Abstract
Arthrobacter oxydans HAP-1 hyperproduces dl-alanine in a non-growth-associated manner. We found that decreased activities of pyruvate dehydrogenase and of the enzyme catalyzing NADH oxidation in the stationary phase are paralleled by a shift of pyruvate metabolism to alanine synthesis by l-alanine dehydrogenase. We propose that this enzyme functions as an electron sink even under aerobic conditions.
Since the discovery of bacterial glutamic-acid production in 1957 (11, 12), several l-amino acids have been produced by fermentative processes (14). However, the commercial production of l-alanine, the simplest l-amino acid, is not carried out in this manner (3). We previously studied the fermentative production of l-alanine and found a dl-alanine-hyperproducing strain of Arthrobacter oxydans HAP-1 (4). Subsequently, mutants lacking alanine racemase were isolated from this strain, and one of them was shown to produce l-alanine at a high yield with high optical purity (5). Furthermore, A. oxydans HAP-1 possessed the activity of l-alanine dehydrogenase (EC 1.4.1.1) (ALD) (4), which catalyzes the reversible, NADH-dependent reductive amination of pyruvate to l-alanine. The finding that ALD-deficient mutants derived from HAP-1 no longer excrete alanine (5) indicated that alanine overproduction by A. oxydans HAP-1 depends on ALD. Although several ALD-possessing microorganisms have been reported (15, 16, 21), this was the first demonstration of the functioning of ALD in l-alanine synthesis in a natural isolate by genetic analysis.
In addition to these traits, alanine production by A. oxydans HAP-1 has another significant characteristic: its growth and amino acid production phases are clearly separated. During the initial stage of cultivation, the bacterium grows vigorously but excretes no alanine. On the contrary, upon cessation of growth, abrupt accumulation of alanine in the medium occurs. This pattern is in marked contrast to the fermentation profiles of the production of other amino acids, such as lysine (7, 20), threonine (8), arginine (22), tryptophan (9), proline (13), or histidine (1), in which production of an amino acid is associated with cell growth. In this study, we investigated the biochemical mechanisms involved in alanine hyperproduction in a non-growth-associated manner.
A. oxydans HAP-1 was cultivated in a 5-liter jar fermentor containing an initial glucose concentration of 5%, to which glucose was fed to a final concentration of 15.5% (5). The pH was automatically maintained at 6.8 by the addition of 10 N NH4OH. Cell growth and metabolites in the medium were measured as previously reported (5). The dissolved oxygen concentration of the medium was continuously monitored with an oxygen electrode (model OE8270G; Toa Electronics, Tokyo, Japan). The exhaust gas of the fermentor was cooled and analyzed by a gas analyzer (model Ex-1562; AIBL, Tokyo, Japan). CO2 production and O2 consumption rates were calculated according to the equations described by Postma et al. (17).
Fermentation profile.
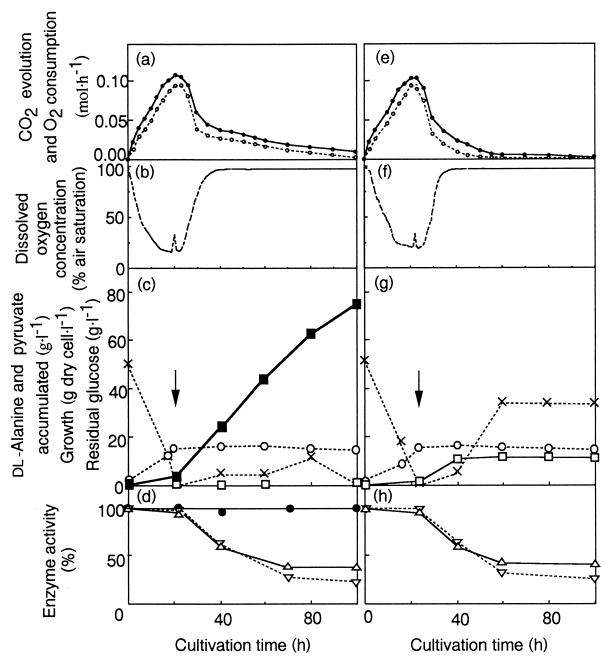
In the growth phase (0 to 20 h), the rate of CO2 production increased while little alanine was accumulated. It was calculated that 0.55 mol of glucose was consumed and 48.8% of carbon derived from glucose was released as CO2 in this phase. Once cell growth was arrested, the rate of CO2 production decreased and alanine production started. In the stationary phase (20 to 80 h), 2.02 mol of glucose was consumed, and CO2 and alanine formation accounted for 21.2 and 51.6% of the carbon from glucose, respectively. Thus, sugar metabolism clearly shifted from CO2 production to alanine synthesis after the shift to the stationary phase. The O2 uptake rate (Fig. 1a) and the changes in dissolved oxygen concentration (Fig. 1b) paralleled the decrease in CO2 production (Fig. 1a). Therefore, cells in the late stage of cultivation (after 40 h) utilize less O2 despite the aerobic conditions.
FIG. 1.
Time course of fed-batch cultivation of A. oxydans HAP-1 (a through d) and A. oxydans DAN 89 (e through h). Cultivations were carried out under aerobic conditions (throughout the cultivation, the airflow and the stirrer speed were maintained at 1.0 liter · min−1 and 600 rpm, respectively). (a and e) Rates of CO2 evolution (●) and O2 consumption (○); (b and f) concentration of dissolved oxygen in the medium; (c and g) growth of cells (dry weight) (○) and concentrations of dl-alanine (■), pyruvate (□), and glucose (×) in the medium. Arrows indicate the point at which additional glucose solution started to be fed continuously to the medium. Feeding for A. oxydans HAP-1 was stopped at 70 h. Feeding for A. oxydans DAN 89 was stopped at 60 h because its glucose consumption stopped earlier (around 50 h). (d and h) Changes in enzymatic activities. Activities are expressed as percentages of the activities observed at the beginning of cultivation. Symbols: ●, ALD (100% corresponds to 0.34 U · mg of protein−1; the ALD activity of DAN 89 was below the detection limit [0.005 U · mg of protein−1]); ▵, PDH (100% corresponds to 0.30 and 0.29 U · mg of protein−1 for HAP-1 and DAN 89, respectively); ▿, NADH oxidase (100% corresponds to 0.9 μmol · min−1 · mg of protein−1 for both strains).
To investigate the biochemical changes accompanying the metabolic shift described above, crude cell extracts were prepared from cells harvested at different stages of cultivation. After centrifugation (at 4,650 × g for 10 min), cells were washed twice with 50 mM potassium phosphate buffer (pH 7.5) and resuspended in the same buffer containing 2 mM EDTA and 1 mM dithiothreitol (PED buffer) at a concentration of 64 g of cells (dry weight) · liter−1. The cells were disrupted as described previously (5), and cellular debris was removed by centrifugation (at 10,500 × g for 45 min) at 4°C. The supernatants were dialyzed against PED buffer (at 4°C for 6 h) and used for the enzyme assays. The amount of protein was determined by the method of Bradford (2) with a Bio-Rad kit.
We measured three enzyme activities: ALD and pyruvate dehydrogenase (PDH), both of which compete for pyruvate, and NADH oxidase, which is essential for aerobic respiration by A. oxydans (10). ALD activity was determined as previously described (4, 5). PDH activity was assayed by the method described by Reed and Willms (18), in which the reaction was coupled to phosphotransacetylase to yield acetylphosphate, which reacted with ferric chloride and was measured spectrophotometrically. One unit of enzyme activity was defined as the amount of protein required to form 1 μmol of acetylphosphate per min at 30°C. The assay for NADH oxidase was performed by essentially the same method as that reported by Sakamoto et al. (19). The dialyzed cell extracts were suspended in 100 mM potassium phosphate buffer (pH 7.0) and flushed with air. The reaction was started by the addition of 5 mM NADH and was immediately monitored with a Hitachi 100-60 spectrophotometer set at 340 nm. The activity was determined from the decrease in the amount of NADH in the first 15 s.
Changes in the three activities during cultivation are shown in Fig. 1d. The activities of PDH and NADH oxidase decreased in the stationary phase and reached 30% of their initial levels at the end of cultivation, whereas the level of ALD activity was essentially unchanged throughout the cultivation. Based on these results, the shift in sugar metabolism was likely not the result of an ALD induction but resulted from the decline in PDH activity and the reduction in respiratory activity due to decreased NADH oxidase activity.
To clarify whether ALD plays a role in sugar metabolism, A. oxydans DAN 89, an ALD-deficient mutant derived from A. oxydans HAP-1 (5), was cultivated and analyzed in the same way as strain HAP-1 (Fig. 1e through h). Cell growth, CO2 production, O2 consumption, and activities of PDH and NADH oxidation of the mutant were similar to those of the parental strain. Significant differences, however, were found in that (i) DAN 89 excreted pyruvate but not alanine (Fig. 1g), as reported previously (5), and (ii) the rate of glucose consumption of the mutant strain was markedly decreased in the stationary phase and reached 0 at around 60 h (Fig. 1g). These results indicate that alanine synthesis by ALD and the maintenance of glycolytic flow are closely related during the stationary phase of A. oxydans HAP-1. Since NADH-oxidizing activity is reduced in the stationary phase, reoxidation of NADH through respiration is apparently insufficient to maintain glycolytic flow. Therefore, the reductive amination of pyruvate by ALD may function as an alternative NADH-reoxidizing reaction, and thus, alanine may be an electron sink. A similar function of ALD has recently been assumed in Mycobacterium smegmatis during adaptation from aerobic growth to the anaerobic dormant state (6), although it remains unclear whether the bacterium overproduces alanine. In the case of A. oxydans HAP-1, the coupling between glycolysis and alanine synthesis, which may be considered a fermentative mechanism, likely results in the high-yield alanine production.
In summary, we propose the following mechanism for alanine overproduction by A. oxydans HAP-1: (i) during the growth phase, the glycolytic end product, pyruvate, is metabolized by the tricarboxylic-acid cycle and respiration because of sufficiently high activities of PDH and NADH oxidase; (ii) when cell growth is arrested, the activities of PDH and NADH oxidase decrease, yielding an excess of pyruvate and NADH. Under these conditions, NADH is oxidized by the reductive amination of ALD. This metabolic shift to a fermentative pathway even under aerobic conditions causes the overproduction of alanine.
Acknowledgments
We thank Naoko Kodama-Oda for her skillful technical assistance.
REFERENCES
- 1.Araki K, Nakayama K. Studies on histidine fermentation. Agric Biol Chem. 1971;35:2081–2088. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Calton G J. The enzymatic preparation of l-alanine. In: Rozzell J D, Wagner E, editors. Biocatalytic production of amino acids and derivatives. New York, N.Y: John Wiley & Sons Inc.; 1992. pp. 60–74. [Google Scholar]
- 4.Hashimoto S, Katsumata R. Overproduction of alanine by Arthrobacter strains with glucose-nonrepressible l-alanine dehydrogenase. Biotechnol Lett. 1993;15:1117–1122. [Google Scholar]
- 5.Hashimoto S, Katsumata R. l-Alanine fermentation by an alanine racemase deficient mutant of the dl-alanine hyperproducing bacterium, Arthrobacter oxydans HAP-1. J Ferment Bioeng. 1998;86:346–351. [Google Scholar]
- 6.Hutter B, Dick T. Increased alanine dehydrogenase activity during dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett. 1998;167:7–11. doi: 10.1111/j.1574-6968.1998.tb13200.x. [DOI] [PubMed] [Google Scholar]
- 7.Jetten M S M, Follettie M T, Sinskey A J. Effect of different levels of aspartokinase on the lysine production by Corynebacterium lactofermentum. Appl Microbiol Biotechnol. 1995;43:76–82. doi: 10.1007/BF00170626. [DOI] [PubMed] [Google Scholar]
- 8.Kase H, Tanaka H, Nakayama K. Studies on l-threonine fermentation. Agric Biol Chem. 1971;35:2089–2096. [Google Scholar]
- 9.Katsumata R, Ikeda M. Hyperproduction of tryptophan in Corynebacterium glutamicum by pathway engineering. Bio/Technology. 1993;11:921–925. [Google Scholar]
- 10.Keddie R M, Collins M D, Jones D. Genus Arthrobacter. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1288–1301. [Google Scholar]
- 11.Kinoshita S, Tanaka K, Udaka S, Akita S. Proceedings of the International Symposium on Enzyme Chemistry Tokyo and Kyoto 1957. Tokyo, Japan: Maruzen Inc.; 1958. Glutamic acid fermentation; pp. 464–468. [Google Scholar]
- 12.Kinoshita S, Udaka S, Simono M. Studies on amino acid fermentation. J Gen Appl Microbiol. 1957;3:193–205. [PubMed] [Google Scholar]
- 13.Masuda M, Takamatsu S, Nishimura N, Komatsubara S, Tosa T. Improvement of culture conditions for l-proline production by a recombinant strain of Serratia marcescens. Appl Biochem Biotechnol. 1993;43:189–197. doi: 10.1007/BF02916452. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama K. Amino acids. In: Reed G, editor. Prescott and Dunn’s industrial microbiology. 4th ed. Westport, Connecticut: Ari Publishing Company Inc.; 1982. pp. 748–801. [Google Scholar]
- 15.Norbert M, Brunhuber W, Blanchard J S. The biochemistry and enzymology of amino acid dehydrogenases. Crit Rev Biochem Mol Biol. 1994;29:415–467. doi: 10.3109/10409239409083486. [DOI] [PubMed] [Google Scholar]
- 16.Ohshima T, Soda K. Purification and characterization of alanine dehydrogenase from Bacillus sphaericus. Eur J Biochem. 1979;100:29–39. doi: 10.1111/j.1432-1033.1979.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 17.Postma E, Verduyn C, Scheffers W A, Van Dijken J P. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed L J, Willms C R. Purification and resolution of the pyruvate dehydrogenase complex (Escherichia coli) Methods Enzymol. 1966;9:247–249. [Google Scholar]
- 19.Sakamoto M, Uchimura T, Komagata K. Comparison of H2O2-forming NADH oxidase from Leuconostoc mesenteroides subsp. mesenteroides NRIC 1541T and H2O2-forming NADH oxidase from Sporolactobacillus inulinus NRIC 1133T. J Ferment Bioeng. 1996;82:531–537. [Google Scholar]
- 20.Sano K, Shiio I. Microbial production of l-lysine. J Gen Appl Microbiol. 1971;17:97–113. [Google Scholar]
- 21.Sawa Y, Tani M, Murata K, Shibata H, Ochiai H. Purification and characterization of alanine dehydrogenase from a cyanobacterium, Phormidium lapileum. J Biochem (Tokyo) 1994;116:995–1000. doi: 10.1093/oxfordjournals.jbchem.a124659. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida H, Araki K, Nakayama K. l-Arginine production by arginine analog-resistant mutants of microorganisms. Agric Biol Chem. 1981;45:959–963. [Google Scholar]