is highly favorable to synthesize non-toxic and environmentally friendly hydrogel as there is no requirement for chemical-based cross-linking agents [135]. In this process, polymer chains are held by weak interactions like hydrophobic interaction, ionic interaction, hydrogen bonding, Van der Waals forces, and π–π interaction [136]. From the literature, it is noted that polysaccharides like dextran, pullulan, carboxymethyl curdlan, and chitosan are used for the synthesis of hydrogels by this method [137].
7.2.1. Synthesis via Freeze-Thaw
Crystallization via the freeze–thaw method is one of the physical processes used to synthesize hydrogel [138]. In this method, crystallization takes place by freezing low molecular solutes or bulk solvents, which enhances the polymer concentration by decreasing the chain gap and allowing the chains to align and join to create a three-dimensional structure [139]. In hydrogels, freeze–thaw cycles give rise to porous structures due to the space created by melting crystals during the thawing stages [140]. By varying the polymer concentration, the freezing temperature, freeze–thaw time duration, and the number of freezing and thawing cycles, the mechanical characteristics of the freeze–thawed hydrogel may be adjusted [139].
Hydrogels synthesized via the freeze–thaw method have greater elastic characteristics in comparison to those synthesized via chemical methods, attracting widespread interest across the world [141]. For instance, poly (vinyl alcohol) (PVA)/carboxy methyl cellulose (CMC) hydrogels are synthesized via the freeze–thaw method and used to absorb heavy metals such as Ni2+, Cu2+, Zn2+ and Ag2+ [139].
7.2.2. Synthesis via Self-Assembling
Self-assembled hydrogels are prepared by monomeric units that spontaneously self-assemble by non-covalent interaction into supramolecular fibers [142]. When such fibers retain proper solvation in liquid (water), they efficiently entangle and immobilize solvent flow, resulting in a 3D mesh structure. The non-covalent interaction stabilizes hydrogel structures by making them softer than those generated by covalently cross-linked material [143]. These interactions provide self-assembled hydrogel with advantages such as tolerance to environmental perturbation and self-healing characteristics [144].
7.2.3. Synthesis via Instantaneous Gelation
Another approach for synthesizing hydrogel quickly after a one-step procedure is instantaneous gelation [39,145]. For example, Zhou et al. synthesized novel chitosan-based magnetic hydrogel beads comprised of amine-functionalized magnetite nanoparticles, carboxylated cellulose nanofibrils, and polyvinyl alcohol incorporated chitosan for adsorption of Pb2+. The synthesized hydrogel beads exhibited an adsorption efficiency of 171.0 mg/g and could be regenerated in a weakly acidic solution with an adsorption efficacy of 90% after 4 cycles [146].
7.2.4. Synthesis via Ionotropic Gelation
Hydrogel synthesis by ionotropic gelation allows the formation of microparticles and nanoparticles via electrostatic bonding among the two ionic species under suitable conditions, one of which must be a polymer [147]. For instance, sodium alginate(SA)/hydroxypropyl cellulose (HPC) hydrogel beads were synthesized with different ratios of 50:50, 75:25, and 100:0 for the removal of Pb2+. According to the results obtained, 75:25 showed better adsorption capacity in comparison to 50:50 and 100:0. After three hours of contact time, hydrogel beads showcased adsorption capacity and adsorption percentage of 47.72 mg/g and 95.45%, respectively [148].
7.2.5. Synthesis via Inverse Emulsion Method
In the inverse emulsion method, the term “water-in-oil” describes the phenomenon in which water-soluble monomer is dispersed in the continuous phase oil (paraffin oil) by using an appropriate stabilizing agent, namely non-ionic surfactant Triton X-100, and after that the systems go through the phase inversion in a coagulation bath to release the monomer and precipitate out the porous film. This method has an advantage over other methods such as fine powdered product is obtained and by altering the reaction condition, the desired particle size can be achieved [149]. For example, a superabsorbent polymer-based hydrogel consisting of acrylic acid and carboxymethyl cellulose was synthesized by inverse emulsion polymerization method by using N, Nʹ methylene bisacrylamide (MBA) as a cross-linking agent and potassium persulfate (KPS) as an initiator. The maximum swelling capacity of 44.0 g/g in 0.9% w/v NaCl solution and 544.95 g/g in deionized water [150].
8. Characterization Techniques of Hydrogel
After the successful synthesis of hydrogel adsorbent, it becomes inevitable to investigate the physical, mechanical, structural, and morphological properties of the hydrogel formed. For this purpose, various characterization techniques such as Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), zeta sizer, and energy-dispersive X-ray (EDX) are used to characterize the hydrogel (Table 5).
Table 5.
Characterization techniques used for hydrogel adsorbent and information obtained from the characterization tools.
| Characterization Techniques | Characteristics |
|---|---|
| Fourier Transform Infrared Spectroscopy (FTIR) | Functional group |
| Field Emission-Scanning Electron Microscopy (FE-SEM) | Surface morphology |
| Thermo Gravimetric Analysis (TGA) | Thermal stability |
| Zeta Sizer | Surface charge |
| Energy Dispersive X-ray (EDX) | Elemental composition |
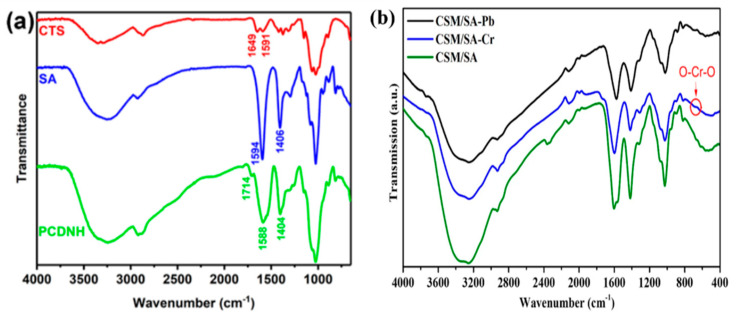
8.1. Functional Group Analysis
The surface functional groups such as hydroxyl, carboxyl, amide, amine, thiol, and amidoxime, etc. can be identified by using FTIR. Tang et al. synthesized chitosan/sodium alginate/calcium ion physically cross-linked double network hydrogel (PCDNH) for scavenging heavy metal ions. Analysis of FTIR spectra of hydrogels reveals that the peaks of chitosan at 1591 cm−1 and 1649 cm−1 (bending vibration of N-H and stretching vibrations for C=O of primary amine) disappear after the synthesis of PCDNH because the −NH2 group is converted to −NH3+. The symmetric and asymmetric stretching vibration peaks of −COO− of sodium alginate at 1406 cm−1 and 1594 cm−1 shift to 1404 cm−1 and 1588 cm−1, indicating, the interaction between −COO− with Ca2+ and −NH3+. A new peak was observed at 1714 cm−1 corresponding to the partial protonation of −COO− after the formation of PCDNH (Figure 6a) [151]. Ablouh et al. investigated the adsorption of Cr6+ and Pb2+ via FTIR analysis for the preparation of Chitosan/Sodium alginate (CSM-SA) hybrid hydrogel beads. It was noticed that the peak of −NH2 or −OH at around 3250 cm−1 shifted to 3245 cm−1, indicating hydrogen bonding among the H atoms in −NH2 groups and the O atoms of oxyanions of Cr6+. In addition, there is a slight shift in the peak of COO from 1600 to 1590 cm−1, indicating the interaction between COO and Cr6+. These shifts correspond to electrostatic interaction between Cr6+ and NH3+, COO, and OH groups. A new peak observed at 682 cm−1 is due to the O-Cr-O band corresponding to Cr species. After the adsorption of Pb2+, the stretching vibration of OH and COO group shows a strong shift from 3250 to 3261 cm−1, and 1600 to 1569 cm−1, respectively. This shift is due to the coordination effect between Pb2+ and O atom, demonstrating ion-exchange among Ca2+ and Pb2+ on the surface of hydrogel (Figure 6b) [152].
Figure 6.
(a) FTIR spectra of chitosan (CTS), sodium alginate (SA), and PCDNH, (Reprinted from Ref. [151], Copyright (2022), with permission from Elsevier). (b) FTIR spectra of CSM/SA hybrid hydrogel beads loaded with Pb2+ and Cr6+ [152].
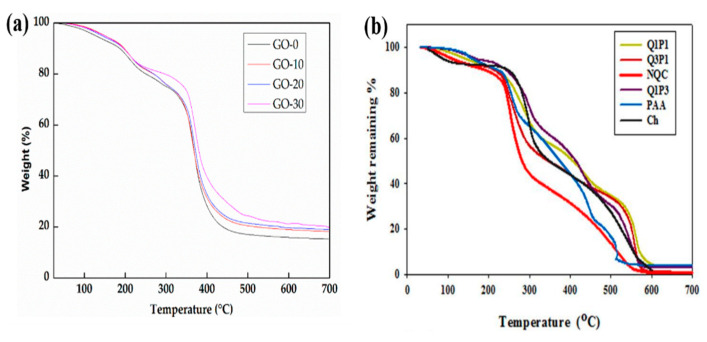
8.2. Thermal Analysis
Thermogravimetric analysis (TGA) is used to determine the thermal stability of a hydrogel. TGA can also be used to evaluate changes in a material’s physical and chemical properties as a function of increasing temperature. For example, Kong et al. studied the thermal stability of the Xylan-g-/p(acrylic acid-co-acrylamide)/graphene oxide (GO) hydrogel. Figure 7a represents the TGA thermogram of the hydrogel with and without GO. The weight loss of samples occurred in four phases when the temperature was raised from room temperature to 700 °C: 25–220 °C, 220–350 °C, 350–400 °C, and 400–700 °C. In the first step, weight loss was due to moisture loss and the decomposition of tiny molecules. The weight loss in the second step was because of the decomposition of long-chain compounds like polyacrylic acid, polyacrylamide, and xylan. The weight of hydrogels remained consistent in the last step, which was due to the carbonation of the hydrogels. Furthermore, the hydrogels with a higher GO loading had a high weight, indicating that GO has a positive effect on hydrogel thermal stability [153]. Mohamed et al. studied the thermal properties of a biodegradable N-quaternized chitosan (NQC)/poly (acrylic acid) (PAA) hydrogel by varying the NQC/PAA ratios to 3:1 (Q1P3), 1:1 (Q1P1), and 1:3 (Q1P3). The TGA thermogram revealed that the initial decomposition temperatures (IDT) of NQC, chitosan, PAA, Q3P1, Q1P1, and Q1P3 were observed at 214, 240, 229, 227,246, and 254 °C, respectively. Q1P3 hydrogel had the greatest IDT, indicating that it was the most thermally stable, owing to greater intermolecular hydrogen bonding between NQC and PAA chains. The thermal stability of hydrogels increased in the sequence: Q1P3 > Q1P1 > chitosan > Q3P1 > PAA > NQC (Figure 7b) [154].
Figure 7.
(a) TGA thermogram of the synthesized hydrogel with and without GO [153], and (b) Table 1. P1, Q1P3, and Q3P1. (Reprinted from Ref. [154], Copyright (2022), with permission from Elsevier).
8.3. SEM Analysis
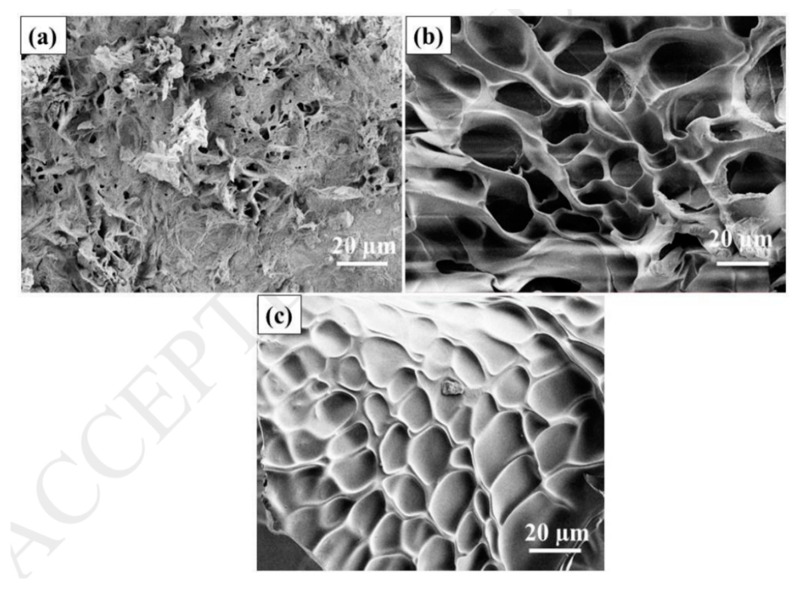
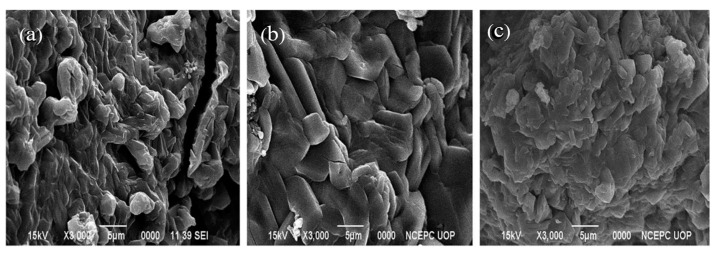
SEM is used to study the surface morphology, topography, and composition of the hydrogels. The porosity of hydrogel is a key factor attributed to its adsorption capacity. For instance, Godiya et al. synthesized bio-based carboxymethyl cellulose (CMC)/poly(acrylamide) (PAM) hydrogel for adsorption of heavy metals. SEM results demonstrated that CMC/PAM hydrogel has a sponge-like, three-dimensional, and highly mesoporous surface morphology (Figure 8b) that significantly differs from CMC hydrogel (Figure 8a). The CMC/PAM hydrogel has a pore size in the range of 5–15 µm in diameter. The pores developed in the hydrogel will permit guest molecules like water and heavy metals to move across the composite structure. The CMC/PAM composite hydrogel retained its structural robustness after the adsorption of Cu2+ (Figure 8c) [92].
Figure 8.
(a) CMC hydrogel, (b) CMC/PAM composite hydrogel, and (c) CMC/PAM composite hydrogel after the adsorption of Cu2+. (Reprinted from Ref. [92], Copyright (2022), with permission from Elsevier).
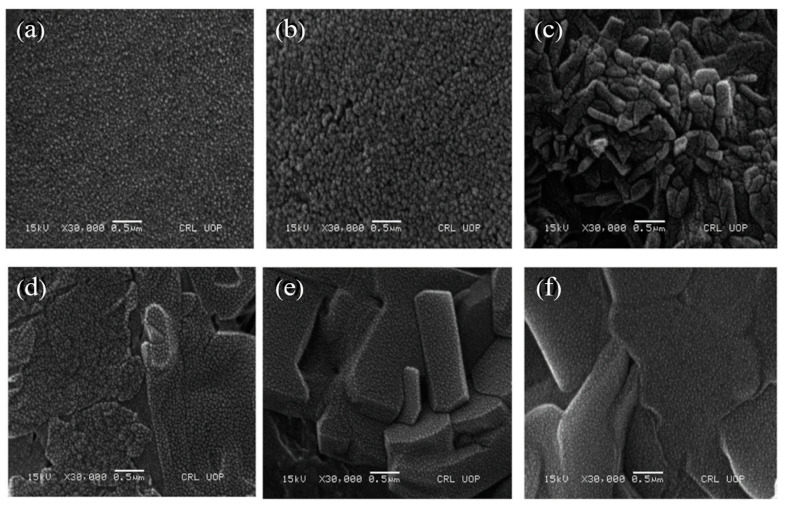
Javed et al. synthesized anionic poly(methacrylic acid)(P(MAA)), neutral poly(acrylamide)(P(AAm)), and cationic poly(3-acrylamidopropyltrimethyl ammonium chloride)(P(APTMACI)) hydrogels and examined surface morphology by using SEM. SEM micrographs revealed that the surface P(MAA) was highly porous and rough compared to P(AAm) and P(APTMACI) (Figure 9a–c). The material with a rougher surface will generally have a higher adsorption capacity. As shown in Figure 10, SEM micrographs of hybrid hydrogels revealed that heavy metals nanoparticles were dispersed throughout the matrix without aggregation [155].
Figure 9.
SEM micrograph of (a) anionic P(MAA), (b) neutral P(AAm), and (c) cationic P(APTMACI) hydrogels [155].
Figure 10.
SEM micrographs of hybrid (a) P(MAA)-Cu, (b) P(MAA)-Ni, (c) P(APTMACI)-Cu, (d) P(APTMACI-Ni, (e) P(AAm)-Cu, and, (f) P(AAm)-Ni hydrogels [155].
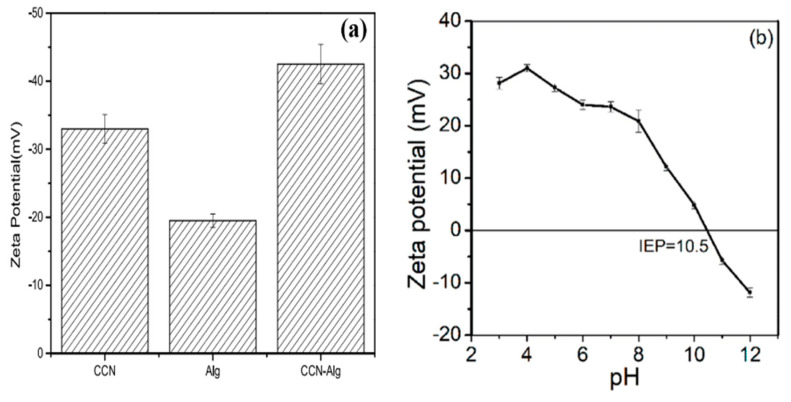
8.4. Zeta Potential Analysis
Zeta potential is useful in determining the surface charge of hydrogel adsorbent. For example, Hu et al. synthesized carboxymethyl cellulose nanocrystals (CCN)/sodium alginate (Alg) hydrogel beads for scavenging Pb2+ and used a zeta-sizer to check the surface charge. Figure 11a depicts the zeta potentials of CCN, Alg, and prepared CCN-ALg were measured at pH 5.2. The zeta potential results revealed that all the three samples had negatively charged surfaces with stable dispersion at pH 5.2, with CCN-Alg being more so than the other two [156]. Bandara et al. studied the surface charge on chitosan/polyethylenimine/graphene oxide hydrogel beads for the abstraction of selenium from wastewater. Positive zeta potentials were noticed across a wide pH range, ranging from acidic pH to the isoelectronic point of 10.5, indicating ideal circumstances for electrostatic interaction with negatively charged species (Figure 11b) [157].
Figure 11.
(a) Zeta potential (mV) of CCN, Alg, and CCN/Alg hydrogel beads. (Reprinted from Ref. [156], with permission from Elsevier), and (b) zeta potential of hydrogel beads varies as a function of pH, showing that negatively charged selenium absorbs at lower pH. (Reprinted from Ref. [158]. Copyright 2022 American Chemical Society).
8.5. EDX Analysis
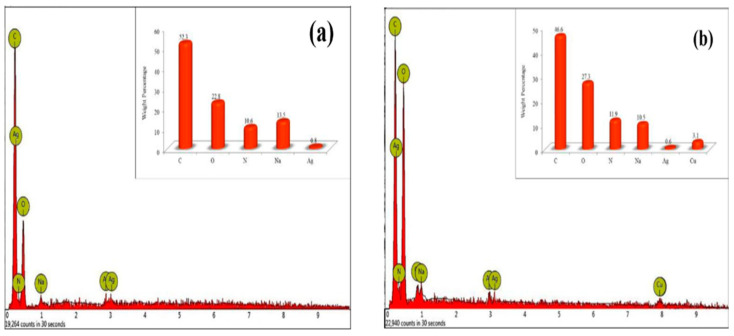
EDX characterization is used to determine the hydrogel’s elemental composition. Dil et al., for example, reported the fabrication of a novel porous gelatin-silver/poly (acrylic acid) (NPGESNC-AcA) nanocomposite hydrogel for Cu2+ removal. Figure 12 represents the element percentage of the synthesized NPGENC-AcA hydrogel before Cu2+ adsorption, which contains 52.3% carbon, 22.8% oxygen, 13.5% sodium, 10.6% nitrogen, 0.8% silver before adsorption of Cu2+. The results showed that silver was deposited in the nanocomposite hydrogel network, with no additional impurity elements detected in the spectrum (Figure 12a). EDX analysis for NPGENC-AcA after Cu2+ adsorption consists of 46.6% carbon, 27.3% oxygen, 11.9% nitrogen, sodium 10.5%, 0.6% silver, and 3.1% copper (Figure 12b) [121].
Figure 12.
EDX spectra of NPGESNC-AcA (a) before Cu2+ adsorption, and (b) after Cu2+ adsorption (Reprinted from Ref. [121], Copyright (2022), with permission from Elsevier).
9. Adsorption Mechanism of Hydrogel
A thorough understanding of the adsorption mechanism and the removal process of various contaminants on different hydrogel-based adsorbents is essential for modifying hydrogels to enhance adsorption efficiency. The interactions such as electrostatic interaction, ion exchange, coordination interaction, and hydrophobic interaction take place depending on the surface functional moieties of hydrogels, provided reaction conditions such as temperature, pH, ligand, salt concentration, etc., and pollutant chemistry [159]. In literature, most hydrogel adsorbents are formed by the combination of interactions that take place simultaneously to form a 3D network. In the case of starch-based hydrogel, chemisorption and physisorption act simultaneously by acid-base interaction, H-bonding, ion exchange, or coordination interaction with heavy metal ions [160,161]. In chitin-based hydrogel, single or combination of multiple interactions occur depending on the operating condition and chemical composition [162]. In cyclodextrin-based hydrogel, complex formation occurs among the cyclodextrin and heavy metals involving host–guest interaction where hydrophobic bonding [163]. The various adsorption/desorption mechanism of heavy metals by hydrogel is discussed in Table 6.
Table 6.
Proposed synthesis and removal mechanism of various hydrogel-based adsorbents.
| Hydrogel Type | Synthesis Method | Mechanism | Heavy Metals Removed | References |
|---|---|---|---|---|
| Carboxymethyl cellulose-graft-poly(acrylic acid)/monmorillonite hydrogel composite | Graft polymerization | Ion exchange and coordination interaction | Zn2+, Pb2+ | [177] |
| Silk sericin/Lignin hydrogel beads | Graft polymerization | Ion exchange or electrostatic interaction | Cr6+ | [178] |
| Chitosan/multiwall carbon nanotube/poly(acrylic acid)/poly(4-aminodiphenyl amine) functional gel | Free radical polymerization and cross-linking reaction | Complexation interaction | Cr6+ | [60] |
| Sugar cane bagasse cellulose and gelatin-based hydrogel composite | Cross-linking | Coordination and electrostatic interaction | Cu2+ | [179] |
| Carboxy methyl cellulose hydrogel | ɤ-raddiation | Coordination interaction | Cu2+ | [180] |
| Chitin/cellulose composite hydrogel | Freeze-thaw method | Electrostatic and coordination interaction | Hg2+, Cu2+, Pb2+ | [181] |
| Carboxy methyl cellulose hydrogel beads | Inverse suspension method | Coordination interaction | Cu2+, Ni2+, Pb2+ | [182] |
| Hydrogel-biochar composite | Free radical polymerization and cross-linking reactions | Chemisorption | As | [101] |
| Pullulan/polydopamine hydrogels | Chemical cross-linking | Electrostatic and coordination interaction | Cu2+ | [158] |
| Jute/poly(acrylic acid) hydrogel | Free radical polymerization | Electrostatic interaction | Cd2+, Pb2+ | [183] |
| Carboxylated chitosan/carboxylated nanocellulose hydrogel beads | Cross-linking | Electrostatic and coordination interaction | Pb2+ | [184] |
9.1. Electrostatic Interaction
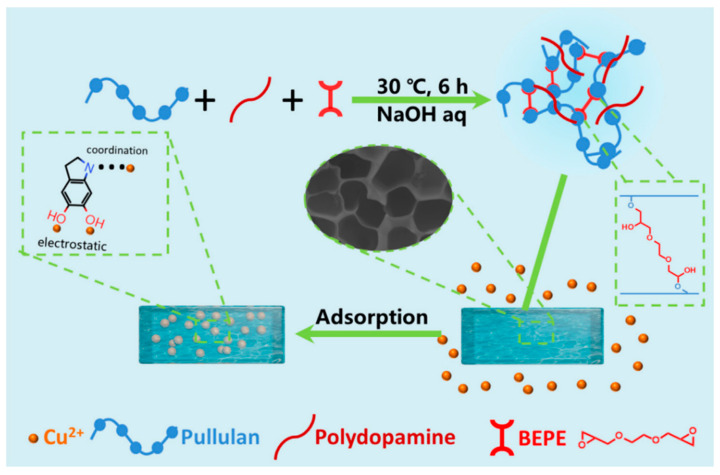
Electrostatic interaction occurs in the hydrogel with specific functional moieties in monomeric units having oppositely charged ions such as cation–anion interaction concerning heavy metals that need to be adsorbed or desorbed [159]. Furthermore, the pH of the solution has a significant impact on the generation of charged ions on the adsorbent (hydrogel) surface [164]. The pH of the solution is represented by pHPZC when there are no charged ions on the surface of the adsorbent [165,166]. When the pH > pHPZC, the surface functional moieties like −OH, −COOH, and −H3PO4 lose the proton due to the higher concentration of OH− ions in the solution that forms anions like −O−, −COO−, −PO43− etc. on the surface of the adsorbent. However, at pH < pHPZC, the surface of the adsorbent is positively charged due to an increase in the concentration of H+ ions, which causes protonation of functional moieties such as −SH, −NH2, etc [167]. According to the studies reported, electrostatic interactions are the dominant adsorption force for heavy metals abstraction in various hydrogels. Yu and co-workers synthesized sodium alginate(SA)/carboxylated nanocrystals cellulose hydrogel beads for the abstraction of Pb2+. The findings in this study reveal that the adsorption mechanism that took place was complexation among −COO and −OH functional moieties and heavy metal (Pb2+) by sharing a pair of electrons. Thereafter, the electrostatic interaction was found to occur between negatively charged hydrogel beads and positively charged Pb2+ ions [156]. Tang et al. synthesized physically cross-linked double network hydrogel (PCDNH) containing chitosan, calcium ion, and sodium alginate. In this study, they reported that chitosan’s cationic NH3+ group reacts with sodium alginate’s anionic −COO− group to construct physically cross-linked hydrogel via electrostatic interaction. In addition, the adsorption of heavy metals (Pb2+ and Cd2+) on the hydrogel surface was due to the electrostatic interaction with PCDNH’s oxygen atom, whereas the adsorption of Cu2+ was primarily due to coordination interaction with PCDNH’s nitrogen atom, besides electrostatic interaction [151]. Zeng et al. prepared pullulan/polydopamine hydrogel for effective elimination of heavy metals (Co2+, Cu2+, and Ni2+). In this research work, hydrogels were prepared by chemically cross-linking pullulan with 1,2-bis (2,3-epoxypropoxy) ethane. Polydopamine was added to the mixture to form a novel hydrogel adsorbent. Polydopamine’s nitrogen atom and catechol group have a high affinity to react with positively charged metal-ion via electrostatic and coordination interaction (Figure 13) [158].
Figure 13.
Schematic representation showcasing electrostatic and coordination interaction between pullulan/polydopamine hydrogel and heavy metals. (Reprinted from Ref. [158], Copyright (2022), with permission from Elsevier).
9.2. Ion-Exchange
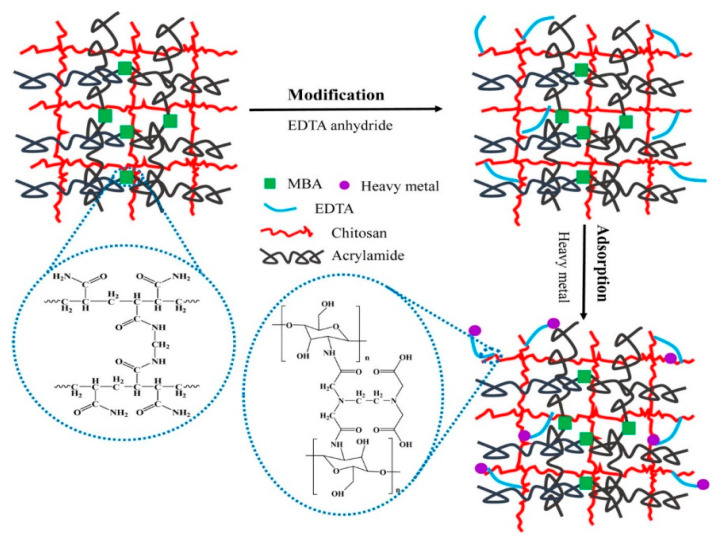
Ion exchange refers to a chemical process whereby the swapping of ions takes place between an insoluble adsorbent (hydrogel) and a liquid phase (wastewater). The unwanted anions or cations dissolved in the wastewater are replaced or removed by the ions of a similar charge present on the hydrogel surface. To maintain the neutrality of the system the number of ions adsorbed by the hydrogel adsorbent must be equal to the number of ions liberated [168]. Ion exchange provides an efficient and convenient route becauser it can distillate and separate distinct contaminants from wastewater [169]. It reduces the degree of harmful load by converting heavy metals waste into a form that can be reused and recycled, leaving behind less hazardous materials in the solution, or by reducing the hydraulic flow of the stream containing toxic heavy metals, allowing for the final release [167]. Ion-exchange mechanisms, like electrostatic interaction, are highly dependent on the pH of the solution. Due to a rise in the concentration of H+ ions at pH<pHPZC, the functional moieties in hydrogel adsorbent become positively charged, leading to cation exchange. However, at pH > pHPZC the functional moieties are negatively charged due to excessive concentration of OH- ions, leading to anion exchange [170]. Saber-Samandari et al. synthesized Cellulose-Graft-Polyacrylamide/Hydroxyapatite hydrogel composite for the removal of Cu2+ ions. In this work, he observed that Cu2+ ions got exchanged with the cations in the hydrogel composite and are attached to the surface of hydroxyapatite by an ion-exchange mechanism [171]. For the treatment of heavy metals from oily wastewater, Xiong et al. prepared a self-cleaning cellulose functionalized titanate microsphere hydrogel via a sol-gel method. The prepared hydrogel microspheres have the combined properties of cellulose and titanate nanotubes that exhibit a high capacity to maintain oily wastewater. At first, Cu2+ got absorbed on the inner surface of cellulose titanate hydrogel by electrostatic interaction. After that, Cu2+ was captured in the layer of titanate nanotubes exhibiting remarkable characteristics for heavy metals under the influence the chemical and physical adsorption [172]. Ma et al. prepared ethylenediaminetetraacetic acid (EDTA) functionalized double network hydrogel for efficient elimination of heavy metals (Cd2+, Pb2+, and Cu2+) from industrial eluents. In this work, a two-step process was conducted in which first polyacrylamide was cross-linked with N, N′ methylene bisacrylamide (MBA), and then EDTA was cross-linked with chitosan to form a double network hydrogel. The hydrogel showed a maximum sorption capacity of 138.41 mg/g, 99.44 mg/g, and 86.00 mg/g for Pb2+, Cu2+, and Cd2+ respectively based on the ion exchange mechanism between carboxylate groups and heavy metal ions (Figure 14) [115].
Figure 14.
The proposed mechanism between chitosan/polyacrylamide hydrogel and heavy metal ions. (Reprinted from Ref. [115]. Copyright 2022 American Chemical Society).
9.3. Hydrophobic Interaction
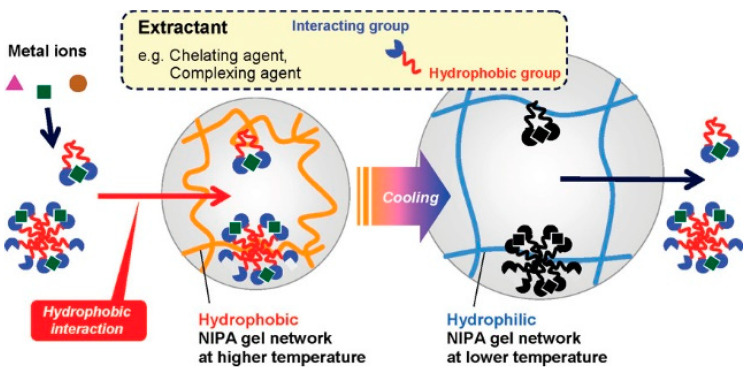
The interaction taking place between water molecules and hydrophobes (non-polar molecules containing long carbon chains that do not react with water molecules because of weak Van-der-Waals forces) is termed hydrophobic interaction [173]. Therefore, a low water-soluble molecule is more likely to be attracted by hydrophobes. For example, Tokuyama et al. prepared superabsorbent hydrogel containing N-isopropyl acrylamide (NIPA) as a thermo-responsive polymer for heavy metals extraction. At first, an aqueous solution of metal ions is complexed with an extractant that has a hydrophobic group and an interacting group. After that, above lower critical solution temperature the complex formed between metal and extractant gets absorbed into the hydrogel via hydrophobic interaction. Finally, after cooling below the low critical solution temperature metal-extractant complexes are extracted from the hydrogel. In this study, Cu2+ is used as a model heavy metal ion [174]. The mechanism for the same is depicted below in Figure 15.
Figure 15.
The proposed mechanism of heavy metal complexed with extractant onto N-isopropyl acrylamide hydrogel. (Reprinted with permission from Ref. [174]. Copyright 2022 American Chemical Society).
9.4. Coordination Interaction
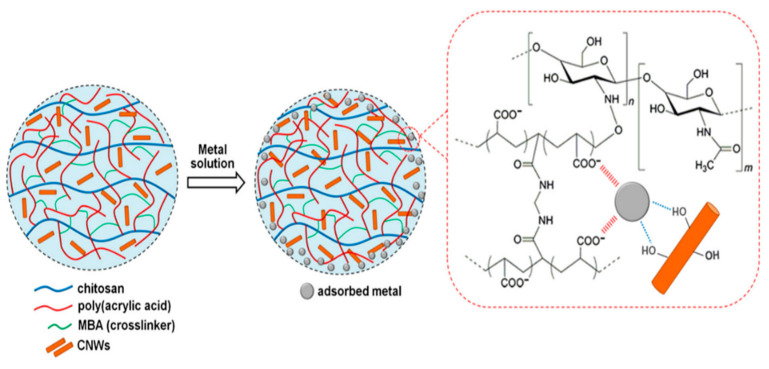
Coordination interaction also known as chelation interaction refers to the formation of covalent bond where a single-atom shares both the electrons. In this interaction, cation (heavy metals) binds with the group containing lone pair electrons, resulting in cation adsorption on the adsorbate surface [167]. Zhaung et al. prepared double network alginate/graphene nanocomposite hydrogel beads for effective extraction of Cu2+ and dichromate (Cr2O72−). He observed that –COOH functional moieties in both graphene and alginate show a high affinity for Cu2+ and Cr2O72− via coordination and complexation. On the contrary, ion exchange takes place between Ca2+ ions in alginate and Cu2+ in the aqueous solution [175]. Rodrigues et al. prepared chitosan-g-poly (acrylic acid)/cellulose nanowhiskers (CNWs) composite hydrogel beads by using N, N′ methylene bisacrylamide as a cross-linker for the adsorption of Cu2+ and Pb2+ from water. FTIR analysis revealed that functional moieties i.e., hydroxyl groups and carboxyl groups act as coordination sites for heavy metal adsorption [176]. The schematic representation depicting the coordination between hydrogel adsorbent and heavy metals is demonstrated in Figure 16.
Figure 16.
The coordination interaction between chitosan-g-poly (acrylic acid)/cellulose nanowhiskers hydrogel beads and adsorbed metal. (Reprinted by permission from Ref. [176]. Copyright (2022), Springer).
10. Recovery, Regeneration, and Reusability of Hydrogel
One of the paramount characteristics of hydrogel other than high adsorption efficiency is their regeneration capacity by desorbing the absorbed heavy metals which further allows it to be reused. The ability to regenerate and reuse an adsorbent material is also an important factor for the practical assessment of its application. Many different ways have been studied by researchers for the effective desorption of heavy metals from the three-dimensional mesh structure of hydrogel after every removal cycle. Changes in the magnetic field, electric field, temperature, pH, etc. will lead to the desorption of heavy metals [185]. The influence of pH on heavy metal desorption from a magnetized cellulose-chitosan hydrogel was reported by Liu et al. [108]. At very low pH values of 1.0–2.0 desorption efficiency of 83–86% was achieved. This represents the merits of using pH-dependent hydrogel for the adsorption of heavy metals such as arsenic and chromium during the elimination process and desorption for the recovery of hydrogels. Moreover, adjusting the required pH is a drawback [38]. According to the literature, studies reported on the recovery of hydrogel adsorbents have used strong as well as weak acids as eluents (HCl, HNO3, CH3COOH, H2SO4, etc.) [37]. Furthermore, the type of acid utilized in the desorption process also has a considerable impact on the durability and desorption capacity of hydrogel [24]. Mohammadi et al. synthesized a chelator-mimetic multi-functionalized hydrogel with a high metal adsorption efficiency (cadmium, lead, and arsenic) and great reusability. By employing a low concentration of hydrochloric acid, the heavy metals absorbed in the hydrogel network were eluted and the hydrogel was regenerated for reuse. After five adsorption/desorption reuse cycles, a removal ratio greater than 60% was obtained [185]. By applying a similar approach, Pourjavadi et al. developed a novel hydrogel containing chitosan, acrylic acid, and an amine-functionalized nano-silica. In this work, 1M hydrochloric acid solution was employed for recovering hydrogel loaded with Pb2+. The hydrogel was then regenerated by filtering and washing with deionized water before being utilized for the next adsorption cycle. After three consecutive cycles, the efficiency of regenerated hydrogel remained around 685–715 mg/g [186].
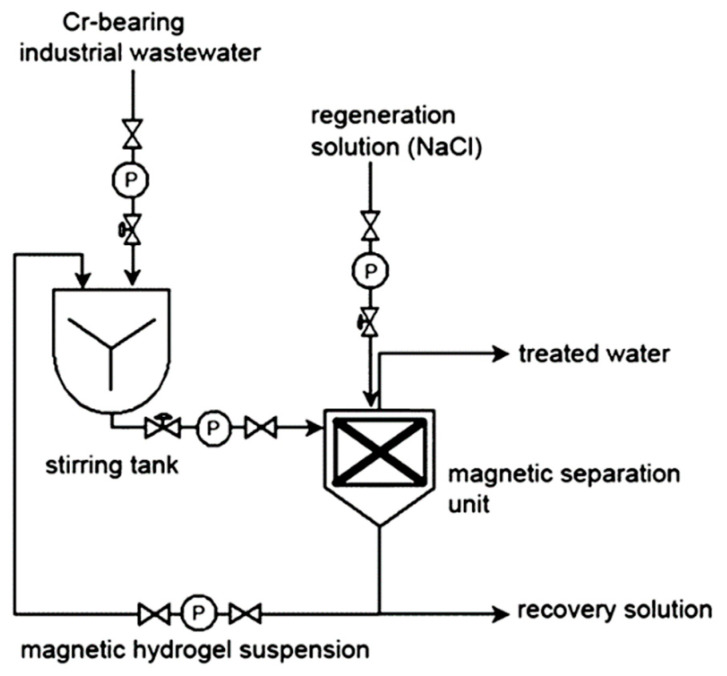
Magnetic hydrogels are one of the most used adsorbent materials for the effective elimination of heavy metals from flowing streams. During the recovery process, the eluent acidity needs to be managed since an excess of acid can damage the magnetic adsorbent. Eluents with high concentrations can damage the binding sites on hydrogels, resulting in lowered adsorption efficiency after numerous sorption cycles [187]. Tang et al. synthesized a magnetic hydrogel with high adsorption efficiency of 200 mg/g for Cr6+ adsorption. The hydrogel possesses an advantage of easy recovery by regenerating in sodium chloride solution (NaCl) [188]. The applicability of any magnetic hydrogel adsorbent in contaminated water is determined by two important factors: an increase in the concentration of heavy metals in that solution and a lower quantity of recovery solution. Tang’s research summarized both the factors in Figure 17. In brief, the treated contaminated water is collected and separated from hydrogel in a magnetic separation unit; NaCl at different concentration is injected for the regeneration of hydrogel, and the leftover solution were collected by separating the magnetic hydrogel. A series of regeneration tests were carried out by step-wise addition of sodium chloride solution. The recovery solution was then collected and further processed by the addition of NaCl solution to it. The results obtained suggested recovery efficiency was maintained for 20 sorption cycles, resulting in the Cr6+ removal capacity of 97–98%. According to the results achieved, the Cr content in the recovery solution reached 500–600 mg/L corresponding to wastewater:recovery volume ratio of 40:1 [188].
Figure 17.
Schematic representation of a wastewater treatment experiment with a magnetic separation unit. (Reprinted with permission from Ref. [188]. Copyright 2022 Americal Chemical Society).
Reusability of hydrogel is one of the most important characteristics for wide-range applications, although it is a challenge for conventional hydrogel adsorbents as they possess poor mechanical strength after swelling in aqueous media. Therefore, increasing mechanical strength plays a crucial role in maintaining the desired adsorption efficiency of the heavy metals-loaded hydrogel. Liu et al. reported that 95% Fe, Pb, and Cu were removed from an aqueous medium through 7 adsorption cycles and hydrogel still can lead to heavy metals removal [108]. Therefore, it proves that hydrogel can be reused many times and lowers the cost of production for heavy metals elimination from an aqueous solution. Tang et al. reported the reusability and regeneration of hydrogel in a column experiment for effective elimination of Cr6+ by a cationic hydrogel. After 6 sorption cycles adsorption efficiency remained constant (27 mg g−1, 90%) and the desorption capacity was 93 percent on average for every cycle [189]. In conclusion, it can be said that low operational and production-cost along with easy separation capabilities and reusability make hydrogel a choice of adsorbent for heavy metal removal from wastewater.
11. Conclusions
Water pollution is one of the serious global problems caused by increasing industrialization and urbanization. In particular, heavy metals discharged into flowing streams have detrimental effects on human health and the natural ecological system. Thus, it is necessary to treat the wastewater containing toxic heavy metals and then discharge it. The adsorption process including various types of adsorbent material is regarded as an efficient, cost-effective, and environment-friendly approach to the treatment of heavy metals. However, the majority of adsorbent materials used for wastewater treatment are non-biodegradable, synthetic, and require post-treatment after use, which prompts researchers’ interest in developing biodegradable, easy to modify, and biocompatible adsorbent materials. Hydrogels as potential adsorbent materials represent the best choice. The present review summarizes the literature concerning hydrogels in the past 25 years, and describes the classification, properties, synthesis, mechanism and recovery, regeneration, and reuse of hydrogel-based material for the elimination of heavy metals.
Although hydrogels have been extensively studied, there are still a few areas that require further investigation.
Currently, the hydrogel-based adsorbent materials used for heavy metal removal are limited to lab scale. Therefore, further research is required to scale up for a large-scale application.
The present research is confined to removing a single type of heavy metal. More research should be undertaken targeting multiple heavy metals.
The research should focus on the ability of the hydrogel to regenerate (for example, the adsorption efficiency of hydrogel drops after five sorption cycles).
To broaden the spectrum of hydrogels application for separation of rare earth metals.
To develop high mechanical strength tailored hydrogel (for example hydrogel membranes) that are easier to separate from the liquid phase for wastewater treatment.
Author Contributions
Conceptualization, Z.D., S.S. and R.G.; methodology, Z.D. and S.S.; software, Z.D., S.S. and R.G.; validation, Z.D. and S.S.; formal analysis, Z.D.; investigation, Z.D., S.S., R.G. and N.S.; resources, S.S., N.S. and I.A.; writing—original draft preparation, Z.D. and S.S.; writing—review and editing, Z.D., S.S., R.G., I.A. and N.S.; supervision, S.S. and R.G.; funding acquisition, N.S., I.A. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Pandit Deendayal Energy University for providing research facilities. This research was supported by the Marine Pollution Special Interest Group, National Defence University of Malaysia via SF0076-UPNM/2019/SF/ICT/6 and Scientific Research Deanship at King Khalid University, Abha, Saudi Arabia through the Large Research Group Project under grant number (RGP.02-205-42).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kabir S., Cueto R., Balamurugan S., Romeo L.D., Kuttruff J.T., Marx B.D., Negulescu I.I. Removal of acid dyes from textile wastewaters using fish scales by absorption process. Clean Technol. 2019;1:311–324. doi: 10.3390/cleantechnol1010021. [DOI] [Google Scholar]
- 2.Hasanpour M., Hatami M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020;309:113094. doi: 10.1016/j.molliq.2020.113094. [DOI] [Google Scholar]
- 3.Hasanpour M., Hatami M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020;284:102247. doi: 10.1016/j.cis.2020.102247. [DOI] [PubMed] [Google Scholar]
- 4.Siti N., Mohd H., Md L.K., Shamsul I. Adsorption process of heavy metals by low-cost adsorbent: A review. World Appl. Sci. J. 2013;28:1518–1530. [Google Scholar]
- 5.Saha J., Dikshit A., Bandyopadhyay M., Saha K. A review of arsenic poisoning and its effects on human health. Crit. Rev. Environ. Sci. Technol. 1999;29:281–313. doi: 10.1080/10643389991259227. [DOI] [Google Scholar]
- 6.Zhou Y., Hu X., Zhang M., Zhuo X., Niu J. Preparation and characterization of modified cellulose for adsorption of Cd (II), Hg (II), and acid fuchsin from aqueous solutions. Ind. Eng. Chem. Res. 2013;52:876–884. doi: 10.1021/ie301742h. [DOI] [Google Scholar]
- 7.Masindi V., Muedi K.L. Environmental contamination by heavy metals. Heavy Met. 2018;10:115–132. [Google Scholar]
- 8.Soleimani M., Amini N., Sadeghian B., Wang D., Fang L. Heavy metals and their source identification in particulate matter (PM2.5) in Isfahan City, Iran. J. Environ. Sci. 2018;72:166–175. doi: 10.1016/j.jes.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Briffa J., Sinagra E., Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6:e04691. doi: 10.1016/j.heliyon.2020.e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Raouf M., Abdul-Raheim A. Removal of Heavy Metals from Industrial Waste Water by Biomass-Based Materials: A Review. J. Pollut. Eff. Control. 2017;5:1000180. [Google Scholar]
- 11.Saxena G., Purchase D., Mulla S.I., Saratale G.D., Bharagava R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2019;249:71–131. doi: 10.1007/398_2019_24. [DOI] [PubMed] [Google Scholar]
- 12.Ali R.M., Hamad H.A., Hussein M.M., Malash G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016;91:317–332. doi: 10.1016/j.ecoleng.2016.03.015. [DOI] [Google Scholar]
- 13.Mthombeni N.H., Mbakop1and S., Onyango M.S. Adsorptive removal of manganese from industrial and mining wastewater; Proceedings of the Sustainable Research and Innovation Conference; Nairobi, Kenya. 4 May 2016; Nairobi, Kenya: College of Engineering and Technology Jomo Kenyatta University of Agriculture and Technology; 2016. pp. 36–45. [Google Scholar]
- 14.Yadav A. Bioremediation of wastewater using various sorbents and vegetable enzymes. Res. Biotechnol. 2015;6:1–15. [Google Scholar]
- 15.Ahmad M., Ahmed S., Swami B.L., Ikram S. Adsorption of heavy metal ions: Role of chitosan and cellulose for water treatment. Langmuir. 2015;79:109–155. [Google Scholar]
- 16.Azimi A., Azari A., Rezakazemi M., Ansarpour M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017;4:37–59. doi: 10.1002/cben.201600010. [DOI] [Google Scholar]
- 17.Rudi N.N., Muhamad M.S., Te Chuan L., Alipal J., Omar S., Hamidon N., Hamid N.H.A., Sunar N.M., Ali R., Harun H. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon. 2020;6:e05049. doi: 10.1016/j.heliyon.2020.e05049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Shafiq M., Alazba A., Amin M. Removal of heavy metals from wastewater using date palm as a biosorbent: A comparative review. Sains Malays. 2018;47:35–49. [Google Scholar]
- 19.Sun C., Chen T., Huang Q., Wang J., Lu S., Yan J. Enhanced adsorption for Pb (II) and Cd (II) of magnetic rice husk biochar by KMnO4 modification. Environ. Sci. Pollut. Res. 2019;26:8902–8913. doi: 10.1007/s11356-019-04321-z. [DOI] [PubMed] [Google Scholar]
- 20.Nuhanović M., Grebo M., Draganović S., Memić M., Smječanin N. Uranium (VI) biosorption by sugar beet pulp: Equilibrium, kinetic and thermodynamic studies. J. Radioanal. Nucl. Chem. 2019;322:2065–2078. doi: 10.1007/s10967-019-06877-z. [DOI] [Google Scholar]
- 21.George R., Bahadur N., Singh N., Singh R., Verma A., Shukla A. Environmentally benign TiO2 nanomaterials for removal of heavy metal ions with interfering ions present in tap water. Mater. Today Proc. 2016;3:162–166. doi: 10.1016/j.matpr.2016.01.051. [DOI] [Google Scholar]
- 22.Manjuladevi M., Sri O. Heavy metals removal from industrial wastewater by nano adsorbent prepared from cucumis melopeel activated carbon. J. Nanomed. Res. 2017;5:1–4. [Google Scholar]
- 23.Es-Sahbany H., Berradi M., Nkhili S., Hsissou R., Allaoui M., Loutfi M., Bassir D., Belfaquir M., El Youbi M. Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. Mater. Today Proc. 2019;13:866–875. doi: 10.1016/j.matpr.2019.04.050. [DOI] [Google Scholar]
- 24.Weerasundara L., Gabriele B., Figoli A., Ok Y.-S., Bundschuh J. Hydrogels: Novel materials for contaminant removal in water—A review. Crit. Rev. Environ. Sci. Technol. 2020;51:1–45. doi: 10.1080/10643389.2020.1776055. [DOI] [Google Scholar]
- 25.Pan Z., An L. Applications of Ion Exchange Materials in the Environment. Springer; Berlin, Germany: 2019. Removal of heavy metal from wastewater using ion exchange membranes; pp. 25–46. [Google Scholar]
- 26.Liu L., Luo X.-B., Ding L., Luo S.-L. Nanomaterials for the Removal of Pollutants and Resource Reutilization. Elsevier; Oxford, UK: 2019. Application of nanotechnology in the removal of heavy metal from water; pp. 83–147. [Google Scholar]
- 27.Teh C.Y., Budiman P.M., Shak K.P.Y., Wu T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016;55:4363–4389. doi: 10.1021/acs.iecr.5b04703. [DOI] [Google Scholar]
- 28.Medina B., Torem M., De Mesquita L. On the kinetics of precipitate flotation of Cr III using sodium dodecylsulfate and ethanol. Miner. Eng. 2005;18:225–231. doi: 10.1016/j.mineng.2004.08.018. [DOI] [Google Scholar]
- 29.Fu F., Wang Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011;92:407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee R., Bhunia P., De S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016;292:284–297. doi: 10.1016/j.cej.2016.02.015. [DOI] [Google Scholar]
- 31.Van Bemmelen J. Das hydrogel und das krystallinische hydrat des kupferoxyds. Z. Anorg. Chem. 1894;5:466–483. doi: 10.1002/zaac.18940050156. [DOI] [Google Scholar]
- 32.Thakur S., Thakur V.K., Arotiba O.A. Hydrogels. Springer; Singapore: 2018. History, classification, properties and application of hydrogels: An overview; pp. 29–50. [Google Scholar]
- 33.Wichterle O., Lim D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. doi: 10.1038/185117a0. [DOI] [Google Scholar]
- 34.Shalla A.H., Yaseen Z., Bhat M.A., Rangreez T.A., Maswal M. Recent review for removal of metal ions by hydrogels. Sep. Sci. Technol. 2019;54:89–100. doi: 10.1080/01496395.2018.1503307. [DOI] [Google Scholar]
- 35.Dai L., Cheng T., Xi X., Nie S., Ke H., Liu Y., Tong S., Chen Z. A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose. 2020;27:915–925. doi: 10.1007/s10570-019-02834-x. [DOI] [Google Scholar]
- 36.Jang S.H., Jeong Y.G., Min B.G., Lyoo W.S., Lee S.C. Preparation and lead ion removal property of hydroxyapatite/polyacrylamide composite hydrogels. J. Hazard. Mater. 2008;159:294–299. doi: 10.1016/j.jhazmat.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Ozay O., Ekici S., Baran Y., Kubilay S., Aktas N., Sahiner N. Utilization of magnetic hydrogels in the separation of toxic metal ions from aqueous environments. Desalination. 2010;260:57–64. doi: 10.1016/j.desal.2010.04.067. [DOI] [Google Scholar]
- 38.Barakat M., Sahiner N. Cationic hydrogels for toxic arsenate removal from aqueous environment. J. Environ. Manag. 2008;88:955–961. doi: 10.1016/j.jenvman.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Ozay O., Ekici S., Baran Y., Aktas N., Sahiner N. Removal of toxic metal ions with magnetic hydrogels. Water Res. 2009;43:4403–4411. doi: 10.1016/j.watres.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 40.Hernández R., Mijangos C. In situ Synthesis of Magnetic Iron Oxide Nanoparticles in Thermally Responsive Alginate-Poly (N-isopropylacrylamide) Semi-Interpenetrating Polymer Networks. Macromol. Rapid Commun. 2009;30:176–181. doi: 10.1002/marc.200800602. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee K.Y., Rowley J.A., Eiselt P., Moy E.M., Bouhadir K.H., Mooney D.J. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules. 2000;33:4291–4294. doi: 10.1021/ma9921347. [DOI] [Google Scholar]
- 43.Sahiner N. Hydrogels of versatile size and architecture for effective environmental applications. Turk. J. Chem. 2008;32:113–123. [Google Scholar]
- 44.Singh A., Sharma P.K., Garg V.K., Garg G. Hydrogels: A review. Int. J. Pharm. Sci. Rev. Res. 2010;4:97–105. [Google Scholar]
- 45.Mahinroosta M., Farsangi Z.J., Allahverdi A., Shakoori Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018;8:42–55. doi: 10.1016/j.mtchem.2018.02.004. [DOI] [Google Scholar]
- 46.Ozay O., Aktas N., Sahiner N. Hydrogels as a potential chromatographic system: Absorption, speciation, and separation of chromium species from aqueous media. Sep. Sci. Technol. 2011;46:1450–1461. doi: 10.1080/01496395.2011.560918. [DOI] [Google Scholar]
- 47.Bashir S., Hina M., Iqbal J., Rajpar A., Mujtaba M., Alghamdi N., Wageh S., Ramesh K., Ramesh S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers. 2020;12:2702. doi: 10.3390/polym12112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu X., Cheng W., Nie W., Shao Z. Synthesis and characterization of a temperature-sensitive hydrogel based on sodium alginate and N-isopropylacrylamide. Polym. Adv. Technol. 2015;26:1340–1345. doi: 10.1002/pat.3682. [DOI] [Google Scholar]
- 49.De S.K., Aluru N.R. A chemo-electro-mechanical mathematical model for simulation of pH sensitive hydrogels. Mech. Mater. 2004;36:395–410. doi: 10.1016/S0167-6636(03)00067-X. [DOI] [Google Scholar]
- 50.Mu R., Liu B., Chen X., Wang N., Yang J. Hydrogel adsorbent in industrial wastewater treatment and ecological environment protection. Environ. Technol. Innov. 2020;20:101107. doi: 10.1016/j.eti.2020.101107. [DOI] [Google Scholar]
- 51.Kuddushi M., Rajput S., Shah A., Mata J., Aswal V.K., El Seoud O., Kumar A., Malek N.I. Stimuli responsive, self-sustainable, and self-healable functionalized hydrogel with dual gelation, load-bearing, and dye-absorbing properties. ACS Appl. Mater. Interfaces. 2019;11:19572–19583. doi: 10.1021/acsami.9b01129. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Y., Wang Y., Li Q., Yu C., Chu W. Natural polymer-based stimuli-responsive hydrogels. Curr. Med. Chem. 2020;27:2631–2657. doi: 10.2174/0929867326666191122144916. [DOI] [PubMed] [Google Scholar]
- 53.Rittikulsittichai S., Kolhatkar A.G., Sarangi S., Vorontsova M.A., Vekilov P.G., Brazdeikis A., Lee T.R. Multi-responsive hybrid particles: Thermo-, pH-, photo-, and magneto-responsive magnetic hydrogel cores with gold nanorod optical triggers. Nanoscale. 2016;8:11851–11861. doi: 10.1039/C5NR09235C. [DOI] [PubMed] [Google Scholar]
- 54.Ullah F., Othman M.B.H., Javed F., Ahmad Z., Akil H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C. 2015;57:414–433. doi: 10.1016/j.msec.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 55.Jing G., Wang L., Yu H., Amer W.A., Zhang L. Recent progress on study of hybrid hydrogels for water treatment. Colloids Surf. A Physicochem. Eng. Asp. 2013;416:86–94. doi: 10.1016/j.colsurfa.2012.09.043. [DOI] [Google Scholar]
- 56.Zhang J., Wang A. Adsorption of Pb (II) from aqueous solution by chitosan-g-poly (acrylic acid)/attapulgite/sodium humate composite hydrogels. J. Chem. Eng. Data. 2010;55:2379–2384. doi: 10.1021/je900813z. [DOI] [Google Scholar]
- 57.Iizawa T., Taketa H., Maruta M., Ishido T., Gotoh T., Sakohara S. Synthesis of porous poly (N-isopropylacrylamide) gel beads by sedimentation polymerization and their morphology. J. Appl. Polym. Sci. 2007;104:842–850. doi: 10.1002/app.25605. [DOI] [Google Scholar]
- 58.Garg S., Garg A., Vishwavidyalaya R. Hydrogel: Classification, properties, preparation and technical features. Asian J. Biomater. Res. 2016;2:163–170. [Google Scholar]
- 59.Yang L., Chu J.S., Fix J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002;235:1–15. doi: 10.1016/S0378-5173(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 60.Kim M.K., Sundaram K.S., Iyengar G.A., Lee K.-P. A novel chitosan functional gel included with multiwall carbon nanotube and substituted polyaniline as adsorbent for efficient removal of chromium ion. Chem. Eng. J. 2015;267:51–64. doi: 10.1016/j.cej.2014.12.091. [DOI] [Google Scholar]
- 61.Singhal R., Gupta K. A review: Tailor-made hydrogel structures (classifications and synthesis parameters) Polym. Plast. Technol. Eng. 2016;55:54–70. doi: 10.1080/03602559.2015.1050520. [DOI] [Google Scholar]
- 62.Kabiri K., Omidian H., Zohuriaan-Mehr M., Doroudiani S. Superabsorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2011;32:277–289. doi: 10.1002/pc.21046. [DOI] [Google Scholar]
- 63.Badsha M.A., Khan M., Wu B., Kumar A., Lo I.M. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021;408:124463. doi: 10.1016/j.jhazmat.2020.124463. [DOI] [PubMed] [Google Scholar]
- 64.Badsha M.A., Lo I.M. An innovative pH-independent magnetically separable hydrogel for the removal of Cu (II) and Ni (II) ions from electroplating wastewater. J. Hazard. Mater. 2020;381:121000. doi: 10.1016/j.jhazmat.2019.121000. [DOI] [PubMed] [Google Scholar]
- 65.He X., Cheng L., Wang Y., Zhao J., Zhang W., Lu C. Aerogels from quaternary ammonium-functionalized cellulose nanofibers for rapid removal of Cr (VI) from water. Carbohydr. Polym. 2014;111:683–687. doi: 10.1016/j.carbpol.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 66.Cyganowski P., Dzimitrowicz A., Jamroz P., Jermakowicz-Bartkowiak D., Pohl P. Polymerization-driven immobilization of dc-apgd synthesized gold nanoparticles into a quaternary ammonium-based hydrogel resulting in a polymeric nanocomposite with heat-transfer applications. Polymers. 2018;10:377. doi: 10.3390/polym10040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He G., Ke W., Chen X., Kong Y., Zheng H., Yin Y., Cai W. Preparation and properties of quaternary ammonium chitosan-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017;111:14–21. doi: 10.1016/j.reactfunctpolym.2016.12.001. [DOI] [Google Scholar]
- 68.Odio O.F., Lartundo-Rojas L., Palacios E.G., Martínez R., Reguera E. Synthesis of a novel poly-thiolated magnetic nano-platform for heavy metal adsorption. Role of thiol and carboxyl functions. Appl. Surf. Sci. 2016;386:160–177. doi: 10.1016/j.apsusc.2016.05.176. [DOI] [Google Scholar]
- 69.Saad D.M., Cukrowska E.M., Tutu H. Selective removal of mercury from aqueous solutions using thiolated cross-linked polyethylenimine. Appl. Water Sci. 2013;3:527–534. doi: 10.1007/s13201-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 70.Mohammadnia E., Hadavifar M., Veisi H. Kinetics and thermodynamics of mercury adsorption onto thiolated graphene oxide nanoparticles. Polyhedron. 2019;173:114139. doi: 10.1016/j.poly.2019.114139. [DOI] [Google Scholar]
- 71.Kumar A.S.K., Jiang S.-J., Tseng W.-L. Facile synthesis and characterization of thiol-functionalized graphene oxide as effective adsorbent for Hg (II) J. Environ. Chem. Eng. 2016;4:2052–2065. doi: 10.1016/j.jece.2016.03.034. [DOI] [Google Scholar]
- 72.El-Hag Ali A. Removal of heavy metals from model wastewater by using carboxymehyl cellulose/2-acrylamido-2-methyl propane sulfonic acid hydrogels. J. Appl. Polym. Sci. 2012;123:763–769. doi: 10.1002/app.34470. [DOI] [Google Scholar]
- 73.Lu W., Dai Z., Li L., Liu J., Wang S., Yang H., Cao C., Liu L., Chen T., Zhu B. Preparation of composite hydrogel (PCG) and its adsorption performance for uranium (VI) J. Mol. Liq. 2020;303:112604. doi: 10.1016/j.molliq.2020.112604. [DOI] [Google Scholar]
- 74.Bai J., Chu J., Yin X., Wang J., Tian W., Huang Q., Jia Z., Wu X., Guo H., Qin Z. Synthesis of amidoximated polyacrylonitrile nanoparticle/graphene composite hydrogel for selective uranium sorption from saline lake brine. Chem. Eng. J. 2020;391:123553. doi: 10.1016/j.cej.2019.123553. [DOI] [Google Scholar]
- 75.Hamza M.F., Roux J.-C., Guibal E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem. Eng. J. 2018;344:124–137. doi: 10.1016/j.cej.2018.03.029. [DOI] [Google Scholar]
- 76.Zeng L., Liu Q., Xu W., Wang G., Xu Y., Liang E. Graft copolymerization of crosslinked polyvinyl alcohol with acrylonitrile and its amidoxime modification as a heavy metal ion adsorbent. J. Polym. Environ. 2020;28:116–122. doi: 10.1007/s10924-019-01590-0. [DOI] [Google Scholar]
- 77.Liao Y., Wang M., Chen D. Electrosorption of uranium (VI) by highly porous phosphate-functionalized graphene hydrogel. Appl. Surf. Sci. 2019;484:83–96. doi: 10.1016/j.apsusc.2019.04.103. [DOI] [Google Scholar]
- 78.Flora G., Mittal M., Flora S.J. Handbook of Arsenic Toxicology. Elsevier; Oxford, UK: 2015. Medical countermeasures—Chelation therapy; pp. 589–626. [Google Scholar]
- 79.Chang Y.-C., Chen D.-H. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu (II) ions. J. Colloid Interface Sci. 2005;283:446–451. doi: 10.1016/j.jcis.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Huang S.-H., Chen D.-H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009;163:174–179. doi: 10.1016/j.jhazmat.2008.06.075. [DOI] [PubMed] [Google Scholar]
- 81.Repo E., Warchoł J.K., Bhatnagar A., Mudhoo A., Sillanpää M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013;47:4812–4832. doi: 10.1016/j.watres.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 82.Atzei D., Ferri T., Sadun C., Sangiorgio P., Caminiti R. Structural characterization of complexes between iminodiacetate blocked on Styrene− Divinylbenzene matrix (Chelex 100 Resin) and Fe (III), Cr (III), and Zn (II) in Solid Phase by Energy-Dispersive X-ray Diffraction. J. Am. Chem. Soc. 2001;123:2552–2558. doi: 10.1021/ja0003728. [DOI] [PubMed] [Google Scholar]
- 83.Huang Y., Wu H., Shao T., Zhao X., Peng H., Gong Y., Wan H. Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chem. Eng. J. 2018;339:322–333. doi: 10.1016/j.cej.2018.01.071. [DOI] [Google Scholar]
- 84.Medina R.P., Nadres E.T., Ballesteros F.C., Rodrigues D.F. Incorporation of graphene oxide into a chitosan–poly (acrylic acid) porous polymer nanocomposite for enhanced lead adsorption. Environ. Sci. Nano. 2016;3:638–646. doi: 10.1039/C6EN00021E. [DOI] [Google Scholar]
- 85.Zhang Y., Li Z. Heavy metals removal using hydrogel-supported nanosized hydrous ferric oxide: Synthesis, characterization, and mechanism. Sci. Total Environ. 2017;580:776–786. doi: 10.1016/j.scitotenv.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 86.Tian R., Liu Q., Zhang W., Zhang Y. Preparation of lignin-based hydrogel and its adsorption on Cu2+ ions and Co2+ ions in wastewaters. J. Inorg. Organomet. Polym. Mater. 2018;28:2545–2553. doi: 10.1007/s10904-018-0943-3. [DOI] [Google Scholar]
- 87.Wu B., Yan D.Y., Khan M., Zhang Z., Lo I.M. Application of magnetic hydrogel for anionic pollutants removal from wastewater with adsorbent regeneration and reuse. J. Hazard. Toxic Radioact. Waste. 2017;21:04016008. doi: 10.1061/(ASCE)HZ.2153-5515.0000325. [DOI] [Google Scholar]
- 88.Yetimoğlu E.K., Kahraman M.V., Bayramoğlu G., Ercan Ö., Apohan N.K. Sulfathiazole-based novel UV-cured hydrogel sorbents for mercury removal from aqueous solutions. Radiat. Phys. Chem. 2009;78:92–97. doi: 10.1016/j.radphyschem.2008.08.011. [DOI] [Google Scholar]
- 89.Saraydın D., Yıldırım E.Ş., Karadağ E., Güven O. Radiation-Synthesized Acrylamide/Crotonic Acid Hydrogels for Selective Mercury (II) Ion Adsorption. Adv. Polym. Technol. 2018;37:822–829. doi: 10.1002/adv.21725. [DOI] [Google Scholar]
- 90.Jiang C., Wang X., Wang G., Hao C., Li X., Li T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos. Part B Eng. 2019;169:45–54. doi: 10.1016/j.compositesb.2019.03.082. [DOI] [Google Scholar]
- 91.Zhang Y., Lin S., Qiao J., Kołodyńska D., Ju Y., Zhang M., Cai M., Deng D., Dionysiou D.D. Malic acid-enhanced chitosan hydrogel beads (mCHBs) for the removal of Cr (VI) and Cu (II) from aqueous solution. Chem. Eng. J. 2018;353:225–236. doi: 10.1016/j.cej.2018.06.143. [DOI] [Google Scholar]
- 92.Godiya C.B., Cheng X., Li D., Chen Z., Lu X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019;364:28–38. doi: 10.1016/j.jhazmat.2018.09.076. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Y., Zheng Y., Wang F., Wang A. Monolithic supermacroporous hydrogel prepared from high internal phase emulsions (HIPEs) for fast removal of Cu2+ and Pb2+ Chem. Eng. J. 2016;284:422–430. doi: 10.1016/j.cej.2015.08.157. [DOI] [Google Scholar]
- 94.Li F., Wang X., Yuan T., Sun R. A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb (II) removal. J. Mater. Chem. A. 2016;4:11888–11896. doi: 10.1039/C6TA03779H. [DOI] [Google Scholar]
- 95.Milani P., França D., Balieiro A.G., Faez R. Polymers and its applications in agriculture. Polímeros. 2017;27:256–266. doi: 10.1590/0104-1428.09316. [DOI] [Google Scholar]
- 96.Kirillova A., Ionov L. Shape-changing polymers for biomedical applications. J. Mater. Chem. B. 2019;7:1597–1624. doi: 10.1039/C8TB02579G. [DOI] [PubMed] [Google Scholar]
- 97.Nasir A., Masood F., Yasin T., Hameed A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 2019;79:29–40. doi: 10.1016/j.jiec.2019.06.052. [DOI] [Google Scholar]
- 98.Mangaraj S., Yadav A., Bal L.M., Dash S., Mahanti N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019;3:77–96. doi: 10.1007/s41783-018-0049-y. [DOI] [Google Scholar]
- 99.Thakur S., Sharma B., Verma A., Chaudhary J., Tamulevicius S., Thakur V.K. Recent approaches in guar gum hydrogel synthesis for water purification. Int. J. Polym. Anal. Charact. 2018;23:621–632. doi: 10.1080/1023666X.2018.1488661. [DOI] [Google Scholar]
- 100.Malik D., Jain C., Yadav A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017;7:2113–2136. doi: 10.1007/s13201-016-0401-8. [DOI] [Google Scholar]
- 101.Sanyang M., Ghani W.A.W.A.K., Idris A., Ahmad M.B. Hydrogel biochar composite for arsenic removal from wastewater. Desalination Water Treat. 2016;57:3674–3688. doi: 10.1080/19443994.2014.989412. [DOI] [Google Scholar]
- 102.Yan E., Cao M., Ren X., Jiang J., An Q., Zhang Z., Gao J., Yang X., Zhang D. Synthesis of Fe3O4 nanoparticles functionalized polyvinyl alcohol/chitosan magnetic composite hydrogel as an efficient adsorbent for chromium (VI) removal. J. Phys. Chem. Solids. 2018;121:102–109. doi: 10.1016/j.jpcs.2018.05.028. [DOI] [Google Scholar]
- 103.Ali A.E.-H., Shawky H., Abd El Rehim H., Hegazy E. Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur. Polym. J. 2003;39:2337–2344. [Google Scholar]
- 104.Pettinelli N., Rodríguez-Llamazares S., Abella V., Barral L., Bouza R., Farrag Y., Lago F. Entrapment of chitosan, pectin or κ-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mater. Sci. Eng. C. 2019;96:583–590. doi: 10.1016/j.msec.2018.11.071. [DOI] [PubMed] [Google Scholar]
- 105.Chern J.M., Lee W.F., Hsieh M.Y. Preparation and swelling characterization of poly (n-isopropylacrylamide)-based porous hydrogels. J. Appl. Polym. Sci. 2004;92:3651–3658. doi: 10.1002/app.20301. [DOI] [Google Scholar]
- 106.Ozay O., Ekici S., Aktas N., Sahiner N. P (4-vinyl pyridine) hydrogel use for the removal of UO22+ and Th4+ from aqueous environments. J. Environ. Manag. 2011;92:3121–3129. doi: 10.1016/j.jenvman.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Sun X., Peng B., Ji Y., Chen J., Li D. Chitosan (chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J. 2009;55:2062–2069. doi: 10.1002/aic.11797. [DOI] [Google Scholar]
- 108.Liu Z., Wang H., Liu C., Jiang Y., Yu G., Mu X., Wang X. Magnetic cellulose–chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem. Commun. 2012;48:7350–7352. doi: 10.1039/c2cc17795a. [DOI] [PubMed] [Google Scholar]
- 109.Yan H., Row K.H. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006;7:155–178. doi: 10.3390/i7050155. [DOI] [Google Scholar]
- 110.Haraguchi K., Li H.-J., Matsuda K., Takehisa T., Elliott E. Mechanism of forming organic/inorganic network structures during in-situ free-radical polymerization in PNIPA− clay nanocomposite hydrogels. Macromolecules. 2005;38:3482–3490. doi: 10.1021/ma047431c. [DOI] [Google Scholar]
- 111.Fu J., Chen L., Li J., Zhang Z. Current status and challenges of ion imprinting. J. Mater. Chem. A. 2015;3:13598–13627. doi: 10.1039/C5TA02421H. [DOI] [Google Scholar]
- 112.Pakdel P.M., Peighambardoust S.J. A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 2018;217:123–143. doi: 10.1016/j.jenvman.2018.03.076. [DOI] [PubMed] [Google Scholar]
- 113.Shah L.A., Khan M., Javed R., Sayed M., Khan M.S., Khan A., Ullah M. Superabsorbent polymer hydrogels with good thermal and mechanical properties for removal of selected heavy metal ions. J. Clean. Prod. 2018;201:78–87. doi: 10.1016/j.jclepro.2018.08.035. [DOI] [Google Scholar]
- 114.Kaşgöz H. New sorbent hydrogels for removal of acidic dyes and metal ions from aqueous solutions. Polym. Bull. 2006;56:517–528. doi: 10.1007/s00289-006-0515-5. [DOI] [Google Scholar]
- 115.Ma J., Zhou G., Chu L., Liu Y., Liu C., Luo S., Wei Y. Efficient removal of heavy metal ions with an EDTA functionalized chitosan/polyacrylamide double network hydrogel. ACS Sustain. Chem. Eng. 2017;5:843–851. doi: 10.1021/acssuschemeng.6b02181. [DOI] [Google Scholar]
- 116.Evren M., Acar I., Güçlü K., Güçlü G. Removal of Cu2+ and Pb2+ ions by N-vinyl 2-pyrrolidone/itaconic acid hydrogels from aqueous solutions. Can. J. Chem. Eng. 2014;92:52–59. doi: 10.1002/cjce.21814. [DOI] [Google Scholar]
- 117.Zhou G., Luo J., Liu C., Chu L., Ma J., Tang Y., Zeng Z., Luo S. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016;89:151–160. doi: 10.1016/j.watres.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 118.Lv Q., Hu X., Zhang X., Huang L., Liu Z., Sun G. Highly efficient removal of trace metal ions by using poly (acrylic acid) hydrogel adsorbent. Mater. Des. 2019;181:107934. doi: 10.1016/j.matdes.2019.107934. [DOI] [Google Scholar]
- 119.Chen Y.-W., Wang J.-L. Removal of cesium from radioactive wastewater using magnetic chitosan beads cross-linked with glutaraldehyde. Nucl. Sci. Tech. 2016;27:43. doi: 10.1007/s41365-016-0033-6. [DOI] [Google Scholar]
- 120.Mishra A., Nath A., Pande P.P., Shankar R. Treatment of gray wastewater and heavy metal removal from aqueous medium using hydrogels based on novel crosslinkers. J. Appl. Polym. Sci. 2021;138:50242. doi: 10.1002/app.50242. [DOI] [Google Scholar]
- 121.Dil N.N., Sadeghi M. Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu (II) metal ions. J. Hazard. Mater. 2018;351:38–53. doi: 10.1016/j.jhazmat.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 122.Fekete T., Borsa J., Takács E., Wojnárovits L. Synthesis of cellulose-based superabsorbent hydrogels by high-energy irradiation in the presence of crosslinking agent. Radiat. Phys. Chem. 2016;118:114–119. doi: 10.1016/j.radphyschem.2015.02.023. [DOI] [Google Scholar]
- 123.Khan M., Lo I.M. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016;106:259–271. doi: 10.1016/j.watres.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 124.Delbecq F., Kono F., Kawai T. Preparation of PVP–PVA–exfoliated graphite cross-linked composite hydrogels for the incorporation of small tin nanoparticles. Eur. Polym. J. 2013;49:2654–2659. doi: 10.1016/j.eurpolymj.2013.06.014. [DOI] [Google Scholar]
- 125.Yang J., Dong X., Gao Y., Zhang W. One-step synthesis of methacrylated POSS cross-linked poly (N-isopropylacrylamide) hydrogels by γ-irradiation. Mater. Lett. 2015;157:81–84. doi: 10.1016/j.matlet.2015.05.091. [DOI] [Google Scholar]
- 126.Abd El-Mohdy H. Water sorption behavior of CMC/PAM hydrogels prepared by γ-irradiation and release of potassium nitrate as agrochemical. React. Funct. Polym. 2007;67:1094–1102. doi: 10.1016/j.reactfunctpolym.2007.07.002. [DOI] [Google Scholar]
- 127.Gaharwar A.K., Rivera C., Wu C.-J., Chan B.K., Schmidt G. Photocrosslinked nanocomposite hydrogels from PEG and silica nanospheres: Structural, mechanical and cell adhesion characteristics. Mater. Sci. Eng. C. 2013;33:1800–1807. doi: 10.1016/j.msec.2012.12.099. [DOI] [PubMed] [Google Scholar]
- 128.Maitra J., Shukla V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014;4:25–31. [Google Scholar]
- 129.Maziad N., Mohsen M., Gomaa E., Mohammed R. Radiation copolymerization of hydrogels based in polyacrylic acid/polyvinyl alcohol applied in water treatment processes. J. Mater. Sci. Eng. A. 2015;5:381–390. [Google Scholar]
- 130.Tran T.H., Okabe H., Hidaka Y., Hara K. Removal of metal ions from aqueous solutions using carboxymethyl cellulose/sodium styrene sulfonate gels prepared by radiation grafting. Carbohydr. Polym. 2017;157:335–343. doi: 10.1016/j.carbpol.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 131.Aly A.A., El-Bisi M.K. Biopolym. Grafting. Elsevier; Amsterdam, The Netherlands: 2018. Grafting of polysaccharides: Recent advances; pp. 469–519. [DOI] [Google Scholar]
- 132.Qi X., Lin L., Shen L., Li Z., Qin T., Qian Y., Wu X., Wei X., Gong Q., Shen J. Efficient decontamination of lead ions from wastewater by salecan polysaccharide-based hydrogels. ACS Sustain. Chem. Eng. 2019;7:11014–11023. doi: 10.1021/acssuschemeng.9b02139. [DOI] [Google Scholar]
- 133.Tian Z., Liu W., Li G. The microstructure and stability of collagen hydrogel cross-linked by glutaraldehyde. Polym. Degrad. Stab. 2016;130:264–270. doi: 10.1016/j.polymdegradstab.2016.06.015. [DOI] [Google Scholar]
- 134.Gennen S., Grignard B., Thomassin J.-M., Gilbert B., Vertruyen B., Jerome C., Detrembleur C. Polyhydroxyurethane hydrogels: Synthesis and characterizations. Eur. Polym. J. 2016;84:849–862. doi: 10.1016/j.eurpolymj.2016.07.013. [DOI] [Google Scholar]
- 135.Kassem I., Kassab Z., Khouloud M., Sehaqui H., Bouhfid R., Jacquemin J., El Achaby M. Phosphoric acid-mediated green preparation of regenerated cellulose spheres and their use for all-cellulose cross-linked superabsorbent hydrogels. Int. J. Biol. Macromol. 2020;162:136–149. doi: 10.1016/j.ijbiomac.2020.06.136. [DOI] [PubMed] [Google Scholar]
- 136.Gong Z., Zhang G., Zeng X., Li J., Li G., Huang W., Sun R., Wong C. High-strength, tough, fatigue resistant, and self-healing hydrogel based on dual physically cross-linked network. ACS Appl. Mater. Interfaces. 2016;8:24030–24037. doi: 10.1021/acsami.6b05627. [DOI] [PubMed] [Google Scholar]
- 137.Akhtar M.F., Hanif M., Ranjha N.M. Methods of synthesis of hydrogels…A review. Saudi Pharm. J. 2016;24:554–559. doi: 10.1016/j.jsps.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang H., Zhang F., Wu J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013;73:923–928. doi: 10.1016/j.reactfunctpolym.2012.12.014. [DOI] [Google Scholar]
- 139.Wang L.-Y., Wang M.-J. Removal of heavy metal ions by poly (vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze–thaw method. ACS Sustain. Chem. Eng. 2016;4:2830–2837. doi: 10.1021/acssuschemeng.6b00336. [DOI] [Google Scholar]
- 140.Guan Y., Bian J., Peng F., Zhang X.-M., Sun R.-C. High strength of hemicelluloses based hydrogels by freeze/thaw technique. Carbohydr. Polym. 2014;101:272–280. doi: 10.1016/j.carbpol.2013.08.085. [DOI] [PubMed] [Google Scholar]
- 141.Qi X., Hu X., Wei W., Yu H., Li J., Zhang J., Dong W. Investigation of Salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015;118:60–69. doi: 10.1016/j.carbpol.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 142.Yang Z., Liang G., Xu B. Enzymatic control of the self-assembly of small molecules: A new way to generate supramolecular hydrogels. Soft Matter. 2007;3:515–520. doi: 10.1039/b700138j. [DOI] [PubMed] [Google Scholar]
- 143.Cui H., Webber M.J., Stupp S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Pept. Sci. Orig. Res. Biomol. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rajbhandary A., Nilsson B.L. GELS HANDBOOK: Fundamentals, Properties and Applications Volume 1: Fundamentals of Hydrogels. World Scientific; Singapore: 2016. Self-Assembling Hydrogels; pp. 219–250. [Google Scholar]
- 145.Feng Y., Gong J.-L., Zeng G.-M., Niu Q.-Y., Zhang H.-Y., Niu C.-G., Deng J.-H., Yan M. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J. 2010;162:487–494. doi: 10.1016/j.cej.2010.05.049. [DOI] [Google Scholar]
- 146.Zhou Y., Fu S., Zhang L., Zhan H., Levit M.V. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb (II) Carbohydr. Polym. 2014;101:75–82. doi: 10.1016/j.carbpol.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 147.Pedroso-Santana S., Fleitas-Salazar N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020;69:443–447. doi: 10.1002/pi.5970. [DOI] [Google Scholar]
- 148.Guerrero R., Acibar C., Alarde C.M., Maslog J., Pacilan C.J. Evaluation of Pb (II) Removal from Water Using Sodium Alginate/Hydroxypropyl Cellulose Beads. E3S Web Conf. 2020;148:02002. doi: 10.1051/e3sconf/202014802002. [DOI] [Google Scholar]
- 149.Omidian H., Zohuriaan-Mehr M., Bouhendi H. Polymerization of sodium acrylate in inverse-suspension stabilized by sorbitan fatty esters. Eur. Polym. J. 2003;39:1013–1018. doi: 10.1016/S0014-3057(02)00352-X. [DOI] [Google Scholar]
- 150.Klinpituksa P., Kosaiyakanon P. Superabsorbent polymer based on sodium carboxymethyl cellulose grafted polyacrylic acid by inverse suspension polymerization. Int. J. Polym. Sci. 2017;2017:3476921. doi: 10.1155/2017/3476921. [DOI] [Google Scholar]
- 151.Tang S., Yang J., Lin L., Peng K., Chen Y., Jin S., Yao W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020;393:124728. doi: 10.1016/j.cej.2020.124728. [DOI] [Google Scholar]
- 152.Ablouh E.-H., Hanani Z., Eladlani N., Rhazi M., Taourirte M. Chitosan microspheres/sodium alginate hybrid beads: An efficient green adsorbent for heavy metals removal from aqueous solutions. Sustain. Environ. Res. 2019;29:5. doi: 10.1186/s42834-019-0004-9. [DOI] [Google Scholar]
- 153.Kong W., Chang M., Zhang C., Liu X., He B., Ren J. Preparation of Xylan-G-/P (AA-co-AM)/GO nanocomposite hydrogel and its adsorption for heavy metal ions. Polymers. 2019;11:621. doi: 10.3390/polym11040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mohamed R.R., Elella M.H.A., Sabaa M.W. Cytotoxicity and metal ions removal using antibacterial biodegradable hydrogels based on N-quaternized chitosan/poly (acrylic acid) Int. J. Biol. Macromol. 2017;98:302–313. doi: 10.1016/j.ijbiomac.2017.01.107. [DOI] [PubMed] [Google Scholar]
- 155.Javed R., Shah L.A., Sayed M., Khan M.S. Uptake of heavy metal ions from aqueous media by hydrogels and their conversion to nanoparticles for generation of a catalyst system: Two-fold application study. RSC Adv. 2018;8:14787–14797. doi: 10.1039/C8RA00578H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu Z.-H., Omer A.M., Ouyang X.K., Yu D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb (II) from aqueous solution. Int. J. Biol. Macromol. 2018;108:149–157. doi: 10.1016/j.ijbiomac.2017.11.171. [DOI] [PubMed] [Google Scholar]
- 157.Bandara P.C., Perez J.V.D., Nadres E.T., Nannapaneni R.G., Krakowiak K.J., Rodrigues D.F. Graphene oxide nanocomposite hydrogel beads for removal of selenium in contaminated water. ACS Appl. Polym. Mater. 2019;1:2668–2679. doi: 10.1021/acsapm.9b00612. [DOI] [Google Scholar]
- 158.Zeng Q., Qi X., Zhang M., Tong X., Jiang N., Pan W., Xiong W., Li Y., Xu J., Shen J. Efficient decontamination of heavy metals from aqueous solution using pullulan/polydopamine hydrogels. Int. J. Biol. Macromol. 2020;145:1049–1058. doi: 10.1016/j.ijbiomac.2019.09.197. [DOI] [PubMed] [Google Scholar]
- 159.Sinha V., Chakma S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019;7:103295. doi: 10.1016/j.jece.2019.103295. [DOI] [Google Scholar]
- 160.Maity S., Naskar N., Lahiri S., Ganguly J. Polysaccharide-derived hydrogel water filter for the rapid and selective removal of arsenic. Environ. Sci. Water Res. Technol. 2019;5:1318–1327. doi: 10.1039/C9EW00247B. [DOI] [Google Scholar]
- 161.Basri S.N., Zainuddin N., Hashim K., Yusof N.A. Preparation and characterization of irradiated carboxymethyl sago starch-acid hydrogel and its application as metal scavenger in aqueous solution. Carbohydr. Polym. 2016;138:34–40. doi: 10.1016/j.carbpol.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 162.Shen X., Shamshina J.L., Berton P., Gurau G., Rogers R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016;18:53–75. doi: 10.1039/C5GC02396C. [DOI] [Google Scholar]
- 163.Liu J., Cheng R., Deng J., Wu Y. Chiral, pH responsive hydrogels constructed by N-Acryloyl-alanine and PEGDA/α-CD inclusion complex: Preparation and chiral release ability. Polym. Adv. Technol. 2016;27:169–177. doi: 10.1002/pat.3615. [DOI] [Google Scholar]
- 164.Li S. Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly (acrylic acid-acrylamide-methacrylate) and amylose. Bioresour. Technol. 2010;101:2197–2202. doi: 10.1016/j.biortech.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 165.Pereira R.C., Anizelli P.R., Di Mauro E., Valezi D.F., da Costa A.C.S., Zaia C.T.B., Zaia D.A. The effect of pH and ionic strength on the adsorption of glyphosate onto ferrihydrite. Geochem. Trans. 2019;20:3. doi: 10.1186/s12932-019-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eskandari S., Dong A., De Castro L.T., Rahman F.B.A., Lipp J., Blom D.A., Regalbuto J.R. Pushing the limits of electrostatic adsorption: Charge enhanced dry impregnation of SBA-15. Catal. Today. 2019;338:60–71. doi: 10.1016/j.cattod.2019.06.082. [DOI] [Google Scholar]
- 167.Akter M., Bhattacharjee M., Dhar A.K., Rahman F.B.A., Haque S., Rashid T.U., Kabir S. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels. 2021;7:30. doi: 10.3390/gels7010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jørgensen S.E. Developments in Environmental Modelling. Volume 14. Elsevier; Amsterdam, The Netherlands: 1989. Adsorption and ion exchange; pp. 65–81. [Google Scholar]
- 169.Wawrzkiewicz M., Hubicki Z. Ion Exchange-Studies and Applications. IntechOpen; London, UK: 2015. Anion exchange resins as effective sorbents for removal of acid, reactive, and direct dyes from textile wastewaters; pp. 37–72. [Google Scholar]
- 170.Kumar P. Fundamentals and Techniques of Biophysics and Molecular Biology. Pathfinder Publication Unit of PAPL; New Delhi, India: 2016. [Google Scholar]
- 171.Saber-Samandari S., Saber-Samandari S., Gazi M. Cellulose-graft-polyacrylamide/hydroxyapatite composite hydrogel with possible application in removal of Cu (II) ions. React. Funct. Polym. 2013;73:1523–1530. doi: 10.1016/j.reactfunctpolym.2013.07.007. [DOI] [Google Scholar]
- 172.Xiong Y., Xu L., Jin C., Sun Q. Cellulose hydrogel functionalized titanate microspheres with self-cleaning for efficient purification of heavy metals in oily wastewater. Cellulose. 2020;27:7751–7763. doi: 10.1007/s10570-020-03329-w. [DOI] [Google Scholar]
- 173.Van Oss C., Good R., Chaudhury M. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986;111:378–390. doi: 10.1016/0021-9797(86)90041-X. [DOI] [Google Scholar]
- 174.Tokuyama H., Iwama T. Temperature-swing solid-phase extraction of heavy metals on a poly (N-isopropylacrylamide) hydrogel. Langmuir. 2007;23:13104–13108. doi: 10.1021/la701728n. [DOI] [PubMed] [Google Scholar]
- 175.Zhuang Y., Yu F., Chen H., Zheng J., Ma J., Chen J. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. J. Mater. Chem. A. 2016;4:10885–10892. doi: 10.1039/C6TA02738E. [DOI] [Google Scholar]
- 176.Rodrigues F.H., de C Magalhães C.E., Medina A.L., Fajardo A.R. Hydrogel composites containing nanocellulose as adsorbents for aqueous removal of heavy metals: Design, optimization, and application. Cellulose. 2019;26:9119–9133. doi: 10.1007/s10570-019-02736-y. [DOI] [Google Scholar]
- 177.Astrini N., Anah L., Haryadi H.R. Adsorption of heavy metal ion from aqueous solution by using cellulose based hydrogel composite. Macromol. Symp. 2015;353:191–197. doi: 10.1002/masy.201550326. [DOI] [Google Scholar]
- 178.Kwak H.W., Shin M., Yun H., Lee K.H. Preparation of silk sericin/lignin blend beads for the removal of hexavalent chromium ions. Int. J. Mol. Sci. 2016;17:1466. doi: 10.3390/ijms17091466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maity J., Ray S.K. Removal of Cu (II) ion from water using sugar cane bagasse cellulose and gelatin based composite hydrogels. Int. J. Biol. Macromol. 2017;97:238–248. doi: 10.1016/j.ijbiomac.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 180.Hara K., Iida M., Yano K., Nishida T. Metal ion absorption of carboxymethylcellulose gel formed by γ-ray irradiation: For the environmental purification. Colloids Surf. B Biointerfaces. 2004;38:227–230. doi: 10.1016/j.colsurfb.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 181.Tang H., Chang C., Zhang L. Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem. Eng. J. 2011;173:689–697. doi: 10.1016/j.cej.2011.07.045. [DOI] [Google Scholar]
- 182.Yang S., Fu S., Liu H., Zhou Y., Li X. Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions. J. Appl. Polym. Sci. 2011;119:1204–1210. doi: 10.1002/app.32822. [DOI] [Google Scholar]
- 183.Zhou G., Luo J., Liu C., Chu L., Crittenden J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018;131:246–254. doi: 10.1016/j.watres.2017.12.067. [DOI] [PubMed] [Google Scholar]
- 184.Xu X., Ouyang X.-K., Yang L.-Y. Adsorption of Pb (II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2021;322:114523. doi: 10.1016/j.molliq.2020.114523. [DOI] [Google Scholar]
- 185.Mohammadi Z., Shangbin S., Berkland C., Liang J.-T. Chelator-mimetic multi-functionalized hydrogel: Highly efficient and reusable sorbent for Cd, Pb, and As removal from waste water. Chem. Eng. J. 2017;307:496–502. doi: 10.1016/j.cej.2016.08.121. [DOI] [Google Scholar]
- 186.Pourjavadi A., Tehrani Z.M., Salimi H., Banazadeh A., Abedini N. Hydrogel nanocomposite based on chitosan-g-acrylic acid and modified nanosilica with high adsorption capacity for heavy metal ion removal. Iran. Polym. J. 2015;24:725–734. doi: 10.1007/s13726-015-0360-1. [DOI] [Google Scholar]
- 187.Zhang K., Luo X., Yang L., Chang Z., Luo S. Progress toward Hydrogels in Removing Heavy Metals from Water: Problems and Solutions—A Review. ACS ES&T Water. 2021;1:1098–1116. [Google Scholar]
- 188.Tang S.C., Yan D.Y., Lo I.M. Sustainable wastewater treatment using microsized magnetic hydrogel with magnetic separation technology. Ind. Eng. Chem. Res. 2014;53:15718–15724. doi: 10.1021/ie502512h. [DOI] [Google Scholar]
- 189.Tang S.C., Yin K., Lo I.M. Column study of Cr (VI) removal by cationic hydrogel for in-situ remediation of contaminated groundwater and soil. J. Contam. Hydrol. 2011;125:39–46. doi: 10.1016/j.jconhyd.2011.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.