Abstract
Recovery from pneumonia takes around 3–6 months in individuals with severe COVID-19. In order to detect the isolated damage caused by COVID-19, the 6-month period must pass after the recoveries. However, to our knowledge, no published study analyzes a comprehensive evaluation of individuals with severe COVID-19 after 6 months. We aimed to evaluate long-term consequences of severe COVID patients by comparing respiratory function, functional capacity, quality of life, fatigue, and balance 6 months after the intensive care unit (ICU) discharge with healthy individuals. Method: 26 post-COVID adult patients and 26 healthy individuals (control group) were included in this study. Physical characteristics of both groups and patients’ ICU data, including APACHE II scores, were recorded. Lung function, respiratory, and peripheral muscle strength were measured. The lower limit of normal (LLN) cutoff points for forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were calculated. A 6-minute walk test (6MWT) was used to assess functional capacity. Time Up and Go test (TUG) with a stadiometer was performed for balance evaluation. Quality of life was evaluated using Nottingham Health Profile (NHP) and St George Respiratory Questionnaire (SGRQ). Results: Percent predicted FVC and FEV1, 6MWT distance, change in oxygen saturation (SpO2) during 6MWT, were lower and NHP, SGRQ, FSS scores and TUG findings were higher in the COVID group than the control group (p < 0.05). The FVC of nine individuals and the FEV1 value of seven individuals in the COVID-19 group were below the LLN values. A moderate correlation was found between ICU length of stay and APACHE II scores with FVC, FEV1, 6MWT distance, and change in SpO2 values in the COVID-19 patients (p < 0.05). Conclusion: Respiratory function, functional capacity, quality of life, and fatigue levels of the individuals with severe COVID-19 infection are impaired at 6 months after ICU discharge. Impaired lung function might be associated with severe inflammation, which starts during the acute infection process and the fibrous tissue during the healing process, impairing lung compliance and diffusion capacity. Infiltration of coronavirus and inflammatory cytokines into the cerebrum and muscle might have increased fatigue and decreased functional capacity. Overall, our study suggests that severe COVID patients need post-discharge care even after 6 months of recovery.
Keywords: lung function, post-COVID-19, fibrosis, functional capacity, fatigue, quality of life
1. Introduction
The new Coronavirus (SARS-CoV-2) causes gradually worsened respiratory problems, acute respiratory distress syndrome (ARDS), septic shock, and/or multi-organ failure, requiring prolonged mechanical ventilation support in 5% of these COVID-19 cases [1]. In addition to respiratory problems, cardiovascular, neurological, musculoskeletal and gastrointestinal system effects, especially in children [2], can also be accompanied in individuals with COVID-19 infection. In particular, patients with invasive mechanical ventilation (IMV) and vasopressor therapy for hypotension and acute hypoxemic respiratory failure are hospitalized and followed up in the intensive care unit (ICU). Circulatory parameters are affected, and vascular inflammation and coagulation disorders can be seen in individuals with advanced age and comorbidities [3]. Negative hematological changes including increased inflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-γ), and interleukin 6 (IL-6) are prominent in cases with severe infection [4,5]. Systemic inflammation caused by the virus also disrupts the coagulant-anticoagulant balance [6] and increases the frequency of acute cerebrovascular diseases, impaired consciousness, and skeletal muscle damage in severe cases of COVID-19 disease [5].
There may be functional loss in patients discharged from ICU after COVID-19 pneumonia due to acute illness, ARDS, and intensive care processes in the post-acute period. Permanent pulmonary damage (alveolar endothelial dysfunction and accompanying pulmonary fibrosis), polyneuropathy, myopathy, weakness, loss of muscle mass, pain, fatigue, depression, anxiety, and occupational problems are frequently observed in hospitalized COVID-19 patients [7,8,9].
Development of a restrictive type of lung disease and reduced diffusion capacity often draws attention to lung function during the post-COVID period, with an average of 1 month after discharge from the hospital or symptoms resolved [10]. Inflammatory biomarker levels return to normal levels in the post-COVID 3-month period, but the damage in the lung continues for more extended periods. Computed tomography (CT) examinations performed 3 months later show an interstitial pattern, fibrosis, and consolidation [11]. It takes 5–6 months for pneumonia to be wholly absorbed, and pulmonary fibrosis develops [12]. The permanent sequelae are not associated with aerobic capacity or respiratory function; they need to be explained by different mechanisms [13].
In order to detect the isolated damage caused by COVID-19, the 6-month period must pass after the recovery. However, today, to our knowledge, there are no studies in the literature that provide a comprehensive assessment of the mechanisms of persistent sequelae in individuals with severe COVID-19 after 6 months. Therefore, our study aimed to evaluate respiratory function, functional capacity, peripheral muscle strength, balance, physical activity, and quality of life in patients with severe COVID-19 infection to give a comprehensive overview of the patients 6 months after severe COVID-19. We evaluated individuals at the 6th month after discharge to show the effect of pulmonary fibrosis alone, which is likely to develop after pneumonia in the lung tissue. In addition, we aimed to investigate whether the length of stay in the ICU and physiological characteristics, including Acute Physiology Chronic Health Evaluation (APACHE) II scores, are related to respiratory function, functional capacity, peripheral muscle strength, balance, physical activity, and quality of life in the COVID-19 group.
2. Materials and Methods
2.1. Participants
A total of 52 individuals, 26 with COVID-19 and 26 healthy subjects, were included in the study. We calculated (G*Power Version 3.1.9.4, Franz Faul, Universitat Kiel, Düsseldorf, Germany) a power of 80% with 0.05 significance, a difference to be detected of 55 m, and a standard deviation of 82 m in 6-minute walk test (6MWT) distance [14], generating a sample of 26 individuals per group.
All individuals’ sociodemographic information and vaccination status were recorded. ICU characteristics, including type of mechanical ventilation (non-invasive or invasive), corticosteroid therapy, blood laboratory results, and APACHE II scores, were recorded after patients were hospitalized. The data were collected from March 2021 to January 2022. COVID-19 pneumonia patients followed up for at least 24 h in the ICU and in the post-COVID 6-months, who did not have any other acute diseases (such as infection and trauma) that would prevent performing the tests and/or interfering with the test results, who could cooperate with the tests to be performed, and who volunteered to participate in the study were included to the COVID-19 group. Individuals with pulmonary, orthopedic, neurological, vestibular, and psychological problems diagnosed before COVID-19 infection; myocarditis developed after COVID-19 infection; and those pregnant were excluded. Age and gender-matched healthy individuals without any acute diseases (such as infection, trauma) that would prevent them from performing the tests and/or that could affect the test results and who were cooperative in the tests to be performed were included in the control group. Individuals who had chronic orthopedic, pulmonary, neurological, and psychological problems preventing them from performing the tests and those pregnant were excluded.
2.2. Experimental Protocol
First, sociodemographic information of all individuals included in the study was obtained, and all questionnaires were conducted. After the questionnaires were performed, balance evaluation, pulmonary function test, respiratory muscle strength measurement, peripheral muscle strength measurement, Time-Up Go (TUG) test, and a 6MWT were performed.
2.2.1. Modified Medical Research Council (MMRC) Dyspnea Scale
Shortness of breath was evaluated using the Modified Medical Research Council (MMRC) dyspnea scale. It is a categorical scale in which individuals choose the most appropriate of the five expressions of dyspnea, between 0–4 points, to define their dyspnea levels [15].
2.2.2. Lung Function Test
Spirometry (Cosmed Pony FX Spirometer, Milan, Italy) was used to measure forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC, peak expiratory flow rate (PEF), and forced expiratory volume from 25–75% (FEF25–75%), based on the European Respiratory Society/American Thoracic Society (ERS/ATS) criteria. The test was performed in the sitting position. At least three technically acceptable measurements were obtained between the two best-measured FEV1 values, with no more than a 5% difference, and the best FEV1 value was selected for analysis. The lower limit of normal (LLN) for FVC and FEV1 was calculated for each subject [16].
2.2.3. Respiratory Muscle Strength
Respiratory muscle strength was measured (Cosmed Pony FX Spirometer, Milan, Italy). For the measurement of maximum inspiratory pressure (MIP), maximum expiration was performed to the person, and immediately the respiratory tract was closed with a valve, and then the person was asked to perform maximum inspiration for 1–3 s. For maximum expiratory pressure (MEP), a maximal inspiration was performed, and then the person was asked to perform a maximal expiration of 2 s against the closed airway. At least three technically acceptable maneuvers were performed, with no more than a 5% difference between the two best-measured values [17].
2.2.4. Peripheral Muscle Strength
Peripheral muscle strength was determined by measuring handgrip strength and quadriceps muscle strength using a digital dynamometer (Jtech Commander Muscle Tester, Midvale, UT, USA). The mean values of the right and left side measurements were obtained. Then, the measurements were recorded in Newton (N) using each side’s measurements [14].
2.2.5. Evaluation of Functional Capacity
The functional capacity of individuals was evaluated using the 6MWT [18]. Before and after 6MWT, heart rate, blood pressure, oxygen saturation (SpO2) (Cosmed Spiropalm 6MWT, Rome, Italy), respiratory frequency were assessed, and fatigue levels during exertion, and dyspnea were evaluated using the Modified Borg Scale [19].
2.2.6. Balance Evaluation
The stabilometer device (HUR Smartbalance 2031, Kokkola, Finland) to be used in the study is a balance mechanism that can move forward-backward, right-left, and 3D in the transverse plane. The device’s reliability has been demonstrated in different studies [20,21]. In addition, the Time-Up Go (TUG) test was used to assess balance. The TUG test is predicted to be an easy-to-apply and reliable test evaluating balance [22].
2.2.7. Hospital Anxiety and Depression Scale (HADS)
Hospital Anxiety and Depression Scale (HADS) is a frequently used scale that the patient fills in themself, in which anxiety and depression symptoms are screened. Our study used the validated and reliable form of HADS adapted to Turkish society [23].
2.2.8. Mini-Mental State Test
Mini-Mental State Test (MMST) was used to evaluate the cognitive functions of the patients. The MMST is a widely used and validated test in evaluating cognitive status [24].
2.2.9. Fatigue Severity Scale (FSS)
Fatigue level of individuals was evaluated using FSS. In our study, the Turkish version of the FSS was used [25].
2.2.10. International Physical Activity Questionnaire (IPAQ) Short Form
IPAQ short form was used to assess participants’ physical activity levels. Severe, moderate-severe, and walking include seven questions that question the time elapsed while doing these, and sitting time is considered a separate question. Metabolic equivalence (MET-minute) score is obtained as a result of calculations. Total MET values are calculated, and those with a total MET value of <600 MET-min/week inactive, 600–3000 MET-min/week of minimum active, and >3000 MET-min/week are classified as very active [26].
2.2.11. St. George Respiratory Questionnaire (SGRQ) and Nottingham Health Profile (NHP)
SGRQ and NHP for quality of life were used. The SGRQ is a disease-specific quality of life questionnaire consisting of 76 items: the symptoms part (29 items), the activity part (9 items), and the impact part (38 items) [27]. The NHP is a frequently used measurement to assess the perceived health status. People’s problems in daily life are questioned in seven sub-categories: pain, emotional reactions, sleep, social isolation, physical activity, and energy [28].
2.3. Statistical Analysis
Statistical analyses and graphs were performed using SPSS Statistics 22.0 (IBM Inc., Armonk, NY, USA) and GraphPad Prism 9.0.0 (GraphPad Software, San Diego, CA, USA). Values are presented as the mean and related standard deviation, median values with minimum and maximum, frequencies, and percentages. The Kolmogorov–Smirnov test was applied to determine the compatibility of the parametric data with the normal distribution. Student’s t-test and the Mann–Whitney U test were used to compare the measured values of COVID-19 and control groups, as appropriate. Spearman correlation analysis was performed to examine the relationship between variables of the COVID-19 group. The descriptive level of significance was set at p < 0.05.
3. Results
3.1. Participant Characteristics
Participants’ characteristics are summarized in Table 1. Age, height, weight, body mass index, and smoking exposure were similar between COVID-19 and control groups (p > 0.05). There were 10 active smokers in the COVID-19 group and 7 in the control group. The MMRC score of the COVID-19 group was found to be significantly higher than that of the control group (p < 0.05). When the individuals in the COVID-19 group were examined, six individuals had never been vaccinated, two individuals had received a single dose, 11 individuals had received two doses, and seven individuals had received three doses. Of these, 13 were Sinovac (CoronaVac, Inactivated, Sinovac Life Sciences, Beijing, China) and 9 were BioNTech (BNT162b2 Vaccine, BioNTech SE, Mainz, Germany) vaccines. All of the individuals in the COVID-19 group were given azithromycin as antibacterial, enoxaparin sodium and acetylsalicylic acid as anticoagulant, and favipiravir as antiviral. Prednisolone and prednisone were given to 15 patients as steroids. In addition, the most common comorbidities encountered were hypertension and anxiety. After discharge, it was determined that four patients were diagnosed with hypertension and six patients were diagnosed with anxiety.
Table 1.
Physical and demographic characteristics and dyspnea in COVID-19 and control groups.
| Parameter a | COVID-19 Group (n = 26) | Control Group (n = 26) | p |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 50.0 ± 13.8 | 47.0 ± 12.6 | 0.41 |
| Height (cm) | 171.2 ± 9.3 | 171.0 ± 7.0 | 0.89 |
| Weight (kg) | 79.1 ± 15.5 | 73.8 ± 13.8 | 0.20 |
| BMI (kg/m2) | 27.1 ± 5.9 | 25.1 ± 3.7 | 0.15 |
| MMRC score | 0.6 ± 0.5 | 0.1 ± 0.3 | <0.001 * |
| Smoking (pack-years) | 19.2 ± 15.3 | 20.0 ± 8.1 | 0.90 |
| Number of active smokers | 10 | 7 |
* The bold values represent statistically significant p < 0.05 values. a Student’s t-test. BMI—Body Mass Index; MMRC—Modified Medical Research Council Dyspnea Scale.
3.2. Lung Function Test
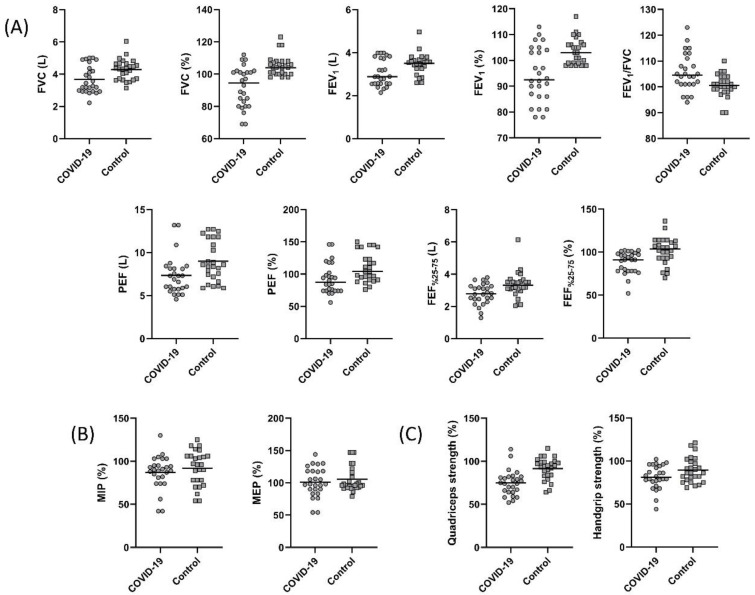
The pulmonary function test parameters are summarized in Figure 1. A statistically significant decrease in FVC, FEV1, PEF, and FEF25–75% was found in the COVID-19 group (p < 0.05, Figure 1A). The FEV1/FVC values were 104.50 (94.00–123.00) and 100.50 (90.00–110.00) in the COVID-19 and control groups, respectively. Although not statistically significant, the FEV1/FVC was low in the COVID-19 group (p > 0.05). The LLN for FVC was 3.51 ± 0.58 L and 3.52 ± 0.54 L for the COVID-19 and the control groups, respectively (p = 0.959). The LLN for measured FEV1 was 2.70 ± 0.44 L and 2.74 ± 0.42 L for the COVID-19 and the control groups, respectively (p = 0.729). The measured FVC value of nine individuals in the COVID-19 group and the measured FEV1 value of seven individuals were below the LLN values of measured FVC and FEV1. However, there was no individual in the control group with FVC and FEV1 values below the LLN value.
Figure 1.
Comparison of (A) forced vital capacity (FVC) (p = 0.005), percent predicted FVC (p < 0.001), forced expiratory volume in one second (FEV1) (p = 0.04), percent predicted FEV1 (p = 0.001), FEV1/FVC (p = 0.255), peak expiratory flow (PEF) (p = 0.01), percent predicted PEF (p = 0.007), forced expiratory volume from 25 to 75% (FEF25–75%) (p = 0.01), percent predicted FEF25–75% (p = 0.001), (B) percent predicted maximum inspiratory pressure (MIP) (p = 0.38), percent predicted maximum expiratory pressure (MEP) (p = 0.46), (C) percent predicted quadriceps strength (p < 0.001), and percent predicted handgrip strength (p = 0.048) between the COVID-19 and control groups.
3.3. Respiratory and Peripheral Muscle Strength
Respiratory and peripheral muscle strength values are shown in Figure 1. MIP and MEP were similar between the groups (p > 0.05, Figure 1B). Handgrip strength and quadriceps muscle strength values were significantly lower in the COVID-19 group (p < 0.05, Figure 1C). The 15 (57.6%) subjects from the COVID-19 group had quadriceps strength lower than the 95% confidence interval of the controls.
3.4. Functional Capacity Evaluation
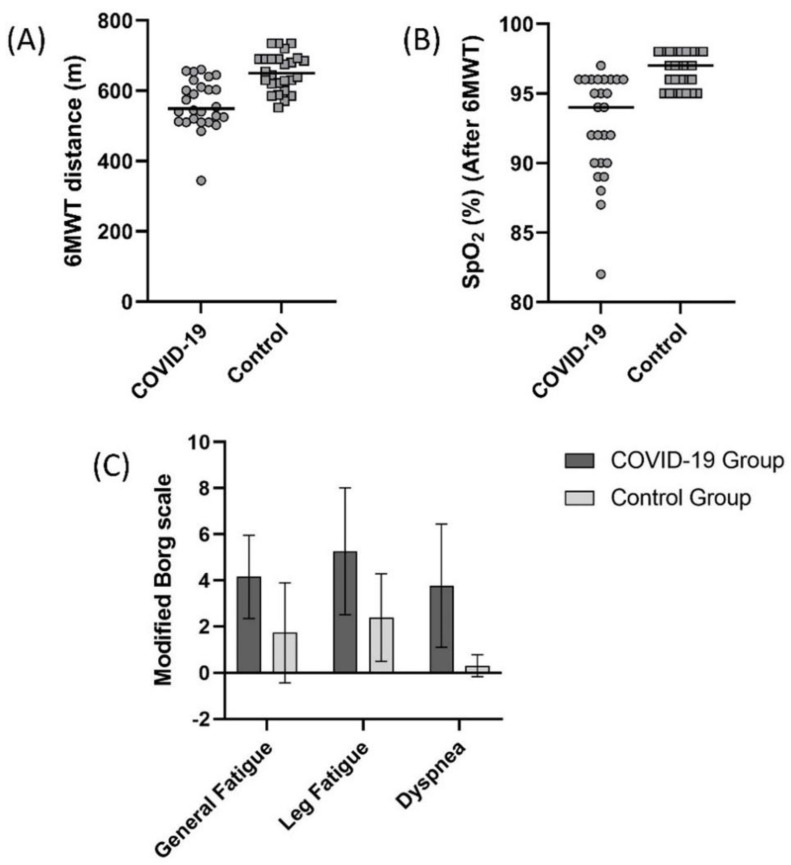
A comparison of 6MWT distance values in the COVID-19 and control group is summarized in Figure 2. In the COVID-19 and control groups, 6MWT distance was 561.1 ± 71.0 m and 652.6 ± 53.4 m, respectively (Figure 2A, p < 0.05). The 6MWT distance of the 21 (80.7%) subjects from the COVID-19 group was lower than the controls’ 95% confidence interval (631.0–674.1 m). SpO2 value measured during 6MWT was significantly lower (Figure 2B), and general fatigue, leg fatigue, and dyspnea scores measured were significantly higher in the COVID-19 group after the test (Figure 2C, p < 0.05).
Figure 2.
Comparison of (A) 6 minute walk test (6MWT) distance (p < 0.001), (B) oxygen saturation (SpO2) after 6MWT (p < 0.001), (C) general fatigue (Borg) (p < 0.001), leg fatigue (Borg) (p < 0.001), and dyspnea (Borg) (p < 0.001) scores between the COVID-19 and control groups.
3.5. Balance Evaluation
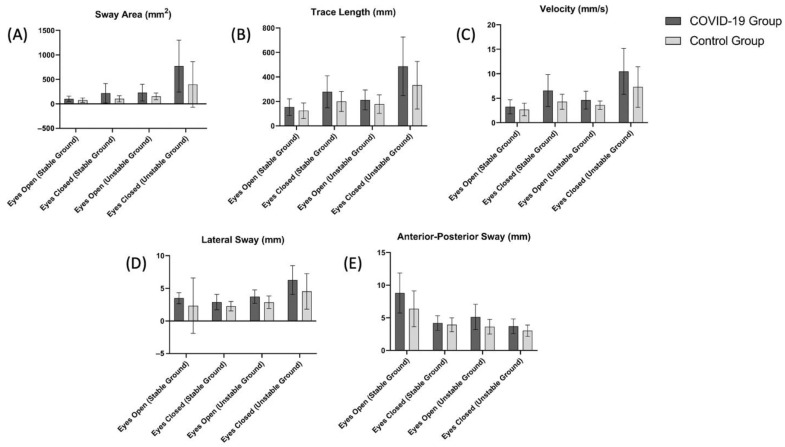
The TUG test results and the balance evaluation using a stabiliometer are summarized in Table 2 and Figure 3, respectively. The TUG test scores of the COVID-19 group were significantly higher than the control group (p < 0.05, Table 2). Based on the scores obtained in the stabiliometer, the sway area (mm2) (Figure 3A) value measured for eyes open (stable ground and unstable ground) and eyes closed (stable ground, unstable ground) was significantly higher in the COVID-19 group (p < 0.05). Trace length (mm) (Figure 3B), velocity (mm/s) (Figure 3C), and lateral sway (mm) (Figure 3D) scores were significantly higher in the COVID-19 group in all measured conditions except for eyes open (stable ground) (p < 0.05).
Table 2.
Comparison of MMST, HADS, FSS, TUG, physical activity, and quality of life in COVID-19 and control groups.
| Variables | COVID-19 Group (n = 26) |
Control Group (n = 26) |
p |
|---|---|---|---|
| Median (Min-Max) | Median (Min-Max) | ||
| HADS a,c | 14.2 ± 7.0 | 5.9 ± 3.6 | <0.001 * |
| FSS a,c | 3.5 ± 1.5 | 1.2 ± 0.7 | <0.001 * |
| MMST total score a,c | 27.7 ± 2.8 | 28.2 ± 1.5 | 0.42 |
| TUG a,c | 7.9 ± 1.2 | 7.2 ± 0.9 | 0.049 * |
| International Physical Activity Questionnaire b | |||
| High intensity (MET-min/week) | 0 (0–3360) | 0 (0–30240) | 0.10 |
| Medium intensity (MET-min/week) | 0 (0–3600) | 240 (0–1440) | 0.23 |
| Walking score (MET-min/week) | 1386 (132–2772) | 792 (0–11088) | 0.14 |
| Sitting score (MET-min/week) | 5 (3–6) | 5 (4–6) | 0.99 |
| Total score (MET-min/week) | 1391 (136–6736) | 929 (324–41333) | 0.08 |
| St George Respiratory Questionnaire b | |||
| Symptom | 30.5 (0–69.7) | 14.0 (0–40.4) | 0.01 * |
| Activity | 38.0 (0–95.6) | 0 (0–62.9) | <0.001 * |
| Impact | 16.7 (0–78.1) | 4.0 (0–15.0) | 0.001 * |
| Total Score | 28.9 (2.1–87.4) | 9.7 (0–30.0) | 0.001 * |
| Nottingham Health Profile b | |||
| Pain | 18.6 (0–89.5) | 0 (0–27.4) | 0.01 * |
| Emotional Reactions | 10.5 (0–92.8) | 0 (0–31.5) | 0.02 * |
| Sleep | 19.9 (0–61.5) | 0 (0–77.6) | 0.42 |
| Social Isolation | 0 (0–77.5) | 0 (0–16.0) | 0.03 * |
| Physical Activity | 16.9 (0–41.9) | 0 (0–22.0) | <0.001 * |
| Energy | 24 (0–100.0) | 0 (0–36.8) | <0.001 * |
| Total Score | 155.6 (0–366.0) | 30.6 (0–130.9) | 0.001 * |
* The bold values represent statistically significant p < 0.05 values. a Student t-test. b Mann–Whitney U test (different tests were used due to distribution difference). c Expressed in mean ± SD. HADS—Hospital Anxiety and Depression Inventory; FSS—Fatigue Severity Scale; MMST—Mini-mental State Test; TUG—Time-Up Go Test.
Figure 3.
Comparison of (A) sway area (eyes open (stable ground), p = 0.049; eyes closed (stable ground), p = 0.009; eyes open (unstable ground), p = 0.038; eyes closed (unstable ground), p = 0.010), (B) trace length (eyes open (stable ground), p = 0.11; eyes closed (stable ground), p = 0.01; eyes open (unstable ground), p = 0.11; eyes closed (unstable ground), p = 0.01), (C) velocity (eyes open (stable ground), p = 0.14; eyes closed (stable ground), p = 0.003; eyes open (unstable ground), p = 0.01; eyes closed (unstable ground), p = 0.01), (D) lateral sway (eyes open (stable ground), p = 0.18; eyes closed (stable ground), p = 0.02; eyes open (unstable ground), p = 0.003; eyes closed (unstable ground), p = 0.01), (E) anterior-posterior sway eyes open (stable ground), p = 0.02; eyes closed (stable ground), p = 0.002; eyes open (unstable ground), p = 0.39; eyes closed (unstable ground), p = 0.004) scores between the COVID-19 and control groups.
3.6. Hospital Anxiety and Depression Scale (HADS), Fatigue Severity Scale (FSS), and Mini-Mental Test (MMST)
HADS, FSS, and MMST scores are shown in Table 2. HADS and FSS scores were found to be significantly higher in the COVID-19 group (p < 0.05), while there was no statistical difference between the groups in MMST scores (p > 0.05).
3.7. International Physical Activity Questionnaire (IPAQ) Short Form, St George Respiratory Questionnaire (SGRQ), and Nottingham Health Profile (NHP)
No significant differences were found in the IPAQ high and medium intensity scores, IPAQ walking and sitting score, and the IPAQ total score between the groups (p > 0.05, Table 2). The total score, symptom, activity, and impact scores of SGRQ were significantly higher in the COVID-19 group (p < 0.05). The total scores, pain, emotional reactions, sleeping, social isolation, physical activity, and energy scores of NHP were statistically significantly higher in the COVID-19 group (p < 0.05).
3.8. Conditions of Those Receiving Corticosteroid and Mechanical Ventilation in the COVID-19 Group
Comparison of respiratory function and functional capacity of the individuals with COVID-19 compared to those who received and did not receive steroid therapy and those who received non-invasive mechanical ventilation (NIMV) and the IMV treatment are summarized in Table 3. The percent predicted FVC and FEV1, FEV1/FVC, 6MWT distance (m), SpO2 (after 6MWT), length of stay in the ICU, and APACHE II values of those who received steroid treatment were found to be significantly lower than those who did not receive steroid treatment (p < 0.05). Similarly, the same parameters except for the length of stay in the ICU and APACHE II in individuals treated with the IMV were statistically significantly lower than those treated with NIMV (p < 0.05).
Table 3.
Comparison of respiratory function and functional capacity of individuals with COVID-19 between those who received and did not receive steroid therapy and those who received NIMV and IMV.
| Parameter | Receiving Steroid Therapy (n = 15) |
Not Receiving Steroid Therapy (n = 11) |
p | NIMV (n = 12) |
IMV (n = 14) |
p |
|---|---|---|---|---|---|---|
| Median (Min-Max) | Median (Min-Max) | Median (Min-Max) | Median (Min-Max) | |||
| FVC (%) b | 83.0 (69.0–102.0) | 101.0 (89.0–112.0) | 0.001 * | 101.0 (80.0–112.0) | 84.0 (69.0–102.0) | 0.004 * |
| FEV1 (%) b | 90.0 (78.0–108.0) | 103.0 (87.0–113.0) | 0.008 * | 100.0 (81.0–113.0) | 90.0 (78.0–108.0) | 0.04 * |
| FEV1/FVC b | 105.0 (101.0–123.0) | 101.0 (94.0–113.0) | 0.01 * | 101.0 (94.0–113.0) | 106.5 (101.0–123.0) | 0.02 * |
| 6MWT distance (m) a,c | 532.7 ± 68.5 | 599.9 ± 56.2 | 0.01 * | 603.2 ± 54.8 | 525.1 ± 64.1 | 0.003 * |
| SpO2 (after 6MWT) (%) a,c | 92.0 (82.0–96.0) | 95.0 (90.0–97.0) | 0.04 * | 95.5 (90.0–97.0) | 91.0 (82.0–96.0) | 0.01 * |
| Length of stay in the ICU (days) a,c | 9.9 ± 7.9 | 4.1 ± 3.9 | 0.02 * | 3.8 ± 3.8 | 10.5 ± 7.8 | 0.01 * |
| APACHE II a,c | 15.5 ± 7.0 | 10.1 ± 5.5 | 0.046 * | 9.9 ± 5.3 | 16 ± 6.9 | 0.02 * |
* The bold values represent statistically significant p < 0.05 values. a Student t-test. b Mann–Whitney U test (different tests were used due to distribution difference). c Expressed as mean ± SD. FVC—Forced vital capacity; FEV1—Forced expiratory volume in one second; 6MWT—6-minute walk test; SpO2—Oxygen saturation.
3.9. Relationship between the Length of ICU Stay, APACHE II, and TUG Scores with the Measured Parameters
Correlation of the length of ICU stay, APACHE II, and TUG scores of the individuals in the COVID-19 group with the measured parameters are shown in Table 4. A correlation was found between the length of stay in the ICU and vaccination status (r = −0.633, p < 0.001), percent predicted FVC (r = −0.564, p = 0.003), percent predicted FEV1 (r = −0.453, p = 0.02), 6MWT distance (r = −0.427, p = 0.03), the change in SpO2 (r = 0.469, p = 0.02), HADS (r = 0.394, p = 0.047), SGRQ symptom (r = 0.551, p = 0.004), SGRQ activity (r = 0.532, p = 0.005), SGRQ impact (r = 0.573, p = 0.002), and SGRQ total scores (r = 0.568, p = 0.002). However, no significant correlation was found between TUG (r = −0.095, p = 0.64), MMRC (r = 0.327, p = 0.10), NHP total score (r = 0.039, p = 0.85), NHP pain (r = 0.232, p = 0.25), NHP emotional reactions (r = −0.118, p = 0.57), NHP social isolation (r = −0.132, p = 0.52), NHP physical activity (r = 0.104, p = 0.61), and NHP energy (r = 0.086, p = 0.69) scores with the length of ICU stay. A correlation was found between the APACHE II scores with vaccination status (r = −0.426, p < 0.03), percent predicted FVC (r = −0.455, p = 0.02), 6MWT distance (r = −0.449, p = 0.02), the change in SpO2 (r = 0.417, p = 0.03), HADS (r = 0.394, p = 0.046), SGRQ activity (r = 0.396, p = 0.045), and SGRQ impact (r = 0.428, p = 0.02) scores. However, no significant correlation was found between TUG (r = 0.015, p = 0.94), MMRC (r = 0.368, p = 0.06), percent predicted FEV1 (r = −0.315, p = 0.11), handgrip strength (r = −0.330, p = 0.10), NHP total score (r = 0.131, p = 0.52), NHP pain (r = 0.169, p = 0.41), NHP emotional reactions (r = 0.052, p = 0.80), NHP sleeping (r = 0.122, p = 0.55), NHP social isolation (r = −0.120, p = 0.55), NHP physical activity (r = 0.259, p = 0.20), NHP energy (r = 0.142, p = 0.49), SGRQ symptom (r = 0.372, p = 0.06), and SGRQ total score (r = 0.372, p = 0.06) with APACHE II scores. A correlation was found between the TUG scores with percent predicted FVC (r = −0.442, p = 0.02), percent predicted FEV1 (r = −0.546, p = 0.004), NHP total score (r = 0.494, p = 0.01), NHP emotional reactions (r = 0.418, p = 0.03), NHP sleeping (r = 0.555, p = 0.003), and NHP physical activity (r = 0.490, p = 0.01) scores. However, no significant correlation was found between vaccination status (r = 0.298, p = 0.14), MMRC (r = 0.221, p = 0.28), 6MWT distance (r = −0.075, p = 0.71), the change in SpO2 (r = −0.242, p = 0.23), handgrip strength (r = −0.295, p = 0.14), HADS (r = 0.102, p = 0.62), NHP pain (r = 0.146, p = 0.48), NHP social isolation (r = −0.030, p = 0.89), and NHP energy (r = 0.353, p = 0.08) scores with the TUG.
Table 4.
Correlation of the length of stay in the intensive care unit and APACHE II scores of the individuals in the COVID-19 group with the measured parameters.
| Parameter d | Length of Stay in the Intensive Care Unit | APACHE II Scores | Time Up Go Test | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Vaccination status | −0.633 | 0.001 * | −0.426 | 0.03 * | 0.298 | 0.14 |
| Time Up Go Test | −0.095 | 0.64 | 0.015 | 0.94 | - | - |
| MMRC | 0.327 | 0.10 | 0.368 | 0.06 | 0.221 | 0.28 |
| FVC | −0.564 | 0.003 * | −0.455 | 0.02 * | −0.442 | 0.02 * |
| FEV1 | −0.453 | 0.02 * | −0.315 | 0.12 | −0.546 | 0.004 * |
| 6MWT distance | −0.427 | 0.03 * | −0.449 | 0.02 * | −0.075 | 0.72 |
| ΔSpO2 | 0.469 | 0.02 * | 0.417 | 0.03 * | −0.242 | 0.23 |
| Handgrip strength | −0.487 | 0.01 * | −0.330 | 0.10 | −0.295 | 0.14 |
| HADS | 0.394 | 0.047 * | 0.394 | 0.046 * | 0.102 | 0.62 |
| NHP Total Score | 0.039 | 0.85 | 0.131 | 0.52 | 0.494 | 0.01 * |
| NHP Pain | 0.232 | 0.25 | 0.169 | 0.41 | 0.146 | 0.48 |
| NHP Emotional Reactions | −0.118 | 0.57 | 0.052 | 0.80 | 0.418 | 0.03 * |
| NHP Sleep | 0.018 | 0.93 | 0.122 | 0.55 | 0.555 | 0.003 * |
| NHP Social Isolation | −0.132 | 0.52 | −0.120 | 0.56 | −0.030 | 0.89 |
| NHP Physical Activity | 0.104 | 0.61 | 0.259 | 0.20 | 0.490 | 0.01 * |
| NHP Energy | 0.086 | 0.67 | 0.142 | 0.49 | 0.353 | 0.08 |
| SGRQ Total Score | 0.568 | 0.002 * | 0.372 | 0.06 | 0.112 | 0.59 |
| SGRQ Symptom | 0.551 | 0.004 * | 0.339 | 0.09 | 0.101 | 0.62 |
| SGRQ Activity | 0.532 | 0.005 * | 0.396 | 0.045 * | 0.133 | 0.52 |
| SGRQ Impact | 0.573 | 0.002 * | 0.428 | 0.03 * | 0.074 | 0.72 |
* The bold values represent statistically significant p < 0.05 values. d Spearman correlation analysis. Vaccination status: Number of vaccine doses of the individuals. MMRC—Modified Medical Research Council; FVC—Forced vital capacity; FEV1—Forced expiratory volume in one second; 6MWT—6-minute walk test; ΔSpO2—Oxygen saturation difference before and after 6MWT; HADS—Hospital Anxiety and Depression Scale; NHP—Nottingham Health Profile; SGRQ—St. George Respiratory Questionnaire.
3.10. Laboratory Changes in Patients with COVID-19
The laboratory changes in patients of COVID-19 are summarized in Table 5. White blood cell (WBC), neutrophil (NE), lymphocyte (LY), and platelet (PLT) values did not change; however, biochemical, coagulation, and inflammatory biomarkers all increased. In addition, although the mean values of WBC and LY were within the reference value range, it was found that WBC values were above the reference values in 8 of the patients, and LY values were below the reference values in 10 of them.
Table 5.
Summary of laboratory findings in patients with COVID-19.
| Parameter | Mean ± SD | Changes with Reference Value |
|---|---|---|
| Hematologic | ||
| White blood cell count (103/µL) | 8.4 ± 3.6 | ↔ |
| Neutrophil (%) | 61.8 ± 10.5 | ↔ |
| Lymphocyte (%) | 18.1 ± 11.5 | ↔ |
| Hemoglobin (g/dL) | 11.0 ± 2.3 | ↓ |
| Hematocrit (%) | 37 ± 4.5 | ↓ |
| Platelet count (103/µL) | 201.7 ± 65.6 | ↔ |
| Biochemical | ||
| Lactate dehydrogenase (U/L) | 350.4 ± 167.2 | ↑ |
| Creatine kinase-MB (U/L) | 33.2 ± 12.9 | ↑ |
| Aspartate aminotransferase (U/L) | 62.9 ± 51.8 | ↑ |
| Alanine aminotransferase (U/L) | 59.0 ± 56.7 | ↑ |
| Troponin T (ng/L) | 30.1 ± 33.4 | ↑ |
| Kreatinin (mg/dL) | 1.4 ± 0.8 | ↑ |
| Coagulation | ||
| Prothrombin time (sn) | 16.8 ± 2.4 | ↑ |
| D-dimer (µg/L) | 829.9 ± 1027.5 | ↑ |
| Inflammatory biomarkers | ||
| Ferritin (µg/L) | 343.5 ± 184.4 | ↑ |
| C-reactive protein (mg/L) | 42.3 ± 46.5 | ↑ |
| Erythrocyte sedimentation rate (mm/h) | 16.8 ± 8.8 | ↑ |
Changes with reference value: ↔ no change, ↑ increase, ↓ decrease.
3.11. Relationship between CRP and LDH with FVC, 6MWT Distance, Dyspnea, and SGRQ Total Score
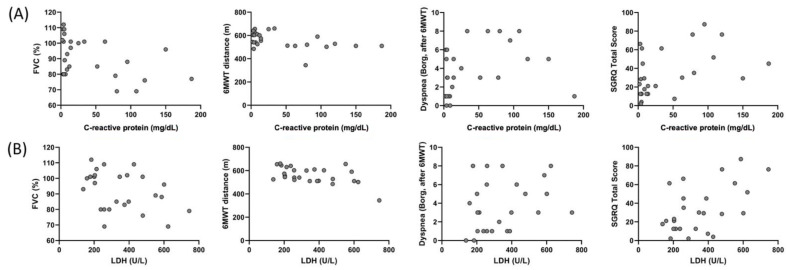
Correlation of CRP and LDH of the individuals in the COVID-19 group with FVC (%), 6MWT distance, dyspnea (after 6MWT), and SGRQ total score are shown Figure 4. A correlation was found between CRP and FVC (%) (r = −0.392, p = 0.048), 6MWT distance (r = −0.460, p = 0.01), dyspnea (Borg, after 6MWT) (r = 0.417, p = 0.03), and SGRQ total score (r = 0.465, p = 0.01), and between LDH and FVC (%) (r = −0.406, p = 0.04), 6MWT distance (r = −0.516, p = 0.007), and SGRQ total score (r = 0.442, p = 0.02) in the COVID-19 group.
Figure 4.
Correlation of the C-reactive protein with (A) FVC (%) (r = −0.392, p = 0.048), 6MWT distance (r = −0.460, p = 0.01), dyspnea (Borg, after 6MWT) (r = 0.417, p = 0.03), and SGRQ total score (r = 0.465, p = 0.01), and correlation of LDH with (B) FVC (%) (r = −0.406, p = 0.04), 6MWT distance (r = −0.516, p = 0.007), dyspnea (Borg, after 6MWT) (r = 0.321, p = 0.11), and SGRQ total score (r = 0.442, p = 0.02) in the COVID-19 group.
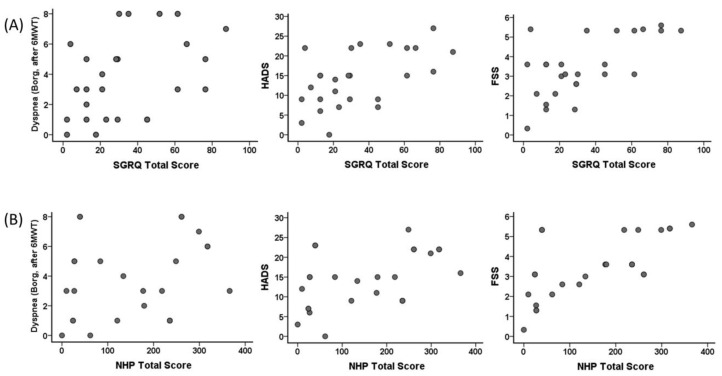
3.12. Relationship between SGRQ Total Score and NHP Total Score with Dyspnea (Borg, after 6MWT), HADS, and FSS
Correlation of SGRQ total score and NHP total score of the individuals in the COVID-19 group with dyspnea (Borg, after 6MWT), HADS, and FSS are shown in Figure 5. A correlation was found between SGRQ total scores and dyspnea (Borg, after 6MWT) (r = 0.448, p = 0.02), HADS (r = 0.543, p = 0.004), and FSS (r = 0.528, p = 0.006). A correlation was found between NHP total score and HADS (r = 0.536, p = 0.005) and FSS (r = 0.723, p < 0.001). However, no significant correlation was found between dyspnea (Borg, after 6MWT) (r = 0.358, p = 0.07) and NHP total score.
Figure 5.
Correlation of the St. George Respiratory Questionnaire (SGRQ) total score with (A) dyspnea (Borg, after 6MWT) (r = 0.448, p = 0.02), Hospital Anxiety and Depression Scale (HADS) (r = 0.543, p = 0.004), and Fatigue Severity Scale (FSS) (r = 0.528, p = 0.006), and correlation of Nottingham Health Profile (NHP) total score with (B) dyspnea (Borg, after 6MWT) (r = 0.358, p = 0.07), HADS (r = 0.536, p = 0.005), and FSS (r = 0.723, p < 0.001) in the COVID-19 group.
4. Discussion
In this paper, we have shown respiratory dysfunction in COVID-19 patients after 6 months of discharge from ICU. Findings favored fibrosis in the lung, low oxygen saturation, desaturation during 6MWT, increased shortness of breath, fatigue, and depression and decreased functional capacity, peripheral muscle strength, quality of life, and balance in individuals with severe COVID-19 even 6 months after recovering from the infection. In addition, we found that length of ICU stay and APACHE II scores were associated with FVC and FEV1, 6MWT distance, change in SpO2, handgrip strength, HADS, and SGRQ total score. In line with these results, we believe that the lungs of individuals with severe COVID-19 have permanent damage that reduces respiratory function, functional capacity, and quality of life.
The inflammatory biomarkers return to typical values in the third month of post-COVID, but lung function gradually deteriorates [11] with consolidation, fibrosis, and interstitial pattern in CT scans taken 3 months later [11]. We found lower FVC, FEV1, PEF, and FEF25–75% of the individuals with COVID-19. FVC and FEV1 values of nine and seven individuals, respectively, were below the LLN stating impaired respiratory function in the COVID-19 group. The higher FEV1/FVC values despite decreased FEV1 and FVC (although not statistically significant, p = 0.25) suggested the development of a restrictive type of lung disease in patients with COVID-19. Together with similar respiratory muscle strength between COVID-19 patients and controls, decreased lung volume was not associated with respiratory muscle weakness expected due to the stay in the ICU. Considering the decrease in all measured volume values, a restrictive type of lung disease may have developed [29] since pneumonia is almost completely cleared from the lung tissue within 5–6 months, and fibrous tissue is formed in this process [12].
Ferrandi et al. [30] argue that some skeletal muscle types may show vulnerability to the COVID-19 virus through angiotensin-converting enzyme-2 (ACE-2). The coronavirus is active in the lungs, the leukocytes infiltrating the lung tissue, and the cytokines (especially IL-6) are secreted by these leukocytes. This disrupts metabolic hemostasis and causes muscle loss by infiltrating into the muscle [30]. In our study, the handgrip and quadriceps muscle strength were lower in the COVID-19 group, which can be attributed to the above mechanism explaining muscle loss. In addition, insufficient oxygen diffusion and transport to peripheral tissues due to fibrosis might explain peripheral muscle weakness.
Pathophysiological changes resulting from ARDS are associated with COVID-19 [31]. Secondary lung injury such as edema, pulmonary inflammation, abnormal surfactant function, decrease in compliance, and deterioration in gas diffusion occur due to prolonged IMV, and these changes may decrease the functional capacity [31]. We found impaired functional capacity measured using 6MWT and peripheral muscle strength in severe COVID-19 patients 6 months after discharge. The loss in peripheral muscle strength may have resulted in the decreased 6MWT distance. Six individuals in the COVID-19 group were desaturated during the test. The changes in SpO2 may suggest that reduced 6MWT distance is primarily due to possible lung fibrosis in the COVID group, as demonstrated in post-COVID-19 CTs [11].
Inflammation and inflammatory cytokines may disrupt the balance by causing damage to the cerebellar tissue [32]. Rudroff et al. showed impaired balance in the COVID-19 patients based on the TUG and the stabiliometer findings [32]. A balance disorder may be due to immobilization restriction during the stay in the ICU. However, we found no correlation between the length of stay in the ICU and TUG results. Therefore, it is difficult to say that the loss of balance is due to immobilization. TUG was associated with NHP and FVC and FEV1. In other words, inflammation and inflammatory cytokines may be related to the damage in cerebellar tissue, as stated by Rudroff et al. [33], and TUG being associated with respiratory parameters indirectly indicates the relationship of balance with inflammation. Further study is needed to identify the mechanisms of balance disorder, including the neuromuscular and psychological covariates in COVID-19 patients.
The factors likely to cause fatigue in COVID-19 are central, psychological, and peripheral [33]. Psychological problems in individuals who have survived the COVID-19 infection are considered to be part of post-traumatic stress disorder [32,34]. Fears such as being exposed to the virus again, being in ICU, and losing relatives cause psychological trauma. In this direction, we evaluated the HADS scores and found higher scores in the COVID-19 group. Hospitalization due to COVID and the strong correlation between the length of stay in the ICU and HADS scores support the idea of post-traumatic stress disorder [34]. Central factors may include penetration of coronaviruses into the central nervous system triggering inflammation by affecting the release of neurotransmitters, and causing permanent problems such as fatigue due to cerebral hypometabolism. Prolonged physical inactivity due to the disease and ICU stay may be one of the factors causing fatigue [33]. We found higher MMRC, FSS, respiratory frequency, general fatigue, leg fatigue, and dyspnea scores after 6MWT in the COVID-19 group. There were high fatigue levels (general fatigue and leg fatigue before 6MWT and FSS findings) even before the test (at rest). The increase in fatigue after 6MWT and the presence of desaturated individuals during the test showed possible gas diffusion problems and central factors [33]. The individuals in the COVID-19 group may not carry enough oxygen to their active muscles during exercise, and the severity of fatigue increases.
Based on the SGRQ and NHP scores, the quality of life of individuals in the COVID-19 group was impaired. From the activity and physical component subscale scores in the COVID-19 patients, one of the critical factors that reduced the quality of life of these individuals may be tiredness. Brugge et al. [35] evaluated COVID-19 patients 6 weeks after discharge and found a decrease in quality of life which is related to diffusion capacity [35]. Considering the presence of desaturation with lower SpO2 values in the COVID-19 group, the quality of life may be affected by the decrease in diffusion capacity, similar to the study of Brugge et al. Similarly, high MMRC and Borg Scale dyspnea scores before and after 6MWT in the COVID-19 group may explain impaired quality of life in this group.
Corticosteroid treatment is administered to individuals with severe COVID-19 infection, especially those with cytokine storm, that is, severe inflammation [36]. The corticosteroid treatment reduces the length of stay in ICU and the mortality rate [37]. In the present study, FVC, FEV1, 6MWT distance, and SpO2 (after 6MWT) values of the patients who received corticosteroid treatment and were followed up with IMV were lower, and the FEV1/FVC value was higher as compared to those who did not (Table 3). In addition, the longer length of stay in the ICU and higher APACHE II scores in those receiving corticosteroid therapy also prove that respiratory function loss is associated with the severe course of the disease (Table 3). In other words, individuals with a more severe infection received corticosteroid treatment and were followed up with IMV. Accordingly, due to the pathological changes, lung compliance, diffusion capacity, and respiratory function decreases more in those with severe COVID-19 infection.
Specific blood laboratory findings are seen in patients with severe COVID-19 [38]. A meta-analysis stated that in patients with severe COVID-19, hemoglobin decreased especially due to hemoglobin destruction resulting increased ferritin and D-dimer due to iron emerging. Ferritin is a marker that shows inflammatory load together with C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) [38]. Similarly, we observed lower HBG and higher D-dimer values (Table 5). CRP and lactate dehydrogenase (LDH) values are associated with inflammation and inflammation-associated cellular damage [38]. More importantly, the finding that CRP, a strong inflammatory marker, was associated with FVC, 6MWT distance, dyspnea, and SGRQ total score (Figure 4) indicated that inflammation in the lung affects respiratory function and functional capacity, even after 6 months of discharge. Similarly, LDH, which is an indicator of cellular damage, was also found to be associated with FVC, 6MWT distance, and SGRQ total score.
We found a relationship between FVC, FEV1, the change in SpO2, handgrip strength, HADS, SGRQ values, and 6MWT distance with the length of stay in ICU even 6 months after discharge. One of the critical points is the SpO2 value. The association of the change in SpO2 with ICU stay is probably due to the severe disease course and pathological processes. The correlation between SGRQ activity and SGRQ impact with APACHE II scores showed that the quality of life parameters of individuals with severe infection was significantly affected by the dimensions associated with physical activity after 6 months post-COVID-19 infection. The association of SGRQ and NHP total scores with dyspnea (Borg score after 6MWT), HADS, and FSS indicated that the continued decline in quality of life even after 6 months post-COVID is associated with increased fatigue, dyspnea, and depression (Figure 4).
Some limitations should be noted when interpreting the findings of this study. Pre-COVID-19 respiratory function, functional capacity, fatigue levels, and quality of life of the individuals were unknown. In that case, the change in these parameters during the process could be determined more clearly. We could have strengthened our obtained data if we had evaluated the disease severity score and had the diffusion capacity and lung CT data 6 months after discharge. Apart from these, it is the first study to functionally evaluate patients 6-months post-COVID.
In the literature, it is generally stated that fibrosis may develop in the lung tissue of patients after COVID-19, based on CT and blood laboratory results. In this study, we tried to prove the hypotheses put forward by studies in the literature with more functional evaluations. In future studies, fibrosis in the lung tissue can be evaluated using more objective evaluation methods, and its relations with similar functional parameters in this study can be investigated. In addition, whatever the underlying factor is, it is an undeniable fact that low oxygen saturation, increased shortness of breath, fatigue, decreased functional capacity, peripheral muscle strength, quality of life and balance, and depression are seen in these patients. Therefore, it should be considered that these patients may be good candidates for pulmonary rehabilitation in previous studies. Studying the effectiveness of pulmonary rehabilitation will make valuable contributions to the literature.
5. Conclusions
From our results, we conclude that patients with COVID-19 infection followed in the ICU due to pulmonary inflammation (indicated by markers of inflammation and cell damage such as CRP and LDH), consolidation, edema, and ARDS who need oxygen support and IMV continue to suffer from loss of respiratory function and decreased muscle strength, functional capacity, dyspnea, oxygen saturation, balance, quality of life, and fatigue even 6 months after discharge. The prolonged symptoms are probably due to fibrosis in the lung tissue during the healing process and the developing interstitial disease. In terms of early intervention for possible interstitial lung disease, it may be essential to follow the pulmonary function tests for at least 6 months in the post-discharge period in patients infected with COVID-19. In addition, these patients’ functional capacity and physiological and perceptional characteristics, primarily their fatigue levels, should be evaluated, and COVID-19 patients should be considered for inclusion in a pulmonary rehabilitation program in the early period.
Author Contributions
Conceptualization, U.S.; methodology, U.S. and D.I.-I.; formal analysis, U.S. and B.K.-V.; investigation, U.S. and C.A.; resources, U.S. and C.A.; writing—original draft preparation, U.S., D.I.-I. and B.K.-V.; writing—review and editing, U.S., D.I.-I., B.K.-V. and C.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Erciyes University Clinical Research Ethics Committee (protocol code 2021/165, approval date: 3 March 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data from this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 Does Not Lead to a ‘Typical’ Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dara N., Hosseini A., Sayyari A.A., Gaman M.A., Fatahi S. Gastrointestinal Manifestations and Dynamics of Liver Enzymes in Children and Adolescents with COVID-19 Infection: A Systematic Review and Meta-Analysis. Iran. J. Pediatr. 2020;30:1–9. doi: 10.5812/ijp.106935. [DOI] [Google Scholar]
- 3.Yin S., Huang M., Li D., Tang N. Difference of Coagulation Features between Severe Pneumonia Induced by SARS-CoV2 and Non-SARS-CoV2. J. Thromb. Thrombolysis. 2021;51:1107–1110. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fara A., Mitrev Z., Rosalia R.A., Assas B.M. Cytokine Storm and COVID-19: A Chronicle of pro-Inflammatory Cytokines. Open. Biol. 2020;10:200160. doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannis D., Ziogas I.A., Gianni P. Coagulation Disorders in Coronavirus Infected Patients: COVID-19, SARS-CoV-1, MERS-CoV and Lessons from the Past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Boon G.J.A.M., Barco S., Endres M., Geelhoed J.J.M., Knauss S., Rezek S.A., Spruit M.A., Vehreschild J., Siegerink B. The Post-COVID-19 Functional Status Scale: A Tool to Measure Functional Status over Time after COVID-19. Eur. Respir. J. 2020;56:2001494. doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eapen M.S., Lu W., Gaikwad A.V., Bhattarai P., Chia C., Hardikar A., Haug G., Sohal S.S. Endothelial to Mesenchymal Transition: A Precursor to Post-COVID-19 Interstitial Pulmonary Fibrosis and Vascular Obliteration? Eur. Respir. J. 2020;56:2003167. doi: 10.1183/13993003.03167-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans G., Van Den Berghe G. Clinical Review: Intensive Care Unit Acquired Weakness. Crit. Care. 2015;5:274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., Solis-Navarro L., Burgos F., Puppo H., Vilaró J. Respiratory Function in Patients Post-Infection by COVID-19: A Systematic Review and Meta-Analysis. Pulmonology. 2021;27:328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mumoli N., Bonaventura A., Colombo A., Vecchié A., Cei M., Vitale J., Pavan L., Mazzone A., Dentali F. Lung Function and Symptoms in Post-COVID-19 Patients: A Single-Center Experience. Mayo Clin. Proc. Inno. Qual. Outcomes. 2021;5:907–915. doi: 10.1016/j.mayocpiqo.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stylemans D., Smet J., Hanon S., Schuermans D., Ilsen B., Vandemeulebroucke J., Vanderhelst E., Verbanck S. Evolution of Lung Function and Chest CT 6 Months after COVID-19 Pneumonia: Real-Life Data from a Belgian University Hospital. Respir. Med. 2021;182:106421. doi: 10.1016/j.rmed.2021.106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harkonen R., Piirtomaa M., Alaranta H. Grip Strength and Hand Position of the Dynamometer in 204 Finnish Adults. J. Hand. Surg. Br. 1993;18:129–132. doi: 10.1016/0266-7681(93)90212-X. [DOI] [PubMed] [Google Scholar]
- 15.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the Medical Research Council (MRC) Dyspnoea Scale as a Measure of Disability in Patients with Chronic Obstructive Pulmonary Disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quanjer P.H., Tammeling G.J., Cotes J.E., Pedersen O.F., Peslin R., Yernault J.C. Lung Volumes and Forced Ventilatory Flows. Eur. Respir. J. 1993;6:5–40. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 17.Society A.T. Standardization of Spirometry, 1994 Update. Am. J. Respir. Crit. Care. Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 18.Hong S.H., Yang H.I., Kim D.-I., Gonzales T.I., Brage S., Jeon J.Y. Validation of Submaximal Step Tests and the 6-Min Walk Test for Predicting Maximal Oxygen Consumption in Young and Healthy Participants. Int. J. Environ. Res. Public Health. 2019;16:4858. doi: 10.3390/ijerph16234858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borg G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982;14:377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Kim M.K., Kong B.S., Yoo K.T. The Effect of Shoe Type on Static and Dynamic Balance during Treadmill Walking in Young Healthy Women. J. Phys. Ther. Sci. 2017;29:1653–1657. doi: 10.1589/jpts.29.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim N.J., Yoo K.T., An H.J., Shin H.J., Koo J.P., Kim B.K., Kim H.R., Choi J.H. The Effects of Balance Exercise on an Unstable Platform and a Stable Platform on Static Balance. J. Int. Acad. Phys. Ther. Res. 2014;5:641–646. doi: 10.5854/JIAPTR.2014.03.31.641. [DOI] [Google Scholar]
- 22.Podsiadlo D., Richardson S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 23.Aydemir Ö., Guvenir T., Kuey L., Kultur S. Validity and Reliability of Turkish Version of Hospital Anxiety and Depression Scale. Turk Psikiyatr. Derg. 1997;8:280–287. [Google Scholar]
- 24.Folstein M.F., Folstein S.E., McHugh P.R. ‘Mini-Mental State’. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Armutlu K., Korkmaz N.C., Keser I., Sumbuloglu V., Akbiyik D.I., Guney Z., Karabudak R. The Validity and Reliability of the Fatigue Severity Scale in Turkish Multiple Sclerosis Patients. Int. J. Rehabil. Res. 2007;30:81–85. doi: 10.1097/MRR.0b013e3280146ec4. [DOI] [PubMed] [Google Scholar]
- 26.Saglam M., Arikan H., Savci S., Inal-Ince D., Bosnak-Guclu M., Karabulut E., Tokgozoglu L. International Physical Activity Questionnaire: Reliability and Validity of the Turkish Version. Percept. Mot. Skills. 2010;111:278–284. doi: 10.2466/06.08.PMS.111.4.278-284. [DOI] [PubMed] [Google Scholar]
- 27.Polatlı M., Yorgancıoğlu A., Aydemir Ö., Demirci N.Y., Kırkıl G., Naycı S.A., Köktürk N., Uysal A., Akdemir S.E., Özgür E.S. St. George Solunum Anketinin Türkçe Geçerlilik ve Güvenilirliği. Tuberk. Toraks. 2013;61:81–87. doi: 10.5578/tt.5404. [DOI] [PubMed] [Google Scholar]
- 28.Kücükdeveci A.A., McKenna S.P., Kutlay S., Gürsel Y., Whalley D., Arasil T. The Development and Psychometric Assessment of the Turkish Version of the Nottingham Health Profile. Int. J. Rehabil. Res. 2000;23:31–38. doi: 10.1097/00004356-200023010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Thillai M., Keith H. In: Understanding ABGs & Lung Function Tests. Mayhew P., editor. Richard Furn; London, UK: 2012. pp. 82–85. [Google Scholar]
- 30.Ferrandi P.J., Alway S.E., Mohamed J.S. The Interaction between SARS-CoV-2 and ACE2 May Have Consequences for Skeletal Muscle Viral Susceptibility and Myopathies. J. Appl. Physiol. 2020;129:864–867. doi: 10.1152/japplphysiol.00321.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt T., Dieterich M. ‘Excess Anxiety’ and ‘Less Anxiety’: Both Depend on Vestibular Function. Curr. Opin. Neurol. 2020;33:136–141. doi: 10.1097/WCO.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 33.Rudroff T., Fietsam A.C., Deters J.R., Bryant A.D., Kamholz J. Post-COVID-19 Fatigue: Potential Contributing Factors. Brain Sci. 2020;10:1012. doi: 10.3390/brainsci10121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., Rubin G.J. The Psychological Impact of Quarantine and How to Reduce It: Rapid Review of the Evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Sar-van der Brugge S., Talman S., Boonman-de Winter L., de Mol M., Hoefman E., van Etten R.W., De Backer I.C. Pulmonary Function and Health-Related Quality of Life after COVID-19 Pneumonia. Respir. Med. 2021;176:106272. doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munch M.W., Myatra S.N., Vijayaraghavan B.K.T., Saseedharan S., Benfield T., Wahlin R.R., Rasmussen B.S., Andreasen A.S., Poulsen L.M., Cioccari L., et al. Effect of 12 Mg vs. 6 Mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dequin P.-F., Heming N., Meziani F., Plantefève G., Voiriot G., Badié J., François B., Aubron C., Ricard J.-D., Ehrmann S., et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry B.M., Oliveira M.H., Benoit S., Plebania M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study are available from the corresponding author on reasonable request.