Abstract
Poly(3-hydroxybutyrate) (PHB) was produced by cultivating several gram-negative bacteria, including Ralstonia eutropha, Alcaligenes latus, and recombinant Escherichia coli. PHB was recovered from these bacteria by two different methods, and the endotoxin levels were determined. When PHB was recovered by the chloroform extraction method, the endotoxin level was less than 10 endotoxin units (EU) per g of PHB irrespective of the bacterial strains employed and the PHB content in the cell. The NaOH digestion method, which was particularly effective for the recovery of PHB from recombinant E. coli, was also examined for endotoxin removal. The endotoxin level present in PHB recovered by 0.2 N NaOH digestion for 1 h at 30°C was higher than 104 EU/g of PHB. Increasing the digestion time or NaOH concentration reduced the endotoxin level to less than 1 EU/g of PHB. It was concluded that PHB with a low endotoxin level, which can be used for various biomedical applications, could be produced by chloroform extraction. Furthermore, PHB with a much lower endotoxin level could be produced from recombinant E. coli by simple NaOH digestion.
Poly(3-hydroxybutyrate) (PHB), the best known member of the polyhydroxyalkanoates (PHA), is an energy and/or carbon storage material synthesized and intracellularly accumulated by numerous microorganisms, usually when an essential nutritional element such as nitrogen, phosphorous, oxygen, sulfur, or potassium is limited in the presence of excess carbon source (1, 9). PHB is a partially crystalline thermoplastic and possesses material properties similar to those of polypropylene (6). It has been drawing much attention as a good candidate for biodegradable and/or biocompatible plastic material which can be produced from renewable raw materials (10). Possible applications of PHB include the following: packaging films and containers, biodegradable carriers for controlled chemical and/or drug release, disposable items, surgical pins and sutures, wound dressings, and bone replacements (6, 9). For biomedical applications, it should be noted that the degradation product of PHB, d(−)-3-hydroxybutyrate, is a common intermediate metabolite present in all higher animals, including humans (14). Furthermore, a low-molecular-weight PHB consisting of 100 to 200 monomer units has also been detected in a relatively large amount in human plasma (14). Therefore, it is highly plausible that implanting highly purified PHB in mammalian tissues or parenteral injection of PHB microspheres will not cause any problems.
Several gram-negative bacteria, including Ralstonia eutropha, Alcaligenes latus, and recombinant Escherichia coli, have been employed for the efficient production of PHB (11). These gram-negative bacteria can release endotoxin (pyrogen), which is in the form of lipopolysaccharides, from the outer cell wall (12). Endotoxin causes fever if introduced into the bloodstream of humans or other animals. As we experienced with recombinant pharmaceutical proteins, a safe parenteral drug (or material) should have an endotoxin level below a set limit. According to the U.S. Food and Drug Administration guideline, the upper limit of the pyrogen level is 5.0 endotoxin units (EU)/kg (body weight) per injection. Since PHB is mostly efficiently produced by gram-negative bacteria as described above, the endotoxin levels present in the purified PHB should be examined for biomedical applications. However, until now, there has been no study of the removal of the endotoxins during the purification of PHB from bacteria. In the present study, PHB was produced by R. eutropha, A. latus, and recombinant E. coli, and the removal of the endotoxins during the purification of PHB was examined. Two different methods were employed for the recovery of PHB from these bacteria. Based on these results, the strategies for producing PHB with a low endotoxin level are discussed.
R. eutropha NCIMB11599 (7), A. latus DSM1123 (16), and recombinant E. coli strain XL1-Blue(pJC4) harboring the A. latus PHA biosynthesis genes (5) were used for the production of PHB. The bacteria were grown for the production of PHB in a chemically defined medium with their respective best carbon sources: glucose for R. eutropha (7) and recombinant E. coli (5) and sucrose for A. latus (16), as previously described. The PHB concentration was determined by gas chromatography (HP5890; Hewlett-Packard, Wilmington, Del.) with benzoic acid as an internal standard (2). PHB content was defined as the percentage of the ratio of PHB to cell dry weight.
Two different methods, chloroform extraction (13) and NaOH digestion (4), were used to recover PHB from the bacterial cells. Endotoxin levels were semiquantitatively determined by the Limulus amebocyte lysate-gel clot method (Associates of Cape Cod, Inc., Falmouth, Mass.). The endotoxin levels are reported as the average values of three repeated experiments.
Chloroform extraction has been widely used to recover PHB with a high degree of purity without polymer degradation during the recovery (13). Therefore, PHB samples were first recovered by chloroform extraction of bacterial cells with different PHB contents. Cells were collected by centrifugation at 4,000 × g for 20 min at 25°C and were washed with hot acetone for 20 min. After being dried, the cells were mixed with 50 volumes of chloroform for 48 h at 30°C. A clear PHB solution was recovered by centrifugation; this was followed by polishing filtration. Finally, pure PHB was obtained by nonsolvent precipitation (five times the volume of chloroform) and filtration. The nonsolvent used was a mixture of methanol and water (7:3 [vol/vol]). The endotoxin levels present in PHB recovered from R. eutropha, A. latus, and recombinant E. coli were almost the same and were <10 EU/g of PHB (Table 1). The PHB content of the cells did not affect the removal of the endotoxin level during the purification of PHB by chloroform extraction. From these data, the chloroform extraction method was found to be efficient not only for the recovery of PHB with a high degree of purity but also for the removal of the endotoxins of gram-negative bacteria. However, large quantities of toxic and/or volatile chloroform were required for the recovery of PHB because the polymer solution containing more than 5% (wt/vol) PHB was very viscous and the processing operation became difficult. To overcome these problems, several other recovery methods have been developed. One of these recovery methods, NaOH digestion, has several advantages (4): (i) NaOH is inexpensive and much more environmentally friendly, (ii) a high degree of purity (>98%) of PHB can be obtained, and (iii) there is no degradation of PHB during recovery.
TABLE 1.
Endotoxin levels present in PHB recovered by the chloroform extraction method
| Strain | PHB content (%) | Endotoxin level (EU/g of PHB) |
|---|---|---|
| R. eutropha | 21.2 | 7.1 ± 0.9 |
| 63.5 | 6.9 ± 0.6 | |
| 82 | 6.1 ± 0.7 | |
| A. latus | 88 | 5.4 ± 0.6 |
| Recombinant E. coli | 22.2 | 2.8 ± 0.7 |
| 61.7 | 3.4 ± 0.4 | |
| 77 | 4.9 ± 0.5 |
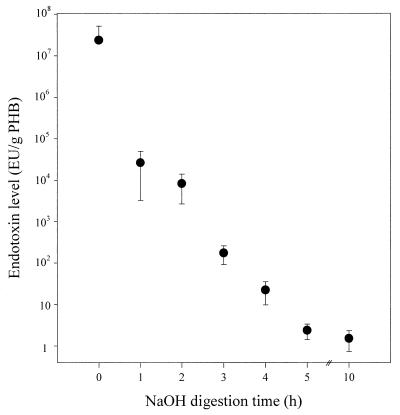
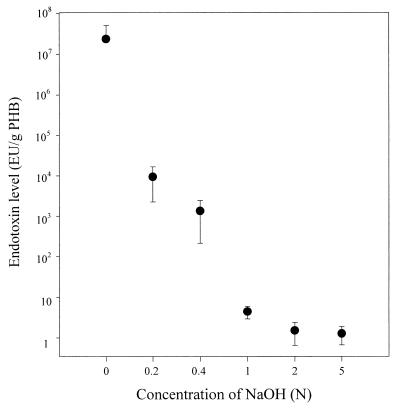
In particular, PHB could be most efficiently recovered by NaOH digestion from recombinant E. coli because these cells, upon accumulating a large amount of PHB, became fragile (4). Cell broth was washed with distilled water and centrifuged. Cells were resuspended in distilled water, and NaOH solutions were added. After the cells were mixed with NaOH solution to digest non-PHB cellular material, PHB granules were separated from the aqueous fraction containing cell debris by centrifugation at 2,500 × g for 20 min. The PHB granules recovered were gently rinsed with distilled water, recentrifuged, and air dried. Right after the NaOH digestion, the pH of the digestion solution was adjusted to pH 7.0 with endotoxin-free HCl solution for the corrective determination of the endotoxin level. Recombinant E. coli cells with a PHB content of 69% were digested with 0.2 N NaOH at 30°C for 1 h, and PHB with a purity of 97% was recovered. The endotoxin level present in purified PHB was higher than 104 EU/g of PHB. The original endotoxin level of E. coli cells was higher than 107 EU/g of PHB. In order to possibly reduce the endotoxin level, the effects of digestion time and NaOH concentration were examined. The endotoxin level could be decreased to 1 EU/g of PHB when the digestion time was increased to 5 h (Fig. 1). The purity of PHB recovered was also increased to 98% by increasing the digestion time. The endotoxin levels present in PHB recovered with NaOH solutions of different concentrations are shown in Fig. 2. When the NaOH concentration was higher than 2 N, the endotoxin level present in PHB was less than 1 EU/g of PHB. The endotoxin level present in PHB obtained by NaOH digestion from recombinant E. coli was lower than that in PHB recovered by chloroform extraction method. This is consistent with the previous finding that NaOH was quite effective for inactivating endotoxins (15).
FIG. 1.
Endotoxin levels present in PHB recovered by 0.2 N NaOH digestion at 30°C for various durations. Recombinant E. coli cells (50 g of cells [dry weight]/liter) with a PHB content of 69% were used.
FIG. 2.
Endotoxin levels present in PHB recovered by digestion with various concentrations of NaOH at 30°C for 2 h. Recombinant E. coli cells (50 g of cells [dry weight]/liter) with a PHB content of 69% were used.
One of the important validation issues to be considered for the biomedical products made by recombinant E. coli and other gram-negative bacteria is the endotoxin removal (15). This study showed for the first time that the endotoxin levels present in PHB, a completely biodegradable polymer produced by several gram-negative bacteria, can be within the allowable limit when purified by chloroform extraction. For small-scale application, therefore, the chloroform extraction method will be useful. For the large-scale production of endotoxin-free PHB, however, a new, more-efficient recovery method must be used (3). In this sense, NaOH digestion method can be efficiently used for the recovery of endotoxin-free PHB produced by recombinant E. coli. Other endotoxin-free PHAs, such as poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (17) and poly(3-hydroxyhexanoate-co-3-hydroxyoctanoate) (8) can also be produced by using recombinant E. coli by NaOH digestion.
Acknowledgments
This work was supported by the Ministry of Science and Technology and by LG Chemicals, Ltd.
REFERENCES
- 1.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunegg G, Sonnleitner B, Lafferty R M. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol. 1978;6:29–37. [Google Scholar]
- 3.Choi J, Lee S Y. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng. 1997;17:335–342. [Google Scholar]
- 4.Choi J, Lee S Y. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol Bioeng. 1999;62:546–553. doi: 10.1002/(sici)1097-0290(19990305)62:5<546::aid-bit6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Lee S Y, Han K. Cloning of the Alcaligenes latus polyhydroxyalkanoate biosynthesis genes and use of these genes for the enhanced production of poly(3-hydroxybutyrate) in Escherichia coli. Appl Environ Microbiol. 1998;64:4897–4903. doi: 10.1128/aem.64.12.4897-4903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes P A. Biologically produced PHA polymers and copolymers. In: Bassett D C, editor. Developments in crystalline polymers. Vol. 2. London, England: Elsevier; 1988. pp. 1–65. [Google Scholar]
- 7.Kim B S, Lee S C, Lee S Y, Chang H N, Chang Y K, Woo S I. Production of poly(3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol Bioeng. 1994;43:892–898. doi: 10.1002/bit.260430908. [DOI] [PubMed] [Google Scholar]
- 8.Langenbach S, Rehm B H A, Steinbüchel A. Functional expression of the PHA synthase gene phaC1 from Pseudomonas aeruginosa in Escherichia coli results in poly(3-hydroxyalkanoate) synthesis. FEMS Microbiol Lett. 1997;150:303–309. doi: 10.1016/s0378-1097(97)00142-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee S Y. Bacterial polyhydroxyalkanoates. Biotechnol Bioeng. 1996;49:1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Lee S Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996;14:431–438. [Google Scholar]
- 11.Lee S Y, Chang H N. Production of poly-(hydroxyalkanoic acid) Adv Biochem Eng Biotechnol. 1995;52:27–58. doi: 10.1007/BFb0102315. [DOI] [PubMed] [Google Scholar]
- 12.Raetz C R H. Bacterial endotoxins: extraordinary lipids that activate eucaryotic signal transduction. J Biotechnol. 1993;175:5745–5753. doi: 10.1128/jb.175.18.5745-5753.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsay J A, Berger E, Voyer R, Chavarie C, Ramsay B A. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol Tech. 1994;8:589–594. [Google Scholar]
- 14.Reusch R N, Sparrow A W, Gardiner J. Transport of poly-β-hydroxybutyrate in human plasma. Biochim Biophys Acta. 1992;1123:33–40. doi: 10.1016/0005-2760(92)90168-u. [DOI] [PubMed] [Google Scholar]
- 15.Sofer G, Hagel L. Handbook of process chromatography: a guide to optimization, scale up, and validation. New York, N.Y: Academic Press, Inc.; 1997. [Google Scholar]
- 16.Wang F, Lee S Y. Poly(3-hydroxybutyrate) production with high polymer content by fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl Environ Microbiol. 1997;63:3703–3706. doi: 10.1128/aem.63.9.3703-3706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yim K S, Lee S Y, Chang H N. Effect of acetic acid on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthesis in recombinant Escherichia coli. Korean J Chem Eng. 1995;12:264–268. [Google Scholar]