Abstract
Background
The Office for Health Improvement and Disparities, part of the UK Government Department of Health and Social Care, highlighted an emerging signal of increased non-COVID-19-related deaths in England between July and October, 2021, with a potentially disproportionate higher increase in people with diabetes. We aimed to substantiate and quantify this apparent excess mortality, and to investigate the association between diabetes routine care delivery and non-COVID-19-related-mortality in people with diabetes before and after the onset of the pandemic.
Methods
In this population-based parallel cohort study, we used the National Diabetes Audit (NDA) to identify people with diabetes in England. The primary outcome was non-COVID-19-related deaths between July 3, 2021, and Oct 15, 2021, in participants in the 2021 COVID-19 cohort (registered in the NDA in the periods Jan 1, 2019, to March 31, 2020, and Jan 1, 2020, to March 31, 2021) compared with deaths between June 29, 2019, and Oct 11, 2019 (the equivalent 15-week period in 2019) in the 2019 pre-COVID-19 comparator cohort (people registered in the NDA in the periods Jan 1, 2017, to March 31, 2018, and Jan 1, 2018 to March 31, 2019). In each cohort, multivariable logistic regression examined whether completion of eight diabetes care processes in each of the two years before the index mortality year was associated with non-COVID-19-related death, adjusting for diabetes type, age, sex, ethnicity, and socioeconomic deprivation.
Findings
There were 3 218 570 people in the 2021 cohort and 2 973 645 people in the 2019 comparator cohort. In the 2021 cohort, there were 30 118 non-COVID-19-related deaths in people with diabetes, compared with 27 132 in the comparator cohort, representing an 11% increase (95% CI 9–13). The unadjusted incidence rate ratio (IRR) for mortality in the 2021 cohort compared to the 2019 cohort was 1·026 (1·009–1·043; p=0·003), which was unchanged after adjustment for age, sex, ethnicity, socioeconomic deprivation, and diabetes type (IRR 1·023 (1·006–1·040); p=0·007). In the 2021 cohort, 853 660 (26·5%) people received all eight care processes in 2020–21 compared with 1 547 240 (48·1%) people in 2019–20; a 44·8% (95% CI 44·7–45·0) relative reduction. In the pre-COVID-19 comparator cohort, 1 370 315 (46·1%) people with diabetes received all eight care processes in 2018–19 compared with 1 437 740 (48·3%) in 2017–18; a 4·7% (95% CI 4·5–4·9) relative decrease. Non-COVID-19-related mortality in the 2021 cohort was highest in people who did not receive all eight care processes in either of the two previous years (OR 2·67 [95% CI 2·56–2·77]; p<0·001) compared with those who received all eight care processes in both previous years. Mortality was also significantly higher in those who received all eight care processes in 2019–20 but not in 2020–21 (OR 1·66 [95% CI 1·59–1·73]; p<0·001) or not in 2019–20 but in 2020–21 (OR 1·27 [1·20–1·35]; p<0·001). This pattern of association was similar in the 2019 pre-COVID-19 cohort.
Interpretation
Our results show an increased risk of mortality in those who did not receive all eight care processes in one or both of the previous two years. Our results provide evidence that the increased rate of non-COVID-19-related mortality in people with diabetes in England observed between July 3, and Oct 15 of 2021 is associated with a reduction in completion of routine diabetes care processes following the pandemic onset in 2020.
Funding
None.
Introduction
Governments and national institutions have been carefully monitoring mortality rates during the COVID-19 pandemic. In October 2021, the UK Government Office for Health Improvement and Disparities, applying weekly data collated by the Office for National Statistics (ONS), highlighted an emerging signal of significant increase in non-COVID-19-related deaths in England from July, 2021, to October, 2021, compared with the average number of deaths over the same period for the 5 years preceding the COVID-19 pandemic.1 Further examination of death certification data suggested a disproportionate increase in non-COVID-19-related deaths in people with diabetes compared to those without diabetes. The COVID-19 pandemic has been associated with a reduction in routine care delivery for many non-COVID-19-related conditions, including many long-term conditions, and there has been an increase in waiting lists for elective surgical procedures.2 Reductions in routine care delivery during the pandemic for those with diabetes in England have been shown.3, 4
Research in context.
Evidence before this study
We searched PubMed and medRxiv from Jan 1, 2020 to Jan 14, 2022. We did not identify any studies assessing associations between non-COVID-19-related mortality and routine care delivery for diabetes or any other long-term condition during the COVID-19 pandemic. Two previous pre-pandemic analyses found associations between delivery of diabetes care processes in England and mortality four and seven years later, respectively. Reduced access to non-COVID-19 health services has been reported in many countries during the pandemic. A global survey of health care professionals from 47 countries reported that diabetes was the chronic condition most impacted by COVID-19 due to disruptions in care. Although the direct risks of COVID-19 on mortality in people with diabetes have been well reported, data on the indirect effects of COVID-19 on mortality due to disruptions in care are sparse.
Added value of this study
To our knowledge, this is the first study to suggest that rates of non-COVID-19-related mortality in people with diabetes are significantly increased in those not receiving comprehensive routine care during the pandemic. The current analyses over a period of 15 weeks in 2021 found associations between mortality and care process delivery in the preceding 3–30 months. However, these data cannot determine how much of the association is directly attributable to the consequences of uncompleted care processes themselves, and how much is related to unmeasured confounding factors that are associated with receipt of the care processes, such as health seeking behaviours, undocumented co-morbidities, fear of contagion in healthcare environments, and reduced access to or interaction with healthcare professionals.
Implications of all the available evidence
The consistent associations between missed routine diabetes care delivery and subsequent mortality, strongly suggest that, irrespective of cause, those affected are a group at high risk of worse outcomes. Further adverse impact on the delivery of routine diabetes care should be minimised during a period when there might potentially be a need to repeatedly repurpose health workforce activities towards both COVID-19 care and COVID-19 vaccination programmes.
We therefore used linked national datasets to quantify this initial signal and, if the apparent excess mortality were confirmed, to explore whether or not a reduction in routine care was a cause. The National Institute for Health and Care Excellence (NICE) recommends the delivery of nine care processes annually for people living with diabetes in England. The National Diabetes Audit (NDA) reports annually the proportion of all people with diabetes, by type, who have received the eight care processes: HbA1c, blood pressure, cholesterol, serum creatinine, urine albumin, foot surveillance, BMI, and smoking status.5 Data on annual retinal screening, the ninth care process, are collected separately, as the delivery of screening is through a national programme outside usual primary and secondary care settings. Associations between care process delivery and subsequent mortality in those with diabetes have been reported previously.6, 7 It is not known whether changes in the proportion of people completing diabetes care processes during the pandemic might also be associated with subsequent mortality.
Using delivery of the eight care processes as a surrogate marker for routine care delivery in people living with diabetes in England, we aimed to assess the associations between changes in routine care delivery before and after onset of the COVID-19 pandemic, in March, 2020, with changes in non-COVID-19-related mortality between the period from July to October, 2021, compared with a similar period in 2019 before the pandemic.
Methods
Study design and data sources
In this population-based parallel cohort study, we used the NDA to identify people with diagnosed diabetes who were registered with general practices in England. The NDA has collated data annually on people with diabetes since 2003, and now has almost complete participation of general practices in England (99% in 2020–21, 99% in 2019–20, 98% in 2018–19, and 98% in 2017–18). For the index cohort, we included those with diabetes who were registered in both 2019–20 and 2020–21 annual audits, and for the comparator cohort, those with diabetes who were registered in both 2017–18 and 2018–19 annual audits.8 These data were then linked by pseudonymised English National Health Service (NHS) number to civil death registrations collated by the ONS.
To fulfil its statutory duties, NHS England and NHS Improvement require access to and linkage of various pseudonymised national datasets, in line with the requirements of the General Data Protection Regulation. The legal basis for the NDA data collection and linkage is a direction from NHS England to NHS Digital according to section 254 of the Health and Social Care Act for England 2012. Data are not extracted if the person has withdrawn their permission to use their record for secondary analyses, which is estimated to apply to 2·6% of records. Furthermore, in March 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 to require organisations to process confidential patient information for the purposes of protecting public health, providing health-care services to the public, and monitoring and managing the COVID-19 outbreak and incidents of exposure.
Covariates
The NDA was used to identify all people with diagnosed diabetes and for each person, whether or not they received all eight annual care processes recommended by NICE between April 1, 2020, and March 31, 2021, and the same dates in 2019–20, 2018–19, and 2017–18. For children aged younger than 12 years, the only care process recorded in the NDA is HbA1c, in line with NDA reporting.5 Individuals included in the index cohort were categorised into four groups on the basis of completion of the annual diabetes care processes over the 2 years before the period of mortality observation (2019–20 and 2020–21): those who had not received all eight annual care processes in either the first year or the second year period, those who had received all eight care processes in the first year period but not the second year period, those who had received all eight care processes in the second year period but not in the first year period, and those who had received all eight care processes in both the first and second year periods. For the comparator cohort, individuals included were similarly divided into four groups on the basis of completion of the annual care processes over the 2 years before the comparator period of mortality observation (2017–18 and 2018–19).
Diabetes type, age, sex, ethnicity, and socioeconomic deprivation were identified as potential confounding factors. Diabetes type was categorised as type 1, type 2, or other. Age was grouped into 5-year age bands (<40, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, or ≥ 75 years). Sex was recorded as male, female, or unknown. Ethnicity was classified as Asian, Black, mixed, other, White, or unknown. Socioeconomic deprivation was defined by indices of multiple deprivation associated with the lower layer super output area9 derived from the individual's home postcode and grouped into quintiles with the first quintile representing most deprived and the fifth quintile representing least deprived.
Outcomes
The primary outcome assessed was non-COVID-19-related deaths over the 15-week period in 2021 (July 3, 2021, to Oct 15, 2021) in individuals with diabetes whose data were recorded in the NDA in both preceding 15-month periods (Jan 1, 2019, to March 31, 2020, and Jan 1, 2020, to March 31, 2021), and who were still alive on July 3, 2021. In the pre-COVID-19 comparator cohort, the outcome assessed was all-cause deaths over the equivalent 15-week period in 2019 (ie, dates that represent weeks 27 to 41 in the corresponding calender year; June 29, 2019, to Oct 11, 2019) in individuals with diabetes whose data were recorded in the NDA in both preceding 15-month periods (Jan 1, 2017, to March 31, 2018, and Jan 1, 2018 to March 31, 2019), and who were still alive on June 29, 2019. Non-COVID-19-related death was defined as death in which an International Classification of Diseases tenth edition (ICD 10) code of U07.1 (COVID-19, virus identified), U07.2 (COVID-19, virus not identified), U09.9 (post-COVID condition, in which the acute infection had ended before the condition immediately causing death occurred), and U10.9 (multisystem inflammatory syndrome associated with COVID-19, a specific uncommon effect of COVID-19 in children) was not recorded as a primary or secondary cause of death. Secondary outcomes included whether completion of the eight care processes in each of the two years before both the index and the comparator mortality years were associated with non-COVID-19-related death, adjusting for age, sex, ethnicity, socioeconomic deprivation, and diabetes type. Additionally, we examined the causes of death in each of the two cohorts.
Statistical analysis
Associations between the independent variables (diabetes type, age, sex, ethnicity, socioeconomic deprivation, and annual eight care process group) and the dependent variable (non-COVID-19-related death over the stated period of observation) were analysed for each of the two cohorts. Unadjusted mortality rates over each 15-week observation period per 100 000 people in each cohort were calculated. Poisson regression analyses were used to test temporal differences in mortality rates in 2021 compared with 2019; the first model only included the time period for each cohort (2021 or 2019) as an independent variable to test temporal differences in unadjusted mortality rates, and the second model included the time period, age, sex, ethnicity, socioeconomic deprivation, and diabetes type as independent variables to test temporal differences in adjusted mortality rates.
Multivariable logistic regression analyses were used in the two parallel cohorts to examine whether completion or incompletion of the eight care processes in each of the two previous 1-year observation periods was associated with non-COVID-19-related death in England during each of the 15-week observation periods, after adjustment for age, sex, ethnicity, socioeconomic deprivation, and diabetes type. The C statistic was used to assess model fit. Interactions between ethnicity and care process group and socioeconomic deprivation and care process group were also considered and tested for significance using the Wald's test. Records with missing values for age, sex, and socioeconomic deprivation were not included in the regression analyses. However, records with unknown ethnicity were included as an unknown category.
An expected number of deaths in 2021 was estimated by applying the proportions of individuals in each of the care process groups in 2019 to the 2021 cohort and applying the 2021 mortality rates to each group. The difference between the expected number of deaths and the observed number of deaths was then used to estimate the number of deaths associated with changes in the proportions in each of the care process groups between 2021 and 2019. A sensitivity analysis was done by extending the analysis period to Dec 17, 2021 (Dec 13, 2019, for the comparator cohort), in order to determine if the observed increases in mortality were restricted in time. Statistical significance was defined as p value less than 0·05 and CIs were set at 95%. All analyses were done with Stata version 16. All data taken directly from the NDA were rounded to the nearest five people to protect patient confidentiality.
Role of the funding source
There was no funding source for this study.
Results
There were 3 218 570 people with diabetes who were included in both the 2019–20 and 2020–21 audits, who were alive as of the July 3, 2021 (2021 cohort). Of those, 243 510 (7·6%) had type 1 diabetes, 2 926 390 (90·9%) had type 2 diabetes, and 48 670 (1·5%) had other types of diabetes (table ). Data were missing for less than 0·1% for age, sex, and socioeconomic deprivation variables, and 8·9% for ethnicity. There were no missing data for diabetes type and annual care process group. In comparison, there were 2 973 645 people with diabetes who were included in both the 2017–18 and 2018–19 audits who were alive as of June 29, 2019 (2019 comparator cohort). Data were missing for <0·1% for age, sex, deprivation and for 11·9% for ethnicity. There were no missing data for diabetes type and annual care process group. As shown in the table, the characteristics of the two parallel cohorts were broadly similar. There were 32 660 deaths in people with diabetes between July and October, 2021. Of those deaths, 2542 (8%) had COVID-19 on the death certificate. There were 30 118 non-COVID-19-related deaths in people with diabetes in 2021 compared with 27 132 in 2019, representing an 11% increase (95% CI 9–13). Unadjusted mortality rates over the 15 weeks per 100 000 people with diabetes were also higher; 936 (95% CI 925–946) in 2021 versus 912 (902–923) in 2019 (table). In a Poisson regression analysis, the unadjusted incidence rate ratio (IRR) for 2021 compared with 2019 was 1·026 (95% CI 1·009–1·043); p=0·003), which was unchanged after adjustment for age, sex, ethnicity, deprivation, and diabetes type (IRR 1·023 [1·006–1·040]; p=0·007; appendix p 1).
Table.
Non-COVID-19-related deaths in people with diabetes and baseline characteristics of participants in the 2021 cohort and the 2019 comparator cohort.
|
2021 |
2019 |
||||||
|---|---|---|---|---|---|---|---|
| Number of people alive as of July 3 | Non-COVID-19-related deaths | Death rate per 100 000 people (95% CI) | Number of people alive as of June 29 | Non-COVID-19-related deaths | Death rate per 100 000 people (95% CI) | ||
| Total | 3 218 570 | 30 118 | 936 (925–946) | 2 973 645 | 27 132 | 912 (902–923) | |
| Annual eight care process groups* | |||||||
| Not received in either first or second year | 1 376 270 (42·8%) | 16 599 (55·1%) | 1206 (1188–1225) | 1 018 855 (34·3%) | 13 160 (48·5%) | 1292 (1270–1314) | |
| Received in first year but not second | 988 645 (30·7%) | 8894 (29·5%) | 900 (881–919) | 584 480 (19·7%) | 5166 (19·0%) | 884 (860–908) | |
| Received in second year but not first | 295 060 (9·2%) | 1664 (5·5%) | 564 (537–592) | 517 050 (17·4%) | 3596 (13·3%) | 695 (673–719) | |
| Received in both years | 558 595 (17·4%) | 2961 (9·8%) | 530 (511–550) | 853 260 (28·7%) | 5210 (19·2%) | 611 (594–627) | |
| Diabetes type | |||||||
| Type 1 diabetes | 243 510 (7·6%) | 963 (3·2%) | 395 (371–421) | 235 455 (7·9%) | 918 (3·4%) | 390 (365–416) | |
| Type 2 diabetes | 2 926 390 (90·9%) | 28 723 (95·4%) | 982 (970–993) | 2 714 685 (91·3%) | 26 063 (96·1%) | 960 (948–972) | |
| Other | 48 670 (1·5%) | 432 (1·4%) | 888 (806–975) | 23 500 (0·8%) | 151 (0·6%) | 643 (544–754) | |
| Age, years | |||||||
| <40 | 215 515 (6·7%) | 121 (0·4%) | 56 (47–67) | 196 925 (6·6·%) | Suppressed | Suppressed | |
| 40–44 | 122 365 (3·8%) | 126 (0·4%) | 103 (86–123) | 112 605 (3·8%) | 100 (0·4%) | 89 (72–108) | |
| 45–49 | 197 655 (6·1%) | 309 (1·0%) | 156 (139–175) | 188 200 (6·3%) | 251 (0·9%) | 133 (117–151) | |
| 50–54 | 288 965 (9·0%) | 578 (1·9%) | 200 (184–217) | 267 345 (9·0%) | 507 (1·9%) | 190 (173–207) | |
| 55–59 | 365 815 (11·4%) | 1013 (3·4%) | 277 (260–295) | 331 225 (11·1%) | 843 (3·1%) | 255 (238–272) | |
| 60–64 | 397 910 (12·4%) | 1556 (5·2%) | 391 (372–411) | 361 050 (12·1%) | 1334 (4·9%) | 369 (350–390) | |
| 65–69 | 404 860 (12·6% | 2448 (8·1%) | 605 (581–629) | 384 240 (12·9%) | 2359 (8·7%) | 614 (589–639) | |
| 70–74 | 429 210 (13·3%) | 3947 (13·1%) | 920 (891–949) | 395 710 (13·3%) | 3483 (12·8%) | 880 (851–910) | |
| ≥75 | 796 280 (24·7%) | 20 020 (66·5%) | 2514 (2479–2549) | 736 345 (24·8%) | 18 136 (66·8%) | 2463 (2427–2499) | |
| Unknown | 5 (<0·1%) | 0 | N/A | 5 (<0·1%) | Suppressed | Suppressed | |
| Sex | |||||||
| Male | 1 796 265 (55·8%) | 16 508 (54·8%) | 919 (905–933) | 1 664 320 (56·0%) | 15 013 (55·3%) | 902 (888–917) | |
| Female | 1 422 285 (44·2%) | 13 610 (45·2%) | 957 (941–973) | 1 309 305 (44·0%) | 12 119 (44·7%) | 926 (909–942) | |
| Unknown | 25 (<0·1%) | 0 | N/A | 20 (<0·1%) | 0 | N/A | |
| Ethnicity | |||||||
| Asian | 435 305 (13·5%) | 1722 (5·7%) | 396 (377–415) | 378 220 (12·7%) | 1488 (5·5%) | 393 (374–414) | |
| Black | 153 325 (4·8%) | 804 (2·7%) | 524 (489–562) | 134 720 (4·5%) | 666 (2·5%) | 494 (458–533) | |
| Mixed | 35 820 (1·1%) | 170 (0·6%) | 475 (406–552) | 30 870 (1·0%) | 153 (0·6%) | 496 (420–581) | |
| Other | 60 295 (1·9%) | 295 (1·0%) | 489 (435–548) | 54 120 (1·8%) | 294 (1·1%) | 543 (483–609) | |
| Unknown | 287 125 (8·9%) | 2532 (8·4%) | 882 (848–917) | 353 315 (11·9%) | 3062 (11·3%) | 867 (836–898) | |
| White | 2 246 705 (69·8%) | 24 595 (81·7%) | 1095 (1081–1108) | 2 022 405 (68·0%) | 21 469 (79·1%) | 1062 (1047–1076) | |
| Deprivation, IMD quintile | |||||||
| 1 | 762 465 (23·7%) | 6988 (23·2%) | 917 (895–938) | 706 720 (23·8%) | 6190 (22·8%) | 876 (854–898) | |
| 2 | 711 915 (22·1%) | 6450 (21·4%) | 906 (884–928) | 653 005 (22·0%) | 5888 (21·7%) | 902 (879–925) | |
| 3 | 662 655 (20·6%) | 6279 (20·8%) | 948 (924–971) | 608 625 (20·5%) | 5699 (21·0%) | 936 (912–961) | |
| 4 | 590 085 (18·3%) | 5805 (19·3%) | 984 (959–1009) | 547 625 (18·4%) | 5158 (19·0%) | 942 (916–968) | |
| 5 | 490 630 (15·2%) | 4584 (15·2%) | 934 (907–962) | 456 800 (15·4%) | 4188 (15·4%) | 917 (889–945) | |
| Unknown | 825 (<0·1%) | 12 (<0·1%) | N/A | 870 (<0·1%) | 9 (<0·1%) | N/A | |
Data are n (%) unless otherwise stated. IMD quintile 1 represents most deprived and IMD quintile 5 represents least deprived. All mortality data referring to between one and five people are suppressed in order to protect patient confidentiality. IMD=indices of multiple deprivation. NA=not assessed.
For 2021 data, first year refers to April 1, 2019, to March 31, 2020, and second year refers to April 1, 2020 to March 31, 2021; for 2019 data, first year refers to April 1, 2017, to March 31, 2018, and second year refers to April 1, 2018, to March 31, 2019.
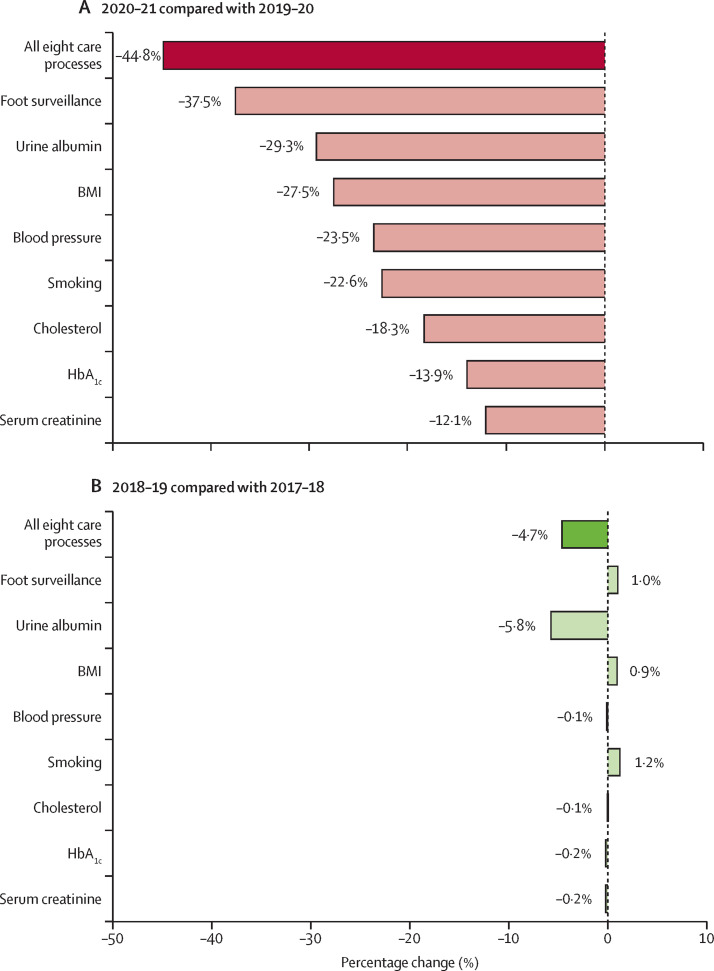
Of the 3 218 570 people with diabetes in the 2021 index cohort, 853 660 (26·5%) received all eight care processes between April 1, 2020, and March 31, 2021 (2020–21), compared to 1 547 240 (48·1%) between April 1, 2019, and March 31, 2020 (2019–20), corresponding to a relative decrease of 44·8% (95% CI 44·7–45·0) following onset of the pandemic (figure 1A ; appendix p 2). There were greater decreases in the number of people receiving all eight care processes in this period in people in the most deprived quintile than there were in people in the least deprived quintile, and greater decreases in those of White ethnicity than in those of non-White ethnicity (appendix p 3). There was significant variation in the change in the percentage of people who received each individual care process in 2020–21 compared with 2019–20, with foot surveillance associated with the largest relative decrease, from 2 494 870 to 1 558 640 (–37·5% [95% CI –37·7 to –37·4%]) and measurement of serum creatinine with the smallest relative decrease, from 2 810 040 to 2 469 510 (–12·1% [95% CI –12·3 to –12·0%]; figure 1A; appendix p 2).
Figure 1.
Percentage change in the proportions of people who received the eight care processes during 2020–21 compared with 2019–20 (A) and during 2018–19 compared with 2017–18 (B)
BMI=body mass index.
Of the 2 973 645 people with diabetes who were included in the 2019 comparator cohort, 1 370 315 (46·1%) received all eight care processes between April 1, 2018, and March 31, 2019 (2018–19), compared with 1 437 740 (48·3%) between April 1, 2017, and March 31, 2018 (2017–18), corresponding to a 4·7% (95% CI 4·5–4·9) decrease (figure 1B; appendix p 2). With the exception of assessment of urine albumin, which had a decrease from 1 779 270 to 1 675 895 (–5·8% [95% CI –6·0 to –5·6%]), there were minimal differences in the percentage of people who received each individual care process in 2018–19 compared with 2017–18 (figure 1B).
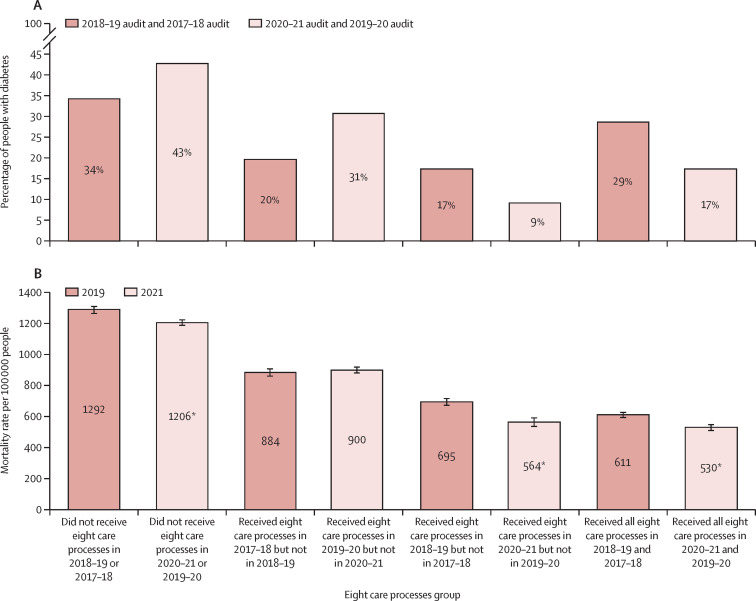
The marked differences in the proportions in each of the four annual care process groups between the two cohorts reflect the reductions in completion of the annual eight care processes in 2020–21, after onset of the COVID-19 pandemic (figure 2A ). In both cohorts, of the individuals who did not receive all eight care processes, there were increased proportions of both those aged under 40 and those with type 1 diabetes; otherwise, characteristics of individuals were broadly similar between cohorts and across each annual care processes group (appendix p 4).
Figure 2.
Percentage of people with diabetes in each eight care processes group (A) and rate of non-COVID-19-related deaths in people with diabetes during the 15-week periods in 2021 and 2019 by eight care processes group (B)
Error bars in B represet 95% CIs. *Non-overlapping CIs compared with previous years.
For both cohorts, the unadjusted mortality rates by annual care process group shows a clear dose-response relationship, in that unadjusted mortality rates were highest in those who did not receive all eight annual care processes in either of the previous two years, intermediate in those who received them in one of the two years, and lowest for those who received them in both of the previous two years (figure 2B). The unadjusted mortality rates per 100 000 in 2021 were significantly lower than those in 2019 for people who did not receive all eight annual care processes in either of the previous two years (1206 vs 1292), for those who received them in the second year but not the first (564 vs 695), and for those who received them in both years (530 vs 611), and there were no significant differences for those who received them in the first year but not the second. Rates by characteristic and eight-care-process group were similar or significantly lower in 2021 than in 2019 (appendix p 5).
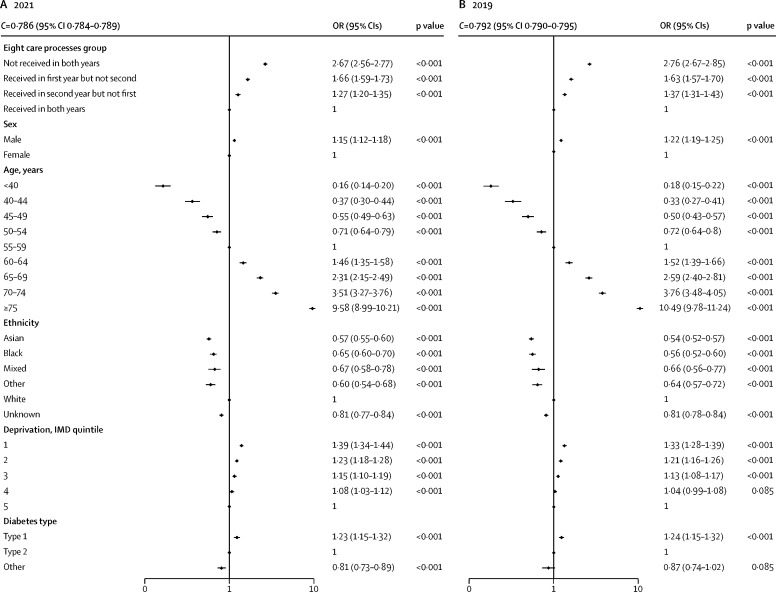
Logistic regression analysis showed that, in both 2021 and 2019, there was an increase in the odds for non-COVID-19-related mortality with increasing age, and odds for non-COVID-19-related mortality was higher for men than for women and for those from the most deprived quintile than those from the least deprived quintile (figure 3 ). Black, Asian, mixed, and other ethnicity groups had lower mortality than the White ethnicity group. Compared with people with type 2 diabetes, people with type 1 diabetes had higher odds of mortality and people with other types of diabetes had lower odds. Adjusted for age, sex, ethnicity, socioeconomic deprivation, and type of diabetes, people who did not receive all eight care processes in either of the two previous years had an odds ratio (OR) of non-COVID-19-related death of 2·67 (95% CI 2·56–2·77; p<0·001) over the 15-week period in 2021 compared with those who received all eight care processes in both previous years. Those who received all eight care processes in 2019–20 but not in 2020–21 had OR 1·66 (1·59–1·73; p<0·001), and those who did not receive all eight care processes in 2019–20 but did in 2020–21 had OR 1·27 (1·20–1·35; p<0·001); the C-statistic was 0·786 (95% CI 0·784–0·789). The pattern of association between receipt of the care processes and all-cause mortality in the pre-COVID-19 cohort (deaths in 2019) was highly similar (figure 3); the C-statistic was 0·792 (95% CI 0·790–0·795). The interaction between annual eight care process group and socioeconomic deprivation quintile on non-COVID-19-related mortality was not significant (p=0·1901) and neither was the interaction between annual eight care process group and ethnicity (p=0·3107; appendix p 6).
Figure 3.
Adjusted ORs for non-COVID-19-related deaths (logarithmic scale) for people with diabetes in the 15-week periods in 2021 (A) and 2019 (B)
IMD is rated on a scale of 1 to 5, with 1 representing most deprived and 5 least deprived. OR=odds ratio. IMD=indices of multiple deprivation.
If the proportions of individuals in each of the eight care process groups had remained the same in 2021 as in 2019, the estimated number of deaths in 2021 would have been 27 043; 3075 fewer than the observed number of deaths in 2021. The estimated rate of mortality would have been 840 (95% CI 830–850) per 100 000 people with diabetes, compared with the observed rate of 936 (95% CI 925–946; appendix p 7).
Increases in the number of deaths in people with diabetes in 2021 compared with 2019 occurred across all causes. For example, 9035 (30·0%) of 30 118 deaths in 2021 were due to cardiovascular disease versus 7859 (29·0%) of 27 132 deaths in 2019 (15% increase), 7694 (25·5%) were due to cancer versus 7261 (26·8%; 6·0% increase), 3232 (10·7%) were due to respiratory disease versus 3102 (11·4%; 4·2% increase), and 1656 (5·5%) were due to diseases of the nervous system versus 1410 (5·2%; 17·4% increase); appendix p 8).
Sensitivity analyses using data from the extended time period of observation to December, 2021, showed a weakened signal for excess risk; there were 50 539 non-COVID-19-related deaths in 2021 and 46 049 in 2019; a 10% (95% CI 8–11%) increase. Non-COVID-19-related death rates per 100 000 people over this extended 24-week period were 1570 (95% CI 1557–1584) in 2021 versus 1549 (1534–1563) in 2019. In a Poisson regression analysis, the unadjusted IRR for 2021 compared with 2019 was 1·014 (95% CI 1·001–1·027; p=0·031), which was no longer significant after adjustment for age, sex, ethnicity, deprivation, and diabetes type (IRR 1·011 [0·998–1·024]; p=0·089; appendix p 9). However, the pattern of association with the eight care processes was unchanged (appendix p 10).
Discussion
We examined mortality over a 15-week period between July and October in two parallel cohorts of people with diabetes in England and found that there was higher non-COVID-19-related mortality in 2021 compared with all-cause mortality in 2019. The association between completion of key diabetes care processes in the previous 2-year period and mortality was very similar in both the 2019 and 2021 cohorts. Mortality rates were highest in those who did not receive all eight annual care processes in either of the previous two years, intermediate in those that received them in one of the previous two years, and lowest for those who received them in both of the previous two years. The higher non-COVID-19-related mortality in 2021 compared with 2019 was associated with a change in the proportion of people completing the annual eight diabetes care processes. Although the proportion of people receiving all eight annual care processes in each of the two years were broadly similar for the pre-COVID-19 comparator cohort, for the 2021 cohort there was a 44·8 % relative decrease in the second year (2020–21) compared to the first (2019–20), reflecting the reduction in routine diabetes care delivery following the onset of the pandemic. There were no other significant differences in the independent variables between the two cohorts.
The care process with the greatest reduction was the one that requires the most in-person contact—foot surveillance—possibly reflecting issues around social distancing, lockdown measures, and the move to remote forms of health-care delivery. Despite this finding, there were significant reductions in major and minor amputation rates during the first wave of the pandemic in England, which we previously speculated might relate to reduced ambulation in the context of home confinement.10 The reduction in routine care following the onset of the pandemic was greater for people living in the most deprived quintile than those living in the least deprived quintile, possibly reflecting the fact that the burden of COVID-19 was relatively greater in more socioeconomically deprived areas.11, 12 However, the opposite was true for ethnicity; although Asian and Black communities had greater risk of more severe COVID-19 outcomes than those of White ethnicity,11, 12 we observed a greater decrease in routine diabetes care delivery and corresponding increases in non-COVID-19-related mortality in those of White ethnicity than in those of Asian and Black ethnicities. We have shown previously that although hazard ratios (HRs) for COVID-19-related mortality in people with diabetes were greater in those of Asian and Black ethnicity than in those of White ethnicity, HRs for non-COVID-19-related mortality during the pandemic were higher for those of White ethnicity.12 Others have previously shown that type 2 diabetes in England is associated with more years of life lost among those of White ethnicity than among those of South Asian or Black ethnicity.13 This current analysis supports the higher risk for non-COVID-19-related mortality in people with diabetes of White ethnicity.
It has been previously shown in a subgroup of the English population that the COVID-19 pandemic has been associated with reductions in delivery of some of the care processes for those with type 2 diabetes,3 as well as reductions in new diagnoses of type 2 diabetes and new prescriptions for metformin.4 Although theoretically we could have assessed whether people with diabetes had reached NICE recommended treatment targets for HbA1c, blood pressure, and cholesterol in order to investigate possible mediation of the effect we observed, this would only have been possible in those people in whom the respective care processes had been measured, which would have been potentially biased by differential measurement in 2020 after the start of the pandemic. We therefore only assessed delivery of care processes as the surrogate for routine care delivery in the current analyses.
Two previous analyses have shown associations between delivery of these same diabetes care processes in England and subsequent mortality, but both were assessed over longer periods of follow-up with means of 4 years7 and 7 years6 respectively between assessment of care process delivery and assessment of mortality. The current analyses report associations between mortality over 15 weeks and care process delivery in the preceding 3 to 30 months, a short follow-up period for effect of care on mortality. As in the previous studies however, we are unable to differentiate how much of this association is attributable to poor delivery of care processes directly, and how much is attributable indirectly via associated unmeasured factors. Some possibilities of relevance to this study, such as health-seeking behaviours and fear of contagion in health-care environments, are associated with the individual, and some, such as access to routine care for long term conditions and some repurposing of the health-care workforce during pandemic times, are associated with the system for health-care delivery. The large size of around three million people with diabetes in each cohort means that the population characteristics are broadly the same, despite COVID-19-related deaths during the latter period being more common in the elderly, in men, in those from more deprived communities, and in those of non-White ethnicity with diabetes.11, 12
A study assessing cause-specific mortality during the first wave of the pandemic in Norway suggested an increase in non-COVID-19-related mortality in those with diabetes. Although overall mortality was not increased in Norway, in which the COVID-19-related mortality was low (only 216 COVID-19 deaths as of May 31, 2020; the time of reporting), the observed death rate from diabetes was 50% higher than predicted.14 In the first pandemic wave in England, we showed that 30–35% of the excess mortality in those with diabetes was not associated with recording of COVID-19 on death certificates.12 However, the access to testing outside hospital environments at that point was poor. By July 2021, there was widespread access to testing in England, so it is unlikely that what we describe as non-COVID-19-related deaths in the current study are due to misclassifications.
The COVID-19 pandemic has posed substantial health-care challenges internationally. Reduced accessibility to non-COVID-19 health services has been shown in many countries.15 A global survey of health-care professionals from 47 countries reported that diabetes was the chronic condition most affected by COVID-19 due to disruptions in care.16 Although the direct risks of COVID-19 in people with diabetes have been well reported, data on the indirect effects of COVID-19 due to disruption in care are sparse. The indirect effects include disruptions in routine care with many patients avoiding or delaying medical attention for routine non-COVID-19-related problems due to fear of contagion, strain on health-care services already overburdened by COVID-19, as well as reduced recall for annual reviews of long-term conditions.17 These indirect effects probably caused varied results over the course of the pandemic, and this might account for the weakening of the signal of excess mortality risk in the sensitivity analysis that included an extended period of observation to December, 2021, although the pattern of association with the eight care processes was unchanged.
Our data, combined with data from other studies, suggests that the pandemic has been associated with a double mortality hit for people with diabetes. Furthermore, the potential effects of disruptions in routine diabetes care will be broader than those realised through increases in mortality alone, and further work to measure associated increases in morbidity is also warranted. Although our analyses will not have captured all the potential predictor variables for non-COVID-19-related mortality, they suggest that a significant proportion of the additional deaths in people with diabetes seen between July, 2021, and October, 2021, in England was associated with the reduction in diabetes routine care delivery following the onset of the pandemic. How much of this association is attributable to poor delivery of diabetes care directly, and how much is attributable indirectly via associated unmeasured factors, is unclear; indeed, the causes of death associated with the increase are diverse. Nonetheless, the consistent finding of associations between missed routine care and subsequent mortality suggests that further impact on the reduction of routine care delivery should be minimised as far as possible during a period when there might potentially be a need to repeatedly repurpose health workforce activities towards both COVID-19 care and COVID-19 vaccination programmes.
Data sharing
Data from the National Diabetes Audit can be requested through the NHS Digital Data Access Request Service process at: https://digital.nhs.uk/services/data-access-request-service-dars/data-access-request-service-dars-process.
Declaration of interests
JV is the national clinical director for diabetes and obesity at National Health Service (NHS) England and NHS Improvement. PK is the national specialty adviser for diabetes and obesity at NHS England and NHS Improvement. CB is an adviser to the NHS Diabetes Programme. BY is clinical lead for the National Diabetes Audit and a trustee of Diabetes UK. NH is funded by Diabetes UK and NHS England and NHS Improvement. KK has been a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, and Merck Sharp & Dohme; has received grants in support of investigator-initiated trials from Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Pfizer, and Boehringer Ingelheim; has served on advisory boards for Novo Nordisk, Sanofi Aventis, Lilly, and Merck Sharp & Dohme; and is supported by the UK National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre. NS has consulted for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, and Sanofi; and received grant support from Boehringer Ingelheim. NW is supported by the Medical Research Council (grant MC_UU_00006/1). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
NHS England and NHS Improvement provided the infrastructures for the data repository and data linkages for these analyses and salaries for the data analysts conducting this work.
Contributors
JV, EB and NW conceived the study. EB and TG managed the data and did the statistical analysis. All the authors collaborated in the interpretation of the results and drafting of the manuscript. EB and TB had access to, and verified, the raw data. All authors had the final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Office for National Statistics Deaths registered weekly in England and Wales, provisional. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/weeklyprovisionalfiguresondeathsregisteredinenglandandwales
- 2.European Observatory on Health Systems and Policies COVID-19 health system response monitor: how are countries reorganizing non-COVID-19 health care service delivery? 2020. https://eurohealthobservatory.who.int/monitors/hsrm/analyses/hsrm/how-are-countries-reorganizing-non-covid-19-health-care-service-delivery
- 3.Carr MJ, Wright AK, Leelarathna L, et al. Impact of COVID-19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: a UK-wide cohort study involving 618 161 people in primary care. BMJ Qual Saf. 2021 doi: 10.1136/bmjqs-2021-013613. published online Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr MJ, Wright AK, Leelarathna L, et al. Impact of COVID-19 on diagnoses, monitoring, and mortality in people with type 2 diabetes in the UK. Lancet Diabetes Endocrinol. 2021;9:413–415. doi: 10.1016/S2213-8587(21)00116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHS Digital Report 1: care processes and treatment targets 2019–20, full report. Aug 12, 2021. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/report-1-care-processes-and-treatment-targets-2019-20
- 6.Holman N, Knighton P, O'Keefe J, et al. Completion of annual diabetes care processes and mortality: a cohort study using the National Diabetes Audit for England and Wales. Diabetes Obes Metab. 2021;23:2728–2740. doi: 10.1111/dom.14528. [DOI] [PubMed] [Google Scholar]
- 7.McKay AJ, Gunn LH, Vamos EP, et al. Associations between attainment of incentivised primary care diabetes indicators and mortality in an English cohort. Diabetes Res Clin Pract. 2021;174 doi: 10.1016/j.diabres.2021.108746. [DOI] [PubMed] [Google Scholar]
- 8.Holman N, Knighton P, Wild SH, et al. Cohort profile: national diabetes audit for England and Wales. Diabet Med. 2021;38 doi: 10.1111/dme.14616. [DOI] [PubMed] [Google Scholar]
- 9.Gov UK. English indices of deprivation. 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019
- 10.Valabhji J, Barron E, Vamos EP, et al. Temporal trends in lower-limb major and minor amputation and revascularisation procedures in people with diabetes in England during the COVID-19 pandemic. Diabetes Care. 2021;44:e133–e135. doi: 10.2337/dc20-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright AK, Kontopantelis E, Emsley R, et al. Life expectancy and cause-specific mortality in type 2 diabetes: a population-based cohort study quantifying relationships in ethnic subgroups. Diabetes Care. 2017;40:338–345. doi: 10.2337/dc16-1616. [DOI] [PubMed] [Google Scholar]
- 14.Raknes G, Strøm MS, Sulo G, Øverland S, Roelants M, Juliusson PB. Lockdown and non-COVID-19 deaths: cause-specific mortality during the first wave of the 2020 pandemic in Norway: a population-based register study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuczyńska M, Matthews-Kozanecka M, Baum E. Accessibility to non-COVID health services in the world during the COVID-19 pandemic: review. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.760795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chudasama YV, Gillies CL, Zaccardi F, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020;14:965–967. doi: 10.1016/j.dsx.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vamos EP, Khunti K. Indirect effects of the COVID-19 pandemic on people with type 2 diabetes: time to urgently move into a recovery phase. BMJ Qual Saf. 2021 doi: 10.1136/bmjqs-2021-014079. published online Oct 22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the National Diabetes Audit can be requested through the NHS Digital Data Access Request Service process at: https://digital.nhs.uk/services/data-access-request-service-dars/data-access-request-service-dars-process.