Abstract
Purpose: The role of erectile dysfunction (ED) has recently shown an association with the risk of stroke and coronary heart disease (CHD) via the atherosclerotic pathway. Cardiovascular disease (CVD)/stroke risk has been widely understood with the help of carotid artery disease (CTAD), a surrogate biomarker for CHD. The proposed study emphasizes artificial intelligence-based frameworks such as machine learning (ML) and deep learning (DL) that can accurately predict the severity of CVD/stroke risk using carotid wall arterial imaging in ED patients. Methods: Using the PRISMA model, 231 of the best studies were selected. The proposed study mainly consists of two components: (i) the pathophysiology of ED and its link with coronary artery disease (COAD) and CHD in the ED framework and (ii) the ultrasonic-image morphological changes in the carotid arterial walls by quantifying the wall parameters and the characterization of the wall tissue by adapting the ML/DL-based methods, both for the prediction of the severity of CVD risk. The proposed study analyzes the hypothesis that ML/DL can lead to an accurate and early diagnosis of the CVD/stroke risk in ED patients. Our finding suggests that the routine ED patient practice can be amended for ML/DL-based CVD/stroke risk assessment using carotid wall arterial imaging leading to fast, reliable, and accurate CVD/stroke risk stratification. Summary: We conclude that ML and DL methods are very powerful tools for the characterization of CVD/stroke in patients with varying ED conditions. We anticipate a rapid growth of these tools for early and better CVD/stroke risk management in ED patients.
Keywords: erectile dysfunction, pathophysiology, atherosclerosis, cardiovascular disease, carotid artery disease, carotid ultrasound-based tissue characterization, machine learning, deep learning, risk assessment
1. Introduction
Erectile dysfunction (ED) is a multi-factorial illness that is characterized by the presence of vascular atherosclerosis and hormonal, lifestyle, age, neurological, and physiological factors, all occurring in a well-coordinated manner [1,2]. Among all of the listed characteristics, vascular disease is the most common cause of ED [3]. Testosterone levels, psychological concerns, such as performance anxiety, and iatrogenesis are all the variables that contribute to ED development [4,5]. According to a variety of demographic studies, ED affects up to 150 million men globally [6,7]. As the world’s population ages, the prevalence of ED is expected to climb to 300 million men by 2025 [8,9]. Males aged 18–75 years in Europe had a prevalence of 19%, but men in the same age range in the UK had a prevalence of 39% for life ED and 26% for current ED [8,10,11].
ED has been linked to future cardiovascular events (CVE) in various studies [12,13], showing a high mortality rate due to CVD and stroke. Various studies have shown that ED patients had a considerably higher CVD risk than non-ED patients [14,15,16]. The most prominent risk factors associated with ED and CVD are diabetes, dyslipidemia, hypertension, smoking, and obesity, which lead to the development of oxidative stress, the primary cause of endothelial dysfunction [11,17]. Due to the reduction in endothelium-dependent vasodilation, there have been changes in structural vascular abnormalities, such as increased carotid intima-media thickness (cIMT) and the formation of atherosclerotic plaques [18,19,20].
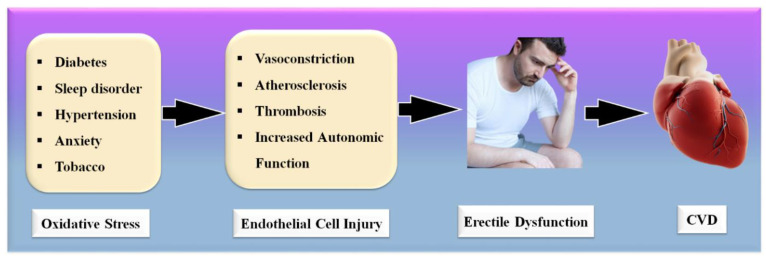
Significantly, the majority of male sexual ED is now recognized to be arterial in origin, with endothelial dysfunction serving as the common link [21,22]. The patient and his spouse are both negatively affected by ED, stressing the need for addressing ED as soon as feasible [23]. Figure 1 indicates the relationship between CVD risk factors and ED. From the above, we conclude that “There is a clear correlation between ED and CVD.” A comprehensive investigation of ED and CVD can be beneficial in the early diagnosis of heart attacks, strokes, and other unfavorable CVE [24,25].
Figure 1.
Relationship between CVD risk factors, ED, and CVD.
Several changes occur as a result of the advancement of ED, including the creation of exudates, bleeding, and other symptoms [26]. These modifications have been implicated in the development of CVD [16]. Patients in the more severe phases of ED have a higher risk of CVD, and once a patient has been diagnosed with a CVD risk, coronary imaging is indicated to stratify the risks [13]. Also essential for visualizing the plaque in COAD, coronary artery imaging (CAI) is vital [27]. Intravascular ultrasonography and coronary angiography are the most frequently used imaging modalities for the visualization of coronary plaque [28,29].
The imaging modalities are costly and difficult to get one’s hands on, especially in underdeveloped nations [30]. As a result, it seems sensible to explore low-cost alternative imaging technologies that can still monitor CTAD in ED patients and risk-stratify them [20,31]. Vascular imaging technologies are useful for the treatment and can save lives before they become life-threatening [19]. Because the carotid artery and the coronary artery have genetically similar compositions, B-mode carotid ultrasonography is a preferred alternative for CTAD imaging of the carotid artery [32]. Image-based phenotypes such as carotid intima-media thickness and carotid total plaque area can be used as CVD surrogates. Further, accurate and automated carotid plaque burden quantification, risk stratification, and early monitoring of atherosclerotic disease in ED patients is therefore required [33].
Artificial intelligence (AI)-based methods have recently played a vital role in computer-aided diagnosis [34,35], especially in the detection and classification of several diseases [36,37]. Machine learning applications in medical imaging have just lately risen to prominence, such as diabetes [38]; the risk stratification of cancer types such as thyroid [39], liver [37,40], prostate [41,42], and ovarian [43]; vascular screening [44]; coronary artery disease risk characterization [45,46]; and surrogate biomarker CTAD imaging and its risk stratification [47,48]. Previously, ML models were developed to predict CVD, as it contains a variety of features from the CVD datasets [49,50,51]. Recently, the DL algorithms have been used to segment the carotid plaque wall thickness [52,53] for CVD risk assessment. As a result, it may be conceivable to use these AI-based solutions to handle CVD and stroke risk stratification in ED patients.
The objective of the proposed review study is to understand (a) the clinical linking between ED and CVD and vice versa, along with the risk factors of CVD in ED patients, and (b) the CVD risk stratification for the severity of heart failure and stroke in ED patients based on AI. One can use the risk factors such as office-based biomarkers (OBBM), laboratory-based biomarkers (LBBM), carotid ultrasound image phenotypes (CUSIP), and medicine usage (MedUSE) combined with ED covariates for designing knowledge-based systems for CVD prediction. Thus, ML and DL solutions can help in establishing the early CVD risk assessment of ED patients who are at a high risk of CVD or ischemic and hemorrhage stroke.
The following is an outline for the proposed review. Section 2 presents the PRISMA model for selecting ED-CVD-based studies. Section 3 presents the evidence of a link between ED and CVD based on the clinical evidence of shared risk factors, while Section 4 explains the biological link between ED and CVD. An AI-based system for a CVD/stroke risk assessment for ED patients is presented in Section. Section 4 presents the recommendations, manifestation, and treatment of ED. A critical 5discussion is presented in Section 5, leading to conclusions in Section 6.
2. Search Strategy
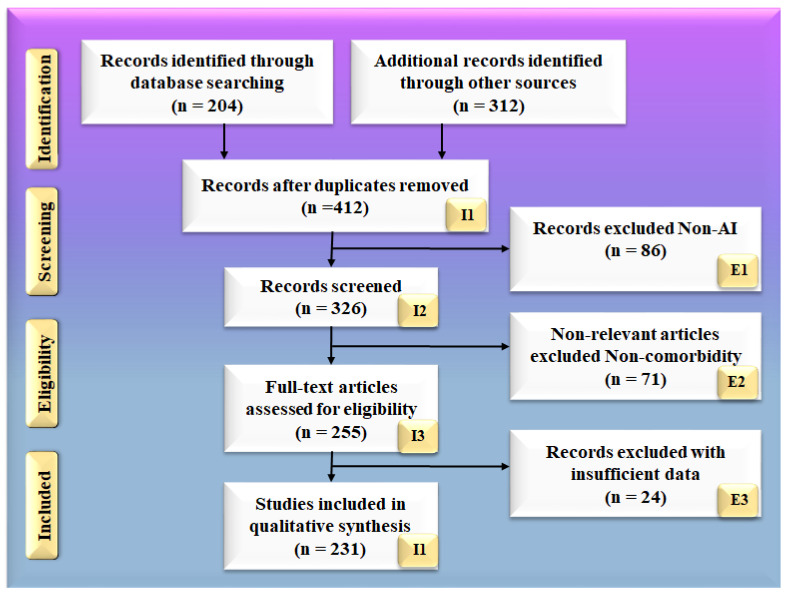
The search approach was based on the PRISMA paradigm, as shown in Figure 2. PubMed and Google Scholar are two major databases that were used to identify and screen relevant papers using keywords such as “cardiovascular disease”, “stroke”, “ED”, “Stroke and CVD”, “Erectile Dysfunction and CVD”, “Erectile Dysfunction and Stroke”, “carotid imaging”, “Erectile Dysfunction and artificial intelligence”, “atherosclerotic tissue classification and characterization in Erectile dysfunction”, “artificial intelligence”, and “Erectile Dysfunction and artificial intelligence”. When searching through the mentioned databases, a total of 204 entries were initially discovered. Furthermore, 312 entries were discovered through additional sources. Following the use of quality custom criteria such as time and relevance, this was reduced to 412 articles. A total of 326 articles were assessed for inclusion in this review, with the majority of them accepted. The three exclusion criteria were as follows: (i) studies that were not connected, (ii) papers that were not relevant, and (iii) research that had inadequate data. This resulted in the exclusion of 86, 71, and 24 studies, denoted by the letters E1, E2, and E3, respectively, resulting in a final selection of 231 studies. These studies, which fall under category (i), are studies that are unrelated to one another. These studies either do not include AI or do not demonstrate risk stratification for CVD/stroke in people with ED.
Figure 2.
PRISMA model for selection of studies.
There were 86 studies that were excluded from the selection process, which are represented by the letter E1 in the PRISMA model. Non-relevant studies are the only ones that do not fall under the umbrella term of ED, CVD, and stroke. They are not concentrating their efforts on the ED–CVD–stroke area. In this study, we are solely interested in studies that discuss the relationship between ED and cardiovascular disease and stroke. (ii) If studies demonstrate a link between ED and diabetes, we will not consider it. There were 71 studies in this category, which is represented by the letter E2 in the PRISMA model. These studies with insufficient data were those that did not provide enough information to be included in our analysis because they did not provide enough information. These studies found no evidence of a relationship between ED and CVD or ED and stroke. There were no attempts to have such conversations. There was no consideration given to the relationship between ED and CVD risk factors, such as LBBM. Furthermore, they did not have adequate AI, CVD, or stroke features from which to choose for analysis, as previously stated. (iii) These AI characteristics may be utilized in the development of an architecture for risk stratification in CVD and stroke. These AI features might be single deep learning (DL) models, hybrid deep learning (HDL) models, or neural network parameters for CVD and stroke risk stratification. We discovered 24 studies with inadequate datasets, represented by the letter E3 in the PRISMA model.
3. Erectile Dysfunction and Cardiovascular Disease Links: Clinical Evidence
The definition of a health risk is “a characteristic or incident that is associated with a higher probability of a certain result, such as the occurrence of a disease.” [54]. The Framingham Heart Study is a major milestone in terms of identifying risk factors for CVD. The FHS’s work has considerably helped preventive medicine. As a result, the focus shifted from treatment to prevention and education [55]. All combined atherosclerotic plaque risk factors should be considered relevant to CVD [56]. Age, gender, a family background of CVD, and ethnicity should be considered as non-modifiable CVD risk factors. Age is an indicator of duration, and it is linked to CVD risk. Age is also the largest indicator affecting cardiovascular outcomes [57,58]. Another well-known CVD risk factor is the male gender. According to the FHS data, women’s CVD mortality is equivalent to that of males 10 years younger [59]. Another well-established, non-modifiable risk factor is a first-degree relative with a history of CVD [60,61]. This link is especially robust in younger people who have a strong family history of premature illness [62,63]. Even though these risk variables are non-modifiable, their identification is important in clinical treatment because it helps in identifying individuals who require more stringent control of modifiable CVD risk factors.
In addition to ischemic heart disease, stroke, and peripheral artery disease, hypertension has been linked to several of the most significant atherosclerotic symptoms, including peripheral artery disease (PAD) [64,65]. In the normal BP range (>115/75 mmHg), there is no solid evidence of a risk threshold for CVD [65]. This link has been seen in people of all ages, and it appears to be greater for systolic BP than diastolic BP [66,67]. Stroke and heart disease fatalities increase more than multiple times for those aged 40–69 years who have an increase in their blood pressure of 20 or 10 mm Hg [65].
Diabetes mellitus (DM) doubles or triples the risk of myocardial infarction or stroke, as well as the risk of CVD mortality [68,69]. This risk rises in proportion to the degree of glycemic change [70]. Intermediate carbohydrate metabolic anomalies have also been linked to a higher CVD risk [71,72]. In contrast to diabetes, diabetic people have a higher risk of CVD due to the existence of additional metabolic abnormalities [73].
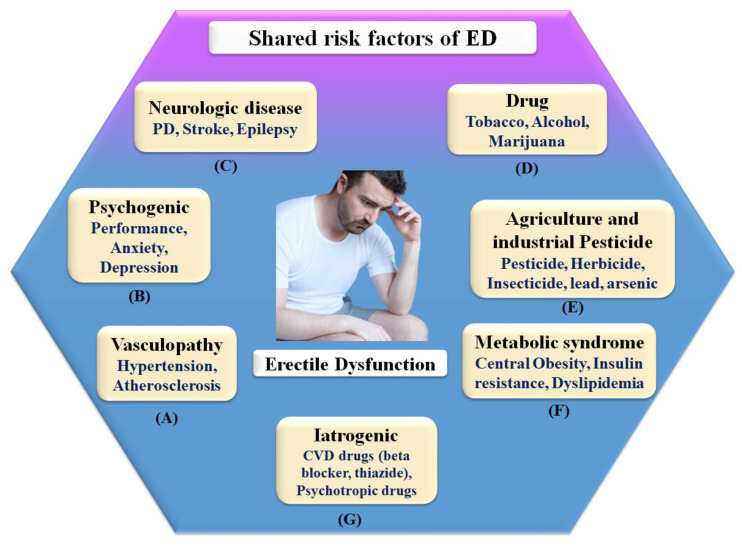
ED is generally referred to as a vascular disease, and it is generally known that it shares several health risks with CVD, including obesity [32,74], chronic renal disease [75], poor socioeconomic status [58], low fruit and vegetable consumption [76], inadequate physical activity [77], metabolic syndrome [78,79], and elevated C-reactive protein levels [80], which are all well-known risk factors for CVD. In this context, a large prospective study evaluating the effect of CVD risk variables on ED over 25 years showed that age, BMI, cholesterol, and triglycerides were all highly associated with ED [79]. Smoking, BMI, hypertension, cholesterol dietary consumption, and unsaturated fat intake have all been linked to an increased risk of ED [78,81]. Figure 3 indicates the shared risk factors of ED.
Figure 3.
Shared risk factors of ED.
Therefore, in connection, ED affects around 75% of diabetes patients over the age of 60 and grows proportionately with the severity of the condition [82]. It is possible that ED and penile atherosclerosis are the common denominators between ED and diabetes [83]. However, the link between these two clinical diseases is complex, and additional pathophysiologic processes, such as autonomic neuropathy and hormonal abnormalities, may be involved in the development of these two clinical conditions [22,84].
3.1. The Pathophysiologic Link between ED and CVD
The pathophysiology of ED is dependent on the integrity of the endothelium [85,86]. Sexual drive induces the production of NO and other endothelial mediators, resulting in stimulating sympathetic stimulation in the veins feeding penile regions and an enhanced blood flow to the penis while blocking the vein discharge [86,87]. These occurrences cause blood to be trapped within the corpora cavernosa. This increase leads to system pressure and an erection [88]. The carotid arteries hypothesized that ED and COAD have the same involvement in the pathogenesis pathway [89]. ED and circulation stenosis may result from exposure to known risk factors. Due to the systematic character of atherosclerosis, all arterial pathways may be harmed to the same amount, but the onset of signs is linked to arterial size [9,90]. Increased vascular tolerance for the same amount of endothelial dysfunction and/or atherosclerotic burden is observed in bigger vessels when compared to smaller arteries [91]. Alongside the more compact ones, penile veins are smaller than other veins in the body [92]. Compared to coronary arteries, they are tiny, (1–2 mm) to (3–4 mm), with endothelial dysfunction at the very same level, and atherosclerosis may cause a greater decline in blood flow [9].
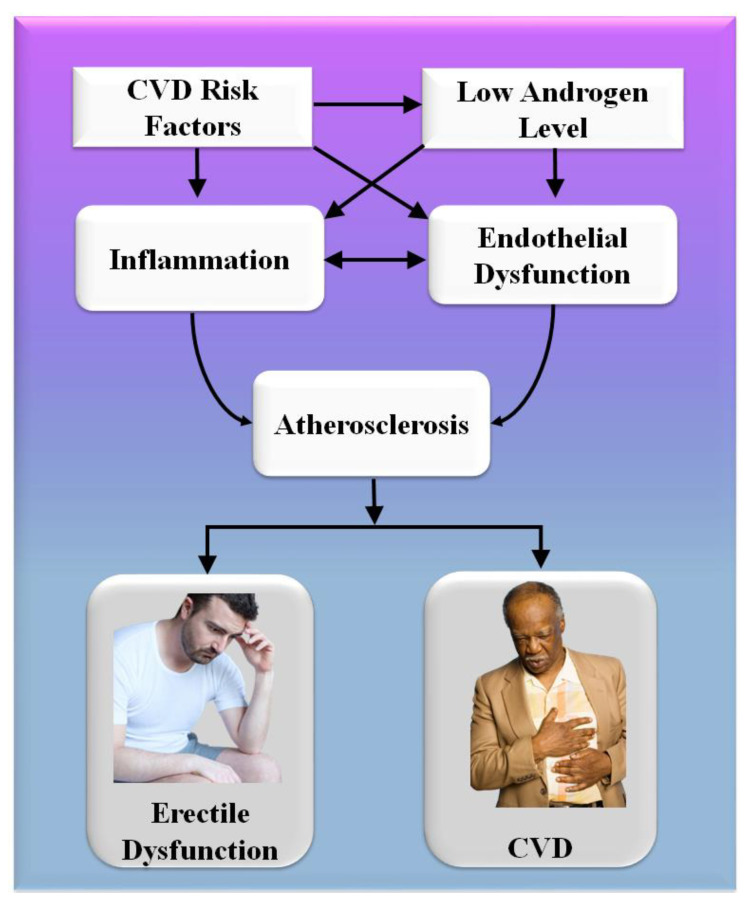
Consequently, the vascular system of the penile organ may serve as an early warning system for a wide range of vascular conditions [93]. Individuals with chronic coronary syndromes (CCS) are more likely to have ED than those without CCS, according to this hypothesis. In this respect, Montorsi et al. [3] explained that for patients with chronic coronary syndrome, ED is common before CAD symptoms appear. Most patients with CCS begin to have sexual dysfunction three years before any cardiac symptoms appear. This contrasts with the rarity of sexual dysfunction in those suffering from acute coronary syndrome [3]. Appropriate arterial penile lesions were found in only 12.9% of the cases, compared to a high frequency of 87% in the coronary system and 77% in the internal iliac artery area [94]. Figure 4 shows the CVD risk factors linked with inflammation, androgen, and endothelial dysfunction.
Figure 4.
CVD risk factors are linked with inflammation, androgen, and endothelial dysfunction.
A comprehensive reformulation of all available evidence revealed that, while the artery-size theory is crucial to understanding the complicated relationship between ED and COAD, vasculogenic ED is also connected with dynamic, macroscopically intangible irregularities linked to endothelial dysfunction and neurogenic hyperactivity [42]. The usual indications of cardiovascular problems are quite often disguised in diabetics, causing a diagnostic lag of COAD and difficulty in altering the disease’s natural history [95]. In diabetes patients, an individual relationship between ED and asymptomatic COAD has indeed been described [96,97]. Endothelial functioning is affected by low-grade inflammatory cytokines, which can lead to a thrombogenic state [98]. Several studies have linked the development and intensity of ED to the elevated expression of inflammation biomarkers [44,45,46,47]. The major targets for androgen actions inside the penile and cardiovascular pathways are endothelium and sleek cells, and congenital hypothyroidism is associated with an increased risk of arteriosclerotic remodeling [99,100].
As a result, it is found that people who have ED and risk factors for cardiovascular disease are more likely to have a “silent COAD.” They should get a full CVD examination.
Mechanism of Penile Erection
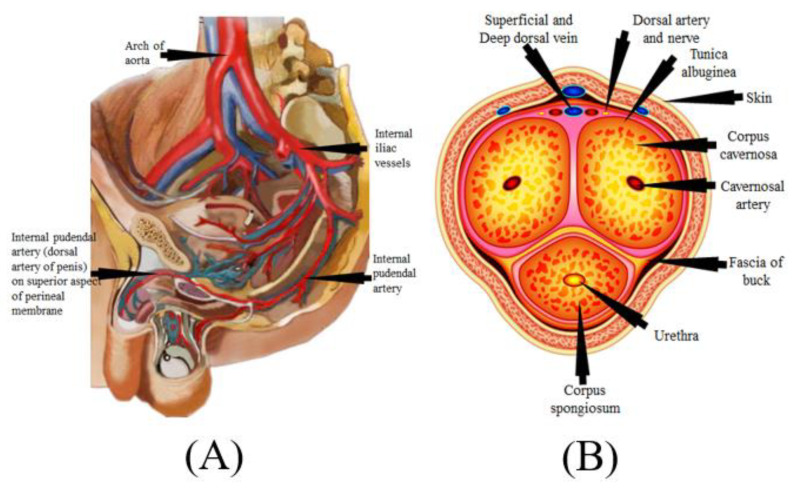
The mechanism of the male penile erection, as well as cross-section, is shown in Figure 5A,B, where the aorta is directly connected to the penal artery. A significant blood input is essential for successful sexual performance [101,102]. As previously stated, normal penile erection is a neurovascular event that causes sexual stimulation and the release of NO hormones from endothelial cells [103,104].
Figure 5.
(A) Mechanics of penile erection (courtesy of AtheropointTM, Roseville, CA, USA). (B) Cross-sectional of the penis (courtesy of AtheropointTM, Roseville, CA, USA).
As a result, strong blood flow from the heart to the penal muscle cells is required for a proper erection [105,106]. All these processes cause blood to be caught inside the corpora cavernosa (Figure 5B), resulting in intracavernous pressure and an erection [107].
3.2. The Effect of SARS-CoV-19 on Erectile Dysfunction
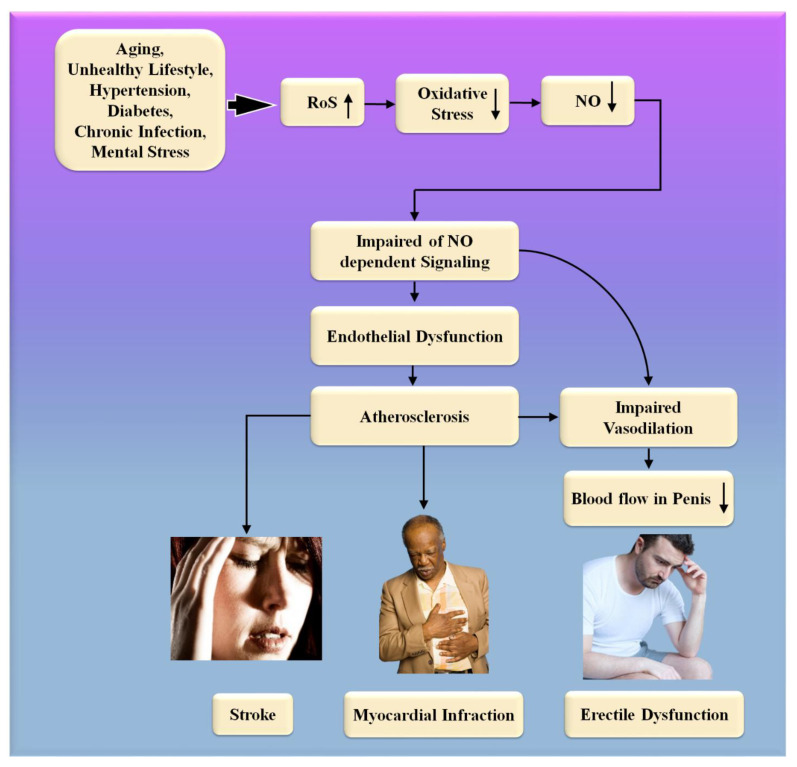
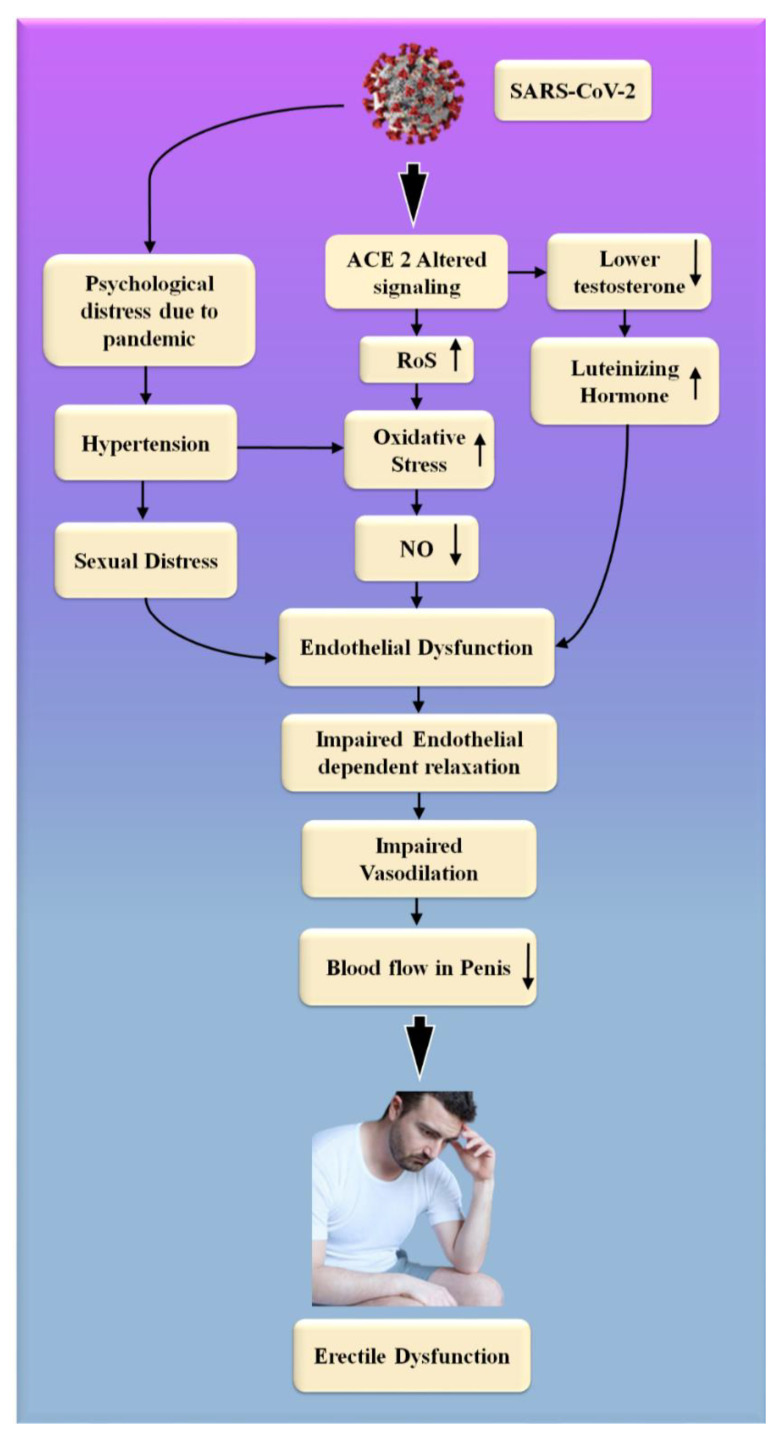
SARS-CoV-2, the interaction of the enhanced ACE2 and the transmembrane protease serine 2 with a component of the spike protein, accelerates binding and transit into vascular endothelium cells [108]. According to the studies, endothelial dysfunction is a significant contributor to COVID-19 symptoms [109,110]. The Table 1 show the relationship between ED with CVD or coronary artery disease. Direct viral invasion of testicular tissue via ACE2 receptors, temperature-related testicular injury resulting from sustained high fever, inflammatory and autoimmune responses, and viral infection-related oxidative stress are some of the suggested causes of this damage [111,112]. Figure 6 explains the biological link between ED and CVD/Stroke and Figure 7 validates the biological link between SARS-CoV-19 with ED.
Table 1.
The studies show the relationship between ED with CVD or coronary artery disease.
| SN | Citations | Relation * | ME | PS | Outcome | Treatment |
|---|---|---|---|---|---|---|
| 1 | Bonetti et al. [113] (2002) |
ED with CVD | LBBM | 45 | ED is a systemic disease that contributes significantly to the advancement of atherosclerosis and its associated complications. There is a need for direct evidence that therapeutic improvements in endothelial function resulted in decreased CVE rates. | NR |
| 2 | Montorsi et al. [9] (2005) |
ED with CAD | LBBM | 34 | Because of the progressive or simultaneous alterations in microvascular and macrovascular function, ED is fundamentally an atherosclerotic disorder in its origin and progression. | NR |
| 3 | Kirby et al. [16] (2005) |
ED with CAD | OBBM | NR | ED and COAD are two distinct clinical manifestations of the same systemic illness, with pathological causes and risk factors that are quite similar to one another. Because of increased understanding of the emergency department as a barometer for cardiovascular health, it is possible to take early action to reduce future CV risk. | NR |
| 4 | Vlachopoulos et al. [114] (2007) |
ED with CAD | LBBM | NR | ED, inflammation, and low testosterone levels in the bloodstream are all risk factors and pathophysiological links that are shared by cardiovascular disease and erectile dysfunction. | NR |
| 5 | Diaconu et al. [115] (2011) |
ED with CVD | OBBM, LBBM | 231 | Both erectile dysfunction and CVD are symptoms of the same illness. ED symptoms often appear three to five years before the onset of symptoms of coronary artery disease, and they may serve as an early warning indication that CVD is on the verge of manifesting itself. As a result, male patients with CVD risk factors should have their erectile dysfunction checked regularly. | phosphodiesterase-5 inhibitors, alprostadil (prostaglandin E1) intracavernous injections, alternatives for the management of ED. |
| 6 | Yannas et al. [54] (2011) |
ED with CVD | OBBM, LBBM | NR | ED is a sign of cardiovascular disease. As a result, guys with ED should be thoroughly evaluated for cardiovascular risk factors to avoid future CVE (MACE). | NR |
| 7 | Gandaglia et al. [82] (2014) | ED With CVD | LBBM | NR | ED and cardiovascular disease (CVD) are two symptoms of the same systemic illness. Atherosclerosis and blood vessel constriction are caused by the interplay of CV risk factors, androgens, and chronic inflammation in the blood vessels. Endothelial dysfunction and autonomic hyperactivity, for example, are both isotropic alterations in the body. | NR |
| 8 | Lim et al. [13] (2018) |
ED with CVD | OBBM, LBBM | 1757 | Distinguishing between symptoms of ED and cardiovascular disease (CVD) demands a distinct strategy. Atherosclerosis and vascular constriction are associated with each other, and this association is generated by the combination of CV risk factors, androgens, and chronic inflammation. Atherosclerosis and autonomic hyperactivity are both apparent alterations that are isotropic. | NR |
| 9 | Roushias et al. [116] (2018) | ED with CVD | OBBM, LBBM | 1768 | Endothelial dysfunction is a common denominator in the pathophysiology of both erectile dysfunction and cardiovascular disease. ED is a warning symptom of endothelial dysfunction and a risk factor for cardiovascular disease. Early detection and assessment of ED redefines the risk of cardiovascular disease and allows for earlier intervention. Patients with cardiovascular disease should be treated and monitored more closely if they develop erectile dysfunction. | NR |
| 10 | Miner et al. [117] (2019) |
ED with COAD | LBBM | 242 | Angiographic studies show that ED patients under the age of 60 had more severe COAD. This connection is independent of COAD and ED risk factors. | NR |
| 11 | Sayadi et al. [118] (2021) |
ED with COAD | OBBM | 100 | COAD is an indicator of atherosclerosis. As a result, the IIEF questionnaire can help diagnose COAD early on. | NR |
| 12 | Kałka et al. [119] (2021) |
ED with COAD | OBBM, LBBM | 751 | Sexual health concerns are crucial in cardiac patients. ED predicts CVD due to shared risk factors and pathophysiology. Hypertension, dyslipidemia, smoking, diabetes, obesity, and a poor diet all contribute to vascular endothelium dysfunction. | NR |
| 13 | Inman et al. [120] (2021) |
ED with COAD | LBBM | 1402 | ED and CAD may be signs of the same vascular illness. In young men, ED increases the risk of future cardiac incidents, but in older men, it appears to have little predictive value. | NR |
| 14 | Imprialos et al. [121] (2021) | ED with CVD | LBBM | NR | Erectile dysfunction is a major health condition that affects many people, and it is more common in people with cardiovascular risk factors or illnesses. Both ED and CVD share pathophysiological pathways. | Patients with or without cardiovascular illness can use phosphodiesterase type 5 inhibitors as first-line ED treatment. |
| 15 | Rinkūnienė et al. [122] (2021) | ED with CVD | LBBM | 171 | ED is common in guys who have had a MI. Men with a history of MI had greater traditional CVD risk factors. Men with ED who have had a MI are more prone to AH. | NR |
* SN: serial number, RELATION: effect of ED on CVD, ME: method of evaluation, PS: patient size, OE: outcome, TE: treatment, NR: not reported, MI: myocardial interaction, OBBM: office-based biomarker, LBBM: lab-based biomarker, NR: not reported.
Figure 6.
The biological link between ED and CVD/Stroke. RoS: reactive oxides stress, NO: nitric oxide, Up Arrow: depicts increase, Down Arrow: depicts decrease.
Figure 7.
The biological link between COVID-19 and ED. RoS: reactive oxides stress, NO: nitric oxide, Up Arrow: depicts increase, Down Arrow: depicts decrease.
Endothelial cells infected with SARS-CoV-2 suffer endothelial damage, which causes thromboembolic vascular lumen alteration in the endothelium, immune thrombosis, and reversal in many organs [123]. These are the ultimate and noticeable consequences of the cells taken by SARS-CoV-2 from the endothelium [124]. ED is one of the most common symptoms of COVID-19, which is caused by endothelial dysfunction [123]. This can result in circulatory problems in numerous organs [109,110]. This includes a reduction in blood supply to the testicles, which can lead to ED. Natural nitric oxide (NO), generated by healthy endothelial cells, is an essential cofactor in the endothelium-dependent phase transition in the corpora cavernosa [125]. Endothelial dysfunction is caused by a decrease in eNOS expression, which results in a decrease in NO production [126,127]. Increased endothelium-bound cavernosal tissue vasodilation is associated with hypertension and diabetes [128].
People were experiencing psychological trauma, as well as the overall feeling of a high degree of uncertainty associated with the COVID-19 global epidemic [129]. The restrictive measures that were implemented during this critical period, in the long term, influenced interpersonal and intimate relationships [130]. Concerns about safe intimate/sexual interplay, the forced separation of intimate partners, the escalation of marital disputes, and degradation in contact are some of the most significant contributors to a person’s experience of sexual troubles and sexual unhappiness at this age [130,131,132].
Sexual desire and expression differences, as well as a lack of privacy while confined, have both been linked to the development of sexual difficulties and dissatisfaction [133,134]. COVID-19 infection, on the other hand, has the potential to negatively impact male sexual function by inducing endothelial damage, which can result in erectile dysfunction, testicular injury, and psychological alterations [134,135].
We hypothesized that erectile dysfunction occurs more frequently in the presence of heart issues when the endothelium and smooth muscle are dysfunctional. Endothelial dysfunction impairs blood flow to the heart and the penis, contributing to the development of atherosclerosis.
4. Artificial Intelligence-Based System for CVD/Stroke Risk Assessment in ED Patients
Machine learning is a powerful framework because it uses a knowledge-based model to create a training system. Several ML-based applications have been developed in healthcare, spanning subfields of medicine, such as diabetes [38,136,137], neonatology [138], gene analysis [139,140], COAD risk stratification [141,142], EEG-based signal classification [143,144], and CTAD symptomatic vs. asymptomatic plaque classification [145,146,147]. When it comes to risk stratification, ML-based strategies have also dominated cancer imaging paradigms, such as thyroid [148,149,150], breast [151], ovarian [41,152], prostate [153], liver [154,155], and other forms of cancer, such as skin [148,149,150,156,157].
The ability of ML to adjust the non-linearity between a set of risk factors (or covariates) and the gold standard is the second major benefit of ML. Such evidence in the context of CVD risk assessment has recently been introduced [28,158,159,160,161]. These risk factors include (i) conventional office-based, (ii) laboratory-based covariates, and (iii) current drug consumption, while the gold standard criteria are heart failure or stroke. In the CVD/stroke risk paradigm, the inclusion of ED covariates can add value to the CVD risk stratification in ED patients.
4.1. Machine and Deep Learning Framework for CVD Risk Assessment in ED Patients
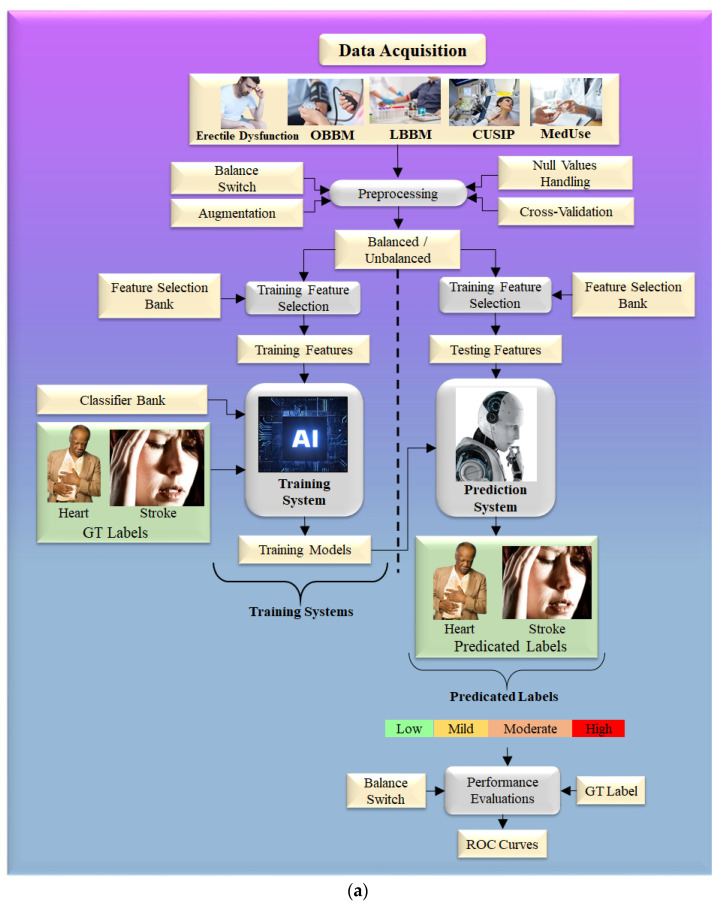
The typical variables included the combination of OBBM, LBBM, CUSIP, and MedUSE [67]. For cost reasons, non-invasive protocols for carotid arteries [162] under minimal noise conditions such as harmonic and compound imaging [47,163] are favored for atherosclerosis imaging. The identification of plaque build-up is aided by automated carotid far-wall segmentation [164,165]. Figure 8a shows the step-by-step approach for risk stratification for CVD and stroke risk stratification in ED patients using the AI framework [166]. On the left-hand side is shown the extraction of features using the training dataset, which is then used for model generation using the conventional classifier, given the gold standard. On the right-hand side of Figure 8a is shown the CVD/stroke risk prediction by transforming the testing features based on the training model. Because the input gold standard consists of multiple risk classes of coronary artery disease, the predicted CVD/stroke risk will also be a CVD/stroke granular risk.
Figure 8.
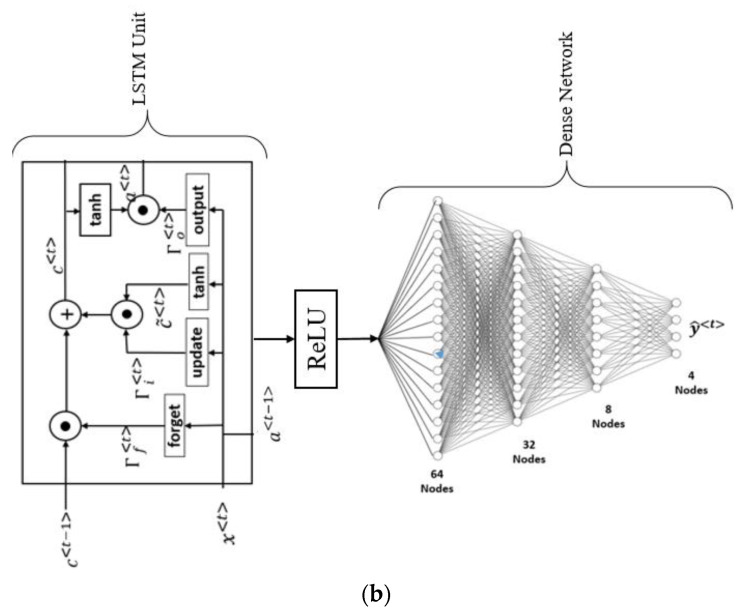
(a). Machine learning model to predict the severity of CVD and stroke in ED framework. (b). The general structure of LSTM architecture.
One can also use deep learning strategies such as LSTM for CVD risk assessment. The main feature of LSTM is the ability to process multiple types of data points, such as a single (image). The main component of the LSTM architecture is a cell, an update gate, an output gate, and a forget gate (Figure 8b). During random intervals, the cell stores the values, and the three gates control the flow of information or features into and out of the cell [167]. LSTM took the place of the recurrent neural network (RNN), which can address the limitation of the RNN (i.e., simple RNN associated with TensorFlow). LSTM is better at formulating long-term dependencies in the data [168]. The LSTM architecture is displayed in Figure 8b, where the LSMT unit has four fully connected dense layers stacked together. The structural configuration of LSTM is similar to an RNN and well suits for CVD risk stratification in ED patients [169,170]. Even though ML is a powerful paradigm, it requires the features to be manually optimized, unlike in DL, where the features are automatically optimized.
4.2. Participating in Studies for CVD Risk Assessment Using AI
Table 2 and Table 3 show six different independent studies for (a) CVD risk prediction and (b) ED prediction, both using the AI framework. There were several different types of ground truth employed in these CVD risk prediction studies, including death, stroke, CHD, and CVD [171,172]. The risk factors that were used were OBBM, LBBM, and CUSIP derived from carotid US scans which are marked as input covariates (IC) in Table 2 and Table 3. As a result, support vector machines (SVMs) were used for the classification, along with logistic regression, a convolution neural network (CNN), an artificial neural network (ANN), a random forest algorithm (RF), and a principal component analysis (PCA). Table 2 and Table 3 contain more information on the characteristics of this classification technique.
Table 2.
Generalized studies for prediction of CVD in AI framework using input covariates.
| SN | Citations | IC | DS | GT | FE | TOC | ML vs. DL | ACC | AUC |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Gorek et al. [176] (1997) | OBBM, LBBM | 30 | Diagnose ED | NR | CNN | DL | 80.79 | 0.80 |
| 2 | Kellner et al. [177] (2000) | OBBM, LBBM | 100 | Diagnose ED | NR | CNN | DL | 72.79 | NA |
| 3 | Glavaš et al. [178] (2015) | OBBM, LBBM | 185 | Diagnose ED | NR | LR, SVN, ANN | ML | 74.40 | 0.812 |
| 4 | Chen et al. [179] (2019) | LBBM | 5664 | Predict ED | NR | LR, ANN, SVM, RF | HDL | 76.65 | 0.817 |
| 5 | Lingli et al. [180] (2018) | OBBM, LBBM | 95 | Diagnose ED | DT | SVM | ML | 96.7 | NR |
| 6 | Jang et al. [181] (2019) | OBBM, LBBM | 187 | ED drugs therapy | NR | ANN | DL | 100.00 | NR |
SN: serial number, IC: input covariates, DS: data size, GT: ground truth, OBBM: office-based biomarker, LBBM: laboratory-base biomarker, FE: feature extraction, TOC: type of classifier, ACC (%): percentage accuracy, US: ultrasound, NR: not reported.
Table 3.
Studies for ED prediction using the AI framework.
| SN | Citations | IC | DS | GT | Classifier | TOC | ML/DL | ACC % | AUC |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Biswas et al. [182] (2018) | OBBM, LBBM (US) | 407 | Stroke, Diabetes | NR | CNN | DL | 99.61 | 0.99 |
| 2 | Jamthikar et al. [158] (2019) | OBBM, LBBM (US) | 395 | CVD | PCA | RF | ML | 95.00 | 0.80 |
| 3 | Kandha et al. [183] (2020) | OBBM, LBBM | 346 | Death | CNN | NB, SVM, KNN, DT | DL | 83.33 | 0.833 |
| 4 | Jamthikar et al. [160] (2020) | OBBM, LBBM, CUSIP | 202 | CVD | SVM | LR, SVN, ANN |
ML | 92.53 | 0.92 |
| 5 | Saba et al. [184] (2020) | OBBM, LBBM, CUSIP | 246 | Death | 6 Models | SVM | HDL | 89.00 | 0.898 |
SN: serial number, IC: input covariates, DS: data size, GT: ground truth, OBBM: office-based biomarker, LBBM: laboratory-based biomarker, FE: feature extraction, TOC: type of classifier, ACC: percentage accuracy, US: ultrasound, NR: not reported.
Atherosclerosis is a systemic inflammatory disease. The plaque in the coronary artery mirrors that in the carotid artery, especially in the bulb or bifurcation area [173]. Numerous studies have shown cholesterol, fibrosis, fibrin, and calcium in both coronary and peripheral arteries [141]. Several studies have found a strong link between carotid artery plaque measures and the risk of COAD and CVD [56,174,175].
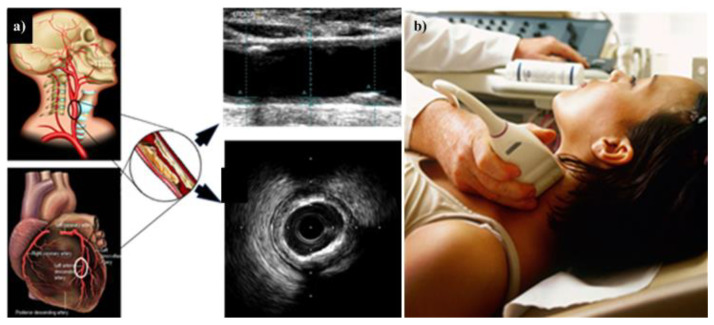
Multiple modalities have been used for imaging the carotid artery. A US is considered more user-friendly, convenient, and cost-effective than an MRI [185]. Figure 9a,b show how the carotid B-mode ultrasound acquisition system can be applied to ED patients [186].
Figure 9.
(a) The CTAD is being investigated as a potential surrogate marker for COAD. (b) Imaging device where the carotid artery is being scanned with the linear ultrasound probe. The middle shows the B-mode carotid longitudinal US scan and IVUS-based coronary artery cross-sectional scan (produced with permission by AtheropointTM, Roseville, CA, USA).
The Carotid IMT and carotid plaque area have been shown in clinical trials [187] to be effective surrogate measures for coronary vascular disease. Additionally, in [188], the authors employed the cIMT on the carotid and coronary arteries in concert with an ultrasound framework. The authors in [189] demonstrated the maximum plaque height (MPH) as a risk factor for COAD. Additionally, the authors in [190,191] demonstrated how the carotid bulb may be used to estimate the risk of COAD.
In a comprehensive risk assessment, we need to be able to automatically and precisely quantify the CUSIP consisting of carotid intima-media thickness, ave., max., and min (cIMTave, cIMTmax, cIMTmin), carotid intima-media thickness variability (cIMTV), morphological total plaque area (mTPA), geometric total plaque area (gTPA), lumen diameter (LD), inter-adventitia diameter (IAD) [184], and composite risk score (CRS) [192]. We require a risk assessment system that can determine the severity of COAD in ED patients. All ED investigations found an increase in cardiovascular illness, which is linked to an increase in phenotypes, such as cIMT, gTPA, mTPA, and CRS [184]. This CUSIP is then used as a covariate in the ML system (Figure 9).
4.3. Plaque Tissue Characterization Using Machine Learning/Deep Learning Paradigms
The presence of bad LDL deposits in the bulb over time due to ED raises plaque load, generating wall sheer stress (WSS) in the artery walls, which can lead to plaque rupture [193,194]. Low-intensity asymptomatic plaques are difficult to detect and can rupture, resulting in death [195,196]. However, they cannot be seen with bare eyes, so we need to find a technique that can characterize the plaque. Bright plaques are simple to detect and identify, although calcium deposits can be deceiving [197,198]. It is quite difficult for ultrasound technicians or radiologists to make rapid judgments on plaque lesion characterization due to the time constraint [199,200]. As a result, there is a strong relationship between COAD and CTAD, and it is simple to obtain image phenotypes using a low-cost, non-invasive B-mode carotid longitudinal US scan.
Endothelium, the inner connecting of the arterial wall, and smooth muscle cells are damaged by ED, resulting in damage to the arterial walls of the coronary artery, causing cardiovascular problems [201]. As a result, normal plaque becomes vulnerable or dangerous plaque over time [202]. Due to this, ED can be an important indicator for symptomatic plaque. Furthermore, plaque growth is a multi-focal illness [203]. It does not occur at a single location in space. As a result, the illness spreads intermittently all over the artery’s sidewalls [204]. ML has been used to identify symptomatic plaque for stroke risk stratification, labeled as AtheromaticTM 1.0 (AtheroPoint LLC, Roseville, CA, USA) [157,182].
4.3.1. PTC Using Machine Learning
To identify the severity of CVD risk in mild ED vs. severe ED patients, ML and DL methodologies for carotid plaque tissue characterization (PTC) approaches are required [182,205]. In the clinical imaging area, popular classifiers such as random forest (RF), support vector machine (SVM), decision tree (DT), and AdaBoost have been commonly implemented. The PTC can serve diagnostic and therapeutic requirements while cutting costs because of advancements in US technology. Saba et al. [206] utilized a polling-based PCA approach in an ML framework to choose dominating characteristics for better performance. International cardiologists mostly use ML for CHD risk stratification before stenting and percutaneous coronary intervention treatments [207]. For CVD risk assessment, this study used a technique that combined intravascular ultrasonography (IVUS) greyscale plaque morphology and cIMT.
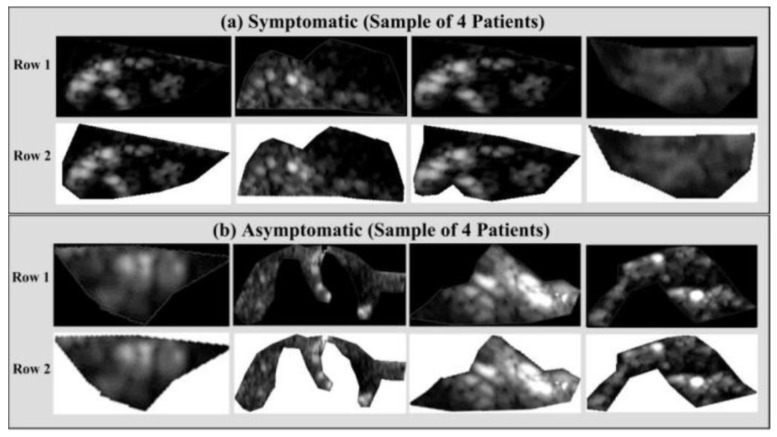
Vascular radiologists can promptly diagnose a patient by using the automated characterization of the symptomatic and asymptomatic plaque from US pictures. Acharya et al. [47] used 346 images of US plaques, and out of that, 196 were symptomatic and 150 asymptomatic. Figure 10a,b illustrate two instances of symptomatic (a) and asymptomatic plaque (b). To extract the features, the photos were pre-processed to eliminate noise, and discrete wavelet transform (DWT) was used.
Figure 10.
Delineated plaque in the B-mode US. (a) Symptomatic plaque and (b) asymptomatic plaque (produced with permission by AtheropointTM, Roseville, CA, USA).
A variety of studies have been conducted in the ML framework to predict the risk of CTAD and COAD [147,184]. Additionally, ML was used to identify individuals with COAD by analyzing the greyscale characteristics of left ventricular ultrasound data [208]. Recently, a deep learning-based technique for predicting the risk of COAD was developed utilizing the carotid artery as a gold standard [183,209,210].
4.3.2. PTC Using Deep Learning
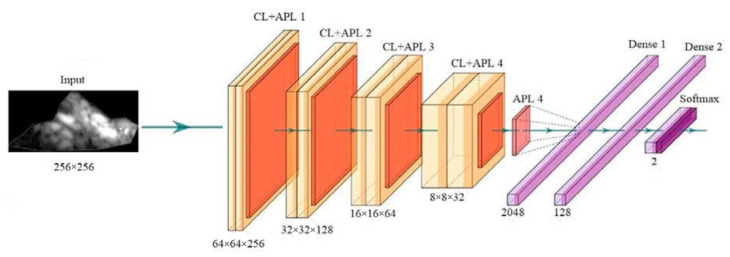
With the help of deep learning, PTC can also be used to predict stroke risk. This strategy can be used to predict coronary risk if the gold standard is taken from the coronary artery. Figure 11 shows a convolution neural network-based deep learning used for enhancing the features or extracting useful information from the input of either images or signals. The feature extraction can be performed in two forms, namely 1D or 2D. The main characteristics of the CNN technology are max pooling, convolution, non-linearity, and classification [211].
Figure 11.
The general structure of CNN architecture (produced with permission by AtheroPointTM, Roseville, CA, USA) [211,212].
A thorough review of various studies reveals that ED patients have a higher risk of CVD. Our observations on the hypothesis showed that “ED has a relationship with CVD/stroke and holds via the vascular atherosclerotic pathway”. We further investigated such a setup in the COVID-19 paradigm. As a result, a low-cost B-mode carotid longitudinal US scan could be used for CVD screening in ED patients to prevent the CVD symptoms from worsening to a cardiovascular event or cerebrovascular event.
4.3.3. Recommendations for ED Patients
With the help of an AI-based non-invasive model, these patients may be successfully monitored, and long-term CVD effects can be prevented. We showed how ML and DL can be integrated for CVD/stroke risk stratification with better sensitivity and specificity for ED patients. Such a strategy will improve better statin control for monitoring the CVD/stroke risk. This can be further customized and personalized for individual patients, which is unique and valuable in today’s healthcare systems. This AI model may be used by physicians to advise ED patients by giving further information on CVD and stroke risk.
4.3.4. Manifestation
ED treatment has changed dramatically since the discovery of sildenafil, a phosphodiesterase type 5 inhibitor, which has enabled many more men to seek assistance [213,214]. Three- and five-cyclic guanosine monophosphate, a second messenger for the relaxing effects of nitric oxide on smooth muscle, is inhibited by phosphodiesterase type 5 inhibitors, which have been shown to be effective in clinical trials [215,216]. As a result of sexual stimulation, endothelial cells and nonadrenergic, noncholinergic neurons release NO, which aids in the relaxation of the trabecular erectile tissues as well as dilation of the helicine artery of the penis by increasing the formation of cyclic guanosine monophosphate [217,218]. In response to the increased blood flow, the sinusoidal gaps of the corpora cavernosa grow swollen and suffocate with blood. Because of the engorgement of the tunica albuginea, the subtunical venules that drain the corpora are compressed, resulting in a decreased venous outflow from the penis [219]. As a result, the penile blood pressure rises, leading to the development of a physiological erection.
As an oral erectile dysfunction medication, Tadalafil is a potent selective phosphodiesterase type 5 inhibitor that is currently being researched and developed [217]. Back discomfort, nasal congestion, myalgia, and flushing are among the most commonly reported treatment-related adverse effects of tadalafil. When taken as needed before sexual activity and with no restrictions on food or drink, the pharmaceutical tadalafil significantly improved erectile function, according to the study. It was successful in restoring normal erection function to a large number of people [220].
5. Critical Discussions
5.1. Principal Findings
We found that ED occurs more frequently in the presence of heart issues when the endothelium and smooth muscle are dysfunctional. Endothelial dysfunction impairs blood flow to the heart and the penis, contributing to the development of atherosclerosis. SARS-CoV-2-infected endothelial cells suffer endothelial damage that results in thromboembolic vascular lumen modification in the endothelium. Moreover, we obtain strong evidence that smoking, BMI, hypertension, cholesterol dietary consumption, and unsaturated fat intake have all been linked to an increased risk of ED. It is feasible to use an AI-based system for the CVD and stroke risk stratification to find the severity of heart failure and stroke in ED patients.
5.2. Benchmarking
Following the analysis of various studies, we discovered a few research studies that examined the link between ED with CVD utilizing OBBM, LBBM, and MedUSE. Only a few papers discuss the significance of AI in the diagnosis of CVD and ED independently. Despite the proposed study, no other study uses the AI model to describe the severity of CVD in the ED framework. Table 4 shows the benchmarking analysis of several studies.
Table 4.
Comparative analysis of studies with CVD and stroke risk stratification in ED patients.
| SN | Citations | Year | Covariates | CVD | Stroke | ED | AI |
|---|---|---|---|---|---|---|---|
| 1 | Bonetti et al. [113] | 2002 | OBBM, LBBM | ✗ | ✓ | ✓ | ✗ |
| 2 | Montorsi et al. [9] | 2005 | OBBM, LBBM | ✓ | ✗ | ✓ | ✗ |
| 3 | Diaconu et al. [115] | 2011 | OBBM, LBBM | ✗ | ✓ | ✓ | ✗ |
| 4 | Gandaglia et al. [82] | 2014 | OBBM, LBBM | ✓ | ✗ | ✓ | ✗ |
| 5 | Miner et al. [117] | 2019 | OBBM, LBBM | ✗ | ✓ | ✓ | ✗ |
| 6 | Mouridsen et al. [221] | 2020 | OBBM, LBBM | ✗ | ✗ | ✓ | ✓ |
| 7 | Jamthikar et al. [30] | 2020 | OBBM, LBBM | ✓ | ✗ | ✗ | ✓ |
| 8 | Bikias et al. [222] | 2021 | LBBM | ✓ | ✗ | ✗ | ✗ |
| 9 | Reva et al. [223] | 2021 | OBBM, LBBM | ✓ | ✗ | ✗ | ✓ |
| 10 | Bermejo et al. [224] | 2021 | OBBM, LBBM | ✓ | ✓ | ✗ | ✗ |
| 11 | Proposed Study | 2022 | OBBM, LBBM, CUSIP | ✗ | ✓ | ✓ | ✗ |
ED: erectile dysfunction, CVD: cardiovascular disease, AI: artificial intelligence, OBBM: office-based, LBBM: laboratory-based, CUSIP: carotid ultrasound image phenotype, ✓: yes, ✗: no.
Bonetti et al. [113] explained the role of ED as a systemic disorder that plays an important role in the progression of atherosclerosis and its consequences. Growing data reveal that endothelial function is not only determined by the properties of currently recognized cardiovascular risk factors. Endothelial integrity, on the other hand, is based on the balance of all cardiovascular risk factors and vasculoprotective aspects in a specific person, including unknown variables and hereditary susceptibility. Endothelial dysfunction can be used as a predictor of an individual’s atherosclerosis risk. In support of this idea, endothelial dysfunction in the coronary or peripheral circulation has been proven to be a lone indicator of a poor cardiovascular outcome, offering predictive information beyond that obtained through traditional risk factor evaluation.
Montorsi et al. [9] focused on vascular illnesses where ED is a concern caused by COAD, high blood pressure, cerebrovascular disease, peripheral arterial disease, and type 2 diabetes Notably, ED is also common in vascular syndromes, such as COAD, hypertension, cerebrovascular disease, PAD, and diabetes mellitus (DM).). Endothelial dysfunction and late obstructive alterations in the vascular system have been found in patients with ED and other cardiovascular diseases. To explain the connection between ED and CTAD, researchers recently proposed the artery-size hypothesis. Because atherosclerosis is a long-term condition, the damage to the major artery beds should have been uniform.
Diaconu et al. [115] described ED as a symptom of vascular disease that is still in its early stages. ED and CVD are both symptoms of the same illness. ED symptoms often present three to five years earlier than indications of COAD and may serve as a warning sign that CVD is on the way. As a result, male patients with cardiovascular risk factors should be examined for ED regularly. In patients with ED, an aggressive treatment strategy targeting the primary cardiovascular risk factors is indicated to avoid CVD complications and improve their prognosis. Gandaglia et al. [82] showed that the systemic relationship of ED with CVD should be treated as such. By the interaction of CVD risk factors, androgens, and chronic inflammation, there is an increase in the formation of atherosclerosis and flow-limiting stenosis. Endothelial dysfunction and autonomic hyperactivity, which are macroscopically undetectable, may help to explain the complicated link between ED and CVD. The diagnosis of ED frequently occurs before the onset of CVD, providing a golden opportunity for risk mitigation. Patients with ED should have a complete cardiologic examination and obtain comprehensive risk factor management, according to procedures devised specifically for them.
Miner et al. described that the responsibility of physicians is stated as the requirement to assess every man over the age of 40 for the presence or absence of ED, particularly those men who are asymptomatic for COAD signs or symptoms. It is suggested for CVD risk stratification in all men with vasculogenic ED. Another study by Rava et al. [225] provided the first AI-based algorithms capable of reliably and effectively measuring collateral flow in individuals suffering from androgen insensitivity syndrome. This automated technique for evaluating collateral filling may improve clinical decision-making for selecting reperfusion-eligible patients by speeding up the clinical process, reducing bias, and assisting in clinical decision-making.
Mouridsen et al. [221] showed that the use of non-contrast CT and MRI can help distinguish between ischemic and hemorrhagic strokes, which are difficult to distinguish based on clinical symptoms alone. Although an MRI has better sensitivity in an emergency, hypodensity on a CT and DWT and hyperintensity on an MRI detect irreversibly harmed tissue. To our understanding, no study has provided significant useful insight into CVD/stroke risk stratification in the ED paradigm.
5.3. A Short Note on Ultrasonography Examination for the Penile Pathology
Of all the causes of impotence, vasculogenic impotence accounts for more than 30%; therefore, ultrasonography is widely preferred for the assessment of penile pathology [226]. Modern ultrasonic examination is based on high-resolution greyscale imaging, which may be used alone or in conjunction with a color and pulsed-wave Doppler. For the examination of vascular reasons in ED, the use of a pharmaceutical stimulant to achieve an erection is currently the standard. Alprostadil (PGE1) and papaverine are the two most often used intracavernous medicines to cause an erection. When phentolamine is combined with these medicines, the amount of stimulant required is reduced, as is the risk of penile discomfort that is occasionally related to PGE1 usage [25,227,228]. Dynamic color–duplex Doppler ultrasonography has been recently proposed for testing high-dose sildenafil [229]. It has fewer false-positive diagnoses and treatments of vascular leakage, but it is time-consuming and requires confirmation in addition to audiovisual sexual excitement [229]. As a result, penile ultrasonography is recommended for the diagnosis of erectile dysfunction.
5.4. A Short Note on Bias in AI Systems
AI systems were introduced as an alternative to conventional CVD risk stratification methods [95,114]. However, AI systems have several challenges, such as a tendency to focus primarily on accuracy while ignoring scientific validation and clinical evaluation [45,49]. The disease severity ratio was determined incorrectly due to a lack of solid ground truth selection, such as CVE, coronary CT score, or angiogram stenosis. It places an abundance of emphasis on AI-system reliability while placing an underabundance of emphasis on AI-system authenticity. It causes a bias in the AI system [49]. It is also worth noting that the database contains specific regional patient features, and as a result, the model may produce an under- or over-estimation of the CVD/stroke results for different ethnicities or comorbidities [230]. Thus, for an improvement in CVD/stroke risk stratification in ED patients, it is vital to detect risk-of-bias (RoB) in AI systems [231] and correct CVD/stroke risk stratification. The performance of the AI-based CVD risk stratification can be further improved significantly by merging components such as mobile, cloud, and e-health infrastructure.
5.5. Strengths, Weakness, and Extensions of This Study
By identifying a correlation between ED with CVD and stroke, the overall cardio-urologic healthcare systems can be improved. Treatment is certainly preferable to prevention. Patients can be not only treated but also prevented from developing CVD severity if they are (i) aware of the relationship between ED with CVD stroke/ and (ii) low-cost screening using AI-based algorithms as well. One restriction we perceive is that no solid AI-assisted strategy has been developed for treating ED patients with CVD and stroke as variables, and additional research is needed in this area.
Although, there is no clear hypothesis that an AI system exists to forecast the risk of CVD and stroke risk stratification in ED patients, several AI models tackle the challenge of diagnosing CVD, stroke, and ED disorders individually. The lack of multi-center data on ED with CVD and stroke as comorbidities is also a challenge. With the pandemic, it is vital to think about how the SARS-CoV-2 virus may affect both diseases. More systematic reviews of ED-based RoB with comorbidities, such as the SARS-CoV-2 virus, CVD, and stroke, are expected. In the future, we would like to explore how understanding the function of large data is critical for eliminating bias in AI models.
6. Conclusions
In this systematic study, the relevance of CVD and stroke risk stratification in ED patients was explored. We also showed how ED problems might lead to vascular and cerebral strokes. As a consequence, recognizing CVD issues in ED patients is crucial. Carotid artery imaging ultrasound has also been found to be a low-cost, non-invasive alternative to traditional imaging modalities for screening CVD and stroke in ED patients. This low-cost B-mode ultrasonography can also be beneficial for the characterization of plaque tissue in ED patients, allowing for better knowledge of CVD and stroke risk stratification in these individuals. Additionally, we showed that AI-based approaches may accurately predict CVD and stroke risk in ED patients. A realistic AI-based model for CVD and stroke stratification in ED patients was described along with the risk of bias in AI. Finally, we discussed the functions of ED in the COVID-19 paradigm, as well as the significance of AI in this context. The study also presented the ED treatment options.
Acronym Table
| SN | Abbreviation | Definition | SN | Abbreviation | Definition |
| 1 | ANS | Autonomic nervous system | 34 | LBBM | Laboratory-based biomarker |
| 2 | ANN | Artificial neural setwork | 35 | LSTM | Long short-term memory |
| 3 | ACE2 | Angiotensin Converting Enzyme 2 | 36 | MedUSE | Medication use |
| 4 | AUC | Area-under-the-curve | 37 | ML | Machine learning |
| 5 | AI | Artificial intelligence | 38 | MI | Myocardial infarction |
| 6 | BMI | Body mass index | 39 | MRI | Magnetic resonance imaging |
| 7 | BP | Blood pressure | 40 | MACE | Major adverse cardiac events |
| 8 | CTAD | Cortaid artery disease | 41 | NPV | Negative predictive value |
| 9 | COAD | Coronary artery disease | 42 | NB | Naive byes |
| 10 | CAS | Coronary artery syndrome | 43 | nOH | Neurogenic orthostatic hypotension |
| 11 | CPD | Chorionic pulmonary disease | 44 | NO | Nitric oxide |
| 12 | CCS | Chronic coronary syndromes | 45 | Non-ML | Non-machine learning |
| 13 | CKD | Chronic kidney disease | 46 | NN | Neural networks |
| 14 | CT | Computed tomography | 47 | OBBM | Office-based biomarker |
| 15 | CUSIP | Carotid ultrasound image phenotype | 48 | OH | Orthostatic hypotension |
| 16 | CV | Cross-validation | 49 | PAD | Peripheral arterial disease |
| 17 | CVD | Cardiovascular disease | 50 | PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| 18 | CNN | Convolution neural network | 51 | PD | Parkinson disease |
| 19 | CHD | Congenital heart defects | 52 | PE | Premature ejaculation |
| 20 | CCS | Chronic coronary syndromes | 53 | PPV | Positive predictive value |
| 21 | DL | Deep learning | 54 | PCA | Principal component analysis |
| 22 | DM | Diabetes mellitus | 55 | pCAD | psoriasis computer-aided diagnosis |
| 23 | DT | Decision tree | 56 | RA | Rheumatoid arthritis |
| 24 | EMG | Electromyography | 57 | RF | Random forest |
| 25 | ED | Erectile dysfunction | 58 | RoB | Risk of bias |
| 26 | FHS | Framingham Heart Study | 59 | ROC | Receiver operating-characteristics |
| 27 | GT | Ground truth | 60 | RoS | Reactive oxygen species |
| 28 | HTN | Hypertension | 61 | RNN | Recurrent neural network |
| 29 | HDL | Hybrid deep learning | 62 | SCORE | Systematic coronary risk evaluation |
| 30 | HDLC | High-density lipoprotein cholesterol | 63 | SMOTE | Synthetic minority over-sampling technique |
| 31 | IMT | Intima-media thickness | 64 | SVM | Support vector machine |
| 32 | IHD | Ischaemic heart disease | 65 | US | Ultrasound |
| 33 | LDLC | Low-density lipoprotein cholesterol | 66 | WSS | Wall shear stress |
Author Contributions
Conceptualization, J.S.S., N.N.K., M.M. (Mahesh Maindarkar) and A.S. (Ajit Saxena); Methodology and software, J.S.S., M.M. (Mahesh Maindarkar), L.S. and P.A. and S.P.; Validation, S.K.S., M.T., E.C.-G., T.O., A.S. (Aditya Sharma), M.M. (Mahesh Maindarkar), I.M.S., G.P., N.N.K. and M.M.K.; Investigation, J.S.S., L.S., S.M., J.R.L., G.P. and M.M. (Martin Miner); Resources, S.P., J.S.S., M.M.F., M.T., M.M. (Mahesh Maindarkar); Data curation, M.M. (Mahesh Maindarkar), L.S., N.N.K., T.O., A.B.B. and J.S.S.; Writing—original draft preparation, M.M. (Mahesh Maindarkar), J.S.S., L.S., M.F. and G.D.K.; Writing—review and editing, S.P., M.M. (Mahesh Maindarkar), L.S., M.T., A.B.B., K.I.P. and J.S.S.; Visualization, N.N.K., P.A., J.S.T., V.A., M.M.F., and J.S.S.; Supervision, S.M., A.S. (Ajit Saxena), A.J., V.A., M.F., G.P., J.R.L. and J.S.S.; Project administration, N.N.K., J.S.T., M.M. (Mahesh Maindarkar) and J.S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Suri is with AtheroPointTM LLC, Roseville, CA, USA, which does cardiovascular and stroke imaging.
Data Availability Statement
No data availability.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nguyen H.M.T., Gabrielson A.T., Hellstrom W.J. Erectile dysfunction in young men—A review of the prevalence and risk factors. J. Sex. Med. Rev. 2017;5:508–520. doi: 10.1016/j.sxmr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig W., Phillips M. Organic causes of erectile dysfunction in men under 40. J. Urol. Int. 2014;92:1–6. doi: 10.1159/000354931. [DOI] [PubMed] [Google Scholar]
- 3.Solomon H., Man J.W., Wierzbicki A.S., Jackson G. Relation of erectile dysfunction to angiographic coronary artery disease. J. Am. J. Cardiol. 2003;91:230. doi: 10.1016/S0002-9149(02)03113-2. [DOI] [PubMed] [Google Scholar]
- 4.Cui X., Zhou J., Qin Z., Liu Z. Acupuncture for erectile dysfunction: A systematic review. J. BioMed. Res. Int. 2016;2016:2171923. doi: 10.1155/2016/2171923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouyanou K., Pither C.E., Wessely S. Iatrogenic factors and chronic pain. J. Psychosom. Med. 1997;59:597–604. doi: 10.1097/00006842-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Johansson M., Ehnvall A., Friberg P., Myredal A. Arterial baroreflex dysfunction in major depressive disorder. J. Clin. Auton. Res. 2010;20:235–240. doi: 10.1007/s10286-010-0053-y. [DOI] [PubMed] [Google Scholar]
- 7.Porst H., Montorsi F., Rosen R.C., Gaynor L., Grupe S., Alexander J. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: Prevalence, comorbidities, and professional help-seeking. J. Eur. Urol. 2007;51:816–824. doi: 10.1016/j.eururo.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Sabino-Carvalho J.L., Falquetto B., Takakura A.C., Vianna L.C. Baroreflex dysfunction in Parkinson’s disease: Integration of central and peripheral mechanisms. J. Neurophysiol. 2021;125:1425–1439. doi: 10.1152/jn.00548.2020. [DOI] [PubMed] [Google Scholar]
- 9.Montorsi P., Ravagnani P.M., Galli S., Rotatori F., Briganti A., Salonia A., Rigatti P., Montorsi F. The artery size hypothesis: A macrovascular link between erectile dysfunction and coronary artery disease. J. Am. J. Cardiol. 2005;96:19–23. doi: 10.1016/j.amjcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Walter B.L. Cardiovascular autonomic dysfunction in patients with movement disorders. J. Clevel. Clin. J. Med. 2008;75:S54. doi: 10.3949/ccjm.75.Suppl_2.S54. [DOI] [PubMed] [Google Scholar]
- 11.Tamler R. Diabetes, obesity, and erectile dysfunction. J. Gend. Med. 2009;6:4–16. doi: 10.1016/j.genm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Gowani Z., Uddin S., Mirbolouk M., Ayyaz D., Billups K.L., Miner M., Feldman D.I., Blaha M.J. Vascular erectile dysfunction and subclinical cardiovascular disease. J. Curr. Sex. Health Rep. 2017;9:305–312. doi: 10.1007/s11930-017-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim G.B. Erectile dysfunction predicts CVD events. J. Nat. Rev. Cardiol. 2018;15:502. doi: 10.1038/s41569-018-0055-3. [DOI] [PubMed] [Google Scholar]
- 14.Ryan J.M., Peterson M.D., Ryan N., Smith K.J., O’connell N.E., Liverani S., Anokye N., Victor C., Allen E. Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. J. Dev. Med. Child Neurol. 2019;61:924–928. doi: 10.1111/dmcn.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osondu C.U., Vo B., Oni E.T., Blaha M.J., Veledar E., Feldman T., Agatston A.S., Nasir K., Aneni E.C. The relationship of erectile dysfunction and subclinical cardiovascular disease: A systematic review and meta-analysis. J. Vasc. Med. 2018;23:9–20. doi: 10.1177/1358863X17725809. [DOI] [PubMed] [Google Scholar]
- 16.Kirby M. The association between erectile dysfunction and CVD. J. Trends Urol. Men’s Health. 2019;10:11–15. doi: 10.1002/tre.682. [DOI] [Google Scholar]
- 17.Choo E.H., Chang K., Lee K.Y., Lee D., Kim J.G., Ahn Y., Kim Y.J., Chae S.C., Cho M.C., Kim C.J. Prognosis and predictors of mortality in patients suffering myocardial infarction with non-obstructive coronary arteries. J. Am. Heart Assoc. 2019;8:e011990. doi: 10.1161/JAHA.119.011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buob A., Winter H., Kindermann M., Becker G., Möller J., Oertel W., Böhm M. Parasympathetic but not sympathetic cardiac dysfunction at early stages of Parkinson’s disease. J. Clin. Res. Cardiol. 2010;99:701–706. doi: 10.1007/s00392-010-0170-6. [DOI] [PubMed] [Google Scholar]
- 19.Shiferaw W.S., Akalu T.Y., Aynalem Y.A. Prevalence of erectile dysfunction in patients with diabetes mellitus and its association with body mass index and Glycated hemoglobin in Africa: A systematic review and meta-analysis. Int. J. Endocrinol. 2020;2020:5148370. doi: 10.1155/2020/5148370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasker G.F., Maley J.H., Kadowitz P.J. A review of the pathophysiology and novel treatments for erectile dysfunction. J. Adv. Pharmacol. Sci. 2010;2010:730861. doi: 10.1155/2010/730861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aschenbach R., Steiner T., Kerl M., Zangos S., Basche S., Vogl T. Endovascular embolisation therapy in men with erectile impotence due to veno-occlusive dysfunction. Eur. J. Radiol. 2013;82:504–507. doi: 10.1016/j.ejrad.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Shamloul R., Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 23.Javaroni V., Neves M.F. Erectile dysfunction and hypertension: Impact on cardiovascular risk and treatment. Int. J. Hypertens. 2012;2012:627278. doi: 10.1155/2012/627278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eardley I. Imaging for erectile dysfunction. J. Curr. Opin. Urol. 2002;12:143–147. doi: 10.1097/00042307-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Aversa A., Sarteschi L.M. The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. J. Sex. Med. 2007;4:1437–1447. doi: 10.1111/j.1743-6109.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 26.Seftel A.D. Erectile dysfunction in the elderly: Epidemiology, etiology and approaches to treatment. J. Urol. 2003;169:1999–2007. doi: 10.1097/01.ju.0000067820.86347.95. [DOI] [PubMed] [Google Scholar]
- 27.Liao K.P., Ananthakrishnan A.N., Kumar V., Xia Z., Cagan A., Gainer V.S., Goryachev S., Chen P., Savova G.K., Agniel D. Methods to develop an electronic medical record phenotype algorithm to compare the risk of coronary artery disease across 3 chronic disease cohorts. PLoS ONE. 2015;10:e0136651. doi: 10.1371/journal.pone.0136651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamthikar A.D., Gupta D., Mantella L.E., Saba L., Laird J.R., Johri A.M., Suri J.S. Multiclass machine learning vs. conventional calculators for stroke/CVD risk assessment using carotid plaque predictors with coronary angiography scores as gold standard: A 500 participants study. Int. J. Cardiovasc. Imaging. 2021;37:1171–1187. doi: 10.1007/s10554-020-02099-7. [DOI] [PubMed] [Google Scholar]
- 29.Rybicki F.J., Melchionna S., Mitsouras D., Coskun A.U., Whitmore A.G., Steigner M., Nallamshetty L., Welt F.G., Bernaschi M., Borkin M. Prediction of coronary artery plaque progression and potential rupture from 320-detector row prospectively ECG-gated single heart beat CT angiography: Lattice Boltzmann evaluation of endothelial shear stress. Int. J. Cardiovasc. Imaging. 2009;25:289–299. doi: 10.1007/s10554-008-9418-x. [DOI] [Google Scholar]
- 30.Jamthikar A.D., Gupta D., Johri A.M., Mantella L.E., Saba L., Kolluri R., Sharma A.M., Viswanathan V., Nicolaides A., Suri J.S. Low-cost office-based cardiovascular risk stratification using machine learning and focused carotid ultrasound in an Asian-Indian cohort. J. Med. Syst. 2020;44:1–15. doi: 10.1007/s10916-020-01675-7. [DOI] [PubMed] [Google Scholar]
- 31.Chiurlia E., D’Amico R., Ratti C., Granata A.R., Romagnoli R., Modena M.G. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J. Am. Coll. Cardiol. 2005;46:1503–1506. doi: 10.1016/j.jacc.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 32.Wilson P.W., Bozeman S.R., Burton T.M., Hoaglin D.C., Ben-Joseph R., Pashos C.L. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. J. Circ. 2008;118:124–130. doi: 10.1161/CIRCULATIONAHA.108.772962. [DOI] [PubMed] [Google Scholar]
- 33.Jackson G. Prevention of cardiovascular disease by the early identification of erectile dysfunction. Int. J. Impot. Res. 2008;20:S9–S14. doi: 10.1038/ijir.2008.47. [DOI] [PubMed] [Google Scholar]
- 34.Tandel G.S., Biswas M., Kakde O.G., Tiwari A., Suri H.S., Turk M., Laird J.R., Asare C.K., Ankrah A.A., Khanna N.J.C. A review on a deep learning perspective in brain cancer classification. Cancers. 2019;11:111. doi: 10.3390/cancers11010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biswas M., Kuppili V., Saba L., Edla D.R., Suri H.S., Cuadrado-Godia E., Laird J.R., Marinhoe R.T., Sanches J.M., Nicolaides A.J.F.B. State-of-the-art review on deep learning in medical imaging. Front. Biosci.—Landmark. 2019;24:392–426. doi: 10.2741/4725. [DOI] [PubMed] [Google Scholar]
- 36.Saba L., Biswas M., Kuppili V., Godia E.C., Suri H.S., Edla D.R., Omerzu T., Laird J.R., Khanna N.N., Mavrogeni S. The present and future of deep learning in radiology. Eur. J. Radiol. 2019;114:14–24. doi: 10.1016/j.ejrad.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Kuppili V., Biswas M., Sreekumar A., Suri H.S., Saba L., Edla D.R., Marinhoe R.T., Sanches J.M., Suri J.S. Extreme learning machine framework for risk stratification of fatty liver disease using ultrasound tissue characterization. J. Med. Syst. 2017;41:152. doi: 10.1007/s10916-017-0797-1. [DOI] [PubMed] [Google Scholar]
- 38.Maniruzzaman M., Rahman M.J., Al-MehediHasan M., Suri H.S., Abedin M.M., El-Baz A., Suri J.S. Accurate diabetes risk stratification using machine learning: Role of missing value and outliers. J. Med. Syst. 2018;42:92. doi: 10.1007/s10916-018-0940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya U.R., Faust O., Sree S.V., Molinari F., Suri J.S. ThyroScreen system: High resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. J. Comput. Methods Programs Biomed. 2012;107:233–241. doi: 10.1016/j.cmpb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Schneider D.F., Chen H. New developments in the diagnosis and treatment of thyroid cancer. J. CA Cancer J. Clin. 2013;63:373–394. doi: 10.3322/caac.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pareek G., Acharya U.R., Sree S.V., Swapna G., Yantri R., Martis R.J., Saba L., Krishnamurthi G., Mallarini G., El-Baz A. Prostate tissue characterization/classification in 144 patient population using wavelet and higher order spectra features from transrectal ultrasound images. J. Technol. Cancer Res. Treat. 2013;12:545–557. doi: 10.7785/tcrt.2012.500346. [DOI] [PubMed] [Google Scholar]
- 42.McClure P., Elnakib A., El-Ghar M.A., Khalifa F., Soliman A., El-Diasty T., Suri J.S., Elmaghraby A., El-Baz A. In-vitro and in-vivo diagnostic techniques for prostate cancer: A review. J. Biomed. Nanotechnol. 2014;10:2747–2777. doi: 10.1166/jbn.2014.1990. [DOI] [PubMed] [Google Scholar]
- 43.Acharya U.R., Saba L., Molinari F., Guerriero S., Suri J.S. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; Piscataway, NJ, USA: 2012. Ovarian tumor characterization and classification: A class of GyneScanTM systems; pp. 4446–4449. [DOI] [PubMed] [Google Scholar]
- 44.Liu K., Suri J.S. Automatic Vessel Indentification for Angiographic Screening. No. 6,845,260. U.S. Patent. 2005 January 18;
- 45.Suri J.S., Bhagawati M., Paul S., Protogeron A., Sfikakis P.P., Kitas G.D., Khanna N.N., Ruzsa Z., Sharma A.M., Saxena S., et al. Understanding the bias in machine learning systems for cardiovascular disease risk assessment: The first of its kind review. Comput. Biol. Med. 2022;142:105204. doi: 10.1016/j.compbiomed.2021.105204. [DOI] [PubMed] [Google Scholar]
- 46.Knuuti J., Bengel F., Bax J.J., Kaufmann P.A., le Guludec D., Filardi P.P., Marcassa C., Marsan N.A., Achenbach S., Kitsiou A. Risks and benefits of cardiac imaging: An analysis of risks related to imaging for coronary artery disease. Eur. Heart J. 2014;35:633–638. doi: 10.1093/eurheartj/eht512. [DOI] [PubMed] [Google Scholar]
- 47.Acharya U.R., Faust O., Sree S.V., Molinari F., Saba L., Nicolaides A., Suri J.S. An accurate and generalized approach to plaque characterization in 346 carotid ultrasound scans. IEEE Trans. Instrum. Meas. 2011;61:1045–1053. doi: 10.1109/TIM.2011.2174897. [DOI] [Google Scholar]
- 48.Soares H.D., Lovestone S. Biomarker utility in Alzheimer’s disease clinical trials. J. Drug Discov. Today Ther. Strateg. 2013;10:e55–e62. doi: 10.1016/j.ddstr.2013.09.002. [DOI] [Google Scholar]
- 49.Paul S., Maindarkar M., Saxena S., Saba L., Turk M., Kalra M., Krishnan P.R., Suri J.S. Bias Investigation in Artificial Intelligence Systems for Early Detection of Parkinson’sDisease: A Narrative Review. Diagnostics. 2022;12:166. doi: 10.3390/diagnostics12010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sibley K.G., Girges C., Hoque E., Foltynie T. Video-based analyses of Parkinson’s disease severity: A brief review. J. Parkinson’s Dis. 2021:1–11. doi: 10.3233/JPD-202402. preprint . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dias A.E., Limongi J.C., Barbosa E.R., Hsing W.T. Voice telerehabilitation in Parkinson’s disease. J. Codas. 2016;28:176–181. doi: 10.1590/2317-1782/20162015161. [DOI] [PubMed] [Google Scholar]
- 52.Agarwal M., Saba L., Gupta S.K., Carriero A., Falaschi Z., Paschè A., Danna P., El-Baz A., Naidu S., Suri J.S. A novel block imaging technique using nine artificial intelligence models for COVID-19 disease classification, characterization and severity measurement in lung computed tomography scans on an Italian cohort. J. Med. Syst. 2021;45:1–30. doi: 10.1007/s10916-021-01707-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suri J.S., Agarwal S., Gupta S.K., Puvvula A., Biswas M., Saba L., Bit A., Tandel G.S., Agarwal M., Patrick A. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput. Biol. Med. 2021;130:104210. doi: 10.1016/j.compbiomed.2021.104210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yannas D., Frizza F., Vignozzi L., Corona G., Maggi M., Rastrelli G. Erectile Dysfunction Is a Hallmark of Cardiovascular Disease: Unavoidable Matter of Fact or Opportunity to Improve Men’s Health? J. Clin. Med. 2021;10:2221. doi: 10.3390/jcm10102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahmood S.S., Levy D., Vasan R.S., Wang T. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. J. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jashari F., Ibrahimi P., Nicoll R., Bajraktari G., Wester P., Henein M.Y. Coronary and carotid atherosclerosis: Similarities and differences. J. Atheroscler. 2013;227:193–200. doi: 10.1016/j.atherosclerosis.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Anderson K.M., Odell P.M., Wilson P.W., Kannel W.B. Cardiovascular disease risk profiles. J. Am. Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-B. [DOI] [PubMed] [Google Scholar]
- 58.Payne R.A. Cardiovascular risk. Br. J. Clin. Pharmacol. 2012;74:396–410. doi: 10.1111/j.1365-2125.2012.04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lerner D.J., Kannel W.B. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am. Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 60.Lloyd-Jones D.M., Nam B.-H., Sr R.B.D., Levy D., Murabito J.M., Wang T.J., Wilson P.W., O’Donnell C. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: A prospective study of parents and offspring. J. JAMA Cardiol. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 61.Bachmann J.M., Willis B.L., Ayers C.R., Khera A., Berry J.D. Association between family history and coronary heart disease death across long-term follow-up in men: The Cooper Center Longitudinal Study. J. Circ. 2012;125:3092–3098. doi: 10.1161/CIRCULATIONAHA.111.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sivapalaratnam S., Boekholdt S.M., Trip M.D., Sandhu M.S., Luben R., Kastelein J.J., Wareham N.J., Khaw K.-T. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. J. Heart. 2010;96:1985–1989. doi: 10.1136/hrt.2010.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoit B.D., Gilpin E., Henning H., Maisel A., Dittrich H., Carlisle J., Ross J., Jr. Myocardial infarction in young patients: An analysis by age subsets. J. Circ. 1986;74:712–721. doi: 10.1161/01.CIR.74.4.712. [DOI] [PubMed] [Google Scholar]
- 64.Bier A., Braun T., Khasbab R., di Segni A., Grossman E., Haberman Y., Leibowitz A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. J. Nutr. 2018;10:1154. doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collaboration P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. J. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 66.Stamler J., Stamler R., Neaton J.D. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. J. Arch. Intern. Med. 1993;153:598–615. doi: 10.1001/archinte.1993.00410050036006. [DOI] [PubMed] [Google Scholar]
- 67.Kannel W.B. Elevated systolic blood pressure as a cardiovascular risk factor. Am. J. Cardiol. 2000;85:251–255. doi: 10.1016/S0002-9149(99)00635-9. [DOI] [PubMed] [Google Scholar]
- 68.Almdal T., Scharling H., Jensen J.S., Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population-based study of 13 000 men and women with 20 years of follow-up. J. Arch. Intern. Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 69.Sheth T., Nair C., Muller J., Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: The effect of age. J. Am. Coll. Cardiol. 1999;33:1916–1919. doi: 10.1016/S0735-1097(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 70.Selvin E., Marinopoulos S., Berkenblit G., Rami T., Brancati F.L., Powe N.R., Golden S.H. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. J. Ann. Intern. Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 71.Huang Y., Cai X., Mai W., Li M., Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. J. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakier J.B. Smoking and cardiovascular disease. Am. J. Med. 1992;93:S8–S12. doi: 10.1016/0002-9343(92)90620-Q. [DOI] [PubMed] [Google Scholar]
- 73.Prescott E., Hippe M., Schnohr P., Hein H.O., Vestbo J. Smoking and risk of myocardial infarction in women and men: Longitudinal population study. J. BMJ. 1998;316:1043. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manna P., Jain S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. J. Metab. Syndr. Relat. Disord. 2015;13:423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., Jafar T.H., Heerspink H.J.L., Mann J.F., Matsushita K., Wen C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. J. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 76.Joshipura K.J., Hu F.B., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Colditz G., Ascherio A., Rosner B., Spiegelman D. The effect of fruit and vegetable intake on risk for coronary heart disease. J. Ann. Intern. Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 77.Powell K.E., Thompson P.D., Caspersen C.J., Kendrick J.S. Physical activity and the incidence of coronary heart disease. J. Annu. Rev. Public Health. 1987;8:253–287. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 78.Feldman H.A., Johannes C.B., Derby C.A., Kleinman K.P., Mohr B.A., Araujo A.B., McKinlay J.B. Erectile dysfunction and coronary risk factors: Prospective results from the Massachusetts male aging study. J. Prev. Med. 2000;30:328–338. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 79.Fung M.M., Bettencourt R., Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2004;43:1405–1411. doi: 10.1016/j.jacc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 80.Ridker P.M., Glynn R.J., Hennekens C.H. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. J. Circ. 1998;97:2007–2011. doi: 10.1161/01.CIR.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 81.Hackett G., Krychman M., Baldwin D., Bennett N., El-Zawahry A., Graziottin A., Lukasiewicz M., McVary K., Sato Y., Incrocci L. Coronary heart disease, diabetes, and sexuality in men. J. Sex. Med. 2016;13:887–904. doi: 10.1016/j.jsxm.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 82.Gandaglia G., Salonia A., Passoni N., Montorsi P., Briganti A., Montorsi F. Erectile dysfunction as a cardiovascular risk factor in patients with diabetes. J. Endocr. 2013;43:285–292. doi: 10.1007/s12020-012-9780-2. [DOI] [PubMed] [Google Scholar]
- 83.Shin D., Pregenzer G., Jr., Gardin J.M. Erectile dysfunction: A disease marker for cardiovascular disease. J. Cardiol. Rev. 2011;19:5–11. doi: 10.1097/CRD.0b013e3181fb7eb8. [DOI] [PubMed] [Google Scholar]
- 84.Ryan J.G., Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J. Diabetes Its Complicat. 2012;26:141–147. doi: 10.1016/j.jdiacomp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Billups K., Bank A., Padma-Nathan H., Katz S., Williams R. Erectile dysfunction as a harbinger for increased cardiometabolic risk. Int. J. Impot. Res. 2008;20:236–242. doi: 10.1038/sj.ijir.3901634. [DOI] [PubMed] [Google Scholar]
- 86.Bedir F., Kocatürk H., Altay M.S., Kocaturk H., Bedir B., Yapanoglu T. Erektil Disfonksiyon Şikayeti ile Üroloji Polikliniğine Başvuran Hastalarda Kardiyovasküler Hastalıkların Değerlendirilmesi. J. Kocaeli Tıp Derg. 2021;10((Suppl. 2)):38–43. [Google Scholar]
- 87.Marwah R.S., Doux J.D., Lee P.Y., Yun A.J. Is atherosclerosis a neurogenic phenomenon? J. Med. Hypotheses. 2007;69:884–887. doi: 10.1016/j.mehy.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 88.Kendirci M., Nowfar S., Hellstrom W.J. The impact of vascular risk factors on erectile function. J. Drugs Today. 2005;41:65. doi: 10.1358/dot.2005.41.1.875779. [DOI] [PubMed] [Google Scholar]
- 89.van Dijk R.A., Rauwerda J.A., Steyn M., Twisk J.W., Stehouwer C.D. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: A 2-year, randomized, placebo-controlled trial. J. Arterioscler. Thromb. Vasc. Biol. 2001;21:2072–2079. doi: 10.1161/hq1201.100223. [DOI] [PubMed] [Google Scholar]
- 90.Kirby M., Jackson G., Betteridge J., Friedli K. Is erectile dysfunction a marker for cardiovascular disease? Int. J. Clin. Pract. 2001;55:614–618. [PubMed] [Google Scholar]
- 91.Blum A., Hadas V., Burke M., Yust I., Kessler A. Viral load of the human immunodeficiency virus could be an independent risk factor for endothelial dysfunction. J. Clin. Cardiol. 2005;28:149–153. doi: 10.1002/clc.4960280311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thum T., Tsikas D., Frölich J.C., Borlak J. Growth hormone induces eNOS expression and nitric oxide release in a cultured human endothelial cell line. J. FEBS Lett. 2003;555:567–571. doi: 10.1016/S0014-5793(03)01356-5. [DOI] [PubMed] [Google Scholar]
- 93.Tycinska A., Mroczko B., Musial W., Sawicki R., Kaminski K., Borowska H., Sobkowicz B., Szmitkowski M. Blood pressure in relation to neurogenic, inflammatory and endothelial dysfunction biomarkers in patients with treated essential arterial hypertension. J. Adv. Med. Sci. 2011;56:80–87. doi: 10.2478/v10039-011-0016-0. [DOI] [PubMed] [Google Scholar]
- 94.Ponholzer A., Stopfer J., Bayer G., Susani M., Steinbacher F., Herbst F., Schramek P., Madersbacher S., Maresch J. Is penile atherosclerosis the link between erectile dysfunction and cardiovascular risk? An autopsy study. Int. J. Impot. Res. 2012;24:137–140. doi: 10.1038/ijir.2012.3. [DOI] [PubMed] [Google Scholar]
- 95.Gandaglia G., Briganti A., Jackson G., Kloner R.A., Montorsi F., Montorsi P., Vlachopoulos C. A systematic review of the association between erectile dysfunction and cardiovascular disease. J. Eur. Urol. 2014;65:968–978. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 96.Gazzaruso C., Giordanetti S., de Amici E., Bertone G., Falcone C., Geroldi D., Fratino P., Solerte S.B., Garzaniti A. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. J. Circ. 2004;110:22–26. doi: 10.1161/01.CIR.0000133278.81226.C9. [DOI] [PubMed] [Google Scholar]
- 97.García-Malpartida K., Mármol R., Jover A., Gómez-Martínez M.J., Solá-Izquierdo E., Victor V.M., Rocha M., Sanmiguel D., Hernández-Mijares A. Relationship between erectile dysfunction and silent myocardial ischemia in type 2 diabetic patients with no known macrovascular complications. J. Sex. Med. 2011;8:2606–2616. doi: 10.1111/j.1743-6109.2011.02365.x. [DOI] [PubMed] [Google Scholar]
- 98.Chironi G.N., Boulanger C.M., Simon A., Dignat-George F., Freyssinet J.-M., Tedgui A. Endothelial microparticles in diseases. J. Cell Tissue Res. 2009;335:143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 99.Mirone V., Imbimbo C., Fusco F., Verze P., Creta M., Tajana G. Androgens and morphologic remodeling at penile and cardiovascular levels: A common piece in complicated puzzles? J. Eur. Urol. 2009;56:309–316. doi: 10.1016/j.eururo.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 100.Rajagopalan V., Gerdes A.M. Role of thyroid hormones in ventricular remodeling. J. Curr. Heart Fail. Rep. 2015;12:141–149. doi: 10.1007/s11897-014-0246-0. [DOI] [PubMed] [Google Scholar]
- 101.Kaynar M., Gomes A.L.Q., Sokolakis I., Gül M. Tip of the iceberg: Erectile dysfunction and COVID-19. Int. J. Impot. Res. 2022;34:152–157. doi: 10.1038/s41443-022-00540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dispenzieri A., Moreno-Aspitia A., Suarez G.A., Lacy M.Q., Colon-Otero G., Tefferi A., Litzow M.R., Roy V., Hogan W.J., Kyle R.A. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. J. Blood. 2004;104:3400–3407. doi: 10.1182/blood-2004-05-2046. [DOI] [PubMed] [Google Scholar]
- 103.Hernández-Cerda J., Bertomeu-González V., Zuazola P., Cordero A. Understanding erectile dysfunction in hypertensive patients: The need for good patient management. J. Vasc. Health Risk Manag. 2020;16:231. doi: 10.2147/VHRM.S223331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeiher A.M., Drexler H., Saurbier B., Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J. Clin. Investig. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aversa A., Isidori A., de Martino M., Caprio M., Fabbrini E., Rocchietti-March M., Frajese G., Fabbri A. Androgens and penile erection: Evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. J. Clin. Endocrinol. 2000;53:517–522. doi: 10.1046/j.1365-2265.2000.01118.x. [DOI] [PubMed] [Google Scholar]
- 106.Lue T.F. Erectile dysfunction. N. Engl. J. Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 107.Jevtich M.J., Khawand N.Y., Vidic B. Clinical significance of ultrastructural findings in the corpora cavernosa of normal and impotent men. J. Urol. 1990;143:289–293. doi: 10.1016/S0022-5347(17)39936-6. [DOI] [PubMed] [Google Scholar]
- 108.Djomkam A.L.Z., Olwal C.O., Sala T.B., Paemka L. Commentary: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. J. Front. Oncol. 2020:1448. doi: 10.3389/fonc.2020.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jung F., Krüger-Genge A., Franke R.-P., Hufert F., Küpper J.-H. COVID-19 and the endothelium. J. Clin. Hemorheol. Microcirc. 2020;75:7–11. doi: 10.3233/CH-209007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. J. Crit. Care. 2020;24:1–8. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fathi M., Vakili K., Aliaghaei A., Nematollahi S., Peirouvi T., Shalizar-Jalali A. Coronavirus disease and male fertility: A systematic review. Middle East Fertil. Soc. J. 2021;26:1–6. doi: 10.1186/s43043-021-00073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pavone C., Giammanco G.M., Baiamonte D., Pinelli M., Bonura C., Montalbano M., Profeta G., Curcurù L., Bonura F. Italian males recovering from mild COVID-19 show no evidence of SARS-CoV-2 in semen despite prolonged nasopharyngeal swab positivity. Int. J. Impot. Res. 2020;32:560–562. doi: 10.1038/s41443-020-00344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. J. Arterioscler. Thromb. Vasc. Biol. 2003;23:168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 114.Vlachopoulos C., Aznaouridis K., Ioakeimidis N., Rokkas K., Vasiliadou C., Alexopoulos N., Stefanadi E., Askitis A., Stefanadis C. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur. Heart J. 2006;27:2640–2648. doi: 10.1093/eurheartj/ehl341. [DOI] [PubMed] [Google Scholar]
- 115.Diaconu C.C., Manea M., Marcu D.R., Socea B., Spinu A.D., Bratu O.G. The erectile dysfunction as a marker of cardiovascular disease: A review. J. Acta Cardiol. 2020;75:286–292. doi: 10.1080/00015385.2019.1590498. [DOI] [PubMed] [Google Scholar]
- 116.Roushias S., Ossei-Gerning N. Sexual function and cardiovascular disease: What the general cardiologist needs to know. J. Heart. 2019;105:160–168. doi: 10.1136/heartjnl-2016-310762. [DOI] [PubMed] [Google Scholar]
- 117.Miner M., Parish S.J., Billups K.L., Paulos M., Sigman M., Blaha M.J. Erectile dysfunction and subclinical cardiovascular disease. J. Sex. Med. Rev. 2019;7:455–463. doi: 10.1016/j.sxmr.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Sayadi M., Elmafshar R., Razeghian-Jahromi I., Zibaeenezhad M.J. Detection of Coronary Artery Disease by an Erectile Dysfunction Questionnaire. J. Cardiol. Res. Pract. 2021;2021:6647995. doi: 10.1155/2021/6647995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kałka D., Gebala J., Biernikiewicz M., Mrozek-Szetela A., Rożek-Piechura K., Sobieszczańska M., Szuster E., Majchrowska M., Miętka A., Rusiecka A. Erectile Dysfunction in Men Burdened with the Familial Occurrence of Coronary Artery Disease. J. Clin. Med. 2021;10:4046. doi: 10.3390/jcm10184046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inman B.A., Sauver J.L.S., Jacobson D.J., McGree M.E., Nehra A., Lieber M.M., Roger V.L., Jacobsen S.J. Mayo Clinic Proceedings. Volume 84. Elsevier; Amsterdam, The Netherlands: 2009. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease; pp. 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imprialos K., Koutsampasopoulos K., Manolis A., Doumas M. Erectile Dysfunction as a Cardiovascular Risk Factor: Time to Step Up? J. Curr. Vasc. Pharmacol. 2021;19:301–312. doi: 10.2174/1570161118666200414102556. [DOI] [PubMed] [Google Scholar]
- 122.Rinkūnienė E., Gimžauskaitė S., Badarienė J., Dženkevičiūtė V., Kovaitė M., Čypienė A. The Prevalence of Erectile Dysfunction and Its Association with Cardiovascular Risk Factors in Patients after Myocardial Infarction. J. Med. 2021;57:1103. doi: 10.3390/medicina57101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ergül E., Yılmaz A.S., Öğütveren M.M., Emlek N., Kostakoğlu U., Çetin M. COVID 19 disease independently predicted endothelial dysfunction measured by flow-mediated dilatation. Int. J. Cardiovasc. Imaging. 2022;38:25–32. doi: 10.1007/s10554-021-02356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. J. Ocul. Surf. 2020;18:537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gur S., Alzweri L., Yilmaz-Oral D., Kaya-Sezginer E., Abdel-Mageed A.B., Dick B., Sikka S.C., Oztekin C.V., Hellstrom W.J. Testosterone positively regulates functional responses and nitric oxide expression in the isolated human corpus cavernosum. J. Androl. 2020;8:1824–1833. doi: 10.1111/andr.12866. [DOI] [PubMed] [Google Scholar]
- 126.Aydinoglu F., Ogulener N. Vascular Effects of Hydrogen Sulfide. Springer; Berlin/Heidelberg, Germany: 2019. The relaxant mechanisms of hydrogen sulfide in corpus cavernosum; pp. 137–150. [DOI] [PubMed] [Google Scholar]
- 127.Bertolo R., Cipriani C., Bove P. Anosmia and ageusia: A piece of the puzzle in the etiology of COVID-19-related transitory erectile dysfunction. J. Endocrinol. Investig. 2021;44:1123–1124. doi: 10.1007/s40618-021-01516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Musicki B., Burnett A. Endothelial dysfunction in diabetic erectile dysfunction. Int. J. Impot. Res. 2007;19:129–138. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- 129.Aksoy Y.E., Koçak V. Psychological effects of nurses and midwives due to COVID-19 outbreak: The case of Turkey. J. Arch. Psychiatr. Nurs. 2020;34:427–433. doi: 10.1016/j.apnu.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carvalho J., Pascoal P.M. Challenges in the practice of sexual medicine in the time of COVID-19 in Portugal. J. Sex. Med. 2020;17:1212–1215. doi: 10.1016/j.jsxm.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karagöz M.A., Gül A., Borg C., Erihan İ.B., Uslu M., Ezer M., Erbağcı A., Çatak B., Bağcıoğlu M. Influence of COVID-19 pandemic on sexuality: A cross-sectional study among couples in Turkey. Int. J. Impot. Res. 2020;33:815–823. doi: 10.1038/s41443-020-00378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Culha M.G., Demir O., Sahin O., Altunrende F. Sexual attitudes of healthcare professionals during the COVID-19 outbreak. Int. J. Impot. Res. 2021;33:102–109. doi: 10.1038/s41443-020-00381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Oliveira L., Carvalho J. Women’s Sexual Health During the Pandemic of COVID-19: Declines in Sexual Function and Sexual Pleasure. J. Curr. Sex. Health Rep. 2021;13:76–88. doi: 10.1007/s11930-021-00309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Healy E., McGuire B., Evans D., Carley S. Sexuality and personal relationships for people with an intellectual disability. Part I: Service-user perspectives. J. Intellect. Disabil. Res. 2009;53:905–912. doi: 10.1111/j.1365-2788.2009.01203.x. [DOI] [PubMed] [Google Scholar]
- 135.Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., Neil D., Hoefer I.E., Fragiadaki M., Waltenberger J. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. J. Cardiovasc. Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kemp K., Griffiths J., Campbell S., Lovell K. An exploration of the follow-up up needs of patients with inflammatory bowel disease. J. Crohn’s Colitis. 2013;7:e386–e395. doi: 10.1016/j.crohns.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 137.Maniruzzaman M., Kumar N., Abedin M.M., Islam M.S., Suri H.S., El-Baz A., Suri J.S. Comparative approaches for classification of diabetes mellitus data: Machine learning paradigm. Comput. Methods Programs Biomed. 2017;152:23–34. doi: 10.1016/j.cmpb.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 138.Maniruzzaman M., Suri H.S., Kumar N., Abedin M.M., Rahman M.J., El-Baz A., Bhoot M., Teji J.S., Suri J.S. Risk factors of neonatal mortality and child mortality in Bangladesh. J. Glob. Health. 2018;8:1. doi: 10.7189/jogh.08.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnson K.B., Wei W.Q., Weeraratne D., Frisse M.E., Misulis K., Rhee K., Zhao J., Snowdon J.L. Precision medicine, AI, and the future of personalized health care. J. Clin. Transl. Sci. 2021;14:86–93. doi: 10.1111/cts.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hurvitz N., Azmanov H., Kesler A., Ilan Y. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur. J. Hum. Genet. 2021;29:1485–1490. doi: 10.1038/s41431-021-00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Banchhor S.K., Londhe N.D., Araki T., Saba L., Radeva P., Laird J.R., Suri J.S. Wall-based measurement features provides an improved IVUS coronary artery risk assessment when fused with plaque texture-based features during machine learning paradigm. J. Comput. Biol. Med. 2017;91:198–212. doi: 10.1016/j.compbiomed.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 142.Yeh C.-H., Chou Y.-J., Tsai T.-H., Hsu P.W.-C., Li C.-H., Chan Y.-H., Tsai S.-F., Ng S.-C., Chou K.-M., Lin Y.-C. Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease. J. Biomed. 2022;10:116. doi: 10.3390/biomedicines10010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Acharya U.R., Sree S.V., Suri J.S. Automatic detection of epileptic EEG signals using higher order cumulant features. Int. J. Neural Syst. 2011;21:403–414. doi: 10.1142/S0129065711002912. [DOI] [PubMed] [Google Scholar]
- 144.Acharya U.R., Sree S.V., Ang P.C.A., Yanti R., Suri J.S. Application of non-linear and wavelet based features for the automated identification of epileptic EEG signals. Int. J. Neural Syst. 2012;22:1250002. doi: 10.1142/S0129065712500025. [DOI] [PubMed] [Google Scholar]
- 145.Acharya U.R., Faust O., Alvin A., Krishnamurthi G., Seabra J.C., Sanches J., Suri J.S. Understanding symptomatology of atherosclerotic plaque by image-based tissue characterization. J. Comput. Methods Programs Biomed. 2013;110:66–75. doi: 10.1016/j.cmpb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 146.Saba L., Jain P.K., Suri H.S., Ikeda N., Araki T., Singh B.K., Nicolaides A., Shafique S., Gupta A., Laird J.R. Plaque tissue morphology-based stroke risk stratification using carotid ultrasound: A polling-based PCA learning paradigm. J. Med. Syst. 2017;41:98. doi: 10.1007/s10916-017-0745-0. [DOI] [PubMed] [Google Scholar]
- 147.Saba L., Sanagala S.S., Gupta S.K., Koppula V.K., Johri A.M., Khanna N.N., Mavrogeni S., Laird J.R., Pareek G., Miner M. Multimodality carotid plaque tissue characterization and classification in the artificial intelligence paradigm: A narrative review for stroke application. J. Ann. Transl. Med. 2021;9:14. doi: 10.21037/atm-20-7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Acharya U.R., Sree S.V., Krishnan M.M.R., Molinari F., Garberoglio R., Suri J.S. Non-invasive automated 3D thyroid lesion classification in ultrasound: A class of ThyroScanTM systems. J. Ultrason. 2012;52:508–520. doi: 10.1016/j.ultras.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 149.Acharya U.R., Sree S.V., Krishnan M.M.R., Molinari F., ZieleŸnik W., Bardales R.H., Witkowska A., Suri J.S. Computer-aided diagnostic system for detection of Hashimoto thyroiditis on ultrasound images from a Polish population. J. Ultrasound Med. 2014;33:245–253. doi: 10.7863/ultra.33.2.245. [DOI] [PubMed] [Google Scholar]
- 150.Acharya U.R., Faust O., Sree S.V., Molinari F., Garberoglio R., Suri J.S. Cost-effective and non-invasive automated benign & malignant thyroid lesion classification in 3D contrast-enhanced ultrasound using combination of wavelets and textures: A class of ThyroScanTM algorithms. J. Technol. Cancer Res. Treat. 2011;10:371–380. doi: 10.7785/tcrt.2012.500214. [DOI] [PubMed] [Google Scholar]
- 151.Huang S.-F., Chang R.-F., Moon W.K., Lee Y.-H., Chen D.-R., Suri J.S. Analysis of tumor vascularity using three-dimensional power Doppler ultrasound images. J. IEEE Trans. Med. Imaging. 2008;27:320–330. doi: 10.1109/TMI.2007.904665. [DOI] [PubMed] [Google Scholar]
- 152.Faskhoudi M.A., Molaei P., Sadrkhanloo M., Orouei S., Hashemi M., Bokaie S., Rashidi M., Entezari M., Zarrabi A., Hushmandi K., et al. Molecular landscape of c-Myc signaling in prostate cancer: A roadmap to clinical translation. Pathol. -Res. Pract. 2022;233:153851. doi: 10.1016/j.prp.2022.153851. [DOI] [PubMed] [Google Scholar]
- 153.Acharya U.R., Sree S.V., Kulshreshtha S., Molinari F., Koh J.E.W., Saba L., Suri J.S. GyneScan: An improved online paradigm for screening of ovarian cancer via tissue characterization. J. Technol. Cancer Res. Treat. 2014;13:529–539. doi: 10.7785/tcrtexpress.2013.600273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nam D., Chapiro J., Paradis V., Paul Seraphin T., Nikolas Kather J. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction. JHEP Rep. 2022;4:100443. doi: 10.1016/j.jhepr.2022.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Biswas M., Kuppili V., Edla D.R., Suri H.S., Saba L., Marinhoe R.T., Sanches J.M., Suri J.S. Symtosis: A liver ultrasound tissue characterization and risk stratification in optimized deep learning paradigm. J. Comput. Methods Programs Biomed. 2018;155:165–177. doi: 10.1016/j.cmpb.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 156.Shrivastava V.K., Londhe N.D., Sonawane R.S., Suri J.S. Reliable and accurate psoriasis disease classification in dermatology images using comprehensive feature space in machine learning paradigm. J. Expert Syst. Appl. 2015;42:6184–6195. doi: 10.1016/j.eswa.2015.03.014. [DOI] [Google Scholar]
- 157.Shrivastava V.K., Londhe N.D., Sonawane R.S., Suri J.S. A novel and robust Bayesian approach for segmentation of psoriasis lesions and its risk stratification. Comput. Methods Programs Biomed. 2017;150:9–22. doi: 10.1016/j.cmpb.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 158.Jamthikar A., Gupta D., Khanna N.N., Saba L., Araki T., Viskovic K., Suri H.S., Gupta A., Mavrogeni S., Turk M. A low-cost machine learning-based cardiovascular/stroke risk assessment system: Integration of conventional factors with image phenotypes. J. Cardiovasc. Diagn. Ther. 2019;9:420. doi: 10.21037/cdt.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jamthikar A., Gupta D., Khanna N.N., Saba L., Laird J.R., Suri J.S. Cardiovascular/stroke risk prevention: A new machine learning framework integrating carotid ultrasound image-based phenotypes and its harmonics with conventional risk factors. Indian Heart J. 2020;72:258–264. doi: 10.1016/j.ihj.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jamthikar A., Gupta D., Saba L., Khanna N.N., Araki T., Viskovic K., Mavrogeni S., Laird J.R., Pareek G., Miner M. Cardiovascular/stroke risk predictive calculators: A comparison between statistical and machine learning models. J. Cardiovasc. Diagn. Ther. 2020;10:919. doi: 10.21037/cdt.2020.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vila M.D.M., Remeseiro B., Grau M., Elosua R., Igual L. Handbook of Artificial Intelligence in Healthcare. Springer; Cham, Switzerland: 2022. Last Advances on Automatic Carotid Artery Analysis in Ultrasound Images: Towards Deep Learning; pp. 215–247. [Google Scholar]
- 162.Sanches J.M., Laine A.F., Suri J.S. Ultrasound Imaging. Springer; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- 163.Molinari F., Liboni W., Giustetto P., Badalamenti S., Suri J.S. Automatic computer-based tracings (ACT) in longitudinal 2-D ultrasound images using different scanners. J. Mech. Med. Biol. 2009;9:481–505. doi: 10.1142/S0219519409003115. [DOI] [Google Scholar]
- 164.Sudeep P., Palanisamy P., Rajan J., Baradaran H., Saba L., Gupta A., Suri J.S. Speckle reduction in medical ultrasound images using an unbiased non-local means method. J. Biomed. Signal Process. Control. 2016;28:1–8. doi: 10.1016/j.bspc.2016.03.001. [DOI] [Google Scholar]
- 165.Pewowaruk R.J., Tedla Y., Korcarz C.E., Tattersall M.C., Stein J.H., Chesler N.C., Gepner A.D. Carotid Artery Stiffening With Aging: Structural Versus Load-Dependent Mechanisms in MESA (the Multi-Ethnic Study of Atherosclerosis) J. Hypertens. 2022;79:150–158. doi: 10.1161/HYPERTENSIONAHA.121.18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Siontis K.C., Noseworthy P.A., Attia Z.I., Friedman P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. J. Nat. Rev. Cardiol. 2021;18:465–478. doi: 10.1038/s41569-020-00503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amin J., Sharif M., Raza M., Saba T., Sial R., Shad S.A. Brain tumor detection: A long short-term memory (LSTM)-based learning model. Neural Comput. Appl. 2020;32:15965–15973. doi: 10.1007/s00521-019-04650-7. [DOI] [Google Scholar]
- 168.Priyanga P., Pattankar V.V., Sridevi S. A hybrid recurrent neural network-logistic chaos-based whale optimization framework for heart disease prediction with electronic health records. J. Comput. Intell. 2021;37:315–343. doi: 10.1111/coin.12405. [DOI] [Google Scholar]
- 169.An Y., Tang K., Wang J. IEEE/ACM Transactions on Computational Biology. IEEE; Piscataway, NJ, USA: 2021. Time-aware multi-type data fusion representation learning framework for risk prediction of cardiovascular diseases. [DOI] [PubMed] [Google Scholar]
- 170.Tan L., Yu K., Bashir A.K., Cheng X., Ming F., Zhao L., Zhou X. Toward real-time and efficient cardiovascular monitoring for COVID-19 patients by 5G-enabled wearable medical devices: A deep learning approach. J. Neural Comput. Appl. 2021 doi: 10.1007/s00521-021-06219-9. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang J., Ding H., Bidgoli F.A., Zhou B., Iribarren C., Molloi S., Baldi P. Detecting cardiovascular disease from mammograms with deep learning. J. IEEE Trans. Med. Imaging. 2017;36:1172–1181. doi: 10.1109/TMI.2017.2655486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Madjid M., Awan I., Willerson J.T., Casscells S.W. Leukocyte count and coronary heart disease: Implications for risk assessment. J. Am. Coll. Cardiol. 2004;44:1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 173.Yahagi K., Kolodgie F.D., Lutter C., Mori H., Romero M.E., Finn A.V., Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. J. Arterioscler. Thromb. Vasc. Biol. 2017;37:191–204. doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Amato M., Montorsi P., Ravani A., Oldani E., Galli S., Ravagnani P.M., Tremoli E., Baldassarre D. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: Correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur. Heart J. 2007;28:2094–2101. doi: 10.1093/eurheartj/ehm244. [DOI] [PubMed] [Google Scholar]
- 175.Mosleh W., Adib K., Natdanai P., Carmona-Rubio A., Karki R., Paily J., Ahmed M.A.-A., Vakkalanka S., Madam N., Gudleski G.D. High-risk carotid plaques identified by CT-angiogram can predict acute myocardial infarction. Int. J. Cardiovasc. Imaging. 2017;33:561–568. doi: 10.1007/s10554-016-1019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gorek M., Stief C., Hartung C., Jonas U. Computer-assisted interpretation of electromyograms of corpora cavernosa using fuzzy logic. World J. Urol. 1997;15:65–70. doi: 10.1007/BF01275159. [DOI] [PubMed] [Google Scholar]
- 177.Kellner B., Stief C.-G., Hinrichs H., Hartung C. Computerized classification of corpus cavernosum electromyogram signals by the use of discriminant analysis and artificial neural networks to support diagnosis of erectile dysfunction. J. Urol. Res. 2000;28:6–13. doi: 10.1007/s002400050002. [DOI] [PubMed] [Google Scholar]
- 178.Glavaš S., ValenćIć L., Trbojević N., Tomašić A.-M., Turćić N., Tibauth S., Ružić A. Erectile function in cardiovascular patients: Its significance and a quick assessment using a visual-scale questionnaire. J. Acta Cardiol. 2015;70:712–719. doi: 10.1080/AC.70.6.3120185. [DOI] [PubMed] [Google Scholar]
- 179.Chen Y.-F., Lin C.-S., Hong C.-F., Lee D.-J., Sun C., Lin H.-H. Design of a clinical decision support system for predicting erectile dysfunction in men using NHIRD dataset. IEEE J. Biomed. Health Inform. 2018;23:2127–2137. doi: 10.1109/JBHI.2018.2877595. [DOI] [PubMed] [Google Scholar]
- 180.Li L., Fan W., Li J., Li Q., Wang J., Fan Y., Ye T., Guo J., Li S., Zhang Y. Abnormal brain structure as a potential biomarker for venous erectile dysfunction: Evidence from multimodal MRI and machine learning. J. Eur. Radiol. 2018;28:3789–3800. doi: 10.1007/s00330-018-5365-7. [DOI] [PubMed] [Google Scholar]
- 181.Jang I., Lee J.-U., Lee J.-M., Kim B.H., Moon B., Hong J., Oh H.B. LC–MS/MS software for screening unknown erectile dysfunction drugs and analogues: Artificial neural network classification, peak-count scoring, simple similarity search, and hybrid similarity search algorithms. J. Anal. Chem. 2019;91:9119–9128. doi: 10.1021/acs.analchem.9b01643. [DOI] [PubMed] [Google Scholar]
- 182.Biswas M., Kuppili V., Saba L., Edla D.R., Suri H.S., Sharma A., Cuadrado-Godia E., Laird J.R., Nicolaides A., Suri J.S. Deep learning fully convolution network for lumen characterization in diabetic patients using carotid ultrasound: A tool for stroke risk. J. Med. Biol. Eng. Comput. 2019;57:543–564. doi: 10.1007/s11517-018-1897-x. [DOI] [PubMed] [Google Scholar]
- 183.Skandha S.S., Gupta S.K., Saba L., Koppula V.K., Johri A.M., Khanna N.N., Mavrogeni S., Laird J.R., Pareek G., Miner M. 3-D optimized classification and characterization artificial intelligence paradigm for cardiovascular/stroke risk stratification using carotid ultrasound-based delineated plaque: AtheromaticTM 2.0. Comput. Biol. Med. 2020;125:103958. doi: 10.1016/j.compbiomed.2020.103958. [DOI] [PubMed] [Google Scholar]
- 184.Saba L., Sanagala S.S., Gupta S.K., Koppula V.K., Laird J.R., Viswanathan V., Sanches M.J., Kitas G.D., Johri A.M., Sharma N. A Multicenter Study on Carotid Ultrasound Plaque Tissue Characterization and Classification Using Six Deep Artificial Intelligence Models: A Stroke Application. J. IEEE Trans. Instrum. Meas. 2021;70:1–12. doi: 10.1109/TIM.2021.3052577. [DOI] [Google Scholar]
- 185.Zaman A.G., Helft G., Worthley S.G., Badimon J.J. The role of plaque rupture and thrombosis in coronary artery disease. J. Atheroscler. 2000;149:251–266. doi: 10.1016/S0021-9150(99)00479-7. [DOI] [PubMed] [Google Scholar]
- 186.Suri J.S., Kathuria C., Molinari F. Atherosclerosis Disease Management. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2010. [Google Scholar]
- 187.Bots M.L., Grobbee D.E. Intima media thickness as a surrogate marker for generalised atherosclerosis. J. Cardiovasc. Drugs Ther. 2002;16:341–351. doi: 10.1023/A:1021738111273. [DOI] [PubMed] [Google Scholar]
- 188.Bots M.L., Grobbee D.E., Hofman A., Witteman J.C. Common carotid intima-media thickness and risk of acute myocardial infarction: The role of lumen diameter. J. Stroke. 2005;36:762–767. doi: 10.1161/01.STR.0000158924.71069.94. [DOI] [PubMed] [Google Scholar]
- 189.Johri A.M., Behl P., Hétu M.F., Haqqi M., Ewart P., Day A.G., Parfrey B., Matangi M.F. Carotid ultrasound maximum plaque height–a sensitive imaging biomarker for the assessment of significant coronary artery disease. J. Echocardiogr. 2016;33:281–289. doi: 10.1111/echo.13007. [DOI] [PubMed] [Google Scholar]
- 190.Johri A.M., Chitty D.W., Matangi M., Malik P., Mousavi P., Day A., Gravett M., Simpson C. Can carotid bulb plaque assessment rule out significant coronary artery disease? A comparison of plaque quantification by two-and three-dimensional ultrasound. J. Am. Soc. Echocardiogr. 2013;26:86–95. doi: 10.1016/j.echo.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 191.Ogata T., Yasaka M., Yamagishi M., Seguchi O., Nagatsuka K., Minematsu K. Atherosclerosis found on carotid ultrasonography is associated with atherosclerosis on coronary intravascular ultrasonography. J. Ultrasound Med. 2005;24:469–474. doi: 10.7863/jum.2005.24.4.469. [DOI] [PubMed] [Google Scholar]
- 192.Jain P.K., Sharma N., Giannopoulos A.A., Saba L., Nicolaides A., Suri J.S. Hybrid deep learning segmentation models for atherosclerotic plaque in internal carotid artery B-mode ultrasound. J. Comput. Biol. Med. 2021;136:104721. doi: 10.1016/j.compbiomed.2021.104721. [DOI] [PubMed] [Google Scholar]
- 193.Kurth T., Everett B.M., Buring J.E., Kase C.S., Ridker P.M., Gaziano J.M. Lipid levels and the risk of ischemic stroke in women. J. Neurol. 2007;68:556–562. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thim T., Hagensen M.K., Falk E., Hørlyck A., Kim W.Y., Niemann A.K., Thrysøe S.A., Drouet L., Paaske W.P., Bøtker H.E. Wall shear stress and local plaque development in stenosed carotid arteries of hypercholesterolemic minipigs. J. Cardiovasc. Dis. Res. 2012;3:76–83. doi: 10.4103/0975-3583.95358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brevetti G., Sirico G., Giugliano G., Lanero S., de Maio J.I., Luciano R., Laurenzano E., Chiariello M. Prevalence of hypoechoic carotid plaques in coronary artery disease: Relationship with coexistent peripheral arterial disease and leukocyte number. J. Vasc. Med. 2009;14:13–19. doi: 10.1177/1358863X08097066. [DOI] [PubMed] [Google Scholar]
- 196.Ho S.S.Y. Current status of carotid ultrasound in atherosclerosis. J. Quant. Imaging Med. Surg. 2016;6:285. doi: 10.21037/qims.2016.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vicenzini E., Ricciardi M.C., Puccinelli F., Altieri M., Vanacore N., di Piero V., Lenzi G.L. Sonographic carotid plaque morphologic characteristics and vascular risk factors: Results from a population study. J. Ultrasound Med. 2008;27:1313–1319. doi: 10.7863/jum.2008.27.9.1313. [DOI] [PubMed] [Google Scholar]
- 198.Saverino A., del Sette M., Conti M., Ermirio D., Ricca M., Rovetta G., Gandolfo C. Hyperechoic plaque: An ultrasound marker for osteoporosis in acute stroke patients with carotid disease. J. Eur. Neurol. 2006;55:31–36. doi: 10.1159/000091423. [DOI] [PubMed] [Google Scholar]
- 199.Meiburger K.M., Caresio C., Salvi M., Molinari F. Automated Techniques for Vessel Detection and Segmentation in Cardiovascular Images. Cardiovascular Computing—Methodologies and Clinical Applications. Springer; Singapore: 2019. pp. 41–161. [Google Scholar]
- 200.Arnold J., Modaresi K., Thomas N., Taylor P., Padayachee T. Carotid plaque characterization by duplex scanning: Observer error may undermine current clinical trials. J. Stroke. 1999;30:61–65. doi: 10.1161/01.STR.30.1.61. [DOI] [PubMed] [Google Scholar]
- 201.Rafieian-Kopaei M., Setorki M., Doudi M., Baradaran A., Nasri H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014;5:927. [PMC free article] [PubMed] [Google Scholar]
- 202.Sadeghi M.M., Glover D.K., Lanza G.M., Fayad Z.A., Johnson L.L. Imaging atherosclerosis and vulnerable plaque. J. Nucl. Med. 2010;51((Suppl. 1)):51S–65S. doi: 10.2967/jnumed.109.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sasayama S., Ishii N., Ishikura F., Kamijima G., Ogawa S., Kanmatsuse K., Kimoto Y., Sakuma I., Nonogi H., Matsumori A. Men’s Health Study Epidemiology of Erectile Dysfunction and Cardiovascular Disease. Circ. J. 2003;67:656–659. doi: 10.1253/circj.67.656. [DOI] [PubMed] [Google Scholar]
- 204.Wang D., Wang Z., Zhang L., Wang Y. Roles of cells from the arterial vessel wall in atherosclerosis. J. Mediat. Inflamm. 2017;2017:8135934. doi: 10.1155/2017/8135934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Giger M.L. Machine learning in medical imaging. J. Am. Coll. Radiol. 2018;15:512–520. doi: 10.1016/j.jacr.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 206.Saba L., Sanfilippo R., Porcu M., Lucatelli P., Montisci R., Zaccagna F., Suri J.S., Anzidei M., Wintermark M. Relationship between white matter hyperintensities volume and the circle of Willis configurations in patients with carotid artery pathology. Eur. J. Radiol. 2017;89:111–116. doi: 10.1016/j.ejrad.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 207.Araki T., Ikeda N., Shukla D., Jain P.K., Londhe N.D., Shrivastava V.K., Banchhor S.K., Saba L., Nicolaides A., Shafique S. PCA-based polling strategy in machine learning framework for coronary artery disease risk assessment in intravascular ultrasound: A link between carotid and coronary grayscale plaque morphology. Comput. Methods Programs Biomed. 2016;128:137–158. doi: 10.1016/j.cmpb.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 208.James S., Fedewa R., Lyden S., Geoffrey D. Attenuation compensation comparison for human carotid plaque characterization using spectral analysis of backscattered ultrasound; Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS); Glasgow, UK. 6–9 October 2019; pp. 41–44. [Google Scholar]
- 209.Saba L., Agarwal M., Sanagala S.S., Gupta S.K., Sinha G., Johri A., Khanna N., Mavrogeni S., Laird J., Pareek G. Brain MRI-based Wilson disease tissue classification: An optimised deep transfer learning approach. J. Electron. Lett. 2020;56:1395–1398. doi: 10.1049/el.2020.2102. [DOI] [Google Scholar]
- 210.Sanagala S.S., Nicolaides A., Gupta S.K., Koppula V.K., Saba L., Agarwal S., Johri A.M., Kalra M.S., Suri J.S. Ten Fast Transfer Learning Models for Carotid Ultrasound Plaque Tissue Characterization in Augmentation Framework Embedded with Heatmaps for Stroke Risk Stratification. J. Diagn. 2021;11:2109. doi: 10.3390/diagnostics11112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shin H.-C., Roth H.R., Gao M., Lu L., Xu Z., Nogues I., Yao J., Mollura D., Summers R.M. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans. Med. Imaging. 2016;35:1285–1298. doi: 10.1109/TMI.2016.2528162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Machacado-Rojas A.M., Aparicio-Pico L.E. Técnicas de inteligencia artificial aplicadas al análisis de imágenes diagnóstico. Eco Matemático. 2021;12:100–111. doi: 10.22463/17948231.3237. [DOI] [Google Scholar]
- 213.Rafailidis V., Li X., Sidhu P.S., Partovi S., Staub D. Contrast imaging ultrasound for the detection and characterization of carotid vulnerable plaque. Cardiovasc. Diagn. Ther. 2020;10:965. doi: 10.21037/cdt.2020.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Goldstein I., Lue T.F., Padma-Nathan H., Rosen R.C., Steers W.D., Wicker P.A. Oral sildenafil in the treatment of erectile dysfunction. N. Engl. J. Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 215.Mobley D.F., Baum N. When patients request the impotence pill: Tips for office evaluation and treatment. J. Postgrad. Med. 1998;104:55–66. doi: 10.3810/pgm.1998.08.561. [DOI] [PubMed] [Google Scholar]
- 216.Lincoln T.M. Cyclic GMP and mechanisms of vasodilation. J. Pharmacol. Ther. 1989;41:479–502. doi: 10.1016/0163-7258(89)90127-7. [DOI] [PubMed] [Google Scholar]
- 217.Carvajal J.A., Germain A.M., Huidobro-Toro J.P., Weiner C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 218.Padma-Nathan H., McMurray J., Pullman W., Whitaker J., Saoud J., Ferguson K., Rosen R. On-demand IC351 (CialisTM) enhances erectile function in patients with erectile dysfunction. Int. J. Impot. Res. 2001;13:2–9. doi: 10.1038/sj.ijir.3900631. [DOI] [PubMed] [Google Scholar]
- 219.Ignarro L.J., Bush P.A., Buga G.M., Wood K.S., Fukuto J.M., Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. J. Biochem. Biophys. Res. Commun. 1990;170:843–850. doi: 10.1016/0006-291X(90)92168-Y. [DOI] [PubMed] [Google Scholar]
- 220.National Institutes of Health, Office of Medical Applications of Research . NIH Consensus Statement. National Institutes of Health, Office of Medical Applications of Research; Hamiliton, MT, USA: 1992. [Google Scholar]
- 221.Statsenko M., Turkina S., Tyschenko I., Kosivtsova M., Kostromeev S. Effect of Tadalafil SZ on endothelial function in patients with erectile dysfunction. J. Urol. 2021;1:50–54. doi: 10.18565/urology.2021.1.50-54. [DOI] [PubMed] [Google Scholar]
- 222.Mouridsen K., Thurner P., Zaharchuk G. Artificial intelligence applications in stroke. J. Stroke. 2020;51:2573–2579. doi: 10.1161/STROKEAHA.119.027479. [DOI] [PubMed] [Google Scholar]
- 223.Bikias T., Iakovakis D., Hadjidimitriou S., Charisis V., Hadjileontiadis L. DeepFoG: An IMU-Based Detection of Freezing of Gait Episodes in Parkinson’s Disease Patients via Deep Learning. J. Front. Robot. 2021;8:537384. doi: 10.3389/frobt.2021.537384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Grassi G., Seravalle G., Quarti-Trevano F., Dell’Oro R., Arenare F., Spaziani D., Mancia G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. J. Hypertens. 2009;53:205–209. doi: 10.1161/HYPERTENSIONAHA.108.121467. [DOI] [PubMed] [Google Scholar]
- 225.Cuenca-Bermejo L., Almela P., Navarro-Zaragoza J., Villalba E.F., González-Cuello A.-M., Laorden M.-L., Herrero M.-T. Cardiac Changes in Parkinson’s Disease: Lessons from Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021;22:13488. doi: 10.3390/ijms222413488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rava R.A., Seymour S.E., Snyder K.V., Waqas M., Davies J.M., Levy E.I., Siddiqui A.H., Ionita C.N. Automated Collateral Flow Assessment in Patients with Acute Ischemic Stroke Using Computed Tomography with Artificial Intelligence Algorithms. J. World Neurosurg. 2021;155:e748–e760. doi: 10.1016/j.wneu.2021.08.136. [DOI] [PubMed] [Google Scholar]
- 227.McCauley J.F., Dean R.C. Diagnostic utility of penile ultrasound in Peyronie’s disease. World J. Urol. 2020;38:263–268. doi: 10.1007/s00345-019-02928-y. [DOI] [PubMed] [Google Scholar]
- 228.Aversa A., Bruzziches R., Spera G. Diagnosing erectile dysfunction: The penile dynamic colour duplex ultrasound revisited. Int. J. Androl. 2005;28:61–63. doi: 10.1111/j.1365-2605.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 229.Aversa A., Isidori A.M., Spera G., Lenzi A., Fabbri A. Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunction. J. Clin. Endocrinol. 2003;58:632–638. doi: 10.1046/j.1365-2265.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- 230.Speel T., Bleumer I., Diemont W., van der Maas M., Wijkstra H., Meuleman E. The value of sildenafil as mode of stimulation in pharmaco-penile duplex ultrasonography. Int. J. Impot. Res. 2001;13:189–191. doi: 10.1038/sj.ijir.3900704. [DOI] [PubMed] [Google Scholar]
- 231.Suri J., Agarwal S., Gupta S., Puvvula A., Viskovic K., Suri N., Alizad A., El-Baz A., Saba L., Fatemi M., et al. Systematic Review of Artificial Intelligence in Acute Respiratory Distress Syndrome for COVID-19 Lung Patients: A Biomedical Imaging Perspective. IEEE J. Biomed. Health Inform. 2021;25:4128–4139. doi: 10.1109/JBHI.2021.3103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data availability.