Abstract
Vibrio parahaemolyticus ATCC 17802, Vibrio vulnificus ATCC 27562, Vibrio cholerae O:1 ATCC 14035, Vibrio cholerae non-O:1 ATCC 14547, Vibrio hollisae ATCC 33564, and Vibrio mimicus ATCC 33653 were treated with 200 to 300 MPa for 5 to 15 min at 25°C. High hydrostatic pressure inactivated all strains of pathogenic Vibrio without triggering a viable but nonculturable (VBNC) state; however, cells already existing in a VBNC state appeared to possess greater pressure resistance.
The demand by consumers for safe, minimally processed foods that retain the appearance, flavor, texture, and nutritional qualities of raw or fresh foods has been a driving force for commercial application of nonthermal food processing methods such as high-hydrostatic-pressure processing (HPP). HPP offers advantages over thermal processing in that microorganisms and detrimental enzymes can be inactivated at ambient or low temperatures without the breaking of covalent bonds which are essential to virtually all flavor, color, and nutritional constituents within a food system (6). Pressure magnitudes between 300 and 600 MPa can inactivate fungi and vegetative bacteria, including most infectious foodborne pathogens (20).
The five Vibrio species that pose a foodborne health risk to humans are V. cholerae, V. parahaemolyticus, V. vulnificus, V. mimicus, and V. hollisae (10). All five have been implicated in food-poisoning outbreaks associated with shellfish, particularly oysters (2, 11), and have been detected in shellfish harvested from the bays and coastal areas of the United States (4, 5, 9). Outbreaks of V. parahaemolyticus gastroenteritis in the United States are usually associated with consumption of contaminated shellfish or crustaceans (10).
Styles et al. (21) eliminated starting inocula of 106 CFU of V. parahaemolyticus per ml after exposure to 1,700 atm (1 MPa ≅ 10 atm) for 10 min in clam juice and 30 min in phosphate buffer. Yukizaki et al. (25) found V. parahaemolyticus, V. mimicus, and V. cholerae non-O:1 to be destroyed by 1,900, 2,900, and 4,800 atm for 10 min in liquid buffer at 0°C, respectively.
Some bacteria in response to certain environmental stresses will remain viable, but lose their ability to grow on media on which they are routinely cultured (14, 17, 18). V. cholerae and Escherichia coli were shown to remain viable following incubation in artificial seawater (ASW), although they lost all ability to produce colonies on media routinely used for their cultivation (24). It has been realized that the viable but not culturable (VBNC) state exhibited by V. cholerae can explain the seasonality and distribution of the organism in regions where cholera is endemic. Several studies showed that the organism was present and viable in many waters from which it could not be cultured (1, 3, 23).
The objectives of this study were to determine the rate of pressure inactivation of pure cultures of V. parahaemolyticus, V. vulnificus, V. cholerae O:1 and non-O:1, V. mimicus, and V. hollisae and to examine the low-temperature-induced nonculturable but viable state in the vibrios related to pressure sensitivity.
Six strains of Vibrio were examined in this study: V. parahaemolyticus ATCC 17802 (Shirasu food-poisoning isolate), V. vulnificus ATCC 27562 (blood isolate), V. cholerae O:1 ATCC 14035 (clinical isolate), V. cholerae non-O:1 ATCC 14547 (fish isolate), V. hollisae ATCC 33564 (human feces), and V. mimicus ATCC 33653 (human ear). All strains were obtained from the American Type Culture Collection (ATCC [Rockville, Md.]) in lyophilized form and revived under ATCC-recommended conditions (all media were from Difco, Detroit, Mich.).
Working stocks were prepared by inoculation of the nonhalophilic Vibrio strains (V. cholerae O:1, V. cholerae non-O:1, and V. mimicus) into 10 ml of tryptic soy broth (TSB) and the halophilic strains (V. parahaemolyticus, V. vulnificus, and V. hollisae) into 10 ml of TSB plus 2.5% NaCl (TSBS). Stock cultures were stored on corresponding agar slants at 17°C and maintained by monthly transfers.
The effect of pressure treatment on the culturability of the Vibrio strains tested was measured by spread plating on tryptic soy agar (TSA) or TSA plus 2.5% NaCl (TSAS) and thiosulfate-citrate-bile salts-sucrose (TCBS) agar. Samples were serially diluted in ASW (400 mM [23.4 g/liter] NaCl, 100 mM [24.6 g/liter] MgSO4 · 7H2O, 20 mM [1.5 g/liter] KCl, 20 mM [2.9 g/liter] CaCl2 · 2H2O; unless otherwise stated, all chemicals and reagents were from Sigma Chemical Co., St. Louis, Mo.). Plates were incubated at 35°C for 20 h, and then bacteria were counted. The difference between the plate counts on TSA(S), nonselective medium, and TCBS, selective Vibrio medium inhibitory to injured cells, represented the extent of injury to the population. All plating was done in triplicate.
Cultures were grown for 6 and 24 h at 35°C by transfer of 0.1 ml of a 20-h culture into 10 ml of TSBS. The final cell concentrations of both 6- and 24-h cultures were approximately 107 CFU/ml in filter-sterilized ASW or TSBS; these were stored at 4°C for up to 2 months. Cell culturability of refrigerated samples was measured at 2-day intervals by spread-plate counts on TSAS and TCBS agar. The cultures were reported as entering the VBNC state when plate counts on TCBS dropped to a nondetectable level (<10 CFU/ml) and plate counts on TSAS dropped below 100 CFU/ml. Resuscitation from the VBNC state was determined by inoculation of 0.1 ml of an VBNC culture into 10 ml of sterile TSBS, followed by incubation for up to 5 days at 25°C. Tubes were examined for turbidity that indicated actively multiplying cells or a resuscitation from the VBNC state. Turbid tubes were streaked onto TSAS and TCBS plates and incubated for up to 24 h at 35°C. In addition, at the end of each experiment, one tube of each of the VBNC cultures was placed at ambient temperature (approximately 23°C) for up to 5 days, and samples were placed daily onto TSAS and TCBS to measure the return of culturability.
The direct viable count (DVC) method of Kogure et al. (8) was adapted. Cells suspended in ASW were mixed with filter-sterilized, nalidixic acid-yeast extract solution (0.02 g of nalidixic acid and 0.5 g of yeast extract dissolved in 100 ml of ASW) in a 9:1 ratio in glass screw-cap tubes. The cell suspensions were incubated for 6 h in the dark at 35°C. After incubation, cell growth was terminated by addition of 0.54 ml of 37% formaldehyde. A volume of cell suspension containing a total of approximately 106 cells/ml was transferred to a sterile tube and mixed with an equal volume of filter-sterilized 1:2,500 acridine orange solution (0.04 g of acridine orange in 100 ml of deionized H2O) and was held at least 2 min. The cells were filtered onto a 25-mm black, polycarbonate membrane filter with a 0.2-μm pore size (Poretics, Livermore, Calif.) by using a 25-mm fritted glass support microanalysis filter holder assembly (Fisher Scientific, Pittsburgh, Pa.) with a vacuum of 0.8 MPa. The traces of sample remaining in the tube were rinsed onto the filter with 20 ml of filter-sterilized 400 mM NaCl solution. The filter was removed from the filter assembly and placed sample side up on a clean glass microscope slide. A drop of Cargill (Minneapolis, Minn.) type FF nonfluorescent immersion oil was placed on the filter followed by a glass 18-mm-diameter coverslip. A drop of Cargill type DF low-fluorescence immersion oil was placed on top of the coverslip before viewing.
The filters were viewed at a ×1,000 magnification with a Bausch & Lomb (Rochester, N.Y.) epifluorescence microscope. For each sample, at least 10 (usually 20) fields of 2 to 200 cells/field were counted. The number of bacteria per milliliter was calculated by using a conversion factor derived from the number of fields in the filtered area and the volume of cell suspension filtered. All of the bacteria-like particles were counted as total direct microscopic counts (DMC). Those cells that elongated or enlarged to the size of freshly cultured bacteria or larger were counted and designated as direct viable counts (DVC).
Treated and control VBNC samples were also spread plated at a 10−1 dilution on TSAS and TCBS plates and incubated at 35°C for up to 24 h. The lack of colony formation showed continued lack of culturability of the cultures.
Slant cultures were grown in 50-ml glass, screw-cap tubes containing 30-ml of TSB (nonhalophilic strains) or TSBS (halophilic strains) to approximately 109 CFU/ml at 35°C for 10 to 12 h. Cultures were aseptically transferred to 50-ml polypropylene centrifuge tubes (Fisher Scientific) and centrifuged at 2,200 × g for 10 min (IEC Centra-4B centrifuge; Needham Heights, Mass.), washed with ASW, and resuspended in 30 ml of ASW. One milliliter of the resuspended cultures was diluted into individual sterile dilution bottles containing 99 ml of ASW with a final cell concentration of approximately 107 CFU/ml. Twenty milliliters of each suspension was aseptically transferred into sterile, heat-sealable Scotchpak pouches (10 by 15 cm) (Kapak 402, Minneapolis, Minn.). The pouches were double sealed in a Scotchpak heat-sealer (Kapak). The pouches were placed in the pressure chamber for treatment.
Each Vibrio strain was treated at 200 and 250 MPa for 5, 10, and 15 min and 300 MPa for 5 min. All of the pressure treatments were done at 25°C. Each pressure-time combination was repeated at least twice for each strain.
Samples were pressurized with an Autoclave Engineers (Erie, Pa.) isostatic press (model IP2-22-60) with a temperature-controlled cylindrical pressure chamber measuring 55.9 by 5.1 cm. Samples were placed into the pressure chamber, which contained a pressure medium consisting of water containing 2% hydraulic fluid (Hydrolubric 142; E. F. Houghton and Co., Valley Forge, Pa.). After pressurization, pouches were removed, rinsed with 70% ethanol, and cut open with a sterile pair of scissors. Appropriate aliquots of the samples were aseptically removed from the pouch and plated directly, further diluted, or held for microscopic examination and recovery studies.
In all of the pure culture experiments, the standard error was calculated for all of the experimental replications. Analysis of variance using the least-squared means was performed by using the SAS program (19) to analyze the comparison between pressure resistance of log-stage cultures and VBNC cells.
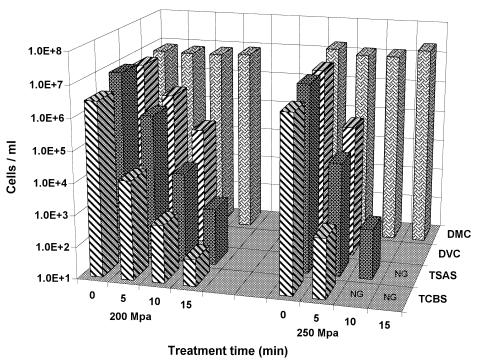
All six cultures of Vibrio responded similarly to greater times of pressurization at 200 and 250 MPa. Figure 1 exemplifies this response with V. vulnificus 27562. The DVC decreased with increased pressure and exposure times to pressurization and dropped below the detection limit (1.6 × 104 cells/ml) at 200 MPa for 15 min and 250 MPa for 10 min. The results indicated an approximate difference of 1 log10 in cell estimation between DVC and TSAS plate counts, suggesting that a portion of the cell population lost culturability but remained viable. This is probably not a development of an induced VBNC state, because of the short amount of time the cell suspensions were exposed to pressure. Instead, this difference can be described as a result of a more sensitive means of assessing sublethal injury. The 1-log10 difference can be assumed to represent the injured subpopulation. No colony formation was found on either plating medium after a 10-min treatment at 250 MPa. The agar plating results showed a trend of HPP inactivation similar to that shown by Styles et al. (21). Styles et al. (21) used TSAS and recorded complete inactivation (an approximate 5-log10 reduction) of V. parahaemolyticus treated in phosphate-buffered saline at 170 MPa for 30 min. HPP at 250 MPa for 15 min or 300 MPa for 5 min (data not shown) at 25°C was sufficient to reduce nonselective plate counts and DVC for all strains to nondetectable levels.
FIG. 1.
Effect of high hydrostatic pressure on Vibrio vulnificus ATCC 27562 treated in ASW at 25°C measured by the four cell enumeration methods described in the text. NG, no colony growth.
Attempts were made to induce a VBNC state in the six strains of Vibrio used in this study, but there was no development of a low-temperature-induced VBNC state in the Vibrio strains in their stationary phase of growth (data not shown). All log-phase cells incubated in TSB(S) at 4°C displayed nonculturability within 22 days compared to ASW-incubated cultures; however, the presence of the TSB interfered with cell enumeration by DVC. V. hollisae 33564 lost culturability within 14 days; however, the culture also dropped to nondetectable levels (<1.6 × 104 cells/ml) for DVC, which prohibited its use. V. cholerae O:1, V. cholerae non-O:1, and V. mimicus did not become completely nonculturable; therefore study of these three strains was discontinued. Wolf and Oliver (22) were unable to induce a VBNC state in strains of V. cholerae, V. mimicus, V. parahaemolyticus, V. natriegens, V. proteolyticus, and V. campbellii used in their study. In our study, V. parahaemolyticus and V. vulnificus both showed acceptable trends of entering the VBNC state under the specified conditions and were used to study the effect of HPP on Vibrio in the VBNC state.
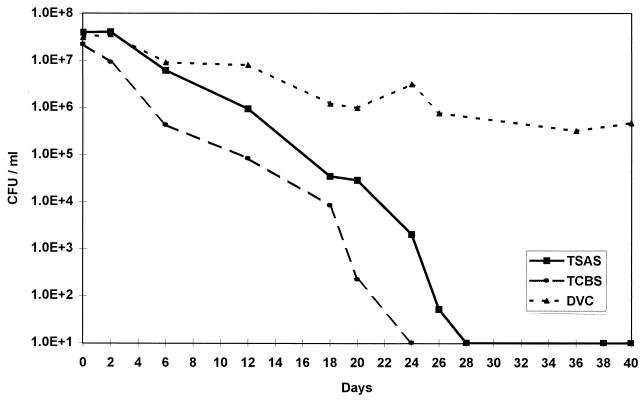
The DMC for V. vulnificus 27562 and V. parahaemolyticus 17802 (data not shown) revealed a perceptible decrease of approximately 1 log10 cells/ml after more than 40 days at 4°C in ASW. Over this period, TCBS counts dropped somewhat more rapidly than counts on TSAS, because of the selectivity of TCBS for viable, noninjured Vibrio cells (Fig. 2) (16). The TSAS counts were used to determine the time of incubation required for each strain to become nonculturable (<10 CFU/ml). V. vulnificus and V. parahaemolyticus were found to become nonculturable after 28 and 38 days of incubation at 4°C in ASW, respectively. The DVCs stayed at approximately 106 cells/ml for the last 10 days of the study for each culture. An important aspect of VBNC cultures is the return of culturability when the conditions of stress are removed. This was examined by placing the post-pressurized VBNC cell suspensions at room temperature (ca. 23°C) in ASW with no additional nutrients added. Plate counts on TSAS and TCBS showed that, after 2 days at room temperature, many small colonies appeared on the TSAS plates for V. parahaemolyticus, but no growth occurred on the TCBS plates or for aliquots of V. vulnificus on either medium. After 4 days at room temperature, typical colonies of Vibrio appeared on the TSAS, and similar but fewer colonies appeared on TCBS for both strains, indicating that the VBNC cultures had returned to culturability. This recovery agrees with the findings of Nilsson et al. (12), who found that V. vulnificus in VBNC returned to full culturability after 3 days of incubation at room temperature in ASW.
FIG. 2.
Induction of a VBNC state in V. vulnificus ATCC 27562 by incubation at 4°C in ASW as measured by plate counts on TSAS and TCBS, as well as DVC.
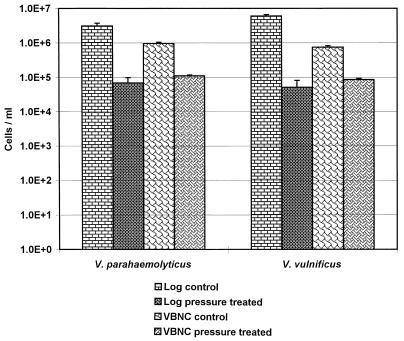
The DVC method was used to determine the extent of inactivation following HPP at 200 and 250 MPa for 10 min at 25°C. Both strains were tested within 10 days of entry into VBNC. Upon exposure to 200 MPa, decreases of 1.7 and 2.1 log10 cells/ml were seen for V. parahaemolyticus and V. vulnificus, respectively (Fig. 3). In comparison, a 1.0-log10 decrease was seen for both VBNC V. parahaemolyticus and V. vulnificus. This apparently enhanced resistance to pressure due to the VBNC state was statistically significant (P > 0.05) only for V. vulnificus; V. parahaemolyticus did not show significance at the P > 0.05 level because of higher than normal variation in control counts. Even with the high variation in the V. parahaemolyticus controls, the general trend of increased pressure resistance of VBNC cells is suggested.
FIG. 3.
Pressure resistance of VBNC cultures of V. parahaemolyticus (ATCC 17802) and V. vulnificus (ATCC 27562) compared to culturable cultures in mid-log stage measured by DVC.
In addition, VBNC and control cultures were pressurized at 250 MPa for 15 min at 25°C. DVC estimates for each strain were below the detection limit of the method following pressure treatment. Survival was measured by the ability of a 0.1-ml aliquot to grow in 10 ml of TSBS at room temperature (23°C) (Table 1). For each of the four cell suspensions, three tubes of TSBS were inoculated before and after pressure treatment. All 12 of the pretreatment tubes became turbid with growth within 3 days at 23°C (data not shown). For the postpressurized samples, none of the tubes inoculated with control samples became turbid after 5 days at 23°C, which indicated complete elimination of the bacteria. Both of the VBNC cultures demonstrated some degree of viability after treatment. The mixed viability results may be explained by considering that VBNC cells may be unable to regain culturability without the presence of at least a small fraction (<0.05 CFU/ml) of culturable cells (13). A few culturable cells may have survived HPP and were able to induce the return to culturability by surviving VBNC cells. When the same cultures were tested at 300 MPa for 15 min at 25°C, no positive (turbid) tubes were observed. In any case, these results lend additional support in suggesting that cells in the VBNC state are more resistant to hydrostatic pressure than are their culturable counterparts.
TABLE 1.
Recovery of pressure-treated, culturable, and VBNC V. parahaemolyticus and V. vulnificus
| HPP sample (250 MPa for 15 min at 25°C) | No. of positive TSBS tubes/no. testeda
|
|
|---|---|---|
| Run 1 | Run 2 | |
| Culturable V. parahaemolyticus | 0/3 | 0/3 |
| Culturable V. vulnificus | 0/3 | 0/3 |
| VBNC V. parahaemolyticus | 2/3 | 1/3 |
| VBNC V. vulnificus | 3/3 | 1/3 |
Each run consisted of three tubes containing 10 ml of TSBS per culture; turbid tubes indicate viability.
The decrease in pressure sensitivity of both strains can be explained at a cellular level. The decrease in cellular size, the change from rod to spherical shape, and the slowing of metabolic activity, all seen in VBNC cells, may play a role in the increased pressure resistance of the cells (13). This also agrees with research done by Jiang et al. (7), who reported that V. parahaemolyticus induced into nonculturability by low temperature and starvation showed an increased resistance to heating, sonication, and storage at −30°C.
The VBNC state may make pathogenic Vibrio more resistant to pressure and conventional food processing methods. Unfortunately virulence does not appear to be eliminated in the VBNC state. Colwell et al. (3) showed that VBNC cells from an attenuated strain of V. cholerae O:1 were revived in vivo in two human volunteers. Colwell et al. (3) have also shown virulence for V. cholerae when nonculturable cells were introduced into ligated rabbit ileal loops. Oliver (13) found that V. vulnificus in the VBNC state retained its virulence in mice when injected with as little as 0.05 CFU of culturable V. vulnificus per ml.
Seafood is often taken from waters or stored under conditions that induce the VBNC state in bacteria (14). Overreliance on conventional plating methods for the microbial analysis of seafoods carries the risk of generating false negatives for process-resistant VBNC Vibrio. This issue places greater emphasis for incorporation of immunological and PCR-based methods as standard analytical techniques for monitoring seafood safety. In addition, even though Vibrio species are relatively pressure sensitive compared to other vegetative bacteria, it may be wise to incorporate elevated temperature during HPP (i.e., 50°C) (15) to ensure complete inactivation of Vibrio in seafood. In a study (data not shown) using homogenated raw eastern oysters (Crassostrea virginica) inoculated with either V. parahaemolyticus 17802 (starting concentration, 1.1 × 107 to 8.1 × 107 CFU/g) or V. vulnificus 27562 (starting concentration, 5.7 × 106 to 2.5 × 107 CFU/g), a pressure treatment of 200 MPa for 10 min at 25°C reduced populations to below the level of detection (<10 CFU/g). Nonetheless, additional safeguards are necessary for commercial application, and use of a higher treatment pressure with elevated temperature has obvious potential to increase safety of this product with regard to pathogenic Vibrio.
ACKNOWLEDGMENT
This project was supported by the Sea Grant College Program (NA16RG0162).
REFERENCES
- 1.Brayton P R, Tamplin M L, Huq A, Colwell R R. Enumeration of Vibrio cholerae O1 in Bangladesh waters by fluorescent-antibody direct viable count. Appl Environ Microbiol. 1987;53:2862–2865. doi: 10.1128/aem.53.12.2862-2865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Food-borne surveillance data for all pathogens in fish/shellfish for years 1978–1987. U.S. Atlanta, Ga: Department of Health and Human Services; 1989. [Google Scholar]
- 3.Colwell R R, Brayton P R, Grimes D J, Roszak S A, Huq S A, Palmer L M. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Bio/Technology. 1985;3:817–820. [Google Scholar]
- 4.Cook D W. Microbiology of bivalve molluscan shellfish. In: Ward D R, Hackney C, editors. Microbiology of marine food products. New York, N.Y: Van Nostrand Reinhold; 1991. pp. 19–39. [Google Scholar]
- 5.DePaola A, Hopkins L H, Peeler J T, Wentz B, McPhearson R M. Incidence of Vibrio parahaemolyticus in U.S. coastal waters and oysters. Appl Environ Microbiol. 1990;56:2299–2302. doi: 10.1128/aem.56.8.2299-2302.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoover D G, Metrick C, Papineau A M, Farkas D F, Knorr D. Biological effects of high hydrostatic pressure on food microorganisms. Food Technol. 1989;43:99–107. [Google Scholar]
- 7.Jiang X, Martin R E, Chai T. 45th Annual Interstate Seafood Seminar Proceedings. Ocean City, Md. 1993. Characterization of Vibrio parahaemolyticus in starvation at low temperature; pp. 205–216. [Google Scholar]
- 8.Kogure K, Shimidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Med. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 9.Madden J M, McCardell B A, Read R B. Vibrio cholerae in shellfish from U.S. coastal waters. Food Technol. 1982;36:93–95. [Google Scholar]
- 10.Morris J G, Black R E. Cholera and other Vibrios in the United States. N Engl J Med. 1985;312:343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- 11.National Advisory Committee on Microbiological Criteria for Foods. Microbiological criteria for raw molluscan shellfish. J Food Prot. 1992;55:463–480. doi: 10.4315/0362-028X-55.6.463. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson L, Oliver J D, Kjelleberg S. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol. 1991;173:5054–5059. doi: 10.1128/jb.173.16.5054-5059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 14.Oliver J D, Wanucha D. Survival of Vibrio vulnificus at reduced temperature and elevated nutrient. J Food Saf. 1989;10:79–86. [Google Scholar]
- 15.Patterson M F, Kilpatrick D J. The combined effect of high hydrostatic pressure and mild heat on inactivation of pathogens in milk and poultry. J Food Prot. 1998;61:432–436. doi: 10.4315/0362-028x-61.4.432. [DOI] [PubMed] [Google Scholar]
- 16.Ray B, Hawkins S M, Hackney C R. Methods for detection of injured Vibrio parahaemolyticus in seafoods. Appl Environ Microbiol. 1978;35:1121–1127. doi: 10.1128/aem.35.6.1121-1127.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roszak D B, Colwell R R. Survival strategies in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SAS. SAS users guide: statistics. Cary, N.C: SAS Institute, Inc.; 1985. [Google Scholar]
- 20.Smelt J P P M. Recent advances in the microbiology of high pressure processing. Trends Food Sci Technol. 1998;9:152–158. [Google Scholar]
- 21.Styles M F, Hoover D G, Farkas D F. Response of Listeria monocytogenes and Vibrio parahaemolyticus to high hydrostatic pressure. J Food Sci. 1991;56:1404–1407. [Google Scholar]
- 22.Wolf P W, Oliver J D. Temperature effects on the viable but nonculturable state of Vibrio vulnificus. FEMS Microbiol Ecol. 1992;101:33–39. [Google Scholar]
- 23.Xu H S, Roberts N C, Adams L B, West P A, Seibeling R J, Huq A, Huq M I, Rahman R, Colwell R R. An indirect fluorescent antibody staining procedure for detection of Vibrio cholerae serovar O1 cells in aquatic environmental samples. J Microb Methods. 1984;2:221–231. [Google Scholar]
- 24.Xu H S, Roberts N C, Singelton E L, Atwell R W, Grimes D J, Colwell R R. Survival and culturability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]
- 25.Yukizaki C, Kawano M, Tsumagari H. The sterilization of sea urchin eggs by high hydrostatic pressure. In: Hayashi R, editor. High pressure bioscience and food science. Kyoto, Japan: San-ei Pub. Co.; 1992. pp. 225–228. [Google Scholar]