Abstract
Why do viruses sometimes not pass through larger pores in track-etch filters? Increasing the salinity (0.8 to 160 mM Na+) decreased φX174 and PRD1 passage through track-etch polycarbonate membranes (sodium dodecyl sulfate coated but not polyvinylpyrrolidone coated) and PRD1 passage through polyester membranes. Undiminished passage when 0.1% Tween 80 was added implied that nonionic virus adsorption occurred and indicated that high levels of salinity decreased virus passage by decreasing electrostatic repulsion that prevented adsorption.
Track-etch membranes (e.g., Nuclepore polycarbonate membranes) are marketed and used as sieve filters. The track-etch process produces pores with approximately cylindrical cross sections and relatively uniform diameters. These thin membranes filter primarily by stereohindrance; i.e., large particles cannot pass through smaller holes. They have been used to determine approximate sizes of some viruses (5, 10). During an investigation of virus sizes in which track-etch filters with different pore sizes were used, it was found that some viruses (e.g., herpes simplex virus type I) in phosphate-buffered saline did not readily pass through pores whose diameters were significantly larger than the virus diameters, as determined by electron microscopy (10). The reason for this apparent discrepancy was suggested by evidence that viruses passed through tortuous-path filters.
During passage through tortuous-path filters (solution-cast membranes, including nitrocellulose membranes), virus particles may adsorb. Wallis et al. (13) concluded that poliovirus adsorption to nitrocellulose filters required the presence of one of several different mono-, di-, or trivalent salts, including NaCl, in the carrier medium. This indicated that there is some ionic aspect to the adsorption process and implied that ions might be directly involved in the adsorption phenomenon. Additional, nonionic (e.g., hydrophobic) aspects are also apparent in adsorption of virus particles to nitrocellulose. Several bacteriophages (including φX174 and PRD1) adsorb via nonionic interactions with nitrocellulose membranes (9, 11). This adsorption can be prevented or reversed by the presence of a nonionic surfactant (4, 9).
One way of explaining the ionic factor is to acknowledge that the filter membrane has a negative charge (7). Since most viruses are negatively charged at neutral pH (2), a negative charge on the membrane might prevent nonionic adsorption because of electrostatic repulsion of the virus particles. The electrostatic forces between charged virus particles and a like-charged membrane can be modified by changing the ionic concentration in the intervening fluid; i.e., adding ions shields the charges (Debye-Hückel screening), thereby decreasing the effective electrostatic force (6). If the ionic concentration is high enough, the electrostatic repulsion could be reduced to the point that nonionic adsorption may occur.
In order to assess how this balance between ionic repulsion and nonionic adsorption might affect the transmission of viruses through track-etch membranes, virus passage was determined as a function of salinity and in the presence and absence of a nonionic surfactant. This was done by passing two bacteriophages, φX174 and PRD1, through track-etch membranes with different nonionic adsorption properties and negative surface charges.
The two bacteriophages were chosen because of their different adsorption properties. φX174 is an approximately spherical virus with a diameter of about 27 nm (3); its bacterial host is Escherichia coli C. With a pI (pH at which the net charge is zero) of 6.6, φX174 has a slight negative charge at neutral pH (1). PRD1 also is an approximately spherical virus and has a diameter of about 63 nm (3), and it is more negatively charged and more strongly adsorptive through nonionic interactions than φX174 is (9); its bacterial host is Salmonella typhimurium LT2. Both viruses were assayed biologically by plaque formation by using the double-agar layer technique (8).
The viruses were suspended in different concentrations of Dulbecco’s phosphate-buffered saline without Ca2+ or Mg2+ (DPBS−), which has a sodium ion (Na+) concentration of 160 mM when it is not diluted. A neutral pH was maintained after dilution, as shown in Table 1. The viruses were diluted to a concentration of about 1,500 PFU/ml with each final salt concentration just before filtration. Because of the possible importance of nonionic adsorption, in some experiments we added 0.1% Tween 80 (Sigma) to the DPBS− to minimize such adsorption (9). Since the virus titers were about the same (±15%) in the presence and absence of surfactant, we believe that virus aggregation was not a significant factor in the filtration results.
TABLE 1.
pH values of DPBS− after dilution with double-distilled water
| Fluid NA+ concn (mM) | pH |
|---|---|
| 0 (ddH2O)a | 7.22 ± 0.12 |
| 1.6 | 7.33 ± 0.07 |
| 16 | 7.32 ± 0.07 |
| 160 | 7.18 ± 0.03 |
ddH2O, double-distilled water.
The following two standard commercially available 0.2-μm-pore-size track-etch membrane filters were used: Nuclepore polycarbonate and Nuclepore polyester membrane filters (Corning Incorporated, Acton, Mass.). The polycarbonate membrane (which was coated by the manufacturer with polyvinylpyrrolidone to create minimal hydrophilic membrane adsorption) had a low negative surface charge (7). The chemistry of the polyester membrane resulted in a higher negative charge. In addition, 0.2-μm-pore-size track-etch Nuclepore polyvinylpyrrolidone-free polycarbonate filters were treated so that they had sodium dodecyl sulfate (SDS)-coated surfaces (12). This treatment was accomplished by dipping the filters into a solution containing 15% isopropyl alcohol and 0.3% SDS in distilled water. The hydrophobic end of the SDS molecule bound to the polycarbonate surface; this left the negatively charged sulfate end free, which resulted in a coated membrane surface, including coated pores (7, 12). The coated filters were thoroughly rinsed by dipping them into distilled water to remove any excess SDS that had not bound to the filters, and then they were air dried. The SDS coating provided hydrophilic filters with a negatively charged surfaces.
To determine the extent of virus passage through a membrane, 3 ml of a virus suspension was passed through the filter by using a 10-ml plastic disposable syringe (Becton Dickinson and Company, Franklin, N.J.) and a syringe pump (model 230; KD Scientific Inc., Boston, Mass.) to provide a constant flow rate of 2 ml/min, and the sample was collected in a glass test tube. To determine the percentage of virus transmission, the virus concentrations in the filtrate and the original suspension were compared.
Effect of salinity on passage through track-etch membranes.
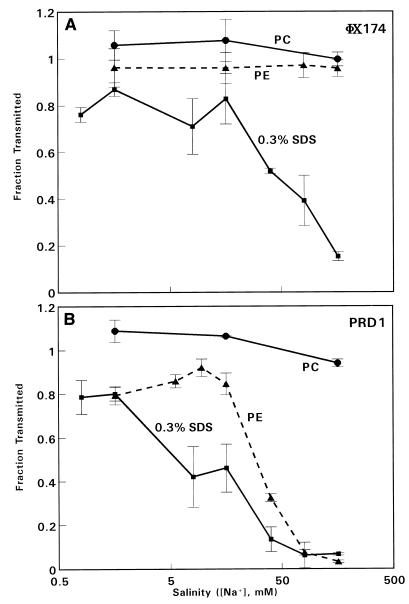
Transmission curves were determined for both viruses as a function of salinity (Na+ concentration). The data for φX174 (Fig. 1A) revealed that essentially complete passage through the two commercial membranes (polycarbonate and polyester) occurred at the Na+ concentrations investigated. This indicates that there was no significant adsorption of φX174 to either commercial membrane and confirmed that there was no evidence of virus aggregation at the Na+ concentrations used. However, φX174 transmission through the SDS-coated membrane was substantially reduced at the higher Na+ concentrations.
FIG. 1.
Transmission of φX174 (A) and PRD1 (B) through track-etch membranes with 0.2-μm-diameter pores in the presence of different Na+ concentrations, prepared by diluting Dulbecco’s phosphate-buffered saline. Symbols: ○, Nuclepore polycarbonate (PC) membrane; ▵, Nuclepore polyester (PE) membrane; □, 0.3% SDS-coated polycarbonate membrane. The flow rate was 2 ml/min. The data are means of values from three to five experiments. The error bars indicate standard errors of the means.
The qualitative data patterns obtained with PRD1 were similar to those obtained with φX174 for the polycarbonate and SDS-coated membranes (Fig. 1B); complete transmission occurred with the former membrane (which confirmed that there was not significant virus aggregation), and saline-dependent reduced transmission occurred with the SDS-coated membrane. On the other hand, passage through the polyester membrane was slightly reduced at low Na+ concentrations and greatly reduced at higher Na+ concentrations (there was a 30-fold reduction at 160 mM Na+). Thus, in physiological saline (160 mM Na+) passage of PRD1 through this commercially available track-etch membrane was only 3% of the expected value based on the physical sizes of the virus and the pores.
Effect of a nonionic surfactant on virus passage.
Increased virus passage in the presence of a nonionic surfactant (e.g., Tween 80) indicates that nonionic adsorption has occurred (9). When virus passage was reduced in the presence of physiological saline (160 mM Na+), 0.1% Tween 80 was added to DPBS− to determine the extent of nonionic adsorption. The results are shown in Table 2. In each case, the presence of the nonionic surfactant increased passage of the virus so that passage was nearly complete, which indicated that nearly all of the adsorption of either virus to either membrane was primarily nonionic in nature.
TABLE 2.
Transmission of viruses in the presence of a nonionic surfactant in physiological saline (160 mM Na+)a
| Track-etch membrane | Virus | % Passage in DPBS− | % Passage in DPBS− containing 0.1% Tween 80 |
|---|---|---|---|
| Polyester | PRD1 | 3.1 ± 0.6 | 80.3 ± 3.9 |
| SDS-coated polycarbonate | φX174 | 15.2 ± 1.9 | 114.5 ± 2.8 |
| PRD1 | 6.6 ± 0.1 | 89.2 ± 6.7 |
The data are the means ± standard errors of data from three to six experiments.
This study provided the first evidence which clearly demonstrated that track-etch membranes are not just simple sieves that indiscriminately pass particles which are smaller than the pores. Virus passage through track-etch pores several times larger than the virus can still be significantly restricted by surface-dependent interactions. Virus passage depended on the Na+ concentration of the carrying fluid and the surface properties of the membrane. The presence of a nonionic surfactant resulted in nearly complete passage, indicating that nonionic adsorption played a primary role in the restriction. This adsorption may explain why the reported levels of passage of some viruses through track-etch membranes were lower than the levels expected based on the virus sizes determined by electron microscopy (10).
As hypothesized, in general, when there was reduced virus transmission, the saline dependencies of the individual transmission curves were consistent with Debye-Hückel screening combined with adsorption through nonionic interactions (i.e., there was less transmission at high Na+ concentrations). This was true even for φX174, which has little charge at neutral pH. The differences in the transmission curves obtained for PRD1 suggest that the balances between nonionic attraction and electrostatic repulsion were different for the polyester and SDS-coated membranes.
In summary, our data demonstrated that transmission of viruses through track-etch membranes can be restricted by factors other than stereohindrance, particularly at physiological levels of saline (160 mM). Nonionic adsorption was apparently the primary means of restricting virus passage, which implied that an increased ion concentration played a secondary role in virus adsorption. This provides an explanation for why certain viruses did not pass through track-etch membranes with pore sizes larger than the viruses. We also demonstrated that a nonionic surfactant (Tween 80) can minimize or even prevent nonionic adsorption.
Acknowledgments
We thank Edward A. Gordon, Center for Devices and Radiological Health, for expert presentation of the graphic material.
REFERENCES
- 1.Ackermann H-W. Cubic, filamentous and pleomorphic phages. In: Ackermann H W, DuBow M S, editors. Viruses in prokaryotes. II. Boca Raton, Fla: CRC Press, Inc.; 1987. pp. 171–218. [Google Scholar]
- 2.Brinton C, Lauffer M. The electrophoresis of viruses, bacteria and cells, and the microscope method of electrophoresis. In: Bier M, editor. Electrophoresis. New York, N.Y: Academic Press, Inc.; 1959. pp. 427–492. [Google Scholar]
- 3.Francki, R. I. B., C. M. Fauquet, D. L. Knudson, and F. Brown. 1991. Classification and nomenclature of viruses. Arch. Virol. Suppl. 2.
- 4.Fujito B T, Lytle C D. Elution of viruses by ionic and nonionic surfactants. Appl Environ Microbiol. 1996;62:3470–3473. doi: 10.1128/aem.62.9.3470-3473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He L-F, Alling D, Popkin T, Shapiro M, Alter H J, Purcell R H. Determining the size of non-A, non-B hepatitis virus by filtration. J Infect Dis. 1987;156:636–640. doi: 10.1093/infdis/156.4.636. [DOI] [PubMed] [Google Scholar]
- 6.Israelachvili J. Intermolecular and surface forces. 2nd ed. San Diego, Calif: Academic Press; 1995. [Google Scholar]
- 7.Keesom W H, Zelenka R L, Radke C J. A zeta-potential model for ionic surfactant adsorption on an ionogenic hydrophobic surface. J Colloid Interface Sci. 1988;125:575–585. [Google Scholar]
- 8.Lytle C D, Budacz A P, Keville E, Miller S A, Prodouz K M. Differential inactivation of surrogate viruses with merocyanine 540. Photochem Photobiol. 1991;54:489–493. doi: 10.1111/j.1751-1097.1991.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 9.Lytle C D, Routson L B. Minimized virus binding for tests of barrier materials. Appl Environ Microbiol. 1995;61:643–649. doi: 10.1128/aem.61.2.643-649.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lytle C D, Tondreau S C, Truscott W, Budacz A P, Kuester R K, Venegas L, Schmukler R E, Cyr W H. Filtration sizes of human immunodeficiency virus type 1 and surrogate viruses used to test barrier materials. Appl Environ Microbiol. 1992;58:747–749. doi: 10.1128/aem.58.2.747-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lytle C D, Truscott W, Budacz A P, Venegas L, Routson L B, Cyr W H. Important factors for testing barrier materials with surrogate viruses. Appl Environ Microbiol. 1991;57:2549–2554. doi: 10.1128/aem.57.9.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmukler, R., and C. D. Lytle. November 1997. Method for viral-proofing a protective barrier. U.S. patent 5,671,754.
- 13.Wallis C, Henderson M, Melnick J L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972;23:476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]