Abstract
Background and Objectives
Ischemic stroke increases the risk of neurodevelopmental disorders; however, the risk of autism is not thoroughly explored. Our aim was to evaluate risk of autism and risk factors for autism in children with pediatric ischemic stroke and in their first-degree relatives.
Methods
In this cohort study, individuals with ischemic stroke from 1969 to 2016, <18 years of age, alive 1 week after stroke, and without prior autism were identified in Swedish national registers. Ten matched controls per index individual and all first-degree relatives of index individuals and controls were identified. Conditional Cox regression was used to calculate the risk of autism. Unconditional logistic regression was performed to analyze sex, gestational age, age at stroke diagnoses, comorbid adverse motor outcome, comorbid epilepsy, and a sibling with autism as risk factors for autism in children with ischemic stroke.
Results
Of the 1,322 index individuals, 46 (3.5%) were diagnosed with autism compared to 161 (1.2%) controls (adjusted hazard ratio [aHR] 3.02, 95% CI 2.15–4.25). There was no significant difference in risk of autism according to age at stroke: perinatal (aHR 2.69, 95% CI 1.44–5.03) and childhood stroke (aHR 3.18, 95% CI 2.12–4.78). The increased risk remained after exclusion of children born preterm or small for gestational age (aHR 3.78, 95% CI 2.55–5.60) and when children with stroke diagnosed from 1997 to 2014 were analyzed (aHR 2.91, 95% CI = 1.95–4.35). Compared to controls, the risk of autism was increased in individuals with ischemic stroke and comorbid epilepsy (aHR 7.05, 95% CI 3.74–13.30), as well as adverse motor outcome (aHR 4.28, 95% CI 2.44–7.51). When individuals with adverse motor outcome and epilepsy were censored, the risk of autism was still increased (aHR 2.37, 95% CI 1.45–3.85). Sex, gestational age, and having a sibling with autism were not associated with autism in individuals with pediatric ischemic stroke.
Discussion
An increased risk of autism was seen after pediatric ischemic stroke, particularly in individuals with comorbid epilepsy, and could not be explained by being born preterm or small for gestational age. The risk was increased also in individuals free from epilepsy and adverse motor outcome, implying that all children with ischemic stroke should be readily screened for autism if the disorder is suspected.
Ischemic stroke is a rare but severe condition with a high risk of neurologic complications such as adverse motor outcome,1-5 epilepsy, and cognitive difficulties.6-11 There are 2 subtypes of pediatric ischemic stroke: perinatal (occurring from 20th gestational week until 28 days of age) and childhood (occurring from 29 days of age). Arteriopathies, cardiac disorders, prothrombotic conditions, infections, inflammations, and metabolic and genetic disorders are risk factors of both childhood and perinatal stroke, whereas in the latter, maternal and perinatal factors are also important.3,12,13
Autism is characterized by varying degrees of difficulties in social interaction and communication in combination with restricted, repetitive, stereotyped behavior, interests, and activities.14 The etiology of autism is complex and has been under intense examination, pointing both to genetic and environmental causes, in some cases shown by abnormal structural development of the brain.15-17 Autism also shares risk factors with pediatric ischemic stroke such as maternal and perinatal complications.3,16,18-20
To the best of our knowledge, only 1 study has investigated the association between stroke and autism in a mixed cohort with hemorrhagic and ischemic perinatal stroke.21 However, other studies have reported impaired theory of mind and cognitive deficits affecting psychosocial functioning in both children and adults who had a stroke.22,23
Several studies have found an association between epilepsy of different etiologies, especially genetic, and autism,24,25 leading to the assumption that both disorders are based partly on an impaired balance between inhibition and excitation.26
An ischemic stroke that occurs before the affected motor function develops often results in cerebral palsy (CP).27 In contrast, a later insult most commonly results in hemiplegia or other paretic conditions. CP, especially with comorbid epilepsy, is strongly associated with autism.28,29 However, no studies have reported the risk of autism in individuals with hemiplegia.
Pediatric ischemic stroke and autism are difficult to study prospectively because of the low incidences, justifying the use of national registers with prospectively collected data on nationwide cohorts. Our aim was to estimate the risk of autism after pediatric ischemic stroke. We assessed the hereditary component by adjusting for autism in first-degree relatives and estimated the risk of autism in first-degree relatives of children with ischemic stroke. In addition, we hypothesized that adverse motor outcome (as a marker for stroke severity) and epilepsy (as an indicator of impaired balance between inhibition and excitation) further increase the risk of autism after pediatric ischemic stroke.
Methods
Data Sources
Data used in this nationwide cohort study were retrieved from the National Patient Register (NPR), the Swedish Medical Birth Register (MBR), the Cause of Death Register, and the Total Population Register, including the Multi-Generation Register.30 The Swedish personal identity number assigned to all residents of Sweden was used to link the registers.31 The NPR includes diagnoses registered in inpatient care at Swedish hospitals from 1964, becoming nationwide in 1987; today, the coverage is 99% of all hospital discharges.32 Beginning in 2001, diagnoses made at outpatient visits are also included. Since 1973, information on antenatal and perinatal factors has been registered in the MBR as variables with high validity.33
Study Cohort
The study cohort comprises children <18 years of age who were diagnosed with ischemic stroke from 1969 to 2016 identified in the NPR, MBR, and Cause of Death Register using ICD codes for ischemic stroke (ICD-8 and ICD-9: 433, 434, 436; ICD-10: I63, I64). A randomly chosen sample of 273 cases found in the NPR and MBR has been validated through chart review, showing a positive predictive value of 89% for ischemic stroke.34 Children who died within the first week after stroke and those with a previous diagnosis of autism were excluded from the main analyses, although not for the analysis of first-degree relatives (Figure 1).
Figure. Flowchart of the Study Cohort.
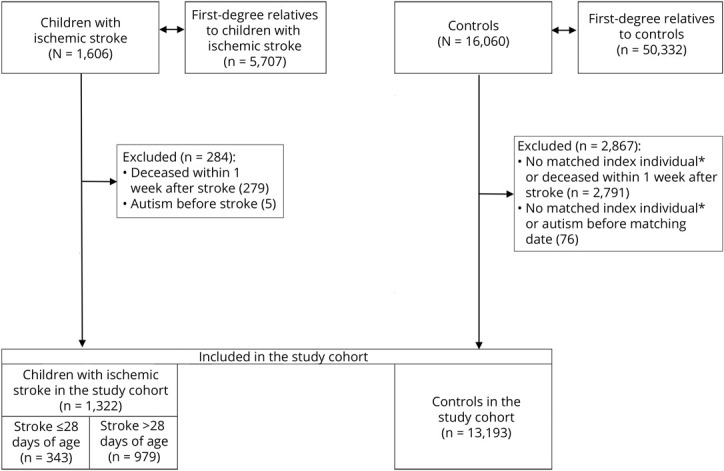
*If 1 index individual was excluded, their controls also were excluded because the analyses were conditioned on a matching set (each individual with a stroke was compared to their controls).
Each child with ischemic stroke was compared to 10 controls matched for sex, year of birth, and county of residence at diagnosis. Controls and first-degree relatives of children with ischemic stroke and their controls were identified from the Total Population and Multi-Generation Registers.
Outcome
The primary outcome was autism, defined by ICD codes (ICD-9: 299A, 299B, 299W, 299X; ICD-10: F84.0-F84.5, F84.8, F84.9) in the NPR from 1987 onward, when ICD-9 was first introduced in Sweden.
Covariates
Preterm birth was defined as <37 completed weeks of gestation, and small for gestational age (SGA) was defined with the Swedish sex-specific estimated fetal growth curves35 as having a birth weight of <2 SDs below the mean birth weight for their gestational age.
Adverse motor outcome was defined as having 1 of the following diagnoses in the NPR: CP, hemiparesis, tetraparesis/paraplegia, and other paresis (ICD-8: 343, 344 [except for 344.B, C, G]; ICD-9: 343, 342, 344 [except for 344.01, 344.03]; ICD-10: G80, G81, G82, G83 [except for G83.0, G83.4–G83.6]). Epilepsy was defined as having 1 of the following diagnoses registered in the NPR: ICD-8 code 345 (except for 345.2), ICD-9 code 345 (except for 345Q), and ICD-10 code G40.
In addition, we stratified for sex, age at diagnosis of stroke (perinatal [≤28 days], childhood [>28 days]), and year of first diagnosis of stroke (1969–1986, 1987–1996, 1997–2016).
Follow-up
Follow-up started at the date of ischemic stroke or corresponding date in matched controls and ended with autism, death, or December 31, 2016, whichever occurred first. Matched controls were censored at an event of ischemic stroke.
For first-degree relatives and their controls, follow-up started in 1987 or with their birth and ended with autism, death, or December 31, 2016.
In a separate analysis of the risk of autism, index individuals were censored at the date of epilepsy or adverse motor outcome diagnosis. A censored individual may or may not be diagnosed with autism thereafter but is then no longer included in the risk calculations.
When stratified according to comorbidity, follow-up started at the diagnosis of epilepsy and adverse motor outcome.
Because autism has been registered only after the implementation of ICD-9 in 1987 in Sweden, we conducted a sensitivity analysis in which we included only individuals diagnosed with stroke from 1997 to 2014 with follow-up until December 31, 2016.
To estimate the association between autism after pediatric ischemic stroke and potential risk factors, only index individuals were included when we compared children with and without comorbid autism.
Statistical Analysis
We used conditional Cox regression to calculate hazard ratios (HRs) to estimate the risk of future autism in individuals with stroke and their first-degree relatives. The analyses were conditioned on a matching set (age, sex, year of birth, and county of residence at the time of stroke). Hence, each individual with a stroke was compared to their controls. Relative risks with 95% CIs that did not include 1.0 were regarded as statistically significant. Analyses of index individuals were in addition adjusted for history of autism in first-degree relatives and stratified according to sex, age at and year of diagnosis, and diagnosis of adverse motor outcome, epilepsy, or both.
In relatives, we adjusted for sex, their index individual's age, and calendar year of diagnosis.
Unconditional logistic regression was used to analyze sex, gestational age, age at stroke diagnoses, comorbid adverse motor outcome, epilepsy, and having a sibling with autism as risk factors for autism in children with ischemic stroke. Adjustments were made for sex, gestational age, age, and calendar year of stroke. The adjustment variable that was used as the outcome in each calculation was excluded.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the regional ethics committee in Linköping (DNR 2017/10–31). Because the authors had access only to data that were already pseudonymized when received from Statistics Sweden and because of the register-based nature of the study, the ethics committee waived the requirement for individual informed consent.36
The reporting of this study is according to Strengthening the Reporting of Observational Studies in Epidemiology guideline.
Data Availability
All data can be requested from the Swedish National Board of Health and Welfare and Statistics Sweden after ethics approval from the Swedish Ethical Review Authority.
Results
Characteristics of the Study Participants
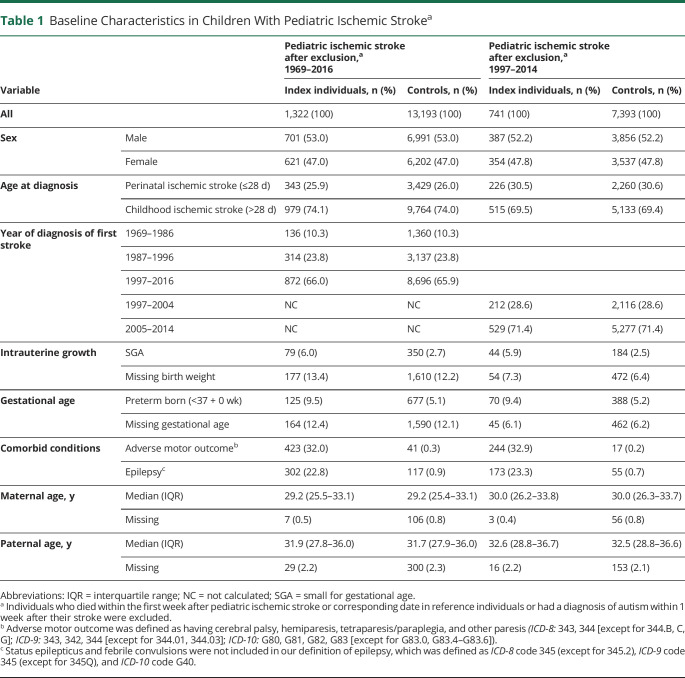
After exclusion of children who died within the first week after stroke or were diagnosed with autism before their stroke, 1,322 index individuals and 13,193 controls remained (Figure 1). In the study cohort, there were more male (53%) than female (47%) participants. Among children with stroke, 79 (6.0%) were born SGA and 125 (9.5%) were born preterm compared to 350 (2.7%) and 677 (5.1%), respectively, among controls. In the study cohort, 872 (65.9) children with ischemic stroke were diagnosed after 1996. Adverse motor outcome was diagnosed in 423 (32%) index individuals, and epilepsy was diagnosed in 302 (22.8%) (Table 1 gives other characteristics).
Table 1.
Baseline Characteristics in Children With Pediatric Ischemic Strokea
Risk of Autism After Pediatric Ischemic Stroke
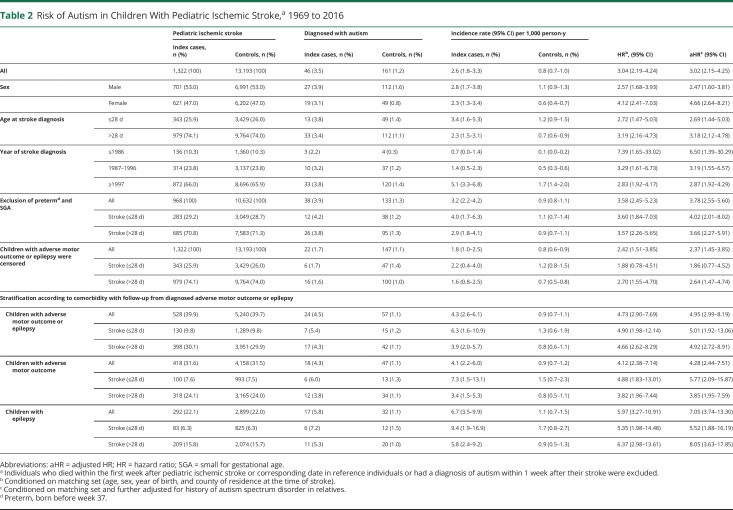
Of index individuals with ischemic stroke, 46 (3.5%) were diagnosed with autism compared to 161 (1.2%) controls, corresponding to an adjusted HR (aHR) of 3.02 (95% CI 2.15–4.25) (Table 2). The risk of autism after perinatal ischemic stroke was aHR = 2.69 (95% CI 1.44–5.03) and after childhood stroke was aHR = 3.18 (95% CI 2.12–4.78). There was no significant difference in risk increase between female and male participants or regarding age at stroke. After exclusion of children born preterm and SGA, the increased risk of autism remained for both perinatal (aHR 4.02, 95% CI 2.01–8.02) and childhood (aHR 3.66, 95% CI 2.27–5.91) stroke (Table 2). In children with stroke and comorbid epilepsy, the risk of autism was aHR = 7.05 (95% CI 3.74–13.30), and when this group was stratified according to age at stroke, the risk of autism in perinatal stroke was aHR = 5.52 (95% CI 1.88–16.19) and in childhood stroke was aHR = 8.05 (95% CI 3.63–17.85). The risk of autism in children with stroke and adverse motor outcome was aHR = 4.28 (95% CI 2.44–7.51); according to age at stroke, the aHR was 5.77 (95% CI 2.09–15.87) for perinatal and 3.85 (95% CI 1.95–7.59) for childhood stroke.
Table 2.
Risk of Autism in Children With Pediatric Ischemic Stroke,a 1969 to 2016
Censoring index individuals diagnosed with epilepsy or adverse motor outcome, we found that the risk of autism was still increased in individuals with pediatric ischemic stroke (aHR 2.37, 95% CI 1.45–3.85). However, after stratification according to age at stroke onset, there was no increased risk of autism in children with perinatal stroke (aHR 1.86, 95% CI 0.77–4.52) in contrast to those with childhood stroke (aHR 2.64, 95% CI 1.47–4.74) (Table 2).
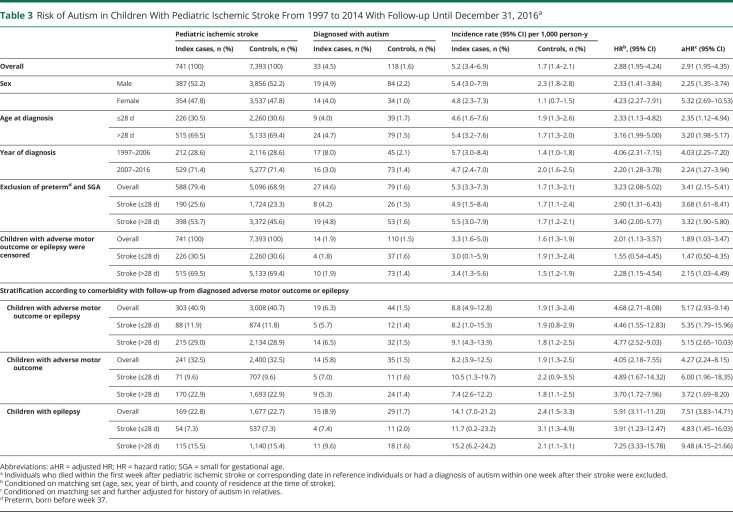
When we included only children with stroke from 1997 to 2014, with follow-up to 2016, the risk estimates for autism remained similarly increased (aHR 2.91, 95% CI 1.95–4.35) (Table 3).
Table 3.
Risk of Autism in Children With Pediatric Ischemic Stroke From 1997 to 2014 With Follow-up Until December 31, 2016a
Association Between Autism After Pediatric Ischemic Stroke and Potential Risk Factors
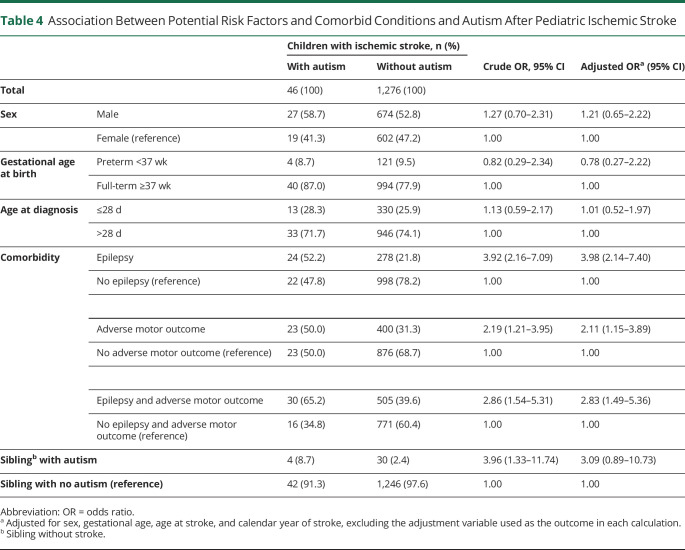
Sex, gestational age at birth, age at stroke diagnosis, and having a sibling with autism were not associated with autism in individuals with pediatric ischemic stroke. Comorbid epilepsy (odds ratio [OR] 3.98, 95% CI 2.14–7.40), adverse motor outcome (OR 2.11, 95% CI 1.15–3.89), and epilepsy in combination with adverse motor outcome (OR 2.83, 95% CI 1.49–5.36) all had a significant association with autism in individuals with pediatric ischemic stroke (Table 4).
Table 4.
Association Between Potential Risk Factors and Comorbid Conditions and Autism After Pediatric Ischemic Stroke
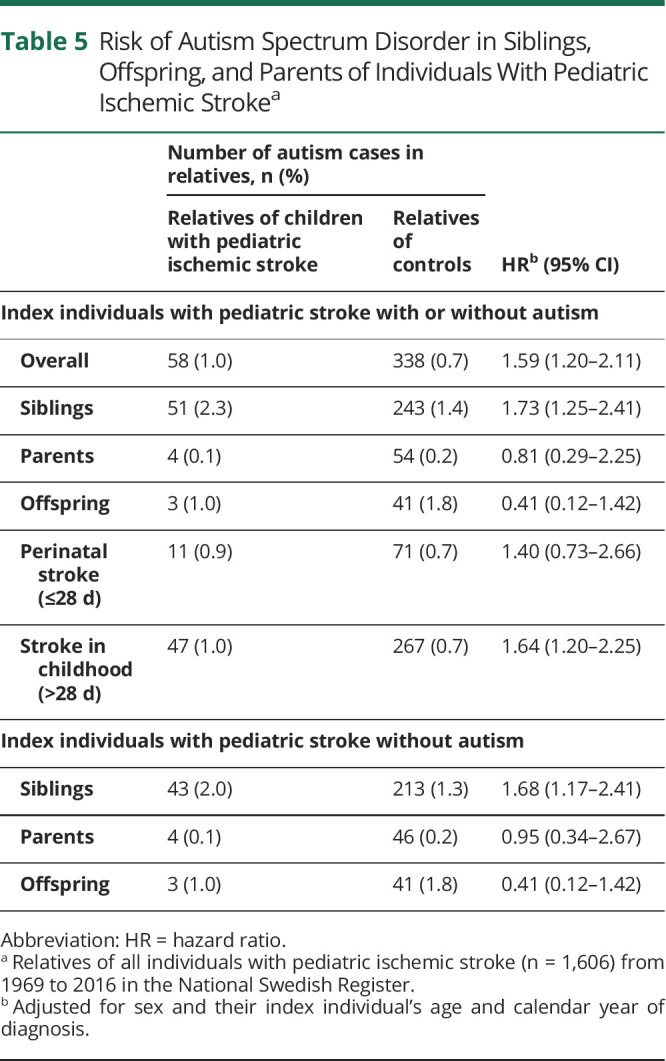
Risk of Autism in First-Degree Relatives
First-degree relatives of children with ischemic stroke had an increased risk of autism compared to relatives of controls (HR 1.59, 95% CI 1.20–2.11), largely due to the increased risk in siblings (HR 1.73, 95% CI 1.25–2.41). The increased risk was significant in relatives of individuals with childhood stroke (stroke onset >28 days of age) (HR 1.64, 95% CI 1.20–2.25) but not with perinatal stroke (HR 1.40, 95% CI 0.73–2.66) (Table 5). The increased risk remained even in siblings to index individuals without autism (Table 5).
Table 5.
Risk of Autism Spectrum Disorder in Siblings, Offspring, and Parents of Individuals With Pediatric Ischemic Strokea
Discussion
In this nationwide cohort study, we found a 3-fold increased risk of future autism after pediatric ischemic stroke, especially in children with comorbid epilepsy, in whom the risk was 7-fold. To the best of our knowledge, there is only 1 previous study on autism after ischemic stroke concerning perinatal stroke.21
The risk of autism after perinatal ischemic stroke was 2.7-fold compared to controls in our study including 343 cases, which is in line with the previous study reporting a prevalence of autism of 11.4% in a smaller cohort (n = 201) including both hemorrhagic and ischemic stroke.21 In childhood stroke, the corresponding risk in our study compared to controls was 3.3-fold, which is in line with the risk after perinatal stroke.
Studies indicate that theory of mind and psychosocial functioning are impaired after stroke.22,23 The insult has been assumed to affect several social cognitive domains, probably regardless of stroke characteristics.23 Although autism cannot be acquired later in life and the main cause of autism is considered genetic, stroke might be an additional factor for reaching the turning point because autism has multiple and complex causes. Although there are some differences in etiology and risk factors between perinatal and childhood stroke, in our study, it seems that the developmental stage of the brain at the insult was not crucial for the risk of developing autism after stroke.
There was a 2.6-fold increased risk of autism in individuals with childhood ischemic stroke without comorbid epilepsy and adverse motor outcome. However, the risk of autism in individuals with perinatal stroke after censoring of those with epilepsy and adverse motor outcome was not significantly increased, possibly because epilepsy is more common after perinatal stroke, rendering only a few cases for this analyses.
Lifestyle factors and hereditary, metabolic, and inflammatory disorders have been linked to ischemic stroke in adults.
Born preterm or SGA is associated with an increased risk of autism.19,37 However, in our cohort, the increased risk of autism remained when individuals born SGA or preterm were excluded.
The risk of autism was 7 times higher for individuals with pediatric ischemic stroke and epilepsy than in controls, probably due to a common underlying mechanism for both epilepsy and autism.24,25 The prevalence of autism in children with CP is almost twice as high in those with an additional diagnosis of epilepsy.28 The risk of autism for individuals <20 years of age with all types of epilepsy is 11.4-fold compared to controls, which is higher than for individuals <18 years of age with pediatric ischemic stroke and epilepsy.24 A possible explanation for this observation could be that some types of childhood epilepsy are part of broader, often genetic, neurodevelopmental disorders that may be independently associated with autism spectrum disorder.38,39 However, the same study showed that individuals with epilepsy with onset later in life with etiologies more often being stroke and dementias had only a slightly higher risk of autism (HR ≈8) than the individuals with pediatric ischemic stroke in our study.24
The association between autism and pediatric ischemic stroke with epilepsy was significantly higher than between autism and pediatric ischemic stroke with adverse motor outcome. Nevertheless, the risk of autism in individuals with pediatric ischemic stroke and adverse motor outcome was 4-fold compared to controls, which is in line with studies on autism in individuals with CP.28 It is possible that the severity of the insult and its location are of greater importance for the risk of autism after stroke. However, the type of CP and gross motor function have been reported not to influence the risk of autism in individuals with CP.28 Affected ability regarding theory of mind and reduced psychosocial functioning after stroke is seen more often after insults on the right side of the brain.23 Adverse motor outcome is used in our study to indicate the severity of the insult because our register data lack information on stroke location and severity. Alterations in the white matter of the brain and many other neuroanatomic markers have been described in individuals with autism.15,16
We found an increased risk of autism in first-degree relatives of children with ischemic stroke, especially in siblings and relatives of individuals with childhood stroke. This finding could indicate a genetic contribution to the cause of autism after ischemic stroke. However, this contention is weak considering that no significant association was noted between having a sibling with autism and being diagnosed with autism after pediatric ischemic stroke. In our subanalyses of relatives of individuals with stroke without autism, we found a significantly increased risk of autism in siblings, supporting our hypothesis of shared risk factors between ischemic stroke and autism.3,12,16-20
One strength of our study is the comparatively large number of individuals with stroke, which allowed us to calculate risk estimates compared to matched controls from the general population. We used a population-based approach incorporating high-quality national registers to minimize selection bias. A substantial part of the cohort was validated through chart review, showing an overall positive predicted value of 89% for pediatric ischemic stroke and 96% and 84% for perinatal and childhood stroke, respectively. We could also adjust our data for any autism in first-degree relatives, although residual confounding can never fully be ruled out in observational studies. The data were collected prospectively before the outcome of the study, thereby minimizing recall bias.33 Birth data were retrieved from a registry with high validity (the Swedish MBR).33
Because we excluded all individuals with autism before ischemic stroke, the true risk estimates might be higher considering that stroke sometimes is registered years after it occurred. However, registering a pediatric stroke diagnosis retrospectively is rare according to our validation of ischemic stroke in the NPR and MBR.34 Perinatal ischemic stroke is sometimes registered at the date of discharge from the neonatal intensive care units instead of the actual date when the stroke occurred and sometimes is not diagnosed until years after the stroke (presumed perinatal stroke), leading to the inclusion of individuals with perinatal ischemic stroke in the group of individuals with childhood stroke. However, in our validation study, only 3.6% of childhood strokes were recategorized as perinatal.34
Autism is registered only after the implementation of ICD-9 in 1987 when the diagnosis was entered in Sweden. Nevertheless, a sensitivity analysis including only children with stroke from 1997 to 2014 found similarly increased risk estimates for autism in all different subcategories. In this study, we excluded children in whom autism was diagnosed before stroke. However, symptoms of autism may be present before the stroke, although the autism diagnosis is established after. This problem cannot be resolved in a study like ours that is based on registry data.
Another limitation is that follow-up might be too short for some children, especially those with perinatal stroke diagnosed in later calendar years, to have been diagnosed with autism, although in our sensitivity analysis, follow-up lasted 2 years after the last day of inclusion. It is unlikely however that it affected our risk estimates given that index individuals were compared only to their matched controls, although this might have influenced the magnitude of the risk of autism after stroke.
Moreover, a Swedish study has shown that 96% of all autism cases identified in the NPR could be confirmed through patient chart review, although there are no data on the proportion of individuals with autism who are not identified in the NPR.40
Epilepsy has been validated in the Swedish Inpatient Register, which is a major part of the NPR, suggesting that most epilepsy cases have been registered.41,42 CP and hemiplegia or other paresis had not been validated in the NPR; however, CP in the Norwegian NPR is correct in only 59.5% of the cases.43 Therefore, we chose to use a broader definition of adverse motor outcome, including CP, hemiparesis, tetraparesis/paraplegia, and other paresis.
Not all patients with autism, epilepsy, and adverse motor outcome are admitted to a hospital and therefore are not registered until the start of the outpatient register in 2001. However, because this is also the case for the controls, it will have only a small impact on our risk estimates.
The lack of information about intellectual disability is a limitation because autism in CP often co-occurs with intellectual disability, which is also the case in epilepsy with comorbid autism.28,44 The register-based data also do not provide information on cognitive profiles, impairment of language, or other functional deficits such as balance or dyscoordination, giving the study a synoptic view, which can be seen as a limitation. This is also the case concerning the lack of information regarding the location, severity, and region of the insult caused by the stroke.
Some studies on ischemic stroke include only arterial ischemic stroke. In contrast, we also included cases of cerebral venous thrombosis because these cannot be distinguished reliably from each other by the 3-character ICD codes used to minimize false-positive cases.34
There is an increased risk of autism after pediatric ischemic stroke, particularly in individuals with comorbid epilepsy, that could not be explained by being born preterm or SGA or having a first-degree relative with autism. Regardless of whether the individual with childhood ischemic stroke was diagnosed with epilepsy or adverse motor outcome, there was an increased risk of autism. In perinatal stroke, the risk was significantly increased only in individuals diagnosed with adverse motor outcome with or without epilepsy. Children with ischemic stroke should readily be screened for autism if the disorder is suspected.
Glossary
- aHR
adjusted HR
- CP
cerebral palsy
- HR
hazard ratio
- ICD
International Classification of Disease
- MBR
Medical Birth Register
- NPR
National Patient Register
- OR
odds ratio
- SGA
small for gestational age
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Editorial, page 784
Study Funding
H.S. received a private donation through the Knut and Alice Wallenberg Foundation for research on stroke and stroke-causing factors in infancy and a grant from Region Östergötland Research Council and supported by Region Stockholm, clinical postdoctoral appointment, 2019-1,138. P.B. received a private donation through the Knut and Alice Wallenberg Foundation for research on stroke and stroke-causing factors in infancy. J.B. is supported by the Jerring foundation, the Crown princess Lovisa's Foundation, the Petrus and Augusta Hedlunds Foundation, Sachs' Children and Youth Hospital, the Samaritan Foundation, and the Linnea and Josef Carlsson Foundation. J.B. also was supported by Region Stockholm (clinical postdoctoral appointment).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Sreenan C, Bhargava R, Robertson CM. Cerebral infarction in the term newborn: clinical presentation and long-term outcome. J Pediatr. 2000;137(3):351-355. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13(6):499-505. [DOI] [PubMed] [Google Scholar]
- 3.Lehman LL, Rivkin MJ. Perinatal arterial ischemic stroke: presentation, risk factors, evaluation, and outcome. Pediatr Neurol. 2014;51(6):760-768. [DOI] [PubMed] [Google Scholar]
- 4.Wagenaar N, Martinez-Biarge M, van der Aa NE, et al. Neurodevelopment after perinatal arterial ischemic stroke. Pediatrics. 2018;142(3):e20174164. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Croen LA, Lindan C, et al. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol. 2005;58(2):303-308. [DOI] [PubMed] [Google Scholar]
- 6.Billinghurst LL, Beslow LA, Abend NS, et al. Incidence and predictors of epilepsy after pediatric arterial ischemic stroke. Neurology. 2017;88(7):630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CK, Mackay MT, Dowling MM, Pergami P, Titomanlio L, Deveber G. Prolonged or recurrent acute seizures after pediatric arterial ischemic stroke are associated with increasing epilepsy risk. Dev Med Child Neurol. 2017;59(1):38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golomb MR, Garg BP, Carvalho KS, Johnson CS, Williams LS. Perinatal stroke and the risk of developing childhood epilepsy. J Pediatr. 2007;151(4):409-412, 413e1-413e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh RK, Zecavati N, Singh J, et al. Seizures in acute childhood stroke. J Pediatr. 2012;160(2):291-296. [DOI] [PubMed] [Google Scholar]
- 10.Lee JC, Lin KL, Wang HS, et al. Seizures in childhood ischemic stroke in Taiwan. Brain Dev. 2009;31(4):294-299. [DOI] [PubMed] [Google Scholar]
- 11.Laugesaar R, Vaher U, Lõo S, et al. Epilepsy after perinatal stroke with different vascular subtypes. Epilepsia Open. 2018;3(2):193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastrangelo M, Giordo L, Ricciardi G, De Michele M, Toni D, Leuzzi V. Acute ischemic stroke in childhood: a comprehensive review. Eur J Pediatr. 2022:181(1):45-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferriero DM, Fullerton HJ, Bernard TJ, et al. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2019;50(3):e51-e96. [DOI] [PubMed] [Google Scholar]
- 14.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896-910. [DOI] [PubMed] [Google Scholar]
- 15.Sato W, Uono S. The atypical social brain network in autism: advances in structural and functional MRI studies. Curr Opin Neurol. 2019;32(4):617-621. [DOI] [PubMed] [Google Scholar]
- 16.Lord C, Brugha TS, Charman T, et al. Autism spectrum disorder. Nat Rev Dis Primers. 2020;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37(1):95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamner T, Shih E, Ichord R, Krivitzky L. Children with perinatal stroke are at increased risk for autism spectrum disorder: prevalence and co-occurring conditions within a clinically followed sample. Clin Neuropsychol. 2021;24:1-12. [DOI] [PubMed] [Google Scholar]
- 22.Lo W, Li X, Hoskinson K, et al. Pediatric stroke impairs theory of mind performance. J Child Neurol. 2020;35(3):228-234. [DOI] [PubMed] [Google Scholar]
- 23.Adams AG, Schweitzer D, Molenberghs P, Henry JD. A meta-analytic review of social cognitive function following stroke. Neurosci Biobehav Rev. 2019;102:400-416. [DOI] [PubMed] [Google Scholar]
- 24.Sundelin HE, Larsson H, Lichtenstein P, et al. Autism and epilepsy: a population-based nationwide cohort study. Neurology. 2016;87(2):192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukmanji S, Manji SA, Kadhim S, et al. The co-occurrence of epilepsy and autism: a systematic review. Epilepsy Behav. 2019;98(pt A):238-248. [DOI] [PubMed] [Google Scholar]
- 26.Bozzi Y, Provenzano G, Casarosa S. Neurobiological bases of autism-epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur J Neurosci. 2018;47(6):534-548. [DOI] [PubMed] [Google Scholar]
- 27.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47(8):571-576. [DOI] [PubMed] [Google Scholar]
- 28.Pahlman M, Gillberg C, Himmelmann K. Autism and attention-deficit/hyperactivity disorder in children with cerebral palsy: high prevalence rates in a population-based study. Dev Med Child Neurol. 2021;63(3):320-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig F, Savino R, Trabacca A. A systematic review of comorbidity between cerebral palsy, autism spectrum disorders and attention deficit hyperactivity disorder. Eur J Paediatr Neurol. 2019;23(1):31-42. [DOI] [PubMed] [Google Scholar]
- 30.Ekbom A. The Swedish Multi-Generation Register. Methods Mol Biol. 2011;675:215-220. [DOI] [PubMed] [Google Scholar]
- 31.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Källén BK K. The Swedish Medical Birth Register: A Summary of Content and Quality. Socialstyrelsen; 2003. [Google Scholar]
- 34.Walas A, Svensson K, Gyris M, Bang P, Sundelin HEK. Paediatric ischaemic stroke is a valid diagnosis in the Swedish National Patient Register. Acta Paediatr. 2021;110(7):2179-2186. [DOI] [PubMed] [Google Scholar]
- 35.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843-848. [DOI] [PubMed] [Google Scholar]
- 36.Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenabi E, Bashirian S, Asali Z, Seyedi M. Association between small for gestational age and risk of autism spectrum disorders: a meta-analysis. Clin Exp Pediatr. 2021;64(10):538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BH, Smith T, Paciorkowski AR. Autism spectrum disorder and epilepsy: disorders with a shared biology. Epilepsy Behav. 2015;47:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Åndell E, Tomson T, Carlsson S, et al. The incidence of unprovoked seizures and occurrence of neurodevelopmental comorbidities in children at the time of their first epileptic seizure and during the subsequent six months. Epilepsy Res. 2015;113:140-150. [DOI] [PubMed] [Google Scholar]
- 40.Idring S, Rai D, Dal H, et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS One. 2012;7(7):e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattsson P, Tomson T, Eriksson O, Brannstrom L, Weitoft GR. Sociodemographic differences in antiepileptic drug prescriptions to adult epilepsy patients. Neurology. 2010;74(4):295-301. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson L, Tomson T, Farahmand BY, Diwan V, Persson PG. Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia. 1997;38(10):1062-1068. [DOI] [PubMed] [Google Scholar]
- 43.Hollung SJ, Vik T, Wiik R, Bakken IJ, Andersen GL. Completeness and correctness of cerebral palsy diagnoses in two health registers: implications for estimating prevalence. Dev Med Child Neurol. 2017;59(4):402-406. [DOI] [PubMed] [Google Scholar]
- 44.Amiet C, Gourfinkel-An I, Laurent C, et al. Does epilepsy in multiplex autism pedigrees define a different subgroup in terms of clinical characteristics and genetic risk? Mol Autism. 2013;4(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data can be requested from the Swedish National Board of Health and Welfare and Statistics Sweden after ethics approval from the Swedish Ethical Review Authority.