Abstract
Background and Objectives
Persons with epilepsy, especially those with drug resistant epilepsy (DRE), may benefit from inpatient services such as admission to the epilepsy monitoring unit (EMU) and epilepsy surgery. The COVID-19 pandemic caused reductions in these services within the US during 2020. This article highlights changes in resources, admissions, and procedures among epilepsy centers accredited by the National Association of Epilepsy Centers (NAEC).
Methods
We compared data reported in 2019, prior to the COVID-19 pandemic, and 2020 from all 260 level 3 and level 4 NAEC accredited epilepsy centers. Data were described using frequency for categorical variables and median for continuous variables and were analyzed by center level, center population category, and geographical location. Qualitative responses from center directors to questions regarding the impact from COVID-19 were summarized utilizing thematic analysis. Responses from the NAEC center annual reports as well as a supplemental COVID-19 survey were included.
Results
EMU admissions declined 23% (-21,515) in 2020, with largest median reductions in level 3 centers [-55 admissions (-44%)] and adult centers [-57 admissions (-39%)]. The drop in admissions was more substantial in the East North Central, East South Central, Mid Atlantic, and New England US Census divisions. Survey respondents attributed reduced admissions to re-assigning EMU beds, restrictions on elective admissions, reduced staffing, and patient reluctance for elective admission. Treatment surgeries declined by 371 cases (5.7%), with the largest reduction occurring in VNS implantations [-486 cases (-19%)] and temporal lobectomies [-227 cases (-16%)]. All other procedure volumes increased, including a 35% (54 cases) increase in corpus callosotomies.
Discussion
In the US, access to care for persons with epilepsy declined during the COVID-19 pandemic in 2020. Adult patients, those relying on level 3 centers for care, and many persons in the eastern half of the US were most affected.
Epilepsy affects an estimated 3.4 million persons in the United States.1 About 30% continue to have seizures despite treatment with antiseizure medications (ASM).2 Persons with drug-resistant epilepsy (DRE) may benefit from surgical intervention, dietary therapy, or access to investigational trials.3,4 Admissions are commonly arranged for long-term EEG monitoring (LTM) for presurgical evaluations. Inpatient elective surgical procedures for epilepsy include surgical implantation of intracranial electrodes for testing and treatment surgeries that may include implantation of neurostimulation devices, resections, disconnections, or ablations. Epilepsy management also includes outpatient palliative procedures, such as implantation of vagus nerve stimulators (VNS) or neurostimulator battery replacement.
Most evaluations and procedures in the United States for DRE are performed at National Association of Epilepsy Center (NAEC) member institutions. The NAEC is a nonprofit association with a membership of more than 260 epilepsy centers. NAEC requires completion of an annual accreditation process by every member center to evaluate certain criteria of specialized epilepsy centers as outlined by NAEC.5 Centers are accredited as level 3 or level 4 centers based on center resources, with level 4 centers serving as regional or national referral sites with comprehensive diagnostic and surgical treatment capabilities.5 In general, level 3 epilepsy centers are facilities with video-EEG (VEEG), neuroimaging, neuropsychology, interdisciplinary epilepsy care, and capability to perform VNS implantation and epilepsy surgeries not requiring invasive monitoring. Level 4 centers are distinguished by expertise with specialized neuroimaging, intracranial VEEG, and more complex surgical techniques.
During the early months of the COVID-19 outbreak in the United States, beginning in early 2020, many hospitals changed practices regarding both elective admissions and procedures, including those for DRE.6 In addition, neurology outpatient visits were transitioned from in-person to telehealth and practices for obtaining outpatient and inpatient EEG were disrupted.7,8 This article describes data trends in 2020 relative to 2019 in the setting of practice changes due, in large part, to the COVID-19 pandemic.
Methods
We analyzed data obtained from annual reports submitted for 2019 and 2020 from all level 3 and level 4 NAEC epilepsy centers. Data trends across NAEC epilepsy centers from 2012 to 2019 were published recently.9
Statistical Methods
Summary statistics included using frequency (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables. Dependent variables were analyzed by center level (level 3 vs level 4) and by center type (adult only, adult/pediatric combined, or pediatric only). Procedure variables were presented in 2 ways due to excessive zeros: frequency (percentage) of centers that performed at least one procedure, as well as median (IQR) of numbers of procedures performed, among these centers (centers with at least 1 procedure).
Two variables were calculated for total intracranial monitoring cases and treatment-related surgical procedures. The intracranial monitoring total was the sum of reported temporal lobectomies with intracranial electrodes, extratemporal resections with intracranial electrodes, and intracranial electrodes with no resection. The treatment surgery total was the sum of reported temporal lobectomies, extratemporal resections, laser interstitial thermal therapy (LITT), corpus callosotomies, hemispherectomies/-otomies, responsive neurostimulation (RNS) implantations, and VNS implantations. In order to keep comparisons between years consistent, DBS implantations were not included due to lack of reliable reporting in 2019. Missing values were ignored in these sums as long as one or more component procedure volume was reported.
Centers were compared by accreditation level, patient demographic, and US census division. The Wilcoxon rank-sum test was used to test for differences in continuous variables between 2019 and 2020. The χ2 or Fisher exact test was used for categorical variables. Percent change from 2019 to 2020 in total epilepsy monitoring unit (EMU) admissions was calculated.
Data from a supplemental survey focused on epilepsy center responses to the COVID-19 pandemic were also included (eAppendix 1, links.lww.com/WNL/B875). The survey was sent in July 2020, with 86/256 (34%) centers responding. Quantitative data were summarized and qualitative responses reviewed.
Qualitative data from annual report free text comment responses and the supplemental survey were manually grouped into themes and subthemes, with 187/260 (72%) annual reports including comments. Questions were open-ended and voluntary, prompting respondents for comments regarding service- or personnel-related changes during the year with regards to the COVID-19 pandemic. Themes were identified by response review, theme development, discussion and consensus, and development of an overarching conceptual framework.
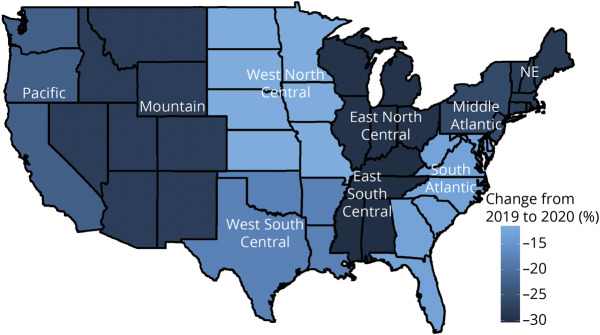
p Values less than 0.05 were considered statistically significant. All analyses were conducted using R 4.0. Figure 1 was generated using the maps package (v3.3.0; R Core Team).
Figure 1. Changes in Aggregate Admissions by US Census Division From 2019 to 2020.
NE = Northeast.
Standard Protocol Approvals, Registrations, and Patient Consents
The ethical standards committee at Nationwide Children's Hospital determined this study exempt from institutional review board approval.
Data Availability
Qualified researchers may request data using the NAEC policy governing the release of member center data.
Results
Reporting centers changed from 256 in 2019 to 260 in 2020. Annual report completion rate was 100% across all years. Data from 2 centers were excluded due to inconsistencies in reporting. The range of data missingness for all variables was <10%.
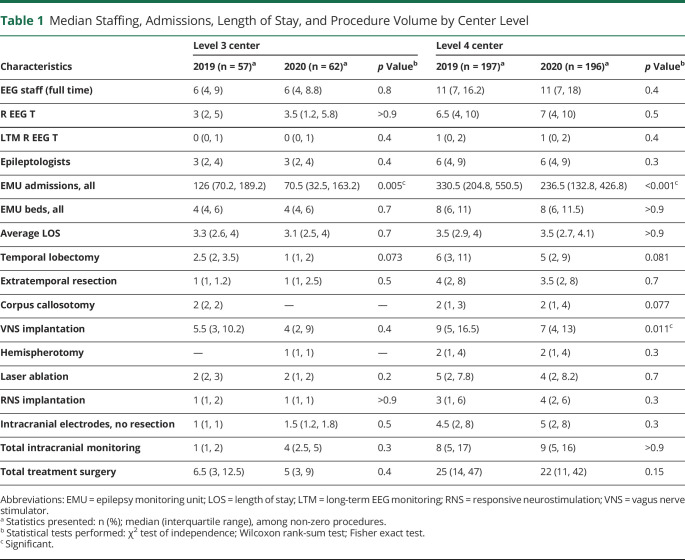
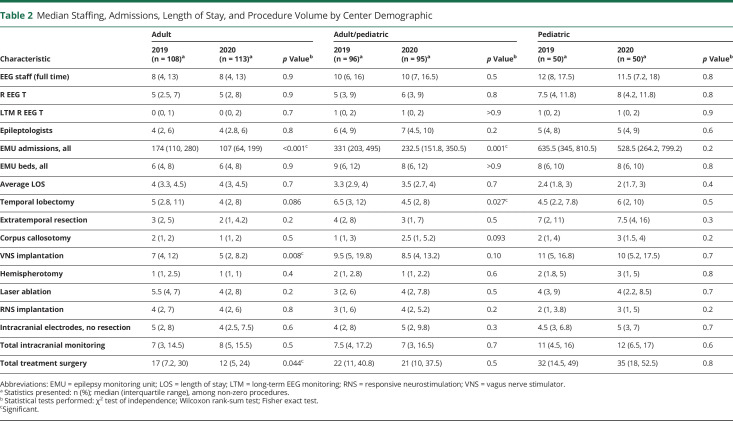
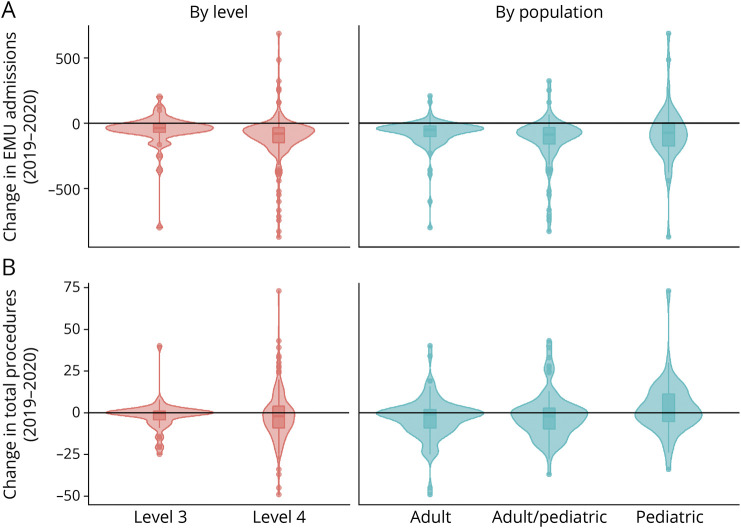
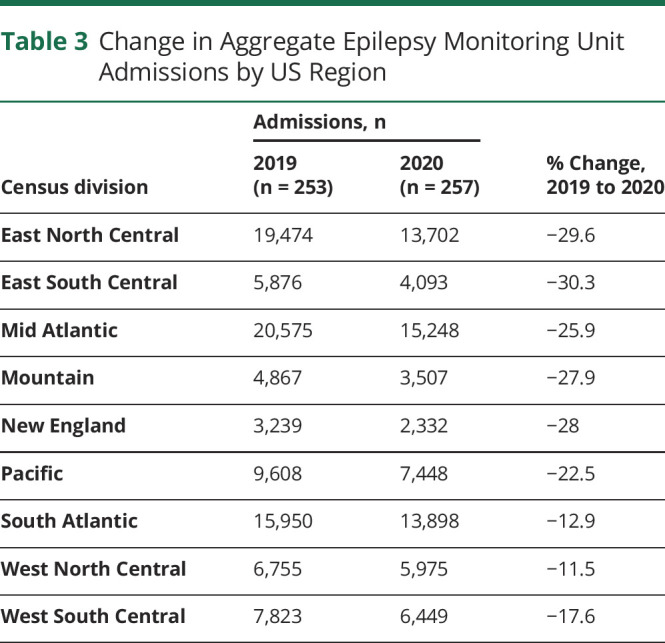
Summary findings for center personnel, EMU admissions, and length of stay (LOS) are detailed by center level (Table 1) and patient population (Table 2). Median EMU admissions significantly declined in all center types except pediatric centers, and aggregate EMU admissions declined 23% (Figure 2). Geographic differences in admissions ranged from an aggregate decline of 30% in the East South Central division to a decline of 12% in the West North Central division (Figure 1). Median admissions per center significantly decreased from 2019 to 2020 in the East North Central (336.5–177.0, p = 0.022), Mid Atlantic (321–220, p = 0.022), and New England (204.5–113.0, p = 0.048) divisions (aggregate admissions per division displayed in Table 3). However, median staffing, EMU beds, and average LOS were unchanged over the study period.
Table 1.
Median Staffing, Admissions, Length of Stay, and Procedure Volume by Center Level
Table 2.
Median Staffing, Admissions, Length of Stay, and Procedure Volume by Center Demographic
Figure 2. Changes in Epilepsy Monitoring Unit Admissions and Total Procedures by NAEC Level or Patient Population.
Changes in epilepsy monitoring unit (EMU) admissions (A) and total procedures (B) displayed by National Association of Epilepsy Center (NAEC) level or patient population. Violin plots by center level and overlaid box plots with median and interquartile range also show the smoothed density at different values. These plots include values of zero, as reported by the respondents.
Table 3.
Change in Aggregate Epilepsy Monitoring Unit Admissions by US Region
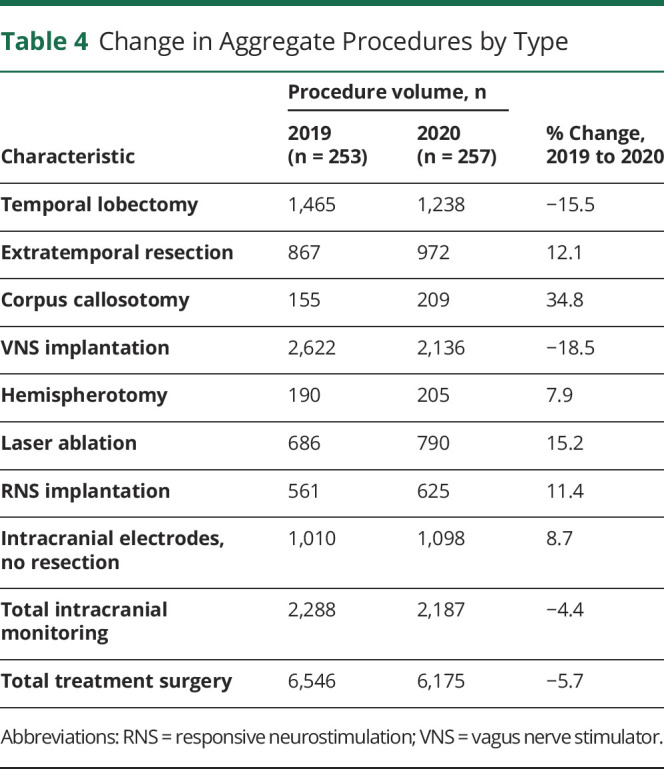
Median surgery volumes did not significantly change across center type or demographic except for VNS implantations, which declined at level 4 and adult centers. Median extratemporal resections significantly declined in the South Atlantic from 6 (IQR 2.5, 8.0) to 3 (IQR 1.8, 4.0) surgeries per year. Otherwise, median procedure volumes by type were not significantly changed within census divisions. Changes in aggregate procedure volumes varied by procedure type (Table 4). For instance, the largest increase occurred in corpus callosotomy (+34.8%). The largest decline was in VNS implantations (−18.5%), although this remained the highest volume single procedure. Intracranial monitoring without resection increased 8.7%; total treatment surgery declined 5.7%
Table 4.
Change in Aggregate Procedures by Type
The supplemental survey was completed by 86 respondents, with 83% level 4 centers and 40% adult, 33% adult/pediatric combined, and 26% pediatric centers. Outpatient visits were within 25% of prepandemic levels in 81% of centers and telehealth with video was utilized for more than half of visits by 54% of respondents (eFigure 1, links.lww.com/WNL/B875). Outpatient EEG volumes were within 25% of prepandemic levels in only 42% of centers.
Three overarching themes emerged: patient-, system-, and staff-related challenges (Table 5). Subthemes included those related to actions taken by patients or institutions to mitigate disease spread, as well as short-term staffing shortages. By late 2020, comments about allocating EMU beds for COVID-19 units on the annual reports were exclusively from adult or combined centers. However, hospital administration-imposed limits on elective admissions and surgeries were reported across all center types. Short-term staffing problems were evident through reports of staff reassignments and furloughs, reduced or stagnant compensation, and quarantines. Patients were perceived as reluctant to undergo elective admissions or procedures and loss of insurance was a reported barrier.
Table 5.
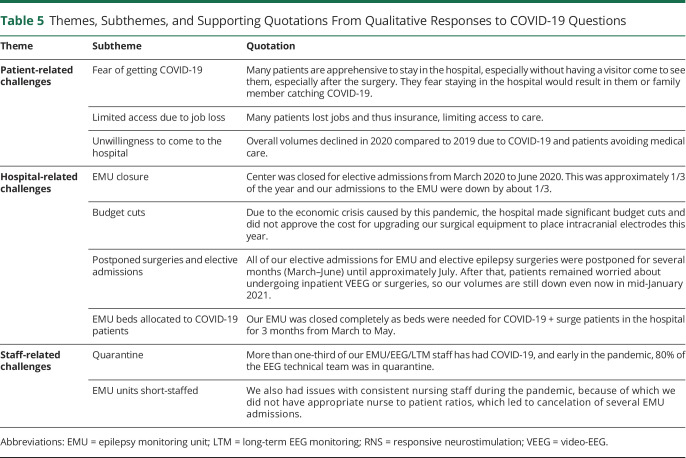
Themes, Subthemes, and Supporting Quotations From Qualitative Responses to COVID-19 Questions
Discussion
This article is the first to examine the effect of the COVID-19 pandemic on inpatient epilepsy care in the United States. Declines in admissions for neurologic conditions, including epilepsy, have been reported for the first half of 2020.8,10-13 Outpatient neurology clinic access was improved with the rapid uptake of telehealth across health care, including neurology.14,15 However, access to outpatient EEG and inpatient VEEG LTM remained restricted. To lessen this effect, the International League Against Epilepsy and International Federation of Clinical Neurophysiology released a consensus statement regarding the importance of continued access to video-EEG monitoring during the COVID-19 pandemic.16 However, improvement in inpatient access was slower and incompletely mitigated by the end of 2020 in specific demographics and regions.
Aggregate admissions to NAEC center EMUs declined 23%. Smaller centers were more negatively affected, with median center admissions declining 44% in level 3 centers compared to a 28% decline in level 4 centers. Pediatric-only centers experienced median declines of 107 admissions per center (17%), although this was not statistically significant due to a wide CI. The decline in admissions was more substantial in the East North Central, Mid Atlantic, and New England divisions. This may have been secondary to differences in state or local restrictions on elective health care testing, admissions, and procedures.
Survey respondents attributed reduced admissions to reassigning EMU beds to become COVID-19 units, administration-imposed restrictions on elective admissions, reduced staffing, and patient reluctance for elective admission. The reduced patient demand is akin to responses provided by adults surveyed in June 2020 when asked if they planned to visit hospitals for emergency or routine care.17 Reduced staffing was largely mitigated by the end of 2020, as EMU employment levels were unchanged from the end of 2019, although continued reduced or stagnant wages and compensation may have effects in 2021 or beyond.
Total procedure volume was slightly lower in 2020 compared to the previous year. The 5.7% and 4.4% decline across total treatment surgeries and total intracranial monitoring surgeries was smaller than expected, given that many EMUs were closed for more than 15% of the year. This more moderate effect may be reflective of prioritization for epilepsy surgery either later in the year or during the restrictions by classifying some types of epilepsy surgery as “nonelective.”
The year-over-year increase of 35% in corpus callosotomies was counter to the decline over the past decade in the United States.9 This may be related to changes in practice. First, corpus callosotomy, traditionally utilized as a palliative surgery for patients with generalized epilepsy and drop seizures, has demonstrated positive effects on infantile spasms and development in West syndrome,18,19 and it is likely that some pediatric centers have expanded indications. Second, increased use of LITT for corpus callosotomy may have attributed to more widespread use due to lower perceived risk by both caregivers and providers.20,21 However, it is likely that relatively few centers were performing corpus callosotomy with LITT in 2020. Finally, some centers utilize corpus callosotomy to palliate other seizure types, such as generalized tonic and generalized tonic-clonic seizures, which may have contributed to the increase. It remains to be seen whether this is a true paradigm shift or anomalous increase in an overall trend towards lower utilization since 2012.9
Aggregate VNS implantation significantly declined 19% and median center implantations significantly declined, continuing the trend from the previous decade.9 This may have been exacerbated by the temporary restriction on outpatient nonurgent procedures. Total temporal lobectomies continued a long-standing downtrend as well,9,22,23 although median center volume did not significantly differ from 2019. In comparison, extratemporal resections increased overall, although they declined in the South Atlantic census division. Regional differences in epilepsy surgery procedures have yet to be explored, so the importance of this finding is unclear.
All other procedure subtypes increased from 2019 to 2020. Thus, the overall decline was driven by the overweight influence of reduced VNS implantations and intracranial monitoring surgeries, based on their larger volumes. The clinical effect on patient outcomes is likely negative based on known benefits of epilepsy surgery,3,4 and the direct effect of deferring other types of surgical procedures was associated with poorer outcomes.24 The financial effect on EMUs will likely negatively affect smaller, independent adult hospitals more than larger centers with academic affiliations.25
The findings are limited primarily by the fact that data were acquired through the NAEC accreditation annual reports, which rely primarily on self-reporting of administrative data. Importantly, the response rate was 100%, with a very low range of missingness, as the annual data are required for NAEC accreditation. Although NAEC member centers do not provide the entirety of epilepsy care in the United States, they likely represent most of the specialized evaluation and procedures for those with DRE. For example, the VA health care system is a large health care provider within the United States, yet performed 4 resections for epilepsy in 2020 and implanted VNS in 8 patients.26 Therefore, our analysis likely reflects accurate data regarding the state of inpatient epilepsy care in the United States in 2020.
The overall implications for reduced EMU admissions for persons with epilepsy in the United States is uncertain and will likely depend on several factors. Influences may include the duration and severity of pandemic-associated changes in hospital admission practices, resource allocation, patient willingness to seek and receive medical care, and staffing fluctuations during 2020. With the COVID-19 pandemic continuing, these effects will likely persist.
Acknowledgment
The authors thank the former NAEC board members and all the medical directors of NAEC-accredited epilepsy centers who submit data annually to NAEC and have supported the strengthening of NAEC’s Accreditation Program to improve the quality of epilepsy care in the United States.
Glossary
- ASM
antiseizure medication
- DRE
drug-resistant epilepsy
- EMU
epilepsy monitoring unit
- IQR
interquartile range
- LITT
laser interstitial thermal therapy
- LOS
length of stay
- LTM
long-term EEG monitoring
- NAEC
National Association of Epilepsy Center
- RNS
responsive neurostimulation
- VEEG
video-EEG
- VNS
vagus nerve stimulator
Appendix. Authors
Footnotes
Study Funding
This study was supported by Award 45141-0001-0321 from Nationwide Children's Hospital and Award 810712-1221-00 from the National Association of Epilepsy Centers.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy: United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. [DOI] [PubMed] [Google Scholar]
- 3.Dwivedi R, Ramanujam B, Chandra PS, et al. . Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377:1639-1647. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and efficiency of surgery for temporal lobe epilepsy study group: a randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. [DOI] [PubMed] [Google Scholar]
- 5.Labiner DM, Bagic AI, Herman ST, et al. . Essential services, personnel, and facilities in specialized epilepsy centers-Revised 2010 guidelines: guidelines for specialized epilepsy centers. Epilepsia. 2010;51(11):2322-2333. [DOI] [PubMed] [Google Scholar]
- 6.Wirrell EC, Grinspan ZM, Knupp KG, et al. . Care delivery for children with epilepsy during the COVID-19 pandemic: an international survey of clinicians. J Child Neurol. 2020;35(13):924-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gélisse P, Rossetti AO, Genton P, Crespel A, Kaplan PW. How to carry out and interpret EEG recordings in COVID-19 patients in ICU? Clin Neurophysiol. 2020;131(8):2023-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zepeda R, Lee Y, Agostini M, et al. . Emergent admissions to the epilepsy monitoring unit in the setting of COVID-19 pandemic-related, state-mandated restrictions: clinical decision making and outcomes. Neurodiagn J. 2021;61(2):95-103. [DOI] [PubMed] [Google Scholar]
- 9.Ostendorf AP, Ahrens SM, Lado FA, et al. . United States epilepsy center characteristics: a data analysis from the National Association of Epilepsy Centers. Neurology. 2022;98(5):e449-e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rameez F, McCarthy P, Cheng Y, et al. . Impact of a stay-at-home order on stroke admission, subtype, and metrics during the COVID-19 pandemic. Cerebrovasc Dis Extra. 2020;10(3):159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacco S, Ricci S, Ornello R, et al. . Reduced admissions for cerebrovascular events during COVID-19 outbreak in Italy. Stroke. 2020;51(12):3746-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esenwa C, Parides MK, Labovitz DL. The effect of COVID-19 on stroke hospitalizations in New York City. J Stroke Cerebrovasc Dis. 2020;29(10):105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinn J, Ramirez A, Hohmann S, Amin A, Nguyen NT. The impact of COVID-19 on volume of inpatient hospitalization through general medicine and medicine subspecialty services at US medical centers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):516-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States. Health Aff. 2021;40(2):349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatcher-Martin JM, Adams JL, Anderson ER, et al. . Telemedicine in neurology: telemedicine work group of the American Academy of Neurology update. Neurology. 2020;94(1):30-38. [DOI] [PubMed] [Google Scholar]
- 16.Beniczky S, Husain A, Ikeda A, et al. . Importance of access to epilepsy monitoring units during the COVID-19 pandemic: consensus statement of the International League Against Epilepsy and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. 2021;132(9):2248-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czeisler MÉ, Marynak K, Clarke KEN, et al. . Delay or avoidance of medical care because of COVID-19-related concerns: United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baba H, Toda K, Ono T, Honda R, Baba S. Surgical and developmental outcomes of corpus callosotomy for West syndrome in patients without MRI lesions. Epilepsia. 2018;59(12):2231-2239. [DOI] [PubMed] [Google Scholar]
- 19.Chan AY, Rolston JD, Lee B, Vadera S, Englot DJ. Rates and predictors of seizure outcome after corpus callosotomy for drug-resistant epilepsy: a meta-analysis. J Neurosurg. 2018;1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roland JL, Akbari SHA, Salehi A, Smyth MD. Corpus callosotomy performed with laser interstitial thermal therapy. J Neurosurg. Epub 2019:1-9. [DOI] [PubMed] [Google Scholar]
- 21.Tao JX, Satzer D, Issa NP, et al. . Stereotactic laser anterior corpus callosotomy for Lennox-Gastaut syndrome. Epilepsia. 2020;61(6):1190-1200. [DOI] [PubMed] [Google Scholar]
- 22.Kaiboriboon K, Malkhachroum AM, Zrik A, et al. . Epilepsy surgery in the United States: analysis of data from the national association of epilepsy centers. Epilepsy Res. 2015;116:105-109. [DOI] [PubMed] [Google Scholar]
- 23.Kwon C-S, Blank L, Mu L, Jetté N. Trends in lobectomy/amygdalohippocampectomy over time and the impact of hospital surgical volume on hospitalization outcomes: a population-based study. Epilepsia. 2020;61(10):2173-2182. [DOI] [PubMed] [Google Scholar]
- 24.Orthopoulos G, Santone E, Izzo F, et al. . Increasing incidence of complicated appendicitis during COVID-19 pandemic. Am J Surg. 2021;221(5):1056-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. 2020;323:2127-2128. [DOI] [PubMed] [Google Scholar]
- 26.Kelly P. Epilepsy Centers of Excellence Annual Report Fiscal Year 2020 [online]. US Department of Veterans Affairs; 2020. Accessed September 6, 2021. epilepsy.va.gov/docs/ECoENational_AnnualReportFY20_web_508c.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request data using the NAEC policy governing the release of member center data.