Abstract
Melon peel is recognized as a source of healthy nutrients and oxidant compounds. Being considered a non-edible part with no profit value, large amounts of melon rinds are discharged by fruit industries. Innovative food ingredients with potential health benefits may arise if these parts were conveniently transformed. The objective was to freeze-dry small melon peel cubes to attain a potential edible matrix. An ozone pre-treatment was applied seeking decontamination purposes and quality retention. The effect of these processes was assessed in terms of physicochemical parameters (moisture content, water activity and color), bioactive compounds (total phenolics, vitamin C and chlorophylls) and antioxidant capacity, during 7 weeks of storage at room temperature. Intrinsic microflora (mesophylls, yeasts and molds) were also monitored. Results showed that the freeze-drying process allowed retention of the most bioactive compounds analyzed, except for total phenolic content. In this case, the ozone pre-treatment was important for phenolics preservation. During the storage period, ozonated samples presented a higher content of bioactive compounds. In terms of microflora, the ozone and freeze-drying effects were not significant. Freeze-drying proved to be a suitable preservation method for melon peel. The ozone impact was not relevant in terms of decontamination.
Keywords: ozone pre-treatment, bioactive compounds, antioxidant activity, total mesophylls, molds and yeasts
1. Introduction
Fruit industries produce waste quantities on a large scale. In the particular case of melon, significant amounts of peel are discharged as residues after juice processing. This is critical from an environmental and economic point of view. Since these wastes are considered rich sources of bioactive compounds, their transformation and reuse are extremely important [1,2]. A study on the characterization of melon (Cucumis melo L. var. reticulatus) showed that the peel contains 32, 30, 27 and 15% of all total phenolics, potassium, antioxidant activity and total carotenoids content, respectively [3]. Therefore, developing new strategies to turn this non-edible part into a valuable and attractive ingredient is of utmost interest [4,5]. Several works have been developed to valorize fruit wastes in the last decade to produce novel nutraceuticals, pharmaceuticals, cosmeceuticals, food ingredients or functional foods. As additives in food products, fruit wastes have been used to preserve and enhance food quality [6], as a supply of bioactive compounds [7], as an encapsulating agent [8], to produce edible packaging material [9] and also in the production of food products with enhanced prebiotic activity [10]. Fruit wastes are also used as biofertilizers [11], in pharma drugs, as a bio-adsorbent for heavy metals removal [12], in biofuel production [13] and as an additive for some skincare products.
Drying is a well-studied process used in the food industry to extend products’ self-life by inhibiting microorganisms’ growth and enzyme activity [14]. Dried food products are considered safe since their water activity (aw) is lower than 0.80, a value at which most bacteria cannot grow. Nevertheless, since yeasts and molds are more tolerant to lower aw values, special attention should be given to this group of microorganisms when drying food products [15].
Among drying processes, freeze-drying is recognized as the one that allows better retention of overall food quality characteristics [2,16]; shape, dimension, appearance, taste, color and flavor are examples. The freeze-dried product is usually highly porous, brittle, hygroscopic and has excellent rehydration capacity [14]. Nevertheless, some sensory and nutritional parameters can be negatively affected.
When conveniently packaged, a dried product has an extended shelf-life and can be considered a suitable transformation of raw materials that can be incorporated into various food matrices. In the particular case of fruit wastes such as melon rinds, drying small pieces of the product or grinding it to attain powders with adequate granulometry may open opportunities to develop new food ingredients while reducing wastes and contributing to a circular economy.
Raw foods, particularly fruits and vegetables, are grown near the soil and their surfaces can be heavily contaminated with microorganisms. Therefore, decontamination treatments are essential to guarantee a safe product for human consumption. Treatments that avoid the negative impact of high temperatures on the quality features of food products are often applied. Ozone is gaining importance in this field, with broad applications in fruit processing. Exposing food products to ozone may allow the inactivation of undesirable microorganisms with positive effects on several bioactive compounds, which are beneficial to health [17,18].
The main objective of this work was to assess the impact of the freeze-drying process on melon peel cubes with and without ozone pre-treatment. Another important goal was to evaluate the effect of these processes during 7 weeks of storage at room temperature. Physicochemical characteristics (moisture content, aw and color), bioactive compounds (total phenolics, vitamin C and total chlorophylls) and antioxidant capacity were assessed weekly. Microbial indicators (total mesophylls and yeasts and molds) were also monitored during the storing period.
Raw melon rinds are non-edible wastes, but a value-added ingredient may be created if conveniently processed and transformed.
2. Materials and Methods
2.1. Sample Preparation
Cantaloupe melons (Cucumis melo L. var. reticulatus) were obtained from a local supermarket at the commercial maturity stage and stored overnight at 4 °C. Fruits selection was based on visual inspection, without physical damage, defects or disease. Using a sharp stainless-steel knife, the melon peel was cut in cubic shapes with dimensions between 0.064 to 0.216 cm3.
Before freeze-drying and storage, part of the melon peel underwent an ozone pre-treatment; the other part did not suffer the ozonation process. As described below, fresh-cut and processed samples were analyzed in terms of physicochemical characteristics, bioactive compounds, antioxidant activity and microbial indicators.
2.2. Ozone Pre-Treatment
Ozone gas (O3) was generated with a corona discharge equipment model (OZ5, SPO3-Sociedade Portuguesa de Ozono, Lda. Porto, Portugal), producing ozone at 5 g/h. Pure oxygen was supplied via an oxygen cylinder at 0.5 bar. Ozone was continuously bubbled for 30 min into an airtight plastic box (12 cm × 27 cm × 17 cm) with a thin film of 300 g of peel cubes, as described by Miller et al. [18]. The corresponding ozone dose attained was 152 ± 71 ppm at a controlled temperature of 15 °C. This treatment time was chosen since previous studies reported more than 1 log cycle reduction of Listeria innocua [18]. Each treatment was carried out three times, independently.
2.3. Freeze-Drying Process
Fresh and ozonized peels (50.0 g) were packed in sterilized plastic flasks (100.0 mL), frozen at −80 °C for 6 to 8 h and then transferred to a Heto Drywinner freeze-dryer (Cambridge Biosystems, Cambridge, United Kingdom). Samples were subjected to a temperature of −50 °C and a pressure ranging from 1.5 to 2 bar, for 80 to 90 h. Three replicates of the drying process were carried out.
2.4. Storage
Dried samples, placed in sterilized plastic flasks (100.0 mL), were kept in the dark, at room temperature, for 7 weeks.
2.5. Physicochemical Analysis
2.5.1. Moisture Content
The moisture content of melon peel was determined according to the oven drying method recommended by the Association of Analytical Chemistry (Method 984.25; AOAC 2002 [19]). Briefly, approximately 10 g of melon peel samples were dehydrated in an air oven (Memmert, Labmetro, Germany) at 105 °C until no weight alteration was detected. Weight measurements were carried out in an analytical balance (Mettler Toledo AE 200, Marshall Scientific, Knutsford, UK) with a precision of ± 0.0001 g. Results were expressed as the difference between fresh and dried peel weights, divided by the fresh wet weight (expressed in %). Moisture content was determined in triplicate.
2.5.2. Water Activity
For water activity (aw) measurements, a dew point hygrometer (Aqualab–Series 3, Decagon Devices Inc., USA) was used at 22 ± 1 °C. Two readings of three replicated samples were carried out.
2.5.3. Color
CIELAB color components of peel samples (L*, a*, b*) were measured using a Minolta CR-400 colorimeter (Konica-Minolta, Osaka, Japan). Total color difference (TCD) was estimated according to Equation (1), which accounts for color changes between fresh and processed samples [20,21]. Higher TCD values indicate more noticeable color alterations.
| (1) |
The index “0” corresponds to the fresh melon peel samples. Two readings of three different replicates were conducted for each sample.
2.6. Bioactive Compounds
2.6.1. Total Phenolic Content
The total phenolic content of peel samples was determined according to the Folin–Ciocalteu method using gallic acid as standard [5]. Extractions were done by mixing 25.0 g of the peel with 50.0 mL of 100% methanol (Merck) [18]. The results were expressed in gallic acid equivalent (GAE) in μg/g of dried melon peel.
2.6.2. Vitamin C
Ascorbic acid (AA) and dehydroascorbic acid (DHA) were determined following the HPLC analytical procedure outlined by Zapata and Dufour [22]. A reverse phase C18-silica analytical column (Waters Spherisorb ODS2 5 µm 4.6 × 250 mm) was used, and mobile phase, standard solutions, and samples were prepared as described by Fundo et al. [3]. Results were reported as mg of vitamin C per 100 g of dried peel.
2.6.3. Chlorophylls
Chlorophylls were extracted, homogenizing 7.0 g of the melon peel with 50.0 mL of 100% methanol with an ultra-turrax. Extracts were then centrifuged at 5000× g at 4 °C for 10 min and filtered using filter paper. The extract absorbance was measured at given wavelengths and the quantification of chlorophylls a and b (µg/mL) was based on Equations (2) and (3) [23]. Total chlorophylls is the sum of chlorophylls a and b.
| (2) |
| (3) |
Results were expressed in µg of chlorophylls per gram of dried peel.
2.7. Antioxidant Activity
Total antioxidant activity was estimated using the ABTS assay, according to Fundo et al. [3]. The results were expressed as μg of AA per gram of dried peel.
2.8. Microbiological Analysis
Melon peel samples (0.5 g) were homogenized with 49.5 mL of buffered peptone water (BPW) in a stomacher for 2 min. Decimal dilutions were carried out in BPW. Total mesophylls were assessed in duplicate using Plate Count Agar–PCA (Lab M, Lancashire, UK). Plates were incubated at 37 °C for 48 h. Molds and yeasts were evaluated in duplicate using Rose Bengal Agar-RBA (Lab M, Lancashire, UK), with the plates incubated at 25 °C for 60 h. Results were expressed as log10 CFU per gram of dried peel.
2.9. Statistical Analysis
Differences between samples were analysed by one-way ANOVA concerning all characteristics studied. Means were compared by Tukey’s test, while normality and homoscedasticity of data were assessed using Shapiro–Wilk and Levene’s tests, respectively. When data normality was not proved, the Kruskal–Wallis test was carried out alternatively to one-way ANOVA. In such cases, the non-parametric Mann–Whitney test was used to detect differences. Data analyses were performed at a significance level of 5% using IBM SPSS Statistics 24 for Windows® (SPSS Inc., Chicago, IL, USA). The results were reported as a mean ± margin of confidence interval at 95%. The confidence interval margin is half of the confidence interval at 95%.
3. Results and Discussion
3.1. Effect of Ozone Pre-Treatment on Dried Samples
3.1.1. Physicochemical Characteristics
The effect of ozone pre-treatment on moisture content, aw and color of dried melon peel can be assessed from the data presented in Table 1. After the drying process, and as expected, moisture content strongly decreased (approximately 88%). The effect of ozone exposure was not evident, with no significant differences observed between ozonized and non-ozonized samples. Akbas and Ozdemir [24] also reported no differences in moisture content of ozonized and non-ozonized figs after drying. Contrarily, the ozone process significantly decreased aw of dried peel samples compared with non-ozonized ones. Based on these results, it is possible to speculate that although ozone exposure did not change the amount of water present in the samples, it decreased the available water for microorganisms’ growth and chemical reactions. This is probably due to the molecular interactions between free water and O3 constituents.
Table 1.
Selected physicochemical characteristics of fresh and freeze-dried melon peel, with and without ozone pre-treatment. The values are mean ± margin of confidence intervals at 95%.
| Physicochemical Characteristic |
Melon Peel | |||
|---|---|---|---|---|
| Fresh | Dried | Ozonated + Dried | ||
| moisture content (%) | 87.51 ± 6.79 b | 12.37 ± 2.79 a | 12.41 ± 2.46 a | |
| aw | 0.99 ± 0.0092 c | 0.06 ± 0.02 b | 0.04 ± 0.01 a | |
| color | L* a* |
47.29 ± 3.24 a −12.79 ± 1.31 a |
66.00 ± 4.24 b −4.82 ± 1.98 b |
63.73 ± 3.61 b −4.69 ± 2.87 b |
| b* | 33.21 ± 2.94 b | 24.65 ± 2.42 a | 21.56 ± 1.49 a | |
| TCD | - | 22.11 ± 10.99 a | 21.12 ± 9.39 a | |
For a given characteristic, values with different letters differ significantly (p < 0.05).
Color alterations frequently occur in dried food matrixes due to non-enzymatic browning reactions [14]. Both non-ozonized and ozonized dried peel samples presented significantly higher L* values, indicating of a brighter color when compared to fresh peel [25]. Dried samples’ greenness (a*) and yellowness (b*) significantly changed. However, no significant differences were observed between non-ozonized and ozonized dried peels on the three color coordinates. The values of TCD were higher than 12 units for both dried and ozonated dried melon peel. According to Drlange [26], this can be considered a very great perceived color difference when compared to fresh samples. Fernandes et al. [27] also attained similar results for freeze-dried pumpkins.
3.1.2. Bioactive Compounds and Oxidant Activity
The results of total phenolic content, vitamin C, total chlorophylls and antioxidant activity are included in Table 2 for fresh, dried and ozonated dried samples.
Table 2.
Bioactive compounds and total antioxidant activity of fresh and freeze-dried melon peels, with and without ozone pre-treatment. The values are mean ± margin of confidence intervals at 95%.
| Compound | Fresh | Dried | Ozonated + Dried |
|---|---|---|---|
| Total phenolic content (μg/g) | 2447.54 ± 241.23 b | 1948.91 ± 307.01 a | 2232.60 ± 262.49 ab |
| Vitamin C (mg/100g) | 423.61.7 ± 236.32 a | 383.27 ± 110.85 a | 452.56 ± 160.18 a |
| Total chlorophylls (μg/g) | 941.61 ± 85.59 a | 877.93 ± 50.01 a | 935.67 ± 148.62 a |
| Total antioxidant activity (μg/g) | 1806.97 ± 236.97 a | 1879.83 ± 327.58 a | 1690.80 ± 255.43 a |
For a given characteristic, values with different letters differ significantly (p < 0.05).
Total phenolics were significantly reduced after the drying process of the non-ozonized peel. Ozone exposure had a positive effect on the retention of these compounds. The drying process might accelerate the release of more bound phenolic compounds due to the breakdown of cellular constituents [28]. The observed ozone positive effect can be attributed to the activation of phenylalanine ammonialyase, one of the key enzymes used to synthesize phenolic compounds in plant tissues. The total phenolic content maintenance might have been caused by peel cell wall modification during ozone exposure [29].
Vitamin C was not affected by the freeze-dried process or ozone exposure. The temperatures involved in the freeze-dried process were not high (around 50 °C), which may not affect the degradation of this water-soluble vitamin [30].
Total chlorophylls were also preserved. This can be explained by low enzymatic activity caused by the processes applied [30].
Antioxidant activity is due to the presence of different bioactive compounds, which were not affected by the freeze-drying processes. The total antioxidant activity of non-ozonized and ozonized dried peel was equivalent to fresh samples.
3.1.3. Intrinsic Microorganisms
The effect of freeze drying on the intrinsic microflora of ozonized and non-ozonized peel is presented in Table 3. No significant differences were observed in total mesophylls, molds and yeasts of fresh and dried samples.
Table 3.
Effect of freeze-drying on the intrinsic microflora of fresh and ozonated melon peel. The values are mean ± margin of confidence intervals at 95%.
| Microflora | Fresh | Dried | Ozonated + Dried |
|---|---|---|---|
| Total mesophylls (Log CFU/mL) | 6.48 ± 1.51 a | 6.26 ± 1.30 a | 5.45 ± 2.14 a |
| Molds and yeasts (Log CFU/mL) | 4.02 ± 2.04 a | 4.00 ± 1.15 a | 3.83 ± 1.15 a |
For a given characteristic, values with different letters differ significantly (p < 0.05).
Since freeze-drying decreased peel aw to values lower than 0.80, in which most bacteria cannot grow, shelf-life studies are essential. The ozone impact was not relevant, being that the microorganisms log counts of dried samples, with or without O3 pre-treatment, were equivalent to the ones enumerated in the fresh melon peel. Although several fruits had similar results, a slight decrease in yeasts and molds counts was observed, after 1 h of ozone treatments [17,31].
3.2. Shelf-Life Assessment of Non-Ozonized and Ozonized Dried Peel
3.2.1. Physicochemical Characteristics
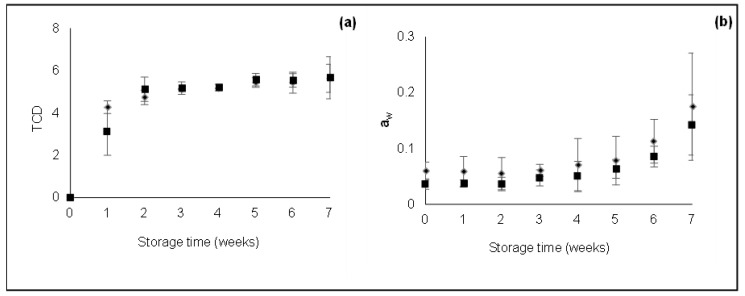
Total color difference and aw of non-ozonized and ozonized dried peel during 7 weeks of storage are shown in Figure 1.
Figure 1.
TCD (a) and aw (b) of non-ozonized (◆) and ozonized (■) dried peels during storage. Data represent mean values and bars represent the limits of confidence intervals at 95%.
The TCD references (index 0 in Equation (1)) were the color coordinates obtained for dried samples. As shown in Figure 1a, TCD increased in the first two weeks of storage. After this period, TCD remained constant until the end of storage time. Similar to these results, Topuz et al. [32] detected dried paprika color alterations in the first 30 days of storage, with no significant differences attained after that period. No significant differences were observed between non-ozonized and ozonized samples.
The aw of non-ozonized and ozonized dried peel during the storage is shown in Figure 1b. During the storage period, no significant differences were observed between ozonized and non-ozonized samples. However, after 5 weeks of being stored, the aw of the dried peel slightly increased. The fact that samples were stored without humidity controlling conditions may explain this increase. This must be taken into consideration when longer storage periods are imposed.
3.2.2. Bioactive Compounds and Antioxidant Activity
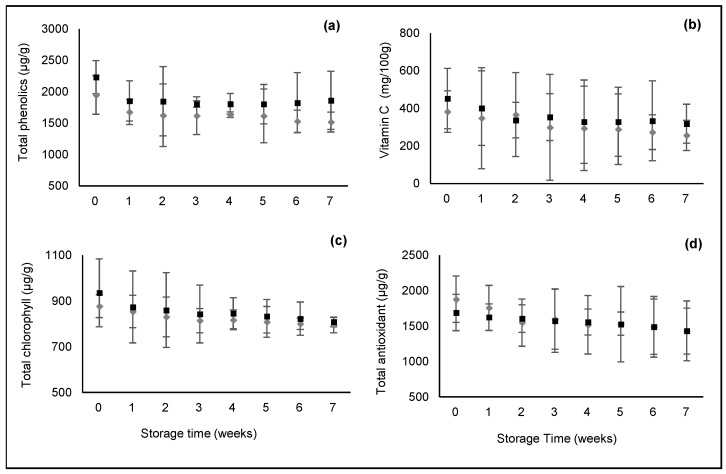
The behavior of the bioactive compounds and total antioxidant activity of non-ozonized and ozonized dried peel during storage are presented in Figure 2. As observed in Figure 2a, the total phenolic content of non-ozonized and ozonized dried samples decreased during the storage period. However, samples with O3 pre-treatment retained better total phenolics throughout the entire storage period. Tzortzakis et al. [33] reported that ozone exposure increased total phenolic content in tomatoes.
Figure 2.
Total phenolic content (a) vitamin C, (b) total chlorophylls content and (c) total antioxidant activity (d) in non-ozonized (◆) and ozonized (■) dried peel. Data represent mean values and bars represent the limits of confidence intervals at 95%.
Vitamin C, in Figure 2b, also decreased during the storage period for both non-ozonized and ozonized dried samples, being slightly higher in ozonated ones. Vitamin C is very susceptible to chemical and enzymatic oxidation during food processing and storage. According to Laing et al. [34], ascorbic acid degradation rates depend on oxygen availability, temperature and moisture content, and the higher the water activity, the greater the vitamin C loss. The increase of aw during the storage period may also explain the occurrence of vitamin C losses.
The green color of fruits peel is mainly due to chlorophylls, which are highly susceptible to degradation during processing and storage [35]. The most abundant type of chlorophyll in melon peel is chlorophyll a (around 530 µg/g), followed by chlorophyll b (about 400 µg/g). Both types of chlorophylls showed a similar slightly degradation during the 7 weeks of storage (both non-ozonized and ozonized dried peels; results not shown). In Figure 2c, it is possible to see the effect of storage time on the total chlorophylls of dried samples. As previously mentioned, ozone may inhibit the enzymes responsible for chlorophyll degradation through the induction of antioxidants that can protect chlorophylls [36]. Although ozonized dried samples seem to better retain these compounds during storage, the difference was not significant. At the end of storage, the value for both ozonized and non-ozonized peels is almost the same.
In terms of total antioxidant activity, differences between ozonated and non-ozonated samples are not significant, as shown in Figure 2d. Both samples presented a similar tendency during the entire storage period. Since the antioxidant activity of fruits may be attributed to the presence of vitamin C, polyphenols and other bioactive compounds, the observed decrease was expected.
3.2.3. Intrinsic Microflora
Regarding total mesophylls counts and at the end of the storage period, 1.40 ± 0.52 and 0.90 ± 1.22 log cycles were reduced in non-ozonized and ozonized samples, respectively. For yeasts and molds, 0.85 ± 1.02 and 1.00 ± 0.48 log cycles reduction were obtained for non-ozonized and ozonized dried peels, respectively. The effect of O3 was not significant in the microflora analyzed.
Total mesophylls, yeasts and molds were the microorganisms chosen as indicators of the drying process’s effectiveness and ozone treatment during storage. For both groups of microorganisms, freeze-drying showed to be effective in controlling microbial growth during the 7 weeks of storage. Freeze-drying at low temperatures may stop microbiological activity, providing food products with better quality [15].
Despite the well-known effect of ozone on microbial inactivation by the progressive oxidation of vital cell components, preventing microbial growth and extending the shelf-life of many fruits [37], this effect was not observed since no significant differences were attained on microbial loads of non-ozonized and ozonized dried peels.
4. Conclusions
Freeze-drying of melon peel proved to be effective in preserving of the bioactive compounds analyzed, except for total phenolic content. Shelf-life studies of dried samples revealed that pre-ozonized samples better retained total phenolics, vitamin C and total chlorophylls.
In terms of microflora inactivation, the effects of ozone and freeze-drying were not significant. However, at the end of storage, a decrease of around 1 log-cycle was observed for the groups of microorganisms studied. Melon rinds exposed to gaseous ozone did not achieve convenient decontamination. Further studies with undesirable target microorganisms are required to attain a safe product.
Freeze-drying can be considered a potential process to transform melon peel into an edible form. When the small cubes of melon rinds were freeze-dried, they became lighter and softer. Grinding these dried materials will also allow for transformation into powders. They can be incorporated into different food products, such as cakes, breads and yogurts, enriching their nutritional value. Moreover, the dried material itself may constitute a novel food ingredient: gluten free flours with a wide range of applications in the food industry, for example. However, a deep study about the toxicity of melon peel compounds should be attained before consuming the products.
Author Contributions
Conceptualization, F.A.M. and J.F.F.; methodology, S.S.; software, T.R.S.B.; validation, F.A.M. and J.F.F.; formal analysis, T.R.S.B.; investigation, S.S.; resources, C.L.M.S.; data curation, F.A.M. and J.F.F.; writing—original draft preparation, F.A.M. and J.F.F.; writing—review and editing, T.R.S.B.; visualization, T.R.S.B.; supervision, C.L.M.S.; funding acquisition, C.L.M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This work was supported by National Funds from FCT-Fundação para a Ciência e a Tecnologia through project UID/Multi/50016/2020.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mallek-Ayadi S., Bahloul N., Kechaou N. Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chem. 2017;221:1691–1697. doi: 10.1016/j.foodchem.2016.10.117. [DOI] [PubMed] [Google Scholar]
- 2.Ramírez-Pulido B., Bas-Bellver C., Betoret N., Barrera C., Seguí L. Valorization of Vegetable Fresh-Processing Residues as Functional Powdered Ingredients. A Review on the Potential Impact of Pretreatments and Drying Methods on Bioactive Compounds and Their Bioaccessibility. Front. Sustain. Food Syst. 2021;5:654313. doi: 10.3389/fsufs.2021.654313. [DOI] [Google Scholar]
- 3.Fundo J.F., Miller F.A., Garcia E., Santos J.R., Silva C.L.M., Brandão T.R.S. Physicochemical characteristics, bioactive compounds and antioxidant activity in juice, pulp, peel and seeds of Cantaloupe melon. J. Food Meas. Charact. 2018;12:292–300. doi: 10.1007/s11694-017-9640-0. [DOI] [Google Scholar]
- 4.Raji Z., Khodaiyan F., Rezaei K., Kiani H., Hosseini S.S. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromol. 2017;98:709–716. doi: 10.1016/j.ijbiomac.2017.01.146. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sayed H.M.A., Ahmed A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013;58:83–95. doi: 10.1016/j.aoas.2013.01.012. [DOI] [Google Scholar]
- 6.Caggia C., Palmeri R., Russo N., Timpone R., Randazzo C.L., Todaro A., Barbagallo S. Employ of Citrus By-Product as Fat Replacer Ingredient for Bakery Confectionery Products. Front. Nutr. 2020;7:46. doi: 10.3389/fnut.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su D.-L., Li P.-J., Quek S.Y., Huang Z.-Q., Yuan Y.-J., Li G.-Y., Shan Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem. 2019;286:1–7. doi: 10.1016/j.foodchem.2019.01.200. [DOI] [PubMed] [Google Scholar]
- 8.Šafranko S., Ćorković I., Jerković I., Jakovljević M., Aladić K., Šubarić D., Jokić S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods. 2021;10:1043. doi: 10.3390/foods10051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayram B., Ozkan G., Kostka T., Capanoglu E., Esatbeyoglu T. Valorization and Application of Fruit and Vegetable Wastes and By-Products for Food Packaging Materials. Molecules. 2021;26:4031. doi: 10.3390/molecules26134031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foti P., Ballistreri G., Timpanaro N., Rapisarda P., Romeo F.V. Prebiotic effects of citrus pectic oligosaccharides. Nat. Prod. Res. 2021:1–4. doi: 10.1080/14786419.2021.1948845. [DOI] [PubMed] [Google Scholar]
- 11.Zema D.A., Calabrò P.S., Folino A., Tamburino V., Zappia G., Zimbone S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018;80:252–273. doi: 10.1016/j.wasman.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Akkaya Sayğılı G., Sayğılı H., Yılmaz C., Güzel F. Lead recovery from aqueous environment by using porous carbon of citrus fruits waste: Equilibrium, kinetics and thermodynamic studies. Sep. Sci. Technol. 2020;55:2699–2712. doi: 10.1080/01496395.2019.1653917. [DOI] [Google Scholar]
- 13.John I., Pola J., Thanabalan M., Appusamy A. Bioethanol Production from Musambi Peel by Acid Catalyzed Steam Pretreatment and Enzymatic Saccharification: Optimization of Delignification Using Taguchi Design. Waste Biomass Valorization. 2020;11:2631–2643. doi: 10.1007/s12649-018-00565-x. [DOI] [Google Scholar]
- 14.Ceballos A.M., Giraldo G.I., Orrego C.E. Effect of freezing rate on quality parameters of freeze dried soursop fruit pulp. J. Food Eng. 2012;111:360–365. doi: 10.1016/j.jfoodeng.2012.02.010. [DOI] [Google Scholar]
- 15.Sagar V.R., Suresh Kumar P. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010;47:15–26. doi: 10.1007/s13197-010-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prosapio V., Norton I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT. 2017;80:401–408. doi: 10.1016/j.lwt.2017.03.012. [DOI] [Google Scholar]
- 17.Guzel-Seydim Z.B., Greene A.K., Seydim A.C. Use of ozone in the food industry. Lebensm Wiss Technol. 2004;37:453–460. doi: 10.1016/j.lwt.2003.10.014. [DOI] [Google Scholar]
- 18.Miller F.A., Fundo J.F., Garcia E., Silva C.L.M., Brandão T.R.S. Effect of Gaseous Ozone Process on Cantaloupe Melon Peel: Assessment of Quality and Antilisterial Indicators. Foods. 2021;10:727. doi: 10.3390/foods10040727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AOAC International . Official Methods of Analysis. Volume 984. AOAC International; Gaithersburg, MD, USA: 2002. pp. 25–1984. [Google Scholar]
- 20.Ihns R., Diamante L.M., Savage G.P., Vanhanen L. Effect of temperature on the drying characteristics, colour, antioxidant and beta-carotene contents of two apricot varieties. Int. J. Food Sci. Technol. 2011;46:275–283. doi: 10.1111/j.1365-2621.2010.02506.x. [DOI] [Google Scholar]
- 21.Alibas I. Microwave, Vacuum, and Air Drying Characteristics of Collard Leaves. Dry. Technol. 2009;27:1266–1273. doi: 10.1080/07373930903267773. [DOI] [Google Scholar]
- 22.Zapata S., Dufour J.P. Ascorbic, dehydroascorbic and isoascorbic acid simultaneous determinations by reverse phase ion interaction HPLC. J. Food Sci. 1992;57:506–510. doi: 10.1111/j.1365-2621.1992.tb05527.x. [DOI] [Google Scholar]
- 23.Lichtenthaler H.K., Buschmann C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001;1:F4.3.1–F4.3.8. doi: 10.1002/0471142913.faf0403s01. [DOI] [Google Scholar]
- 24.Akbas M.Y., Ozdemir M. Application of gaseous ozone to control populations of Escherichia coli, Bacillus cereus and Bacillus cereus spores in dried figs. Food Microbiol. 2008;25:386–391. doi: 10.1016/j.fm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Youssef K.M., Mokhtar S.M. Effect of Drying Methods on the Antioxidant Capacity, Color and Phytochemicals of Portulaca oleracea L. Leaves. J. Nutr. Food Sci. 2014;4:1–6. doi: 10.4172/2155-9600.1000322. [DOI] [Google Scholar]
- 26.Drlange . Colour Review. Drlange; St. Louis, MO, USA: 1994. Drlange Application Report No.8.0e. [Google Scholar]
- 27.Fernandes F.A.N., Rodrigues S., Law C.L., Mujumdar A.S. Drying of Exotic Tropical Fruits: A Comprehensive Review. Food Bioprocess Technol. 2011;4:163–185. doi: 10.1007/s11947-010-0323-7. [DOI] [Google Scholar]
- 28.Das A., Raychaudhuri U., Chakraborty R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int. J. Food Sci. Nutr. 2012;63:718–721. doi: 10.3109/09637486.2011.644769. [DOI] [PubMed] [Google Scholar]
- 29.Ali A., Ong M.K., Forney C.F. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014;142:19–26. doi: 10.1016/j.foodchem.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Cui Z.-W., Xu S.-Y., Sun D.-W. Effect of Microwave-Vacuum Drying on the Carotenoids Retention of Carrot Slices and Chlorophyll Retention of Chinese Chive Leaves. Dry. Technol. 2004;22:563–575. doi: 10.1081/DRT-120030001. [DOI] [Google Scholar]
- 31.Najafi M.B.H., Khodaparast M.H.H. Efficacy of ozone to reduce microbial populations in date fruits. Food Control. 2009;20:27–30. doi: 10.1016/j.foodcont.2008.01.010. [DOI] [Google Scholar]
- 32.Topuz A., Feng H., Kushad M. The effect of drying method and storage on color characteristics of paprika. LWT—Food Sci. Technol. 2009;42:1667–1673. doi: 10.1016/j.lwt.2009.05.014. [DOI] [Google Scholar]
- 33.Tzortzakis N., Borland A., Singleton I., Barnes J. Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biol. Technol. 2007;.45:317–325. doi: 10.1016/j.postharvbio.2007.03.004. [DOI] [Google Scholar]
- 34.Laing B.M., Schlueter D.L., Labuza T.P. Degradation Kinetics of Ascorbic Acid at high Temperature and Water Activity. J. Food Sci. 1978;43:1440–1443. doi: 10.1111/j.1365-2621.1978.tb02515.x. [DOI] [Google Scholar]
- 35.An-Erl King V., Liu C.-F., Liu Y.-J. Chlorophyll stability in spinach dehydrated by freeze-drying and controlled low-temperature vacuum dehydration. Food Res. Int. 2001;34:167–175. doi: 10.1016/S0963-9969(00)00148-4. [DOI] [Google Scholar]
- 36.Forney C.F. Postharvest Response of Horticultural Products to Ozone. In: Hodges D.M., editor. Postharvest Oxidative Stress in Horticultural Crops. Food Products Press; New York, NY, USA: 2003. pp. 13–54. [Google Scholar]
- 37.Aguayo E., Escalona V.H., Artés F. Effect of cyclic exposure to ozone gas on physicochemical, sensorial and microbial quality of whole and sliced tomatoes. Postharvest Biol. Technol. 2006;39:169–177. doi: 10.1016/j.postharvbio.2005.11.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.