Abstract
Background
Studies of outcomes among adults with congenital heart defects (CHDs) have focused on those receiving cardiac care, limiting generalizability. The Congenital Heart Survey To Recognize Outcomes, Needs, and well-beinG (CH STRONG) will assess comorbidities, health care utilization, quality of life, and social and educational outcomes from a US population-based sample of young adults living with CHD.
Methods
Individuals with CHD born between 1980 and 1997 were identified using active, population-based birth defects surveillance systems from 3 US locations (Arkansas [AR]; Arizona [AZ]; and Atlanta, Georgia [GA]) linked to death records. Individuals with current contact information responded to mailed survey materials during 2016 to 2019. Respondents and nonrespondents were compared using χ2 tests.
Results
Sites obtained contact information for 74.6% of the 9,312 eligible individuals alive at recruitment. Of those, 1,656 returned surveys, either online (18.1%) or via paper (81.9%), for a response rate of 23.9% (AR: 18.3%; AZ: 30.7%; Atlanta, GA: 28.0%; P value < .01). For 20.0% of respondents, a proxy completed the survey, with 63.9% reporting that the individual with CHD was mentally unable. Among respondents and nonrespondents, respectively, sex (female: 54.0% and 47.3%), maternal race/ethnicity (non-Hispanic white: 74.3% and 63.0%), CHD severity (severe: 33.8% and 27.9%), and noncardiac congenital anomalies (34.8% and 38.9%) differed significantly (P value < .01); birth year (1991–1997: 56.0% and 57.5%) and presence of Down syndrome (9.2% and 8.9%) did not differ.
Conclusions
CH STRONG will provide the first multisite, population-based findings on long-term outcomes among the growing population of US adults with CHD.
Congenital heart defects (CHDs) are the most common type of structural birth defects, affecting approximately 1% of all births in the United States.1 As a result of cumulative advances in medical and surgical management, survival of those born with CHD has improved markedly, with 85% expected to survive to adulthood.2–7 Based on data from 2010, there are >2.4 million persons with CHD in the United States, more than half of whom are adults.3 Although much is known about the long-term survival and mortality of individuals with CHD in the United States,8 less is known about other long-term outcomes, such as health care utilization, chronic conditions, quality of life, and educational attainment.9
The lack of a national population-based, longitudinal surveillance system of all adults with CHD in the United States hinders the ability to investigate long-term outcomes in this population. Current and prior projects conducted to investigate such questions have focused on adults receiving care, often at specialized adult CHD (ACHD) centers,10–15 or linking existing clinical databases and identifying individuals through CHD-related International Classification of Diseases, Ninth Revision, Clinical Modification, codes.16 However, those approaches likely do not capture half of individuals with CHD who are lost to cardiac follow-up throughout childhood and adolescence or the approximately 6 of 10 adults with CHD who do not receive cardiac follow-up in adulthood.17
Although no US surveillance system exists to provide long-term outcome data among adults with CHD in and out of care, the childhood cancer community has a model to study similar concerns in survivors of childhood cancer. In the Childhood Cancer Survivor Study (CCSS), begun in 1994 and still ongoing, clinicians and researchers surveyed survivors of childhood cancer and their siblings to determine the effects of childhood cancer on long-term outcomes and to identify factors that improved those outcomes.18,19 With the CCSS as a model, the Centers for Disease Control and Prevention (CDC) and the March of Dimes embarked on a similar project for adults living with CHD: Congenital Heart Survey To Recognize Outcomes, Needs, and well-beinG (CH STRONG). Using population-based birth defect surveillance systems from 3 US locations, the project aimed to survey young adults with CHD about their health care utilization, quality of life, and educational and social outcomes. These cross-sectional findings, in turn, can help individuals living with CHD and their families understand the potential long-term expectations and may also inform clinicians, public health professionals, and policymakers about the needs of adults living with CHD. In this article, we describe the methods for the design, implementation, and analysis phases of this project and provide some preliminary results.
Materials and methods
Individuals recruited for CH STRONG were identified through population-based birth defects surveillance systems with active case-finding methods in Arizona (AZ); Arkansas (AR); and Atlanta, Georgia (GA). All 3 sites code birth defects using a 6-digit CDC-modified version of the British Paediatric Association (BPA) codes which provide more specificity for birth defects than the International Classification of Disease, Ninth Revision, Clinical Modification, hereafter referred to as CDC/BPA codes. Inclusion codes for CH STRONG were within the range of 745.000–747.9XX, excluding patent foramen ovale (PFO) and some nonspecific codes (Appendix 1). Because of the active case ascertainment with medical record review at each site and use of 6-digit CDC/BPA codes, atrial septal defects could be differentiated from PFOs. Individuals with PFOs were not recruited for CH STRONG. Each site had to identify at least 2,500 children born in their catchment area in a 10-year period between January 1, 1980, and December 31, 1997, with 1 or more eligible CHD CDC/BPA codes. A brief description of each surveillance system is provided below.
The Arizona Birth Defects Monitoring Program (ABDMP), established in 1986, includes data on individuals with a structural, genetic, or biochemical birth defect or other specified birth outcome that can adversely affect an infant’s health and development. The ABDMP includes only individuals diagnosed in the first year of life whose mothers were AZ residents at time of delivery. After deduplication of cases from multiple data sources, the ABDMP staff reviewed medical records to validate cases and abstract information. Individuals from ABDMP included in CH STRONG were born between January 1, 1986, and December 31, 1997. More information about ABDMP is located at http://www.azdhs.gov/preparedness/public-health-statistics/birth-defects-monitoring/index.php.
The Arkansas Reproductive Health Monitoring System (ARHMS), founded in 1980, uses population-based active surveillance to monitor birth defects diagnosed prenatally and before 2 years of age in children whose mothers were AR residents at time of delivery. Trained staff abstracted information about the child’s birth defect from medical records in more than 43 birth facilities across the state, including the state’s only pediatric hospital and high-risk obstetric hospital. Individuals identified through ARHMS and included in CH STRONG were born between January 1, 1980, and December 31, 1997. More information about ARHMS is located at https://www.archildrens.org/research/research-programs-and-centers/arkansas-reproductive-health-monitoring-system/arhms.
Begun in 1968, the Metropolitan Atlanta Congenital Defects Program (MACDP) captured information on children whose major birth defect was diagnosed before the child’s sixth birthday and whose mother resided in 1 of 5 metropolitan Atlanta counties at delivery as determined by the mother’s medical record or vital records.20 Trained abstractors visited hospitals across the 5 counties to find information on infants with birth defects that met the MACDP case definition. Clinicians then reviewed the information for accuracy. Individuals from MACDP (hereafter referred to as individuals from Atlanta, GA) included in CH STRONG were born between January 1, 1980, and December 31, 1997. More information about MACDP is located at https://www.cdc.gov/ncbddd/birthdefects/macdp.html.
Using probabilistic matching by date of birth, sex, and name, sites linked these individuals to their respective state death records through December 31, 2015, to identify those individuals who would be presumed to be alive at time of recruitment. Individuals were not eligible for CH STRONG if they were incarcerated at time of survey recruitment or could not complete the survey in English or Spanish. Another person (eg, caregiver, parent) could complete the survey for an individual with a CHD who could not complete the survey themselves (eg, physically or mentally unable or unavailable).
Once sites identified CH STRONG-eligible individuals who were presumed alive on January 1, 2016, they used available information from the surveillance system and vital records (name, date of birth, mother’s and father’s names, and address at delivery) to search tracing databases (eg, LexisNexis Accurint or Whitepages) to locate current contact information for each eligible individual. If current contact information was not available for the person, sites sought to obtain contact information from the individual’s mother. In those cases, a letter; a child contact information form; and a self-addressed, stamped envelope were sent to the mother explaining the project and asking for the eligible individual’s current contact information. Each site then attempted to recruit those individuals for whom the mother provided current contact information.
The CH STRONG cross-sectional survey (Appendix 2) consisted of 81 questions on type of CHD, health care access and use, quality of life, general and reproductive health, education, and work history. The survey included 39 questions from US national and state-based surveys, including the National Health Interview Survey (NHIS; https://www.cdc.gov/nchs/nhis/index.htm), National Health and Nutrition Examination Surveys (https://www.cdc.gov/nchs/nhanes/index.htm), Behavioral Risk Factor Surveillance System (https://www.cdc.gov/brfss/index.html), and American Community Survey (https://www.census.gov/programs-surveys/acs/). This will make it possible to compare prevalence estimates from the CH STRONG population to a population-based national or state estimate.
Recruitment occurred in phases from October 2016 to December 2018, when eligible survey respondents were 19 to 38 years of age. To initiate recruitment, sites sent a letter to participants informing them about the survey. Three weeks later, the sites sent participants survey materials including a letter explaining the reasons for conducting the survey, a passive consent form, the paper survey, a gift card for a national retailer as incentive, and a postage-paid envelope to return the completed paper survey to the CDC. The letter included in the survey materials additionally provided participants with the Web address to the CH STRONG informational Web site (www.chstrong.org) and informed participants of the option to complete the survey online by entering their participant ID and passcode. Over an additional 4-week period, sites sent 2 reminder postcards and another hardcopy survey, without incentive, if no completed survey was received. When individuals completed the survey, online or on paper, the participant received a thank you letter with another gift card.
CDC received all completed surveys. Paper surveys were double-entered into a database by separate individuals, and inconsistencies were corrected. Information from the birth defects surveillance system (eg, type of CHD and presence of other birth defects, Down syndrome diagnosis) and birth certificates (eg, year of birth, maternal race) was linked to the survey responses. Investigators also geocoded residence at birth and at time of survey completion and linked census information from corresponding time periods to survey responses. County-level census information at birth came from the decennial census occurring nearest the individual’s birth date (ie, survey responses from individuals born in 1980–1984 were linked to 1980 census data; births occurring in 1985–1994 were linked to 1990 census data21; and births occurring in 1995–1997 were linked to 2000 census data). Tract-level information at time of survey completion came from the 2017 American Community Survey (https://www.census.gov/programs-surveys/acs/).
We examined total number of eligible CHD cases, vital status at recruitment, whether current contact information was found, and response rates overall and compared differences by site using χ2 tests. We calculated response rates in 2 ways: (1) among all eligible individuals not known to be deceased at recruitment (overall response rate) and (2) among all eligible individuals not known to be deceased at recruitment for whom current contact information was found (survey response rate). To understand the potential magnitude of nonresponse bias, we also compared demographic characteristics of survey respondents to nonrespondents. Demographic and health characteristics from the birth defects surveillance system and birth certificate included year of birth; sex; maternal race/ethnicity; CHD severity; presence of noncardiac congenital anomaly (a co-occurring birth defect or chromosomal anomaly, including Down syndrome, falling outside of the CDC/BPA 745.000–747.9XX code range); and, specifically, Down syndrome (CDC/BPA 758.0XX). CHD severity was based on an established algorithm that categorized CHD into 1 of 5 mutually exclusive hierarchical categories (severe, shunt + valve, shunt, valve, and other) and modified by CH STRONG clinicians for use with CDC/BPA codes16 (Appendix 1). We compared demographic and health characteristics of respondents and nonrespondents using χ2 tests. To inform CH STRONG analyses, as well as the research methods of future studies on the adult CHD population, we also examined characteristics of survey completion: mode of completion (paper or online); person who completed the survey (eg, self, parent, spouse); and, if completed by a proxy, the reason the individual with CHD could not complete the survey (eg, physically unable, mentally unable, not interested), overall and by site using χ2 tests. All analyses were independently conducted and results verified by 2 analysts.
CH STRONG was funded by the CDC and approved by the Institutional Review Boards at the CDC and the University of Arkansas for Medical Sciences. The University of Arizona deferred to the CDC Institutional Review Board. The US Office of Management and Budget approved CH STRONG data collection activities (OMB number: 0920–1122).
Results
A total of 11,695 individuals were identified from the 3 birth defects surveillance programs who were born alive with CHD that met inclusion criteria (Figure 1). Of those, 20.4% (n = 2,383) were deceased (n = 2,376) or incarcerated (n = 7) at time of survey completion. Overall, sites found current contact information on 74.6% of individuals (AR: 78.5%, AZ: 71.0%; Atlanta, GA: 71.1%), all of whom were mailed survey materials. Of those, 1,656 returned surveys, for a survey response rate of 23.9% (AR: 18.3%; AZ: 30.7%; Atlanta, GA: 28.0%). Among all 9,312 individuals presumed alive at survey completion, irrespective of having current contact information, the overall response rate was 17.8% (AR: 14.4%; AZ: 21.8%; Atlanta, GA: 19.9%).
Figure 1.
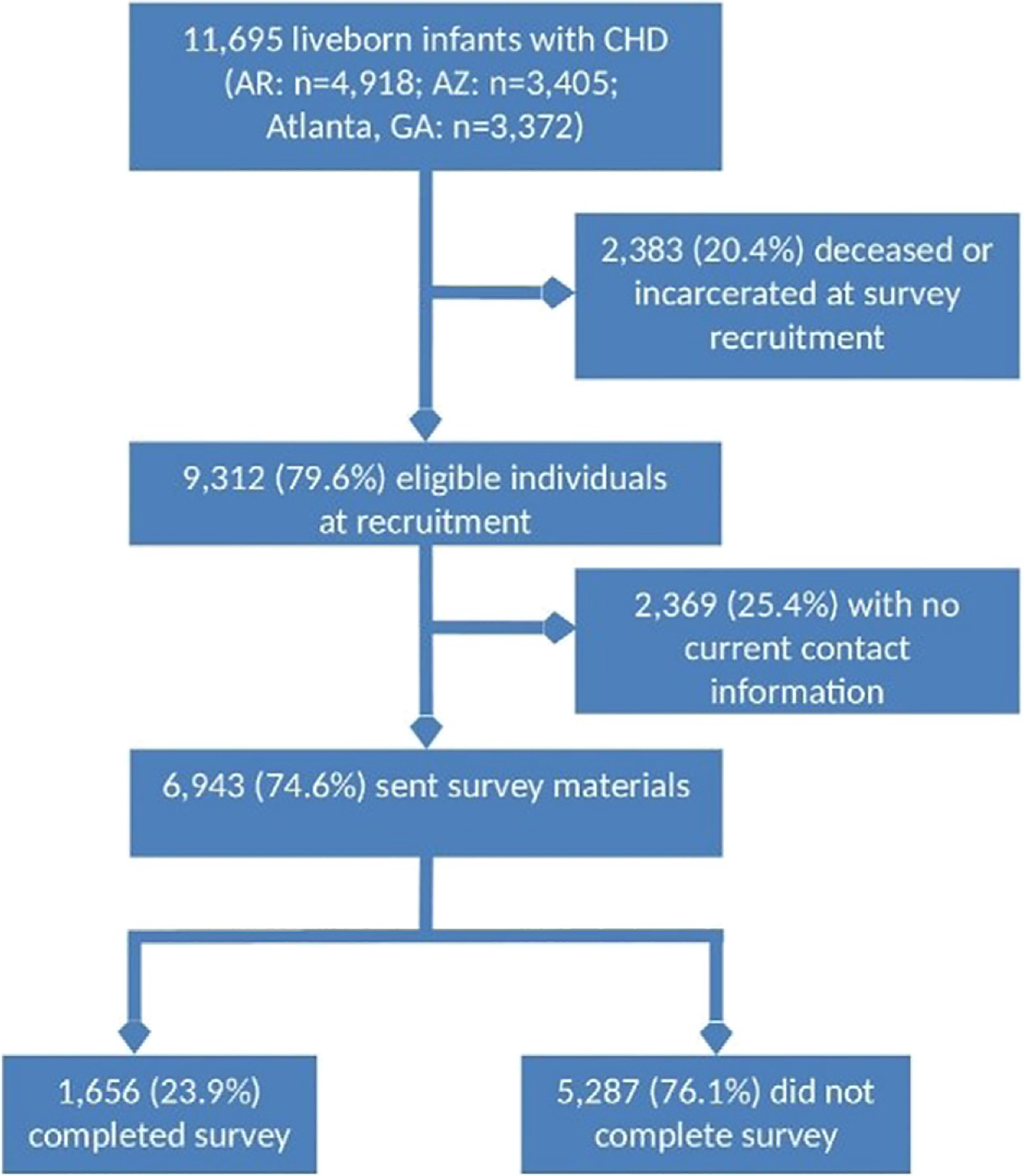
Identification and recruitment of participants for CH STRONG.
A little over a third of respondents were born in AR (38.2%), with equal percentages born in AZ (30.9%) and Atlanta, GA (30.9%) (Table I). Most respondents were female (54%), born during 1991 and 1997 (56.0%), and non-Hispanic white (74.3%). About one third (33.8%) had a severe CHD, but this percentage differed by site (AR: 22.5%, AZ: 48.0%, GA: 33.6%; Appendix 3). Of respondents, 34.8% and 9.2% had Down syndrome specifically. Distributions of sex, maternal race/ethnicity, CHD severity, and presence of noncardiac congenital anomalies differed between respondents and nonrespondents (P < .01 for all). Compared to nonrespondents, respondents tended to be more commonly female, non-Hispanic white, with severe CHD, and did not have noncardiac congenital anomalies. Of respondents, the most common CHDs were ventricular septal defect, atrial septal defect, and pulmonary valve stenosis. However, >15% had more than 1 primary CHD (eg, tetralogy of Fallot with atrioventricular canal defect).
Table I.
Demographic and health characteristics of respondents and nonrespondents, CH STRONG
| CH STRONG respondents | CH STRONG nonrespondents* | Total eligible for CH STRONG | χ2 P value† | ||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| 1656 | (17.8%) | 7656 | (82.2%) | 9312 | (100%) | ||
| Site | <.001 | ||||||
| AR | 632 | (38.2) | 3756 | (49.1) | 4388 | (47.1) | |
| AZ | 512 | (30.9) | 1840 | (24.0) | 2352 | (25.3) | |
| Atlanta, GA | 512 | (30.9) | 2060 | (26.9) | 2572 | (27.6) | |
| Sex | <.001‡ | ||||||
| Female | 894 | (54.0) | 3621 | (47.3) | 4515 | (48.5) | |
| Male | 762 | (46.0) | 4023 | (52.5) | 4785 | (51.4) | |
| Unknown/ambiguous | 0 | 0 | 12 | (0.1) | 12 | (0.1) | |
| Birth year | 230 | (13.9) | 1110 | (14.5) | 1340 | (14.4) | .23 |
| 1980–1985 | |||||||
| 1986–1990 | 499 | (30.1) | 2145 | (28.0) | 2644 | (28.4) | |
| 1991–1997 | 927 | (56.0) | 4401 | (57.5) | 5328 | (57.2) | |
| Maternal race/ethnicity | <.001 | ||||||
| Non-Hispanic white | 1231 | (74.3) | 4824 | (63.0) | 6055 | (65.0) | |
| Non-Hispanic black or African American | 230 | (13.9) | 1712 | (22.4) | 1942 | (20.9) | |
| Hispanic | 124 | (7.5) | 689 | (9.0) | 813 | (8.7) | |
| Non-Hispanic American Indian or Alaska Native | 26 | (1.6) | 219 | (2.9) | 245 | (2.6) | |
| Non-Hispanic Asian or Pacific Islander | 20 | (1.2) | 84 | (1.1) | 104 | (1.1) | |
| Other, unknown, or missing | 25 | (1.5) | 128 | (1.7) | 153 | (1.6) | |
| Severity§ | <.001 | ||||||
| Severe | 560 | (33.8) | 2136 | (27.9) | 2696 | (29.0) | |
| Shunt + valve | 141 | (8.5) | 698 | (9.1) | 839 | (9.0) | |
| Shunt | 717 | (43.3) | 3608 | (47.1) | 4325 | (46.4) | |
| Valve | 181 | (10.9) | 962 | (12.6) | 1143 | (12.3) | |
| Other | 57 | (3.4) | 252 | (3.3) | 309 | (3.3) | |
| Has a noncardiac congenital anomaly | <.01 | ||||||
| Yes | 577 | (34.8) | 2982 | (38.9) | 3559 | (38.2) | |
| No | 1079 | (65.2) | 4674 | (61.1) | 5753 | (61.8) | |
| Has Down syndrome | .68 | ||||||
| Yes | 152 | (9.2) | 678 | (8.9) | 830 | (8.9) | |
| No | 1504 | (90.8) | 6978 | (91.1) | 8482 | (91.1) | |
Include 2,369 individuals for whom no current contact information was found and 5,287 individuals who were sent survey materials but did not respond.
Comparing respondents to nonrespondents.
χ2 test limited to men and women.
CDC/BPA codes corresponding to each category are described in Appendix 1.
Most respondents (81.9%) completed the survey on paper rather than online (Table II), although frequencies differed by site (percent completing paper surveys: AZ: 72.5%; AR: 85.4%; Atlanta, GA: 87.1%; P < .001). Most people completing the survey were the individuals with CHD (78.0%), followed by a parent (16.2%), sibling or other family member (1.9%), spouse or partner (1.3%), and other individual (0.6%). Survey completion by the individual with CHD also differed by site, with 85.5% of respondents in Atlanta, GA, completing the survey themselves compared to 74.2% in AR and 75.0% in AZ (P = .002). Of those surveys completed by a proxy, the most commonly reported reason that someone else completed the survey for the individual with CHD was that the person was mentally unable (63.9%).
Table II.
Characteristics of survey completion, overall and by birth defects program, CH STRONG
| Total | AR | AZ | Atlanta, GA | |||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Survey type* | ||||||||
| Online | 299 | (18.1) | 92 | (14.6) | 141 | (27.5) | 66 | (12.9) |
| Paper | 1357 | (81.9) | 540 | (85.4) | 371 | (72.5) | 446 | (87.1) |
| Person completing survey* | ||||||||
| Self | 1291 | (78.0) | 469 | (74.2) | 384 | (75.0) | 438 | (85.5) |
| Partner/spouse | 21 | (1.3) | 8 | (1.3) | 7 | (1.4) | 6 | (1.2) |
| Parent | 269 | (16.2) | 119 | (18.8) | 97 | (18.9) | 53 | (10.4) |
| Sibling/other family member | 32 | (1.9) | 18 | (2.8) | 9 | (1.8) | 5 | (1.0) |
| Unrelated caregiver/other | 10 | (0.6) | 4 | (0.6) | 4 | (0.8) | 2 | (0.4) |
| Missing | 33 | (2.0) | 14 | (2.2) | 11 | (2.1) | 8 | (1.6) |
| Reason person cannot complete survey† | ||||||||
| Physically unable | 22 | (6.6) | 13 | (8.7) | 8 | (6.8) | 1 | (1.5) |
| Mentally unable | 212 | (63.9) | 94 | (63.1) | 81 | (69.2) | 37 | (56.1) |
| Unavailable | 72 | (21.7) | 30 | (20.1) | 19 | (16.2) | 23 | (34.9) |
| Not interested | 3 | (0.9) | 1 | (0.9) | 2 | (1.7) | 0 | 0 |
| Not managing their own care | 12 | (3.6) | 4 | (2.7) | 6 | (5.1) | 2 | (3.0) |
| Other | 7 | (2.1) | 4 | (2.7) | 1 | (0.9) | 2 | (3.0) |
| Missing | 4 | (1.2) | 3 | (2.0) | 0 | (0.0) | 1 | (1.5) |
χ2 P value < .05 comparing AR; AZ; and Atlanta, GA.
Limited to 332 individuals who had information on person completing survey and responded other than “self.”
Discussion
CH STRONG provides the first multisite population-based survey data on long-term outcomes among adults with CHD in the United States. With information from >1,650 adults ages 19 to 38 years of age with CHD from AZ, AR, and metropolitan Atlanta, GA, CH STRONG data will be used to examine health care access and barriers to care, quality of life, educational and social outcomes, reproductive health, and comorbidities, including obesity. Using CH STRONG data, we can examine differences in these outcomes between individuals with CHD and national or state populations, as well as differences by site, sex, race/ethnicity, and CHD type or severity.
We found that the large majority of CH STRONG respondents completed the hardcopy rather than online survey. This is likely because the hardcopy survey was sent to the individual’s home and individuals were not recruited via e-mail. We were able to recruit individuals who could not complete the survey themselves, the large majority of whom were mentally unable to complete the survey. We also have survey information on a substantial number of individuals with noncardiac congenital anomalies, including Down syndrome, allowing us to examine differences by these characteristics. Inclusion of adults with CHD who could not complete the survey themselves increases the generalizability of the findings and provides more accurate estimates for certain outcomes, such as disability and need for special education services.
Several prior or existing CHD-focused networks and registries have provided information on long-term outcomes among adults with CHD.10–13 They identified individuals with CHD who received care, often at a specialized ACHD center, and followed them retrospectively or prospectively to examine clinical or patient-reported outcomes, such as stroke and physical activity.22,23 Other CHD-focused databases have linked existing data sources, such as clinical and surgical data, electronic health records, health care claims data, and vital records, to examine health care utilization, and cardiac and noncardiac outcomes and survival among individuals with CHD.8,15,16,24 Many of these networks and databases are limited to individuals who received cardiac care as adults and/or whose CHD was documented in their adult health care records. Although all are based on clinical records and/or health care claims data, one is a population-based sample of current health care data,16 and another examined long-term survival among all individuals who received cardiac surgery as children at specific centers.8
CH STRONG has many advantages over other ACHD databases. First, CH STRONG used population-based, active ascertainment birth defects surveillance systems as the source for identifying individuals with CHD rather than recruiting via specialized ACHD centers, clinical records, or health care claims databases. This strategy will enable the study of important questions related to access to care among the entire ACHD population, including those who are not receiving specialty care or any type of health care. In addition, using active ascertainment birth defects surveillance systems provides accurate information on type of CHD diagnosed in infancy and early childhood as well as other clinical information at birth, such as gestational age at birth. Based on data from ACHD clinics, 42% of patients at their first presentation had over a 3-year gap in cardiac care, lesser severity of disease predicted falling out of care, and more than half sought care at an ACHD clinic because of new onset of symptoms.25 Therefore, adults seeking cardiac care at ACHD centers or elsewhere may have better access to care, more severe defects, symptomatic disease, or different health care utilization patterns than adults with CHD not receiving cardiac care at ACHD clinics or at all. These differences between adults receiving care for their CHD and others may lead to biased results not generalizable to the larger population of individuals with CHD. Studies have shown that even those with mild CHD may be impacted by long-term medical and nonmedical outcomes8,26,27; CH STRONG will provide information on those with severe and nonsevere CHD. Secondly, outcomes from CH STRONG are directly reported by the patients, or their proxies, instead of identified in health care records or claims data. This self-reported approach will allow us to understand outcomes as perceived by patients, as has been done in studies of clinical populations,10 although generalizable to those out of cardiac care. Self-reported data also allow us to answer questions related to quality of life, undiagnosed depression, and educational and work history, information not found in medical records. Finally, many questions included in CH STRONG, such as those on comorbidities, quality of life, disability, and health care access, are derived from national or state-based population surveys. Therefore, we can compare CH STRONG respondents to nationally representative and state-representative samples of young adults to determine how individuals with CHD fare relative to their counterparts without CHD. Overall, CH STRONG complements multicenter studies based on clinical or health care data16 and will help bridge the gap between clinical research and public health surveillance in the United States.28
CH STRONG has limitations as well. First, although the selection of the CH STRONG population was based on active ascertainment birth defects surveillance systems, the systems differed in terms of date the surveillance system was founded (AZ: 1986, AR: 1980, GA: 1968) and upper age at case identification (up to age 1 in AZ, age 2 in AR, and age 6 in GA). Therefore, the age range and CHD severity of eligible individuals differed by site. Access to health care and health outcomes may also differ between sites and will be examined in future analyses. Second, although self-reported outcomes have the advantages mentioned above, they may limit the validity of certain outcomes. For example, the survey asks patients about certain medical comorbidities such as cardiac dysrhythmias or diabetes. However, some patients may not be aware of the full extent of their medical conditions. Third, without extensive clinical records, CH STRONG is limited in its ability to analyze the association of various clinical risk factors, types of surgical procedures, or other interventions on long-term outcomes. Although the survey included questions on number of surgeries at different ages, self-reported information may not be as accurate as clinical registries such as the Society of Thoracic Surgeons database (https://www.sts.org) or the Pediatric Cardiac Care Consortium registry (https://pcccweb.com). Finally, as with any study, selection bias is an important consideration. Fortunately, we were able to find current contact information via various tracking and tracing techniques for approximately 8 out of 10 eligible individuals, despite having only birth information from 19 to 38 years ago. Unfortunately, of those with contact information, less than 1 out of 4 returned a completed survey. This CH STRONG survey response rate is lower than that of the CCSS (81%) or national population-based surveys such as NHIS (70%). However, for CCSS, 22% of individuals were <18 years of age at recruitment, >60% could be located, and the hospital where treatment occurred sent the survey materials.18,29 For NHIS, data were collected by personal household interview (https://www.cdc.gov/nchs/nhis/about_nhis.htm). Although sites did not receive undeliverable survey materials and postcards for any of the 6,943 individuals with current contact information, it is possible that contact information was incorrect for a portion of those individuals and the survey response rate is therefore underestimated. CH STRONG response rates differed by site, sex, maternal race/ethnicity, CHD severity, and presence of a noncardiac defect. However, subgroup differences between respondents and nonrespondents were approximately 11 percentage points or less, and sample sizes for several characteristics will allow us to stratify results to minimize nonresponse bias (eg, by sex). However, it is unknown whether current health status affected response rates.
CH STRONG used novel methods to identify a population-based sample of young adults with CHD, based on their diagnosis at birth, irrespective of their health care use. Findings from CH STRONG will identify differences in health care access and utilization between young adults with CHD and nationally representative samples of young adults from the general population. We can also use CH STRONG data to examine nonclinical outcomes of interest, such as quality of life and social and educational outcomes, overall and by state of birth, and compare those to national and state-based estimates. Furthermore, among individuals with CHD, CH STRONG will examine how these outcomes vary by race/ethnicity, sex, CHD type, and receipt of cardiac care.
Supplementary Material
Acknowledgements
The authors thank Margaret A. Honein, PhD; Jodi Jackson, PhD; Regina Simeone, MPH; Ginnie Abarbanell, MD; and Pam Costa, MS, for help with conceptualizing the project; Dr Nancy McClung for MACDP project coordination; the Geospatial Research, Analysis, and Services Program, a division of the Agency for Toxic Substances and Disease Registry at CDC, for providing material support for this research; Rebecca Russell and Sammi Cardoso for their commitment to and ongoing support for CH STRONG; Dr Ann Mertens, a former program manager for the Childhood Cancer Survivor Study, for guidance in the design of CH STRONG; the University of Arizona’s Pediatric Multidisciplinary Research Unit and Division of Pediatric Cardiology for their support and help in this project; Mr Dan Sobkoviak, Senior Database Programmer, for assisting with the data extractions and merges required to complete this project; and Dr James Robbins, Professor of Pediatrics, for his contributions as Co-PI of this project before his retirement.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Footnotes
Declarations of interest: none.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2019.12.021.
References
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, et al. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr 2008;153(6):807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003;126(5):1385–96. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation 2016;134(2):101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kogon B, Grudziak J, Sahu A, et al. Surgery in adults with congenital heart disease: risk factors for morbidity and mortality. Ann Thorac Surg 2013;95(4):1377–82 discussion 1382. [DOI] [PubMed] [Google Scholar]
- 5.Mahle WT, Clancy RR, Moss EM, et al. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics 2000;105(5):1082–9. [DOI] [PubMed] [Google Scholar]
- 6.Mahle WT, Lu M, Ohye RG, et al. A predictive model for neurodevelopmental outcome after the Norwood procedure. Pediatr Cardiol 2013;34(2):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012;126(9):1143–72. [DOI] [PubMed] [Google Scholar]
- 8.Spector LG, Menk JS, Knight JH, et al. Trends in long-term mortality after congenital heart surgery. J Am Coll Cardiol 2018;71(21): 2434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oster ME, Riehle-Colarusso T, Simeone RM, et al. Public health science agenda for congenital heart defects: report from a Centers for Disease Control and Prevention experts meeting. J Am Heart Assoc 2013;2(5), e000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apers S, Kovacs AH, Luyckx K, et al. Assessment of patterns of patient-reported outcomes in adults with congenital heart disease—international study (APPROACH-IS): rationale, design. and methods Int J Cardiol 2015;179:334–42. [DOI] [PubMed] [Google Scholar]
- 11.Khairy P, Hosn JA, Broberg C, et al. Multicenter research in adult congenital heart disease. Int J Cardiol 2008;129(2):155–9. [DOI] [PubMed] [Google Scholar]
- 12.Strange G, Stewart S, Farthing M, et al. Living with, and caring for, congenital heart disease in Australia: insights from the Congenital Heart Alliance of Australia and New Zealand Online Survey. Heart Lung Circ; 2019. [DOI] [PubMed] [Google Scholar]
- 13.van der Velde ET, Vriend JW, Mannens MM, et al. CONCOR, an initiative towards a national registry and DNA-bank of patients with congenital heart disease in the Netherlands: rationale, design. and first results Eur J Epidemiol 2005;20(6):549–57. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs JP, Shahian DM, D’Agostino RS, et al. The Society of Thoracic Surgeons national database 2018 annual report. Ann Thorac Surg 2018;106(6):1603–11. [DOI] [PubMed] [Google Scholar]
- 15.Pyles LA, Hills CM, Larson VE, et al. Pediatric Cardiac Care Consortium: an instrument for evidence-based clinical decision support. J Cardiovasc Transl Res 2009;2(2):219–24. [DOI] [PubMed] [Google Scholar]
- 16.Glidewell J, Book W, Raskind-Hood C, et al. Population-based surveillance of congenital heart defects among adolescents and adults: surveillance methodology. Birth Defects Res 2018;110(19): 1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackie AS, Ionescu-Ittu R, Therrien J, et al. Children and adults with congenital heart disease lost to follow-up: who and when? Circulation 2009;120(4):302–9. [DOI] [PubMed] [Google Scholar]
- 18.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute–supported resource for outcome and intervention research. J Clin Oncol 2009;27(14):2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol 2002;38(4):229–39. [DOI] [PubMed] [Google Scholar]
- 20.Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol 2007;79(2):65–186. [DOI] [PubMed] [Google Scholar]
- 21.Berghammer M, Dellborg M, Ekman I. Young adults experiences of living with congenital heart disease. Int J Cardiol 2006;110(3):340–7. [DOI] [PubMed] [Google Scholar]
- 22.Bokma JP, Zegstroo I, Kuijpers JM, et al. Factors associated with coronary artery disease and stroke in adults with congenital heart disease. Heart 2018;104(7):574–80. [DOI] [PubMed] [Google Scholar]
- 23.Ko JM, White KS, Kovacs AH, et al. Physical activity-related drivers of perceived health status in adults with congenital heart disease. Am J Cardiol 2018;122(8):1437–42. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs ML, Jacobs JP, Hill KD, et al. The Society of Thoracic Surgeons congenital heart surgery database: 2018 update on research. Ann Thorac Surg 2018;106(3):654–63. [DOI] [PubMed] [Google Scholar]
- 25.Gurvitz M, Valente AM, Broberg C, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol 2013;61(21):2180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riehle-Colarusso T, Autry A, Razzaghi H, et al. Congenital heart defects and receipt of special education services. Pediatrics 2015;136(3):496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha P, Potiny P, Rigdon J, et al. Substantial cardiovascular morbidity in adults with lower-complexity congenital heart disease. Circulation 2019;139(16):1889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins KJ, Botto LD, Correa A, et al. Public health approach to improve outcomes for congenital heart disease across the life span. J Am Heart Assoc 2019;8(8), e009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertens AC, Walls RS, Taylor L, et al. Characteristics of childhood cancer survivors predicted their successful tracing. J Clin Epidemiol 2004;57(9):933–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


