Abstract
N1-methyladenosine (m1A) is a prevalent and reversible post-transcriptional RNA modification that decorates tRNA, rRNA and mRNA. Recent studies based on technical advances in analytical chemistry and high-throughput sequencing methods have revealed the crucial roles of m1A RNA modification in gene regulation and biological processes. In this review, we focus on progress in the study of m1A methyltransferases, m1A demethylases and m1A-dependent RNA-binding proteins and highlight the biological mechanisms and functions of m1A RNA modification, as well as its association with human disease. We also summarize the current understanding of detection approaches for m1A RNA modification.
Keywords: N1-methyladenosine(m1A), RNA modification, gene expression
1. Introduction
Cellular RNAs contain more than 170 different types of chemical modifications across species [1]. N1-methyladenosine(m1A) is a reversible methylation involving the addition of a methyl group at the N1 position of adenosine in cellular transcripts [2]. The methyl group can block the normal Watson–Crick base pairing of A:T or A:U, resulting in an unstable mismatch with other nucleosides by forming Hoogsteen base pairs [3]. The secondary structure and RNA–protein interaction of m1A-modified RNAs are also altered under physiological conditions [4]. As a dynamic and reversible post-transcriptional RNA modification, m1A can be installed by methyltransferases, removed by demethylases and recognized by m1A-dependent RNA-binding proteins [2,5]. m1A RNA modification affects RNA metabolism, including RNA structure, stability and mRNA translation, thereby regulating gene expression and several fundamental cellular processes [6].
m1A RNA modification has been found with high abundance in transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs) but at low levels in messenger RNAs (mRNAs) [7,8,9,10,11,12]. It occurs in the tRNA of bacteria, archaea and eukaryotes at positions 9, 14, 16, 22, 57 and 58 (m1A9, m1A14, m1A16, m1A22, m1A57, and m1A58, respectively) [13]. In cytosolic (cyt) tRNAs, m1A RNA modification occurs at five different positions (9, 14, 22, 57, and 58) [14,15]. Among them, m1A14 has only been identified in cyt(tRNA)Phe from mammals, m1A22 has only been identified in bacteria tRNAs, and m1A57 has been identified in archaea existing only transiently as an intermediate of 1-methylinosine (m1I) [14,15]. In mitochondria, m1A9 is quite abundant and found in 14 species of mt-tRNA, while m1A58 is a minor modification with a 17% frequency found in four species of mt-tRNAs [16]. Additionally, m1A16 is unique to human mt-tRNAArg, and its frequency is approximately 20% [16]. For rRNAs, the nuclear-encoded large subunit rRNA m1A645 in 25S rRNA and m1A1322 in 28S rRNA located in the peptidyl transfer center of the ribosome are conserved in budding yeast and humans, respectively [17,18,19], and m1A is conserved at position 947 of 16S rRNA in the mitochondrial ribosome of vertebrates [20]. Regarding mRNAs, m1A in mRNA accounts for approximately 0.015–0.054% of all adenosines in mammalian cell lines and 0.05–0.16% in mammalian tissues [9,10,21]. m1A sites are usually located near the translation start site and the first splice site of mRNA, and they are associated with the translation of coding transcripts [9,10].
In this review, we describe mammalian m1A RNA-modifying proteins that specifically install, remove, and bind to the m1A base. We also discuss the recent progress in understanding the biological mechanisms involving m1A in post-transcriptional gene expression regulation and the biological functions of m1A. Further, we review the current approaches for transcriptome-wide and single-base resolution m1A detection.
2. m1A RNA-Modifying Proteins
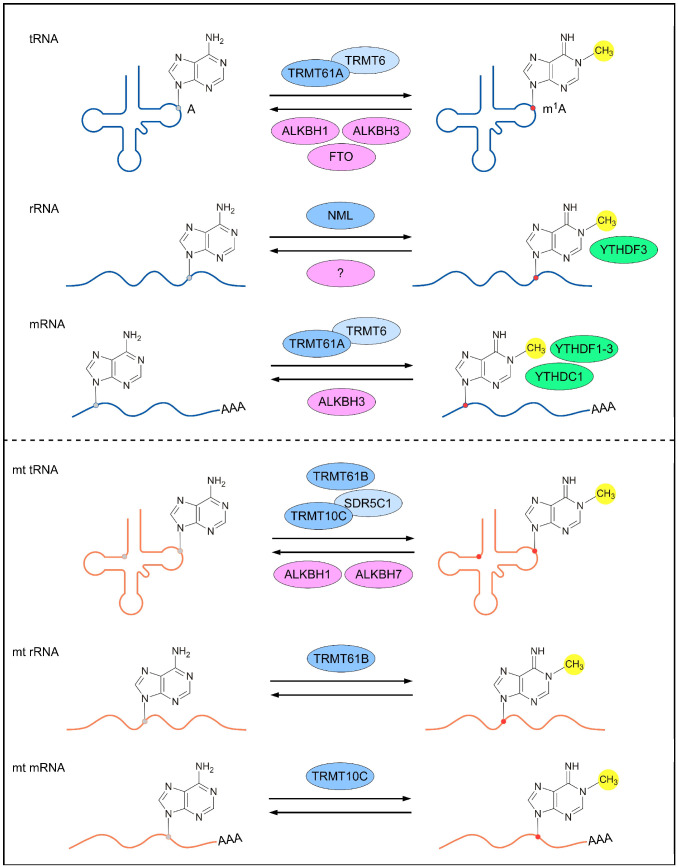
Reversible m1A methylomes in nuclear- and mitochondrial-encoded transcripts are achieved via the dynamic regulation of m1A RNA-modifying proteins (m1A methyltransferases, m1A demethylases and m1A-dependent RNA-binding proteins). The characterization of m1A-modifying proteins is crucial for understanding the mechanisms underlying m1A-mediated gene regulation and the biological roles of m1A RNA modification. To date, several m1A RNA-modifying proteins responsible for nuclear- and mitochondrial-encoded transcripts have been identified in humans (Figure 1).
Figure 1.
m1A-modifying proteins for different types of RNAs. The nuclear-encoded (top panel) and mitochondrial (bottom panel) RNAs are reversibly methylated by m1A methyltransferases (blue; dark blue represents catalytic core of the methylase complex), demethylased by m1A demethylases (pink), and bound by m1A-dependent RNA-binding proteins (green). A, adenosine; m1A, N1-methyladenosine; TRMT, tRNA (adenine (58)-N (1))-methyltransferase subunit; ALKBH, α-ketoglutarate-dependent dioxygenase alkB homolog; FTO, α-ketoglutarate-dependent dioxygenase alkB homolog FTO; NML, nucleomethylin; YTHDF, YTH domain-containing family protein; YTHDC1, YTH domain-containing protein 1; SDR5C1, 3-hydroxyacyl-CoA dehydrogenase type-2.
2.1. m1A-Modifying Proteins for Nuclear-Encoded Transcripts
m1A is highly abundant in tRNAs, and data on the modifying proteins for m1A58 are clearer than those for other m1A sites in cytoplasmic tRNAs. The tRNA m1A58 methyltransferase complex was first identified in Saccharomyces cerevisiae, which contains a non-catalytic subunit and a catalytic subunit encoded by tRNA (adenine(58)-N(1))-methyltransferase non-catalytic subunit TRM6 (Trm6) and Trm61, respectively [22]. Trm61 is critical for AdoMet binding and catalytic function, while Trm6 is responsible for tRNA binding [23]. The conserved homolog of this complex in eukaryotes comprises TRMT61A and TRMT6, which mediate the m1A58 modification in cytoplasmic tRNAs [24]. Demethylases for nuclear-encoded tRNAs include α-ketoglutarate-dependent dioxygenase alkB homolog 1 (ALKBH1), ALKBH3 and α-ketoglutarate-dependent dioxygenase FTO (FTO), all belonging to the AlkB family of Fe(II)/α-ketoglutarate-dependent dioxygenases [25,26].
rRNAs act as scaffolds for ribosomal proteins and as ribozymes for peptide bond formation in ribosomes; these functions are partly regulated by post-transcriptional modifications [26]. The m1A modification has been founded in the large subunit of nuclear-encoded rRNAs in yeast and humans [27]. Yeast 25S rRNA (adenine(645)-N(1))-methyltransferase (Rrp8) and 25S rRNA (adenine(2142)-N(1))-methyltransferase (Bmt2) were reported to be the methyltransferases responsible for the m1A645 and m1A2142 modifications, respectively, of 25S rRNA [28,29]. The homolog of yeast Rrp8 in humans is nucleomethylin (NML), a nucleolar factor that catalyzes the m1A modification of 28S rRNA in human and mouse cells [30,31]. NML contains a Rossmann-fold methyltransferase-like domain that binds to S-adenosyl-L-methionine, which acts as a methyl donor [30]. YTH domain-containing family protein 3 (YTHDF3) was identified as an m1A-dependent RNA-binding protein in HEK293T and RAW264.7 cells, because it showed the highest ability to bind to a human 28S rRNA-derived m1A probe. A subsequent RNA immunoprecipitation analysis confirmed that m1A-modified RNA was bound by YTHDF3, but not YTHDF1/2, in trophoblast HTR8/SVneo cells [32].
Studies have shown that tRNA m1A-modifying proteins are also responsible for the m1A modification of mRNAs. For example, the TRMT6/TRMT61A complex mediates the m1A addition to mRNA. TRMT6/61A also requires a consensus GUUCRA motif and a tRNA T-loop-like structure for substrate recognition in mRNA, which is similar to tRNA recognition [12]. In contrast, m1A in mRNA can be demethylated by ALKBH3 [9,10,33]. In total, 774 m1A peaks were reported to be specifically detected in ALKBH3-knockout HEK293T cells, and these peaks were enriched in the 5′UTR [9]. Stable isotope labeling by amino acid in cell culture (SILAC)-based quantitative proteomics have identified that YTH domain-containing proteins (YTHDF1-3 and YTHDC1) act as m1A-dependent RNA-binding proteins in mRNA; these proteins can directly bind to an m1A-modified RNA probe (sequence from the human transcription factor SOX-18 mRNA) in HEK293T cells. It was also found that Trp432 in YTHDF2 is a key binding residue [34]. m1A RNA modification was reported to favor a GA-rich motif, and transcripts containing this motif were increased after ALKBH3 depletion [9,11]. Therefore, the researchers of another study designed a biotin-labeled RNA probe in which m1A RNA modification was placed in a GAGGm1AG sequence. It could be recognized by YTHDF1 and YTHDF2 in HeLa cells, but YTHDC1 was not detected in this system [35].
2.2. m1A-Modifying Proteins for Mitochondrial RNAs
tRNA methyltransferase 10 homolog C (TRMT10C) acts as a catalytic core in the TRMT10C/SDR5C1 (also known as HSD17B10, hydroxysteroid 17-β dehydrogenase 10) methylase complex, which is responsible for the m1A9 of mt tRNALys and m1A1374 of mitochondrial NADH–ubiquinone oxidoreductase chain 5 (ND5) mRNA [12,36]. In addition, TRMT10C is also able to catalyze the m1G9 modification in mt tRNAs [36]. TRMT61B catalyzes the m1A58 of mt tRNALeu (UUR) and m1A947 of mt 16S rRNA [20,37]. Two demethylases, ALKBH1 and ALKBH7, have been shown to remove m1A sites from mt RNAs. The level of m1A16 of mt-tRNAArg and m1A58 of mt-tRNALys was found to be increased upon ALKBH1 knockout, indicating the role for ALKBH1 in the m1A of mt RNAs [38]. The human ALKBH7 has been reported to demethylate m1A in Ile and Leu1 pre-tRNA in the mitochondria [39].
3. Biological Functions of m1A RNA Modification
Since the discovery of m1A RNA modification as a chemical modification of RNAs, efforts have been taken to understand the functional characterization of this dynamic methylation in RNA metabolism and gene expression regulation.
3.1. m1A RNA Modification in RNA Metabolism
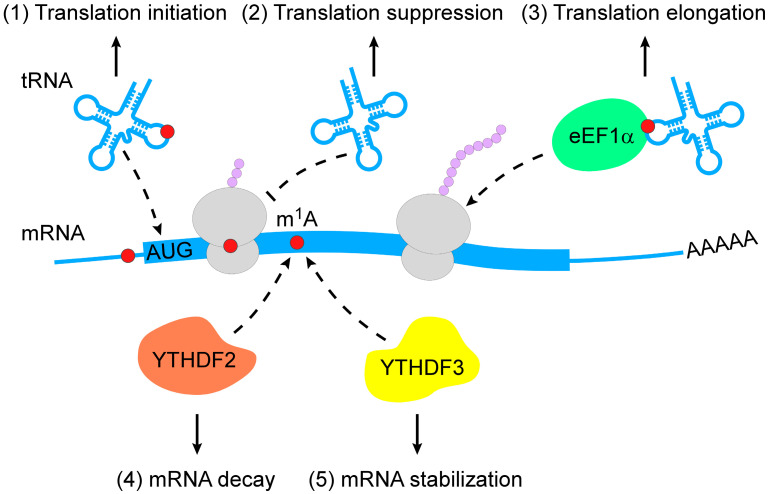
m1A RNA modification is a pivotal regulator of RNA metabolism, including RNA structure alteration, decay and translation (Figure 2).
Figure 2.
Action mechanisms of m1A in RNA metabolism. m1A RNA modification regulates RNA metabolism in multiple layers (from top to bottom: (1) m1A RNA modification stabilizes tRNAs to promote translation initiation; (2) m1A-modified mRNAs interfere with Watson–Crick base-pairing with tRNA to suppress translation; (3) m1A-modified tRNAs are coupled with eEF1α to polysomes to promote translation elongation; (4) m1A-modified mRNAs are subjected to degradation by interacting with YTHDF2; (5) m1A-modified mRNAs become stable when they bind to YTHDF3). m1A, N1-methyladenosine; eEF1α, eukaryotic elongation factor 1-α; YTHDF, YTH domain-containing family protein.
The chemical properties of m1A RNA modification enable changes in RNA secondary structure. For instance, m1A9 and m1A58 in tRNAs are required for the conformational shift of mitochondrial tRNALys and tRNAiMet, respectively, which contribute to the stabilization of alternative native structures [40,41,42,43]. The loss of m1A645 has been shown to affect the topological structure of 28S rRNA and alter the RNA interactome [31]. m1A was also found to favor the hairpin structure of palindromic RNA sequences, wherein m1A can stably localize within apical loops [44]. A recent study revealed that m1A RNA modification controlled RNA conformational equilibrium by blocking base-pairing to modulate the RNA duplex [3].
The regulation of m1A-modified mRNA decay is mediated by m1A-dependent RNA-binding proteins. Limited evidence suggests that the knockdown of YTHDF2 increases the abundance of 7 out of 8 m1A-modified transcripts and 2 out of 3 transcripts that bear only the m1A but not m6A (N6-methyladenosine) modification [35]. In addition to YTHDF2, YTHDF3 overexpression has been reported to decrease the abundance and decay rate of insulin like growth factor 1 receptor (IGF1R) mRNA [32].
Translational regulation by m1A modification varies among different RNA types. The m1A demethylases ALKBH1 and FTO have been reported to control specific tRNA m1A demethylation and decrease translation initiation [45,46]. Eukaryotic elongation factor 1-α (eEF1α) immunoprecipitation was used to reveal that m1A-methylated tRNAs are enriched in polysomes, indicating the role of m1A RNA modification in translation activation [45]. During retroviral reverse transcription in early human immunodeficiency virus 1 (HIV-1) replication, TRMT6-mediated m1A58 of tRNA3Lys acted as a stop site that contributed to genome integration [47]. Further, mRNAs carrying m1A undergo translation repression because of interfered Watson–Crick base pairing [8,12,48].
3.2. m1A RNA Modification in Biological Processes
Post-transcriptional modifications are involved in various biological processes, and recent evidence showed the importance of m1A RNA modification in this field. In a high-temperature-sensitive Thermococcus kodakarensis strain, decreased m1A58 and melting temperature of tRNA were observed, suggesting the relevance of m1A58 and the growth ability of this strain at high temperatures [49]. m1A RNA modification was found to exhibit its protective ability of RNAs under stress conditions. During heat shock, m1A-harbouring transcripts were found to preferentially accumulate in stress granules, subsequently resulting in a shorter time to restore the translation state during recovery [50]. Alkylating agents induced m1A modification in RNAs and orchestrated translational suppression by recruiting the ASCC damage repair complex (activating signal cointegrator 1 complex) [51]. The tRNA modification profiles of the Aplysia central nervous system showed increased m1A RNA modification levels in animals after behavioral training [52]; this was the first study to characterize the variable pattern of m1A RNA modification during defensive reflex-associated behavioral sensitization. Petunia TRMT61A catalyzed m1A RNA modification in mRNAs, and the knockdown of TRMT61A decreased the chlorophyll content and changed chlorotic and wrinkled leaf phenotype [53]. A recent study showed that the m1A demethylase ALKBH3 functioned as a negative regulator of ciliogenesis by removing the m1A sites on Aurora A mRNA (a key regulator of cilia disassembly) in mammalian cells, which was further involved in cilia-associated developmental processes in zebrafish [54].
4. m1A RNA Modification in Diseases
The limited exploration of m1A RNA modification as a pathological feature has mainly focused on tumor progression (Table 1). It was reported that the knockdown of m1A demethylase ALKBH3 increased the abundance of m1A RNA modification in small RNAs (< 200 nucleotides) along with suppressed nascent protein in pancreatic cancer cells [55]. The ALKBH3-dependent m1A demethylation of macrophage colony-stimulating factor 1 (CSF1) mRNA enhanced its mRNA stability and thus promoted the invasion of breast and ovarian cancer cells [56]. In addition, ALKBH3 removed the m1A RNA modification of tRNAGlyGCC to promote tRNA cleavage by angiogenin. The generation of excessive tRNA-derived small RNAs may affect ribosome assembly and apoptosis in HeLa cells [57]. Furthermore, ALKBH3 promoter CpG island hypermethylation and transcriptional silencing were found in Hodgkin lymphoma cells, which were identified as a potential prognostic biomarker associated with poor clinical outcomes in patients with Hodgkin lymphoma [58]. A recent study found that levels of tRNA m1A modification were upregulated in hepatocellular carcinoma (HCC) tissues. The TRMT6/TRMT61A complex mediated increased m1A58 levels in tRNA, which then triggered peroxisome proliferator-activated receptor delta (PPARδ) mRNA translation in HCC stem cells. PPARδ promoted cholesterol biogenesis to activate the Hedgehog pathway, thereby initiating the self-renewal of HCC stem cells [59].
Table 1.
Dysregulation of m1A RNA modification in human cancers.
| Cancers | m1A-Modifying Proteins | Roles | Targets | Mechanisms | Refs |
|---|---|---|---|---|---|
| Pancreatic cancer | ALKBH3 | Oncogene | small RNAs | Unknown | [55] |
| Breast and ovarian cancer | ALKBH3 | Oncogene | CSF1 | mRNA decay | [56] |
| Cervical cancer | ALKBH3 | Oncogene | tRNAs | tRNA cleavage | [57] |
| Hodgkin lymphoma | ALKBH3 | Tumor suppressor |
COL1A1, COL1A2 | Unknown | [58] |
| Hepatocellular carcinoma | TRMT6/TRMT61A | Oncogene | tRNAs | Unknown | [59] |
ALKBH, α-ketoglutarate-dependent dioxygenase alkB homolog; TRMT, tRNA (adenine(58)-N(1))-methyltransferase subunit; CSF-1, macrophage colony-stimulating factor 1; COL1A1, collagen α-1(I) chain; COL1A2, collagen α-2(I) chain.
5. Approaches for m1A RNA-Modification Detection
Several methods have been developed for the detection of m1A modifications in RNAs. m1A can be detected by bi-dimensional thin-layer chromatography (2D-TLC) or high-pressure liquid chromatography (HPLC), both of which utilize the differential retention properties for the separation of various nucleosides [4]. Furthermore, the coupling of liquid chromatography to mass spectrometry (LC–MS/MS) can provide a more sensitive approach for quantifying the m1A level in RNAs [4]. The drawback of these methods is that the location information of m1A is generally lost.
Recent advances in high-throughput technologies have revealed the global m1A distribution map and dynamics in the transcriptome, and developed tools provide easy ways for m1A site-specific detection. Here, we summarize the high-throughput sequencing approaches for m1A RNA-modification detection (Table 2).
Table 2.
Detection methods for mapping m1A RNA modification.
| Methods | Features | RNA Substrate | Antibody Dependent | Resolution | Cell Line |
|---|---|---|---|---|---|
| ARM-seq [7] | AlkB treatment | tRNA | No | Fragment | BY4741, GM05372, GM12878 |
| m1A-quant-seq [11] | Spike-in m1A RNA; AlkB treatment; RT-1306-mediated RT mutation |
tRNA, rRNA, mRNA, lncRNA | No | Single base | HEK293T |
| m1A-seq [10] | m1A-RNA immunoprecipitation; Dimroth rearrangement |
rRNA, mRNA | Yes | Fragment | BY4741, Sp1, MEF, mESC, HeLa |
| m1A-ID-seq [9] | m1A-RNA immunoprecipitation; AlkB treatment; AMV-mediated RT truncation |
rRNA, mRNA, lncRNA | Yes | Fragment | HEK293T |
| m1A-seq-SS [12] | m1A-RNA immunoprecipitation; Dimroth rearrangement; SS-mediated RT truncation |
tRNA, rRNA, mRNA, lncRNA | Yes | Single base | HEK293T |
| m1A-seq-TGIRT [12] | m1A-RNA immunoprecipitation; Dimroth rearrangement; TGIRT-mediated RT readthrough |
tRNA, rRNA, mRNA, lncRNA | Yes | Single base | HEK293T |
| m1A-MAP [8] | m1A-RNA immunoprecipitation; AlkB treatment; TGIRT-mediated RT read-through |
tRNA, rRNA, mRNA, lncRNA | Yes | Single base | HEK293T |
| m1A-IP-seq [11] | m1A-RNA immunoprecipitation; AlkB treatment; RT-1306-mediated RT mutation |
tRNA, rRNA, mRNA, lncRNA | Yes | Single base | HEK293T |
AlkB, α-ketoglutarate-dependent dioxygenase; RT, reverse transcription; AMV, avian myeloblastosis virus; SS, SuperScript III; TGIRT, thermostable group II intron reverse transcriptase; BY4741, saccharomyces cerevisiae BY4741 strains; GM05372, human B lymphocyte-derived GM05372 cell; GM12878, human B lymphocyte-derived GM12878 cell; HEK293T, human embryonic kidney 293T cell; Sp1, schizosaccharomyces pombe Sp1 cells; MEF, mouse embryonic fibroblasts; mESC, mouse embryonic stem cell; HeLa, human HeLa cell.
5.1. m1A Antibody-Independent Detection Methods
Several enzyme-based strategies have been developed for detecting m1A RNA modification. Escherichia coli α-ketoglutarate-dependent dioxygenase, AlkB, can demethylase several post-translational modifications to avoid unexpected termination during reverse transcription. In ARM-seq (AlkB-facilitated RNA methylation sequencing), the removal of m1A, m3C (N3-methylcytidine), or m1G (N1-methylguanosine) modifications by AlkB treatment facilitates the production of full-length cDNAs from previously modified templates, producing a ratio of reads in treated versus untreated samples that can be used to identify methylated RNAs [7]. RT-1306 is an evolved HIV-1 reverse transcriptase that can induce m1A-specific base mutations, thus providing a direct and rapid method for mapping m1A modification [11]. One possible drawback is that the accuracy is dependent on the fidelity and specificity of the enzymes used in these approaches.
5.2. m1A Antibody-Dependent Detection Methods
m1A antibody-dependent RNA immunoprecipitation has several advantages in m1A-modification detection. m1A antibody-dependent methylated RNA immunoprecipitation sequencing (m1A-MeRIP-seq) combined with AlkB demethylation (m1A-ID-seq) has been used to describe m1A dynamics in the human transcriptome [9]. Dominissini and colleagues also developed a method termed ‘m1A-seq’ that combined MeRIP-seq and Dimroth rearrangement to obtain the mRNA m1A methylome in human cells [10]. Moreover, combining advanced reverse transcriptase TGIRT (thermostable group II intron reverse transcriptase) and SuperScript III—which enable efficient RT misincorporation and truncation at m1A sites—with m1A antibody was shown to provide a feasible way to map m1A at a single-base resolution (m1A-seq-TGIRT and m1A-seq-SS) [12]. The misincorporation-assisted profiling of m1A (m1A-MAP) has improved the previous m1A-ID-seq by utilizing TGIRT and AlkB treatment. m1A-MAP has been used to identify a total of 740 m1A sites in the transcriptome and showed a GUUCRA consensus motif located in a subset of tRNA substrates of the TRMT6/61A complex [8]. A major drawback of these approaches is the non-specific binding of m1A antibodies, which may cause false positives.
5.3. Site-Specific m1A Detection
Based on m1A-induced Watson–Crick base-pairing disruption, some approaches have been developed for site-specific m1A evaluation. m1A induces the truncation or mutation of cDNA during reverse transcription, so primer extension can be applied to compare m1A modification levels based on the intensity of the truncated and full-length cDNA bands [60]. The clustered regularly interspaced short palindromic repeat (CRISPR)-associated endoribonuclease Cas13 can mediate the cleavage of both target and collateral RNAs after the formation of the Cas13/crRNA/target RNA complex. Based on this phenomenon, a reporter system with CRISPR/Cas13a can produce a high-fluorescence signal by cleaving quenched fluorescent RNAs with the correct Watson–Crick base-pairing of crRNA or target RNA but not m1A-modified RNAs [61]. Another method takes advantage of the different nick ligation efficiencies by T3 DNA ligases at the adenosine and m1A base. It can easily detect the m1A state at a specific site by analyzing the PCR amplification products from nick ligation [62]. These methods are suitable for RNA with high abundance and known sequence of m1A sites rather than the identification of novel modification sites.
6. Future Perspectives
Although findings related to the biological functions of the m1A modification have been reported, some key questions remain to be addressed. For instance, studies on m1A-dependent RNA-binding proteins are limited. With the advances in the sensitivity and throughput of proteomics techniques, it is critical to further characterize m1A-dependent RNA-binding proteins involved in m1A recognition and RNA metabolism, as well as to determine how they recognize the m1A sites in their target transcripts. In addition, the reported methyltransferases and demethylases may target different transcripts in different cell types or biological processes; therefore, the context-dependent roles and regulatory mechanisms of m1A-modifying proteins are also interesting and important to explore. Further, optimized technologies are still needed for transcriptome-wide and single-base resolution m1A detection. These will greatly improve our understanding of the biological roles of m1A in RNA. Finally, more work is needed to understand the roles of the m1A RNA modification in human diseases.
Author Contributions
H.J. wrote the manuscript and prepared the figures; C.H. prepared the figures and tables; T.Z. revised the manuscript; S.X. conceptualized and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Chinese National Natural Scientific Foundation Grants, grant number U21A20197.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boccaletto P., Stefaniak F., Ray A., Cappannini A., Mukherjee S., Purta E., Kurkowska M., Shirvanizadeh N., Destefanis E., Groza P., et al. MODOMICS: A database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022;50:D231–D235. doi: 10.1093/nar/gkab1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiener D., Schwartz S. The epitranscriptome beyond m6A. Nat. Rev. Genet. 2021;22:119–131. doi: 10.1038/s41576-020-00295-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H., Kimsey I.J., Nikolova E.N., Sathyamoorthy B., Grazioli G., McSally J., Bai T., Wunderlich C.H., Kreutz C., Andricioaei I., et al. m1A and m1G disrupt A-RNA structure through the intrinsic instability of Hoogsteen base pairs. Nat. Struct. Mol. Biol. 2016;23:803–810. doi: 10.1038/nsmb.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong X., Li X., Yi C. N1-methyladenosine methylome in messenger RNA and non-coding RNA. Curr. Opin. Chem. Biol. 2018;45:179–186. doi: 10.1016/j.cbpa.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Xu G.L., Bochtler M. Reversal of nucleobase methylation by dioxygenases. Nat. Chem. Biol. 2020;16:1160–1169. doi: 10.1038/s41589-020-00675-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozen A.E., Quartley E., Holmes A.D., Hrabeta-Robinson E., Phizicky E.M., Lowe T.M. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods. 2015;12:879–884. doi: 10.1038/nmeth.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., Xiong X., Zhang M., Wang K., Chen Y., Zhou J., Mao Y., Lv J., Yi D., Chen X.W., et al. Base-Resolution Mapping Reveals Distinct m1A Methylome in Nuclear- and Mitochondrial-Encoded Transcripts. Mol. Cell. 2017;68:993–1005. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Xiong X., Wang K., Wang L., Shu X., Ma S., Yi C. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome. Nat. Chem. Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 10.Dominissini D., Nachtergaele S., Moshitch-Moshkovitz S., Peer E., Kol N., Ben-Haim M.S., Dai Q., Di Segni A., Salmon-Divon M., Clark W.C., et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H., Rauch S., Dai Q., Cui X., Zhang Z., Nachtergaele S., Sepich C., He C., Dickinson B.C. Evolution of a reverse transcriptase to map N1-methyladenosine in human messenger RNA. Nat. Methods. 2019;16:1281–1288. doi: 10.1038/s41592-019-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safra M., Sas-Chen A., Nir R., Winkler R., Nachshon A., Bar-Yaacov D., Erlacher M., Rossmanith W., Stern-Ginossar N., Schwartz S. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 2021;22:375–392. doi: 10.1038/s41580-021-00342-0. [DOI] [PubMed] [Google Scholar]
- 14.Oerum S., Dégut C., Barraud P., Tisné C. m1A post-transcriptional modification in tRNAs. Biomolecules. 2017;7:20. doi: 10.3390/biom7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motorin Y., Helm M. RNA nucleotide methylation: 2021 update. Wiley Interdiscip. Rev. RNA. 2022;13:e1691. doi: 10.1002/wrna.1691. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T., Yashiro Y., Kikuchi I., Ishigami Y., Saito H., Matsuzawa I., Okada S., Mito M., Iwasaki S., Ma D., et al. Complete chemical structures of human mitochondrial tRNAs. Nat. Commun. 2020;11:4269. doi: 10.1038/s41467-020-18068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S., Lafontaine D.L.J. ‘View from a bridge’: A new perspective on eukaryotic rRNA base modification. Trends Biochem. Sci. 2015;40:560–575. doi: 10.1016/j.tibs.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Sloan K.E., Warda A.S., Sharma S., Entian K.D., Lafontaine D.L.J., Bohnsack M.T. Tuning the ribosome: The influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biol. 2017;14:1138–1152. doi: 10.1080/15476286.2016.1259781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sergiev P.V., Aleksashin N.A., Chugunova A.A., Polikanov Y.S., Dontsova O.A. Structural and evolutionary insights into ribosomal RNA methylation. Nat. Chem. Biol. 2018;14:226–235. doi: 10.1038/nchembio.2569. [DOI] [PubMed] [Google Scholar]
- 20.Bar-Yaacov D., Frumkin I., Yashiro Y., Chujo T., Ishigami Y., Chemla Y., Blumberg A., Schlesinger O., Bieri P., Greber B., et al. Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biol. 2016;14:e1002557. doi: 10.1371/journal.pbio.1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legrand C., Tuorto F., Hartmann M., Liebers R., Jacob D., Helm M., Lyko F. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res. 2017;27:1589–1596. doi: 10.1101/gr.210666.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinnebusch A.G. The essential Gcd10p–Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson J., Phan L., Hinnebusch A.G. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1-methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2000;97:5173–5178. doi: 10.1073/pnas.090102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozanick S. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA. 2005;11:1281–1290. doi: 10.1261/rna.5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu B., Liu D., Wang Z., Tian R., Zuo Y. Multi-substrate selectivity based on key loops and non-homologous domains: New insight into ALKBH family. Cell Mol. Life Sci. 2020;78:129–141. doi: 10.1007/s00018-020-03594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinge S., Woolford J.L. Ribosome assembly coming into focus. Nat. Rev. Mol. Cell Biol. 2019;20:116–131. doi: 10.1038/s41580-018-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lirussi L., Demir O., You P., Sarno A., Amaro R.E., Nilsen H. RNA Metabolism Guided by RNA Modifications: The Role of SMUG1 in rRNA Quality Control. Biomolecules. 2021;11:76. doi: 10.3390/biom11010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peifer C., Sharma S., Watzinger P., Lamberth S., Kotter P., Entian K.D. Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Res. 2013;41:1151–1163. doi: 10.1093/nar/gks1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma S., Watzinger P., Kotter P., Entian K.D. Identification of a novel methyltransferase, Bmt2, responsible for the N-1-methyl-adenosine base modification of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2013;41:5428–5443. doi: 10.1093/nar/gkt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waku T., Nakajima Y., Yokoyama W., Nomura N., Kako K., Kobayashi A., Shimizu T., Fukamizu A. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J. Cell Sci. 2016;129:2382–2393. doi: 10.1242/jcs.183723. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S., Hartmann J.D., Watzinger P., Klepper A., Peifer C., Kotter P., Lafontaine D.L.J., Entian K.D. A single N1-methyladenosine on the large ribosomal subunit rRNA impacts locally its structure and the translation of key metabolic enzymes. Sci. Rep. 2018;8:11904. doi: 10.1038/s41598-018-30383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Q., Gan H., Yang F., Yao Y., Hao F., Hong L., Jin L. Cytoplasmic m1A reader YTHDF3 inhibits trophoblast invasion by downregulation of m1A-methylated IGF1R. Cell Discov. 2020;6:12. doi: 10.1038/s41421-020-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie S.S., Jin H., Yang F., Zheng H., Chang Y.X., Liao Y., Zhang Y., Zhou T.H., Li Y. Programmable RNA N1-methyladenosine demethylation by a Cas13d-directed demethylase. Angew Chem. Int. Ed. Engl. 2021;60:19592–19597. doi: 10.1002/anie.202105253. [DOI] [PubMed] [Google Scholar]
- 34.Dai X.X., Wang T.X., Gonzalez G., Wang Y.S. Identification of YTH domain-containing proteins as the readers for N1-methyladenosine in RNA. Anal. Chem. 2018;90:6380–6384. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo K.W., Kleiner R.E. YTHDF2 recognition of N1-methyladenosine (m1A)-modified RNA is associated with transcript destabilization. ACS Chem. Biol. 2020;15:132–139. doi: 10.1021/acschembio.9b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilardo E., Nachbagauer C., Buzet A., Taschner A., Holzmann J., Rossmanith W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase-extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40:11583–11593. doi: 10.1093/nar/gks910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chujo T., Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18:2269–2276. doi: 10.1261/rna.035600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawarada L., Suzuki T., Ohira T., Hirata S., Miyauchi K., Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L.S., Xiong Q.P., Pena Perez S., Liu C., Wei J., Le C., Zhang L., Harada B.T., Dai Q., Feng X., et al. ALKBH7-mediated demethylation regulates mitochondrial polycistronic RNA processing. Nat. Cell Biol. 2021;23:684–691. doi: 10.1038/s41556-021-00709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helm M., Giegé R., Florentz C. A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38:13338–13346. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 41.Voigts-Hoffmann F., Hengesbach M., Kobitski A.Y., Aerschot A.V., Herdewijn P., Nienhaus G.U., Helm M. A methyl group controls conformational equilibrium in Human mitochondrial tRNALys. J. Am. Chem. Soc. 2007;129:13382–13383. doi: 10.1021/ja075520+. [DOI] [PubMed] [Google Scholar]
- 42.Wang X., Jia H., Jankowsky E., Anderson J.T. Degradation of hypomodified tRNAiMet in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA. 2008;14:107–116. doi: 10.1261/rna.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richter U., Evans M.E., Clark W.C., Marttinen P., Shoubridge E.A., Suomalainen A., Wredenberg A., Wedell A., Pan T., Battersby B.J. RNA modification landscape of the human mitochondrial tRNALys regulates protein synthesis. Nat. Commun. 2018;9:3966. doi: 10.1038/s41467-018-06471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang T., Cheong A., Mai X., Zou S., Woon E.C. A methylation-switchable conformational probe for the sensitive and selective detection of RNA demethylase activity. Chem. Commun. 2016;52:6181–6184. doi: 10.1039/C6CC01045H. [DOI] [PubMed] [Google Scholar]
- 45.Liu F., Clark W., Luo G., Wang X., Fu Y., Wei J., Wang X., Hao Z., Dai Q., Zheng G., et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:816–828. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei J., Liu F., Lu Z., Fei Q., Ai Y., He P.C., Shi H., Cui X., Su R., Klungland A., et al. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell. 2018;71:973–985. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda H., Chujo T., Wei F.Y., Shi S.L., Hirayama M., Kaitsuka T., Yamamoto T., Oshiumi H., Tomizawa K. Cooperative methylation of human tRNA3Lys at positions A58 and U54 drives the early and late steps of HIV-1 replication. Nucleic Acids Res. 2021;49:11855–11867. doi: 10.1093/nar/gkab879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas E.N., Kim K.Q., McHugh E.P., Marcinkiewicz T., Zaher H.S. Alkylative damage of mRNA leads to ribosome stalling and rescue by trans translation in bacteria. eLife. 2020;9:e61984. doi: 10.7554/eLife.61984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orita I., Futatsuishi R., Adachi K., Ohira T., Kaneko A., Minowa K., Suzuki M., Tamura T., Nakamura S., Imanaka T., et al. Random mutagenesis of a hyperthermophilic archaeon identified tRNA modifications associated with cellular hyperthermotolerance. Nucleic Acids Res. 2019;47:1964–1976. doi: 10.1093/nar/gky1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alriquet M., Calloni G., Martinez-Limon A., Delli Ponti R., Hanspach G., Hengesbach M., Tartaglia G.G., Vabulas R.M. The protective role of m1A during stress-induced granulation. J. Mol. Cell Biol. 2020;12:870–880. doi: 10.1093/jmcb/mjaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsao N., Brickner J.R., Rodell R., Ganguly A., Wood M., Oyeniran C., Ahmad T., Sun H., Bacolla A., Zhang L., et al. Aberrant RNA methylation triggers recruitment of an alkylation repair complex. Mol. Cell. 2021;81:4228–4242. doi: 10.1016/j.molcel.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark K.D., Lee C., Gillette R., Sweedler J.V. Characterization of neuronal RNA modifications during non-associative learning in Aplysia reveals key roles for tRNAs in behavioral sensitization. ACS Cent. Sci. 2021;7:1183–1190. doi: 10.1021/acscentsci.1c00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W., Meng J., Liu J., Ding B., Tan T., Wei Q., Yu Y. The N1-methyladenosine methylome of petunia messenger RNA. Plant. Physiol. 2020;183:1710–1724. doi: 10.1104/pp.20.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuang W., Jin H., Yang F., Chen X., Liu J., Li T., Chang Y., Liu M., Xu Z., Huo C., et al. ALKBH3-dependent m1A demethylation of Aurora A mRNA inhibits ciliogenesis. Cell Discov. 2022;8:25. doi: 10.1038/s41421-022-00385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ueda Y., Ooshio I., Fusamae Y., Kitae K., Kawaguchi M., Jingushi K., Hase H., Harada K., Hirata K., Tsujikawa K. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci. Rep. 2017;7:42271. doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo H.H., Chambers S.K. Human ALKBH3-induced m1A demethylation increases the CSF-1 mRNA stability in breast and ovarian cancer cells. Biochim. Biophys. Acta. Gene Regul. Mech. 2019;1862:35–46. doi: 10.1016/j.bbagrm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z., Qi M., Shen B., Luo G., Wu Y., Li J., Lu Z., Zheng Z., Dai Q., Wang H. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esteve-Puig R., Climent F., Pieyro D., Domingo-Domenech E., Davalos V., Encuentra M., Rea A., Espejo-Herrera N., Soler M., Lopez M., et al. Epigenetic loss of m1A RNA demethylase ALKBH3 in Hodgkin Lymphoma targets collagen conferring poor clinical outcome. Blood. 2020;137:994–999. doi: 10.1182/blood.2020005823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Wang J., Li X., Xiong X., Wang J., Zhou Z., Zhu X., Gu Y., Dominissini D., He L., et al. N1-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat. Commun. 2021;12:6314. doi: 10.1038/s41467-021-26718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moshitch-Moshkovitz S., Dominissini D., Rechavi G. The epitranscriptome toolbox. Cell. 2022;185:764–776. doi: 10.1016/j.cell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y., Yang S., Peng S., Li W., Wu F., Yao Q., Wang F., Weng X., Zhou X. N1-Methyladenosine detection with CRISPR-Cas13a/C2c2. Chem. Sci. 2019;10:2975–2979. doi: 10.1039/C8SC03408G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding J.H., Ma C.J., Chen M.Y., Chen B., Yuan B.F., Feng Y.Q. Quantification and Single-Base Resolution Analysis of N1-Methyladenosine in mRNA by Ligation-Assisted Differentiation. Anal. Chem. 2020;92:2612–2619. doi: 10.1021/acs.analchem.9b04454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.