Abstract
Interaction of Listeria monocytogenes with mammalian intestinal cells is believed to be an important first step in Listeria pathogenesis. Transposon (Tn916) mutagenesis provided strong evidence that a 104-kDa surface protein, designated the Listeria adhesion protein (LAP), was involved in adherence of L. monocytogenes to a human enterocyte-like Caco-2 cell line (V. Pandiripally, D. Westbrook, G. Sunki, and A. Bhunia, J. Med. Microbiol. 48:117–124, 1999). In this study, expression of LAP in L. monocytogenes at various growth temperatures (25, 37, and 42°C) and in various growth phases was determined by performing an enzyme-linked immunoassay (ELISA) and Western blotting with a specific monoclonal antibody (monoclonal antibody H7). The ELISA and Western blot results indicated that there was a significant increase in LAP expression over time only at 37 and 42°C and that the level of LAP expression was low during the exponential phase and high during the stationary phase. In contrast, there were not significant differences in LAP expression between the exponential and stationary phases at 25°C. Examination of the adhesion of L. monocytogenes cells from exponential-phase (12-h) or stationary-phase (24-h) cultures grown at 37°C to Caco-2 cells revealed that there were not significant differences in adhesion. Although expression of L. monocytogenes LAP was different at different growth temperatures and in different growth phases, enhanced expression did not result in increased adhesion, possibly because only a few LAP molecules were sufficient to initiate binding to Caco-2 cells.
Listeria monocytogenes is an invasive food-borne pathogen that severely affects immunocompromised individuals. L. monocytogenes survives the acidic environment in the stomach and passes through this barrier into the intestinal tract. Bacteria possess several distinct and alternative means of cell attachment that can be manifested under different environmental and host cell conditions (12). Adherence to host cells is an essential first step for L. monocytogenes to cause disease. After attachment to host cells, L. monocytogenes can enter the cells either by phagocytosis or by induced endocytosis (i.e., invasion) (11). Once inside the cells, the bacteria can infect adjacent cells, reach the bloodstream, target organs, and cause liver abscesses, meningitis, and encephalitis.
The surface protein internalin has been shown to mediate adhesion and penetration of L. monocytogenes into epithelial cells and hepatocytes (9, 13). Internalin mutants still have some adhesion capabilities, which is an indication that other proteins or factors may be involved in the adhesion process (13). An extracellular protein, p60, has also been reported to participate in adhesion and invasion of L. monocytogenes into mammalian fibroblast cells (4, 15). Recently, Alvarez-Dominguez et al. (1) reported that actin polymerization protein also might be responsible for adhesion to mammalian cells. Other Listeria spp. have adherence capabilities independent of invasion of human epithelial cells (RPMI-4788 and HT-29 cells) (19), suggesting that multiple adherence factors may be inherent in members of the genus Listeria.
The results of recent studies in our laboratory suggested that a 104-kDa surface protein, designated Listeria adhesion protein (LAP), is also responsible for adhesion of L. monocytogenes to intestinal cells (3, 21). Mutant strain AAMU572, which lacks the 104-kDa protein, exhibited very low levels of adhesion to human enterocyte-like Caco-2 cells (21).
Temperature and growth state are important factors that influence the expression of virulence genes in many bacterial species (18). For L. monocytogenes, it has been well-documented that the expression of virulence factors, such as transcriptional activator protein (PrfA), internalin, listeriolysin, phospholipases, metalloprotease, and actin polymerization proteins, is influenced by bacterial growth phase and temperature (5, 6, 10, 17, 22, 24). Considering the potential significance of LAP in pathogenesis, we thought that it was essential to study the expression of LAP at clinically important growth temperatures and in different growth phases.
L. monocytogenes can grow at a wide range of temperatures (3 to 45°C). Therefore, the purpose of this research was to study the effects of some selected growth temperatures (25, 37, and 42°C) on the expression of LAP. A growth temperature of 25°C was selected because many foods are handled at this temperature and often L. monocytogenes may be present on surfaces and utensils and be a major source of contamination. A growth temperature of 37°C was selected because listerial pathogenesis depends on the ability of the microorganism to grow in the human body and cause tissue damage. A temperature of 42°C was chosen because food may be subjected to temperature abuse during food handling or storage and this temperature can stress the microorganism (20); thus, we wanted to determine if L. monocytogenes could express LAP in this stressful environment. In addition, 37°C cultures at two growth phases, representing high and low levels of LAP expression, were examined to determine their adhesion to Caco-2 cells.
Bacterial strains and growth curves.
The L. monocytogenes strains used in this study were wild-type strain F4244 (serotype 4b; Centers for Disease Control and Prevention, Atlanta, Ga.), which was erythromycin resistant, and strain AAMU572 (lap::Tn916), which lacked the 104-kDa LAP and was erythromycin and tetracycline resistant. Stocks of these cultures were kept frozen in brain heart infusion (BHI) broth (Difco) containing 10% glycerol at −80°C. For experimental purposes, the wild-type strain was cultured in BHI broth containing erythromycin (Sigma) at a concentration of 10 μg/ml, while AAMU572 was grown in medium containing erythromycin (10 μg/ml) and tetracycline (10 μg/ml; Sigma).
For growth experiments, the wild-type and AAMU572 cultures were inoculated (1%) into BHI broth media containing the appropriate antibiotics and incubated at 25, 37, and 42°C, and absorbance at 595 nm was determined at 2-h intervals by using a microplate reader (Bio-Rad, Hercules, Calif.). In some cases, bacterial counts were determined at each time point by plating preparations onto BHI agar plates.
Growth curves were generated by using absorbance values (data not shown). From these curves the approximate mid-exponential- and stationary-phase points were determined and used as reference times to perform the LAP expression experiments. The median time points for the exponential-phase cultures grown at 25, 37, and 42°C were determined to be about 13, 12, and 7 h, respectively, and the median time points for the stationary-phase cultures grown at these temperatures were 24, 20, and 12 h, respectively.
In general, mutant growth was slower than wild-type growth at all temperatures (data not shown), possibly because of the presence of an additional antibiotic (tetracycline). Antibiotic stress has been shown to slow bacterial growth as well as other factors, such as storage conditions, preincubation temperature, and inoculum concentration, which can affect bacterial growth (14).
LAP expression at different temperatures and in different growth phases.
Cultures of L. monocytogenes wild-type strain F4244 and strain AAMU572 were incubated at 25, 37, or 42°C until the log, stationary, and death phases. The cell mass of each sample (1 to 20 ml) obtained at different temperatures and in different growth phases was adjusted to an absorbance at 595 nm of 0.3 so that all of the samples contained approximately the same number of cells. LAP expression in these adjusted cultures was monitored by performing indirect enzyme-linked immunosorbent assays (ELISA) and Western blot assays.
To perform the indirect ELISA, several 1.5-ml aliquots of bacterial cultures were removed at 12-h intervals for 48 h and centrifuged (8,160 × g, 10 min). The cell pellets were resuspended with 1.5 ml of 0.1 M carbonate-bicarbonate coating buffer (pH 9.6). Aliquots (100 μl) of each absorbance-adjusted cell suspension were placed in several wells of a microtiter plate (Immulon 1; Dynatech, Chantilly, Va.) and stored at 4°C until they were used. The plates were washed four times with rinse buffer (40 mM phosphate-buffered saline [PBS] [pH 7.0], 0.5% Tween 20) and immunoprobed with a 104-kDa LAP-specific monoclonal antibody (MAb), MAb H7 (3), as described by Bhunia et al. (2).
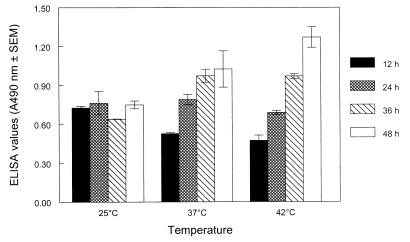
The ELISA data (Fig. 1) indicated that LAP expression in the wild-type L. monocytogenes cultures continued to increase over time when the cultures were grown at 37 and 42°C. At 37°C, the average ELISA absorbance value for exponential-phase (12-h) cultures was 0.53, and for the stationary-phase (24-h) cultures the average ELISA absorbance value was 0.79, which represents a significant increase in expression (P < 0.05). Similar results were obtained at 42°C. However, a different trend was observed at 25°C; at this temperature the absorbance values (0.73 to 0.75) were not significantly different during the 48-h study period. This study indicated that during the exponential phase, the highest level of LAP expression occurred in cultures grown at 25°C, followed by cultures grown at 37 and 42°C. During the stationary phase, the highest level of expression occurred in cultures grown at 37 and 25°C, followed by cultures grown at 42°C. In addition, a very high level of LAP expression occurred in cultures beyond the stationary phase grown at 37 and 42°C (Fig. 1).
FIG. 1.
Analysis of 104-kDa LAP expression in the L. monocytogenes wild-type strain by an indirect ELISA at different growth temperatures (25, 37, and 42°C) and after growth for 12 h (■), 24 h (▩), 36 h (▧), and 48 h (□). Growth for 12 h, growth for 24 h, and growth for 36 to 48 h at 25 and 37°C roughly represent the exponential, stationary, and death phases, respectively, whereas growth for 12 h and growth for 24 to 48 h at 42°C represent the stationary and death phases, respectively. The ELISA absorbance values are the averages of values from three experiments based on absorbance-adjusted bacterial cell populations analyzed in triplicate; means ± standard errors of the means are shown. A490, absorbance at 490 nm.
For sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blot analyses, 20-ml aliquots of absorbance-adjusted bacterial cultures were centrifuged (12,100 × g, 10 min) at 4°C, and the surface proteins were extracted from the bacterial cells by resuspending the pellets with 0.25 ml of SDS sample solvent (2, 16). The samples were incubated at 37°C for 1 h and then centrifuged (16,000 × g, 10 min). The supernatants containing the protein extracts were collected and loaded (25 μl/well) onto duplicate SDS–8% polyacrylamide gels. After electrophoresis, one gel was stained with Coomassie blue and the protein of the remaining gel was transferred to an Immobilon-P membrane (Millipore, Bedford, Mass.). The membrane was immunoprobed with MAb H7 (2, 3), and the reaction intensity (sum intensity) of MAb H7 with LAP bands was analyzed with a Kodak 1D Image Analysis System (Eastman Kodak Co., Rochester, N.Y.).
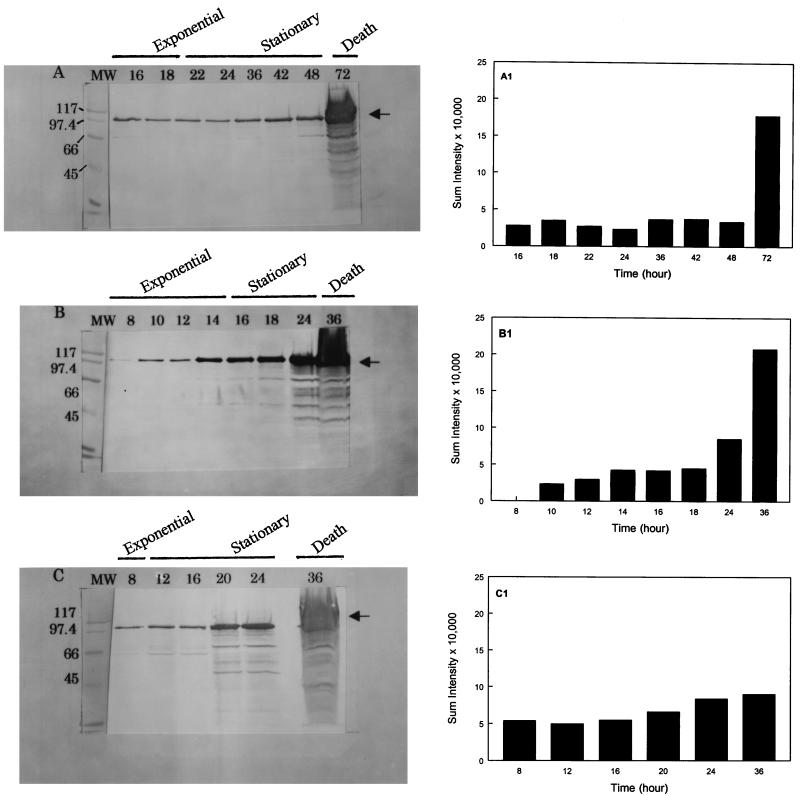
Western blot data (Fig. 2) indicated that LAP expression was dependent on the bacterial growth temperature and growth phase, similar to the results obtained in the indirect ELISA. At 25°C the expression of LAP remained essentially constant from 16 to 24 h, and there was a slight increase in expression towards the end of the stationary phase (36 to 48 h). However, at 37°C the levels of protein expression increased from 14 to 36 h. At 42°C a similar pattern of expression was observed from 20 to 36 h. The Western blot results confirmed that LAP expression is related to both growth temperature and growth phase. Substantial amounts of LAP were detected towards the end of the stationary phase and beyond (i.e., 24 to 36 h) in cultures grown at 37 or 42°C and in a 72-h culture grown at 25°C (Fig. 2). These large amounts may have represented surface-associated and intracellular or membrane-bound 104-kDa proteins. During this period the cultures usually entered the death phase, in which the bacterial cell walls became very fragile because of bacterial autolysins (23). When sample solvent containing SDS was added to the bacterial suspensions, it caused overt lysis of the cells, which resulted in increased release of 104-kDa protein in the preparation (Fig. 2). Also during this phase (the death phase), several other bands reacted with the antibody, indicating that the 104-kDa protein was probably degraded by cellular proteolytic enzymes.
FIG. 2.
Western blot analyses of 104-kDa LAP from L. monocytogenes performed with MAb H7. Bacterial proteins were extracted at incubation temperatures of 25°C (A), 37°C (B), and 42°C (C) and after growth for different periods of time. The lengths of the growth periods (in hours) are indicated above the lanes, as are the growth phases. The arrows indicate the location of the 104-kDa protein. Lanes MW contained standards, whose molecular masses (in kilodaltons) are indicated on the left. (A1, B1, and C1) Reaction intensities (sum intensities) of MAb H7 with the 104-kDa LAP band as measured by the Kodak 1D Image Analysis Program at 25, 37, and 42°C, respectively.
As a control, our analysis of mutant strain AAMU572 in which LAP expression was determined at different growth temperatures and in different growth phases did not reveal any reactions with MAb H7, as determined by either the ELISA or the Western blot assay (data not shown). This indicates that AAMU572 did not express LAP at different growth temperatures and in different growth phases.
Collectively, the ELISA and Western blot analysis results indicated that the level of LAP expression in the L. monocytogenes wild-type strain was higher at 37 and 42°C than at 25°C. Similarly, it has been reported that expression of other virulent proteins, like PrfA, internalin, and listeriolysin, is also influenced by growth temperature and that a higher level of expression occurs at 37°C than at 20 to 26°C (8, 10, 17, 22).
Bacterial adherence assay.
We compared the abilities of L. monocytogenes F4244 and AAMU572 grown at 37°C for 12 h (exponential phase) and 24 h (stationary phase), which expressed low and high levels of LAP, respectively, to adhere to human colon carcinoma cell line Caco-2. The Caco-2 cells (catalog no. TIB37; American Type Culture Collection) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (D10F) (Atlanta Biologicals, Norcross, Ga.) and were incubated at 37°C with 7% CO2 in a humidified incubator. The cell monolayers in 24-well plates were washed three times and supplemented with 0.4 ml of D10F. Bacterial cultures (5 ml) that had been grown for 12 and 24 h at 37°C were treated with 50 μl of 10× protease inhibitor cocktail (Sigma) for 30 min to inhibit bacterial proteases, and the absorbance at 595 nm was adjusted to 0.3. Portions (1 ml) of the cultures were centrifuged (8,160 × g, 5 min) and washed twice, and each pellet was resuspended in 1 ml of D10F. An aliquot of each bacterial suspension was serially diluted and plated onto BHI agar plates to determine bacterial counts. The wells containing Caco-2 cells were inoculated in quadruplicate with 100-μl portions of the bacterial cells and incubated at 37°C for 30 min (7). The monolayers were washed five times with PBS to remove nonadherent bacteria and then treated with 0.5 ml of PBS containing 1% Triton X-100 (Sigma) for 10 min. A 100-μl sample from each well was serially diluted, and bacterial counts were determined on BHI agar plates.
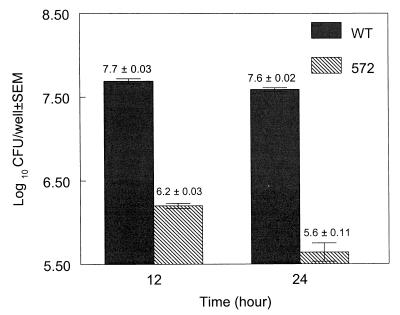
In general, the adhesion results revealed that there was a significant difference between the wild-type strain and mutant strain AAMU572 (P < 0.05) (Fig. 3). The adherence of wild-type strain F4244 to Caco-2 cells was about 2 log units greater than the adherence of the mutant strain when cultures grown for 24 h were used, which is consistent with the results reported by Pandiripally et al. (21). The ratio at which L. monocytogenes cells (6.5 × 107 cells/well) were added to Caco-2 cells (1.6 × 105 cells/well) was about 400:1. Following the adhesion analyses, we estimated that for exponential-phase cultures the adhesion ratios were about 320:1 for the wild-type strain and 10:1 for the mutant strain. For the stationary-phase cultures, the calculated ratios were 250:1 for the wild-type strain and 3:1 for the mutant strain (Fig. 3). These data indicated that there were not significant differences in adhesion between exponential-phase wild-type cultures and stationary-phase wild-type cultures at 37°C (P < 0.05). However, the ELISA and Western blot results indicated that the level of LAP expression was significantly higher during the stationary phase than during the exponential phase. Therefore, we expected that the increase in adherence of the stationary-phase culture would be greater than the increase in adherence of the exponential-phase culture. A possible explanation for the decrease in adhesion is that the L. monocytogenes cells produced few LAP molecules on their surfaces during the exponential phase, yet these molecules may have been sufficient to initiate contact with and binding to most of the receptors present on the Caco-2 cells. In contrast, despite that fact that the same number of bacterial cells was present in stationary-phase cultures, expressing higher number of LAP molecules on their surfaces, there was no qualitative increase in adhesion to the Caco-2 cell receptors. Therefore, an increase in adhesion was not observed in spite of the increased expression of LAP.
FIG. 3.
Adhesion of L. monocytogenes wild-type (WT) and AAMU572 (572) cells obtained from exponential-phase (12-h) and stationary-phase (24-h) cultures grown at 37°C to Caco-2 cells. The log10 CFU/well values are the averages of values from two experiments analyzed in quadruplicate; means ± standard errors of the means (P < 0.05) are shown.
It has been demonstrated previously that the ability of a wild-type strain to enter cultured mammalian cells is influenced by the growth phase of the bacterium (10). Maximum entry was observed with exponential-phase cultures (about 12 h) grown at 37°C, probably because entry into the cells was mediated by internalin, whose expression was maximal during the exponential phase (10). In contrast, Conte et al. (6) showed that growth temperature had no influence on entry of L. monocytogenes into Caco-2 cells.
In our previous report (21) we stated that besides the 104-kDa protein, other factors might be associated with adhesion. This observation was supported by data obtained in adhesion experiments performed with mutant strain AAMU572. The adhesion count for AAMU572 was slightly higher (1.6 × 106 cells/well) during the exponential phase than during the stationary phase (4.4 × 105 cells/well). The difference in adhesion indicates that additional factors (internalin, Act A, or p60) whose levels of expression are higher during the exponential phase than during the stationary phase may be involved (1, 10, 15).
In conclusion, the ELISA and Western blot results demonstrated that there were significant increases in LAP expression over time on the surfaces of L. monocytogenes wild-type cells grown at 37 or 42°C and that the level of expression was higher during the stationary phase than during the exponential phase (P < 0.05). In contrast, the levels of LAP expression at 25°C during the exponential and stationary phases were not significantly different. Additionally, as expected, LAP expression was not observed in mutant strain AAMU572 at different growth temperatures or in different growth phases. Even though the L. monocytogenes wild-type strain exhibited a high level of expression of LAP during the stationary phase at 37°C, it did not exhibit a high level of binding to Caco-2 cells, suggesting that only a few LAP molecules were required to establish adhesion of bacteria to the target host cells.
Acknowledgments
This work was supported in part by funds from the U.S. Department of Education under Title III for strengthening the graduate program in food science at Alabama A&M University and by funds from the Department of Food Science at Purdue University.
We thank Maribeth Cousin and Suzanne Nielsen for critical reading of the manuscript and Bruce Hamaker and Adam Aboubacar for assistance with scanning and image analysis of Western blot membranes.
REFERENCES
- 1.Alvarez-Dominguez C, Vazquez-Boland J A, Carrasco-Marin E, Lopez-Mato P, Leyva-Cobian F. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhunia A K, Ball P H, Fuad A T, Kurz B, Emerson J W, Johnson M G. Development and characterization of a monoclonal antibody specific for Listeria monocytogenes and Listeria innocua. Infect Immun. 1991;59:3176–3184. doi: 10.1128/iai.59.9.3176-3184.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhunia A K, Westbrook D G, Story R, Johnson M G. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Monoclonal antibody mediated blockage of Listeria adhesion to mammalian cells, abstr. P-79; p. 396. [Google Scholar]
- 4.Bubert A, Kuhn M, Goebel W, Kohler S. Structural and functional properties of the p60 proteins from different Listeriaspecies. J Bacteriol. 1992;174:8166–8171. doi: 10.1128/jb.174.24.8166-8171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buncic S, Avery S M. Relationship between variations in pathogenicity and lag phase at 37°C of Listeria monocytogenespreviously stored at 4°C. Lett Appl Microbiol. 1996;23:18–22. doi: 10.1111/j.1472-765x.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 6.Conte M P, Longhi C, Petrone G, Polidoro M, Valenti P, Seganti L. Listeria monocytogenesinfection of Caco-2 cells: role of growth temperature. Res Microbiol. 1994;145:677–682. doi: 10.1016/0923-2508(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 7.Cowart R E, Lashmet J, McIntosh M E, Adams T J. Adherence of a virulent strain of L. monocytogenesto the surface of hepatocarcinoma cell line via lectin-substrate interaction. Arch Microbiol. 1990;153:282–286. doi: 10.1007/BF00249083. [DOI] [PubMed] [Google Scholar]
- 8.Datta A, Kothary M H. Effects of glucose, growth temperature, and pH on listeriolysin O production in Listeria monocytogenes. Appl Environ Microbiol. 1993;59:3495–3497. doi: 10.1128/aem.59.10.3495-3497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of L. monocytogenesinto hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 10.Dramsi S, Kocks C, Forestier C, Cossart P. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and pleiotropic activator prfA. Mol Microbiol. 1993;9:931–941. doi: 10.1111/j.1365-2958.1993.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 11.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 12.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenesinto cells is mediated by internalin, a repeat protein reminiscent of surface antigens from Gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 14.Gay M, Cerf O, Davey K R. Significance of pre-incubation temperature and inoculum concentration on subsequent growth of Listeria monocytogenesat 14°C. J Appl Bacteriol. 1996;81:433–438. doi: 10.1111/j.1365-2672.1996.tb03530.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn M, Goebel W. Identification of an extracellular protein of Listeria monocytogenespossibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989;57:55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Leimeister-Wachter M, Domann E, Chakraborty T. The expression of virulence genes in Listeria monocytogenesis thermoregulated. J Bacteriol. 1992;174:947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer D H, Bunduki M, Beliveau C M, Donnelly C W. Differences in invasion and adherence of Listeria monocytogeneswith mammalian gut cells. Food Microbiol. 1992;9:115–126. [Google Scholar]
- 20.Morange M, Hevin B, Fauve R M. Differential heat shock protein synthesis and response to stress in three avirulent and virulent Listeriaspecies. Res Immunol. 1993;144:667–677. doi: 10.1016/s0923-2494(93)80050-9. [DOI] [PubMed] [Google Scholar]
- 21.Pandiripally V, Westbrook D G, Sunki G R, Bhunia A K. Surface protein p104 is involved in adhesion of Listeria monocytogenesto human intestinal cell line, Caco-2. J Med Microbiol. 1999;48:117–124. doi: 10.1099/00222615-48-2-117. [DOI] [PubMed] [Google Scholar]
- 22.Renzoni A, Klarsfeld A, Dramsi S, Cossart P. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect Immun. 1997;65:1515–1518. doi: 10.1128/iai.65.4.1515-1518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers H J, Perkins H R, Ward J B. Microbial cell walls and membranes. London, United Kingdom: Chapman and Hall; 1980. [Google Scholar]
- 24.Sokolovic Z, Riedel J, Wuenscher M, Goebel W. Surface-associated, PrfA-regulated proteins of Listeria monocytogenessynthesized under stress conditions. Mol Microbiol. 1993;8:219–227. doi: 10.1111/j.1365-2958.1993.tb01566.x. [DOI] [PubMed] [Google Scholar]