Abstract
The MYD88 gene has a physiological role in the innate immune system. Somatic mutations in MYD88, including the most common L265P, have been associated with the development of certain types of lymphoma. MYD88L265P is present in more than 90% of patients with Waldenström’s macroglobulinemia (WM) and IgM monoclonal gammopathy of undetermined significance (IgM-MGUS). The absence of MYD88 mutations in WM patients has been associated with a higher risk of transformation into aggressive lymphoma, resistance to certain therapies (BTK inhibitors), and shorter overall survival. The MyD88 signaling pathway has also been used as a target for specific therapies. In this review, we summarize the clinical applications of MYD88 testing in the diagnosis, prognosis, follow-up, and treatment of patients. Although MYD88L265P is not specific to WM, few tumors present a single causative mutation in a recurrent position. The role of the oncogene in the pathogenesis of WM is still unclear, especially considering that the mutation can be found in normal B cells of patients, as recently reported. This may have important implications for early lymphoma detection in healthy elderly individuals and for the treatment response assessment based on a MYD88L265P analysis.
Keywords: signal transduction, hematologic neoplasms, targeted therapy, Waldenström’s macroglobulinemia
1. Introduction
The myeloid differentiation primary response 88 (MYD88) gene encodes a cytosolic adaptor protein that plays a central role in the innate and adaptive immune responses mediated by the interleukin (IL) 1 receptor (IL-1R) family and toll-like receptors (TLRs) [1]. The MyD88 protein has a modular structure composed of three main domains: an N-terminal death domain (DD), responsible for binding to the IL-1R-associated kinase (IRAK) complex and for further signaling through the pathway [2], a toll-interleukin-1 receptor (TIR) domain, located at the C-terminus and that binds to the receptors [3], and an intermediary domain (INT), which separates both [1]. Based on its unique structure, MyD88 serves as the central link that connects TLR-IL-1R family members to IRAKs (IRAK1, IRAK2, and IRAK4) [4,5], leading to the activation of the nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and interferon regulatory factor (IRF) 5/7 signaling pathways and the downstream production of type I interferons and proinflammatory cytokines, including IL-1, IL-6, IL-12, and tumor necrosis factor (TNF)-α [2,6,7,8,9,10].
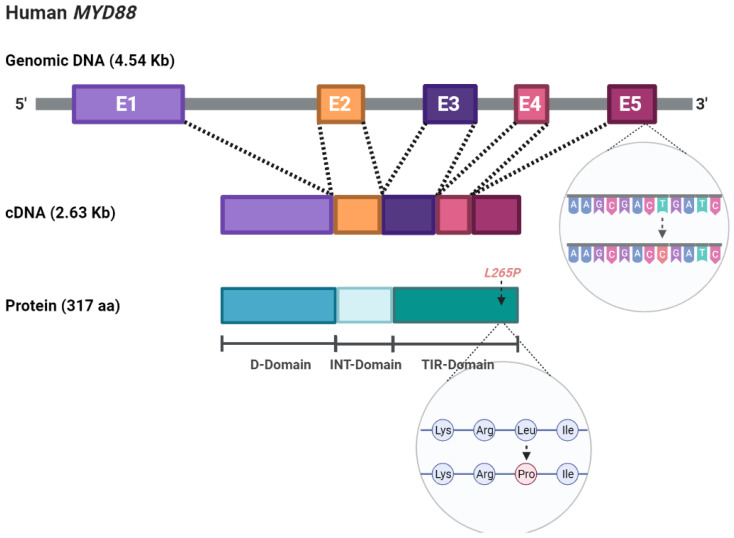
In addition to its physiological role in the immune response, MYD88 can also act as an oncogene associated with lymphoma development, and somatic mutations have been identified in numerous patients. The most recurrent, a single nucleotide change from T to C, resulting in a change from leucine to proline at position 265 (L265P) (Figure 1), is present in more than 90% of Waldenström’s macroglobulinemia (WM) patients [11,12,13,14,15], in whom it has been shown to have important clinical and therapeutic implications [16]. However, its role in the disease pathogenesis has not yet been fully elucidated. In this paper, we will provide a summary view of the gene function in normal and pathological conditions, the clinical applications, including diagnosis and therapy, and will comment on the latest findings about MYD88L265P not being exclusive to tumor cells and the potential implications derived from this.
Figure 1.
Schematic representation of the MYD88 gene structure and the L265P mutation at the DNA and protein levels.
2. Signaling Pathway and Molecular Alterations
In normal physiology, MyD88 is required for full function of the innate immune response as a signaling adaptor in the canonical NF-κB pathway [17]. Upon ligand binding, MyD88 dimerizes, through its death domain (DD), and recruits IRAK4, followed by IRAK1/2, resulting in the assembly of a large complex called ‘Myddosome’, which consists of six MyD88 molecules, four IRAK4 molecules, and four IRAK1/2 molecules [8,18,19]. IRAK1/2 can subsequently interact with TNF receptor-associated factor 6 (TRAF6) to activate transforming growth factor beta-activated kinase 1 (TAK1), which initiates a phosphorylation cascade, resulting in the release of the NF-κB subunits RELA [p65]-p50 and RELB-p52, which migrate to the nucleus, bind to the DNA, and increase the expression of the target genes [19].
In the context of cancer and, particularly, in hematology, one can hardly speak about MyD88 without thinking of WM. WM is an indolent hematological malignancy that makes up approximately 1% of non-Hodgkin’s lymphomas (NHL). Patients present both abnormal cells in their bone marrow biopsy and abnormal IgM macroglobulin in their blood [20]. Such as other low-grade lymphoproliferative malignancies, WM is an incurable disease, although with a variable clinical course. Thus, there are patients who remain asymptomatic for 5–10 years, while high-risk patients have a median survival of around 3 years [21,22]. Until 2012, very little was known about the genomics underlying the disease. The discovery of the gain-of-function MYD88L265P mutation was a turning point for WM, providing valuable insights into the signaling pathways involved in this malignancy [11].
The L265P mutation makes the TIR domain of MyD88 more activate compared to the wild-type protein, which increases the formation of the ‘Myddosome’ complex and the downstream signaling [23,24,25]. Thus, the functional effects of MYD88L265P include increased NF-κB activity; Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling; and the production of proinflammatory cytokines, such as IL-6, IL-10 and interferon (IFN)-β, as well as the enhanced growth and survival of lymphoma cells [23,26].
In addition to the NF-κB pathway, the B-cell receptor (BCR) pathway also plays an important role in the oncogenesis of B-NHL with MYD88 mutations. In normal conditions, the BCR signaling activates NF-κB, phosphoinositide 3-kinase (PI3K), MAPK, and nuclear factor of activated T cells (NFAT) pathways. Bruton’s tyrosine kinase (BTK), a protein of the BCR signaling cascade, has been shown to preferentially form complexes with mutated MyD88 and not with wild-type MyD88 in WM cells. MYD88L265P triggers BTK and downstream NF-kB signaling independent of IRAK1 and -4 [26]. In addition, MYD88L265P is common in patients with mutations in BCR-associated protein CD79B, and these patients benefit most from the treatment with BTK inhibitors [27,28,29]. The mechanism of combined MyD88 and BCR pathway activation may be explained by the existence of a ‘MyD88-TLR9-BCR (My-T-BCR) supercomplex’ that encompasses mutated MyD88 and BCR components and contributes to broader signaling, including the mammalian target of rapamycin (mTOR) and NF-κB pathways, thereby promoting lymphomagenesis [30].
There is also evidence of cross-communication with the BCR pathway triggered by mutated MyD88 through the activation of the BCR-signaling component SYK (spleen tyrosine kinase) in WM and activated B-cell diffuse large B-cell lymphoma (ABC DLBCL) cells [31]. SYK can also be activated by the hematopoietic cell kinase (HCK) in MyD88-mutated lymphoma cells [32], and, at the same time, HCK can be triggered either by mutated MyD88 directly or through IL-6. For its part, the HCK protein promotes lymphomagenesis via multiple signaling pathways, including BTK, PI3K, and MAPK [33].
To complete the picture, NF-κB activity not only activates B-cell proliferation and survival-related genes but also results in IL-6 and Il-10 autocrine signaling, which, via JAK/STAT or HCK, increases the transcription of genes involved in several signaling cascades, such as PI3K/AKT/mTOR, JAK/STAT, or NF-κB [34].
The MYD88 alteration has been found at high frequencies in cutaneous DLBCL (69%), primary testicular lymphoma (68%), primary central nervous system lymphoma (38–86%), or ABC DLBCL (30%), indicating its role in the pathogenesis of lymphoid neoplasia [23,35,36,37,38,39]. In WM, a small number of patients (7%) lack MYD88 mutations. These patients present a different genomic landscape, including other NF-κB-activating mutations, epigenomic dysregulation, and impaired DNA damage repair, which leads to inferior outcomes compared to those with MyD88 mutations—specifically, a shorter overall survival (OS) and a higher risk of histological transformation [40,41,42,43]. These findings have prompted some authors to consider the disease with the MYD88 wild-type genotype to be an entirely separate entity, proposing the presence of the MyD88 mutation as a WM-defining feature [44,45]. Few cancers have a single amino acid substitution in one gene present in most cases, making WM paradigmatic for studying the role of a single causative mutation in oncogenesis. The presence of MYD88L265P in IgM-MGUS suggests that it is an early oncogenic factor, but most IgM-MGUS patients never progress to WM or other lymphoproliferative disorders, so this mutation cannot be considered a unique pathogenic factor in WM [46,47]. This is consistent with animal models, where MYD88 mutations are not sufficient for the development of WM or other MyD88-driven lymphomas, and additional alterations in tumor cells and/or the host response are needed [48,49].
3. Clinical Applications
The MYD88L265P mutation has become the hallmark of WM, as it is present in more than 90% of patients. Therefore, numerous clinical applications have emerged concerning its diagnosis, management, and treatment.
3.1. Diagnosis
Currently, the diagnosis of WM is contingent on demonstrating a lymphoplasmacytic cell infiltrate, usually by a bone marrow biopsy, which has several disadvantages, such as patient discomfort, unforeseen complications, high cost, and delay in the diagnosis. In contrast to WM and IgM-MGUS (>90%) [12,13,14,15,46], MYD88L265P is absent or less frequent in other related B-cell (including IgM-secreting) disorders, such as mucosa-associated lymphoid tissue lymphoma, splenic marginal zone lymphoma, nodal marginal zone lymphoma, IgM-secreting multiple myeloma, and chronic lymphocytic leukemia, therefore representing a useful tool for discriminating WM from other related B-cell disorders at diagnosis (Table 1) [23,24,50,51,52,53,54,55,56]. In addition, several studies support the feasibility of using allele-specific polymerase chain reaction (AS-PCR), digital PCR (dPCR), and next-generation sequencing assays for MYD88L265P detection in peripheral blood and circulating tumor DNA, thereby providing convenient and less invasive methods for the diagnosis of WM and IgM-MGUS [57,58,59,60,61], similar to other mutations associated with hematological conditions, e.g., chronic myeloid leukemia (BCR-ABL), polycythemia vera (JAK2V617F), and hairy cell leukemia (BRAFV600E) [62,63,64].
Table 1.
The frequency of MYD88L265P in B-cell lymphoproliferative disorders.
| Entity | N | MYD88L265P Range | References |
|---|---|---|---|
| Waldenström’s macroglobulinemia | 470 | 67–100% | [11,12,13,14,15,50,51,52,56] |
| IgM-MGUS | 164 | 10–87% | [11,12,13,14,15,46] |
| MALT lymphoma | 105 | 0–9% | [23,50,54] |
| MZL | 325 | 0–21% | [11,12,13,14,15,50,53,54] |
| Multiple myeloma (including IgM) | 188 | 0% | [11,13,14,15,51,53,55,56] |
| Chronic lymphocytic leukemia | 412 | 0–43% 1 | [13,14,15,24,52,53,56] |
1 Forty-three percent in a series of CLL with an IgM component. IgM-MGUS, IgM monoclonal gammopathy of undetermined significance; MALT, mucosa-associated lymphoid tissue; MZL, marginal zone lymphoma.
MYD88L265P detection in the cerebrospinal fluid by dPCR is also useful to diagnose Bing-Neel syndrome and central nervous system (CNS) lymphomas as an alternative to a cerebral or retinal biopsy [65,66], since, in clinical practice, the collection of tumor tissue is a highly invasive procedure hampered by the risk of severe complications. Remarkably, the MyD88 mutation never occurs in tissue biopsies from nonhematologic brain tumors, such as a glioblastoma, or in solid metastatic tumors, suggesting it is a sensitive and specific biomarker for the differentiation of primary central nervous system lymphoma from other CNS cancers [67]. Furthermore, the high frequency of MYD88L265P in these diseases makes this mutation a perfect candidate for liquid biopsy to enter clinical practice [35].
3.2. Follow-Up
Although minimal residual disease (MRD) monitoring in certain lymphomas, such as follicular lymphoma, has been gradually established, it has only started to be explored in WM [68]. Several studies have shown the role of MYD88L265P as a predictive biomarker of the therapy response in the bone marrow and peripheral blood compartments [13,15,57]. The application of MYD88L265P testing has been demonstrated to be more useful than serum IgM in estimating the underlying disease burden, especially with agents affecting the serum IgM levels, either by inducing an IgM flare or by blocking IgM secretion, such as rituximab, bortezomib, everolimus, and ibrutinib [69]. Increased serum IgM can be mistaken for disease progression, leading to a drug change, whereas a blockage in IgM secretion out of proportion to the tumor load lends to underestimating the posttreatment disease burden, missing the disease progression in some instances. Therefore, the use of peripheral blood MYD88L265P testing to estimate the underlying disease burden in patients undergoing those treatments could help guide the clinical management and avoid the repetition of bone marrow biopsies to clarify IgM discordance [57]. Nevertheless, the value of using MYD88L265P in the response assessment still needs to be validated in a larger series of treated WM patients and, ideally, across multiple therapeutic regimens. In addition to peripheral blood samples, circulating tumor DNA may represent another attractive, noninvasive alternative to bone marrow [68].
3.3. Prognosis
Data regarding the prognostic value of MyD88 mutation are quite controversial. The initial studies showed that MYD88L265P was associated with a higher risk of disease progression in IgM-MGUS and asymptomatic WM [11,12,13,70,71]. However, other authors have identified the absence of the alteration as an independent risk factor for progression to WM or other lymphoproliferative disorders, although, in some studies, this observation did not reach statistical significance, likely as a reflection of the small sample size [72,73]. There is also another perspective that considers that those patients who progressed were precursors of WM rather than transformations from IgM-MGUS to WM, thus suggesting that the acquisition of MYD88L265P would not represent a transformation event [46]. Therefore, further studies are needed to clear up this matter. An assessment of the MYD88 status should be included in the initial work-up of all patients with IgM-MGUS/asymptomatic WM to help clarify whether mutated patients show a higher risk of evolution to WM. Furthermore, patients with wild-type MYD88 should be followed closely because of their higher risk of histological transformation and development of therapy-related myelodysplastic syndrome [41,42,43,44,74].
The impact of MYD88 mutation on the disease outcome is also unclear. Although it is fairly well-established that the wild-type genotype exhibits an increased risk of death (estimated 10-year survival is 73% for MYD88wild-type vs. 90% for mutated MYD88) [40,75], other groups have observed similar OS and similar times to the next treatment after the frontline therapy in MYD88L265P and MYD88wild-type patient populations [44,76].
The prognostic significance of the MYD88 status in other diseases has not been fully elucidated either. This is very well-exemplified by CLL, where studies have shown strong contradictions, both in the frequency of MYD88 mutations (from 1.5 to 10% of patients) [24,77] and in the prognostic significance: from favorable [78] to neutral [79] or unfavorable [80]. In ABC DLBCL, a negative effect of MYD88L265P on patient survival seems evident, but it is still considered a matter of debate [39,81,82,83].
3.4. Treatment
There are multiple treatment options for WM patients, such as chemotherapy, monoclonal antibodies, proteasome inhibitors, and BTK inhibitors. The choice for therapy should consider the clinical presentation, comorbidities, and preferences. However, there is increasing evidence that the genomic profile may provide insightful information for treatment selection. Thus, the detection of MYD88L265P may help identify those patients who are more suitable for treatments targeting the MyD88-driven pathways, as described below.
Thanks to the discovery of the MyD88 mutation in WM, BTK inhibitors have become an important treatment option. Much of their efficacy is due to the presence of this alteration, which has been shown to serve as a predictor of the response in patients treated with a BTK inhibitor-based regimen. In previously treated WM, the major response rate to ibrutinib therapy was found to be substantially higher for MYD88L265P-CXCR4wild-type (97%) and MYD88L265P-CXCR4WHIM (68%) compared to patients with the MYD88 wild-type genotype (0%) [43,84,85]. In treatment-naïve patients, the overall response rate and major response rate were, respectively, 100% and 94% for MYD88L265P-CXCR4wild-type patients [86]. Nevertheless, another study observed no influence of the genotype in the response rate and time to respond when comparing ibrutinib–rituximab vs. placebo–rituximab [87]. Zanubrutinib, a second-generation BTK inhibitor, has demonstrated high-quality responses in MYD88wild-type WM patients, including 27% of the very good partial responses and 50% of the major responses [88]. The overall response rate was similar regardless of the genotype. However, the proportions of a very good partial response and complete response were still different, since they were higher for MYD88L265P-CXCR4WHIM patients (59%) compared to MYD88wild-type (25%) [89]. Acalabrutinib, another second-generation BTK inhibitor, also provides a good response rate in WM, although no sufficient data are yet known about the potential influence of genomics [90].
The National Comprehensive Cancer Network® Guidelines for WM (version 2.2022) include MYD88L265P testing of the bone marrow and a genomic-based treatment approach to symptomatic treatment-naïve and relapsed or refractory WM [91]. Concordantly, the last international workshop on a WM consensus panel recommended against the use of ibrutinib monotherapy in MYD88wild-type patients [16].
In ABC DLBCL, the MYD88/CD79B double mutant shows an intense sensitivity to BTK inhibitors, while patients with mutated MyD88 but without BCR mutations (i.e., CD79A or CD79B) are not responsive [28,92]. The molecular basis of this observation might be the induction by abnormal MyD88 of a chronically active BCR signaling through the formation of the ‘My-T-BCR supercomplex’, thus mitigating its sensitivity to ibrutinib [30]. In WM, alterations concerning the key negative regulators of BTK, MyD88, and NF-κB, as well as the ubiquitin ligase and TLR pathway regulators, are involved in ibrutinib resistance [93,94].
3.5. Therapeutic Target
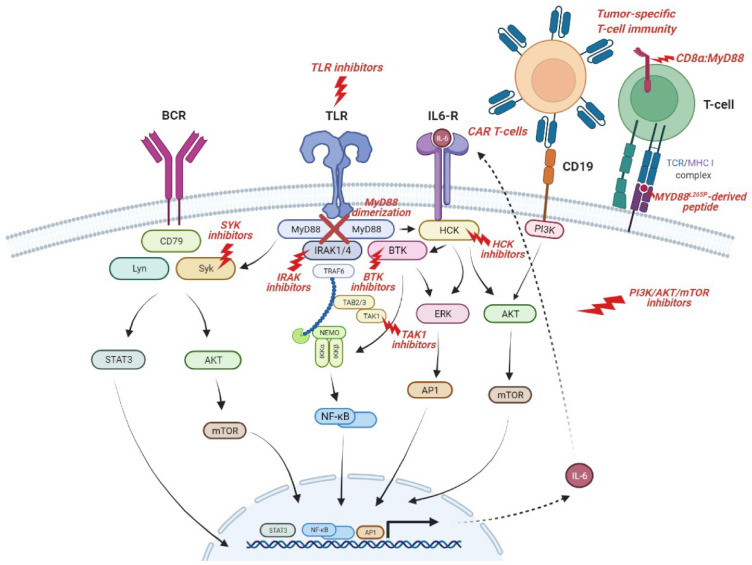
MYD88L265P is also significant given the interest in therapies targeting the components of pathways activated by this mutation (Figure 2). Therapeutic targets that have been, or are currently being, investigated include BTK in the BCR pathway (as already reviewed); TLRs, IL-1R, and their ligands; IRAK1 and IRAK4 in the ‘Myddosome’ complex; TAK1 in downstream signaling; and components of the HCK and PI3K/AKT/mTOR pathways [95,96,97,98,99,100,101].
Figure 2.
Representation of the MyD88 and related pathways that can be targeted by specific drugs. Components of the pathways activated by MYD88L265P mutation, such as BTK, IRAKs, and HCK, have proven to be relevant targets for lymphomas. MyD88 is also being used for T-cell immunotherapy as part of CAR T cells, synthetic coreceptors (CD8α:MyD88), or peptides that can induce T-cell responses.
The blockade of TLRs ligand activation results in cell signaling inhibition, tumor growth reduction, and the induction of apoptosis in MYD88L265P WM cells. Oligonucleotides that inhibit TLR7/8/9 have already been tested in clinical trials for MYD88-mutated DLBCL and WM patients [102,103]. Nevertheless, it is possible that L265P induces NF-κB activation completely independently of any upstream receptor, so that therapeutic options targeting the downstream pathway may be mechanistically more effective.
MyD88 is involved in the IRAK-mediated activation of TLR signaling. The initial IRAK inhibitors showed promising results, blocking IRAK4 in vitro and in xenografts with human DLBCL cell lines [95,97]. Recent studies have demonstrated the potential superior antitumor activity of these compounds in combination with ibrutinib, bortezomib, or venetoclax in preclinical models of WM, MYD88-mutated DLBCL, and CLL [104,105,106,107,108]. However, IRAKs are not involved in all MyD88-dependent signaling, so that MyD88 dimerization may represent a more favorable target. Mini-peptides that compete with MyD88 TIR domain interactions prevent MyD88 dimerization and ‘Myddosome’ signaling, leading to the inhibition of NF-κB activity and cell survival [109,110,111]. L265P-mutant cell lines have been shown to be much more sensitive to this inhibition than wild-type cells [112].
HCK activation is triggered by mutated MyD88 and promotes malignant cell growth and survival through BTK. KIN-8194 is a novel dual inhibitor of HCK and BTK that has shown the potent and selective in vitro killing of MYD88-mutated lymphoma cells, including ibrutinib-resistant BTKC481S-expressing cells, and demonstrated excellent bioavailability, pharmacokinetic parameters, and good tolerance at active doses [113]. Pirtobrutinib, a third-generation noncovalent BTK inhibitor, has a different binding site in BTK, being able to overcome the resistance associated with mutations at C481. Pirtobrutinib inhibits growth and can trigger the apoptosis of MyD88-mutated lymphoma cells in a highly selective manner, thus improving their efficacy and tolerance [114,115].
A novel personalized therapeutic strategy are MYD88L265P-derived peptides. These tumor-specific neoepitopes are identified as foreign by the immune system, inducing tumor-specific T-cell immunity. It has been demonstrated that healthy individuals harbor T cells with specific T-cell receptors (TCRs) that are able to recognize L265P-containing neoantigens and elicit human leukocyte antigen (HLA) class I-restricted cytotoxic T-cell responses (when presented by HLA-B*07 and -B*15), supporting the potential for TCR-based immunotherapy. The peptide-specific cytolytic activity of CD8+ T cells was not observed when wild-type MyD88 peptides were presented [116,117]. Nevertheless, further studies are needed to better understand the functional properties of MYD88L265P-specific T cells in the tumor niche, identify specific major histocompatibility complex haplotypes for TCRs that could be used for immunotherapy, and quantify the extent to which mutant MyD88 is a target of specific immunity [118,119].
Finally, chimeric antigen receptor (CAR) T cells have provided a new opportunity to specifically exploit T-cell-intrinsic TLR functions, i.e., activating TLR signaling in tumor-recognizing T cells [120,121,122,123]. This can be achieved by expressing TLR signaling or MyD88 domains within or alongside the CAR. CAR-modified T-cell therapy using a second-generation CAR derived from a CD19-directed antibody fused to the ζ chain of CD3 and the intracellular signaling domain of CD28 (19-28z) has shown robust preclinical activity against WM cells [124]. In another study, MyD88 was employed alongside CD40 in an inducible costimulatory complex consisting of a chemical inducer of the dimerization-binding domain and co-expressed with a first-generation CAR construct in T cells [125]. These inducible MyD88/CD40 CAR T cells exhibited superior T-cell proliferation, cytokine production, and tumor killing ability compared to second-generation CAR T cells that did not contain the inducible MyD88/CD40 molecule. CD19- and CD123-targeting MyD88/CD40 CAR T cells have also been tested [122]. In addition to CARs, MyD88 domains are being successfully used in other synthetic T-cell-stimulatory molecules. CD8α:MyD88, a synthetic coreceptor that joins the extracellular and transmembrane domains of CD8α and the intermediate and death domains of MyD88, is able to activate the TLR-signaling pathway in T cells. The CD8α portion interacts with the TCR, leading to TLR pathway activation through the fused MyD88 intracellular domain and resulting in increased effector function and decreased T-cell exhaustion [126].
4. Current State-of-the-Art of MyD88
The MYD88L265P mutation is a disease-defining genetic alteration of WM (95–97%) and IgM-MGUS (90%) that can be used for the diagnosis and monitoring of the disease [11,12,13,14,15]. Despite being a unifying event, its role in the disease pathogenesis is not entirely clear. Its presence in the precursor condition (IgM-MGUS) suggests that is a ‘driver mutation’ or tumor-initiating event, which might provide the early tumor clone a competitive growth advantage and predispose it toward further genetic alterations, since it is insufficient by itself for the full development of lymphomas [48,49]. It has also been considered a progression event, although no other potential driver events (i.e., highly prevalent mutations or genetic abnormalities) have been identified [11].
Recent works have provided interesting and unexpected findings about the mutation. MYD88L265P was shown to be present in B-cell precursors in 7/10 patients and in residual normal B cells in 6/6 patients [127]. Another study also reported that MYD88 mutations were present in pre-B progenitors, being detected in more than 20% of the progenitor cells, and in the nonclonal B cells of WM patients [118]. These data demonstrate that the MYD88 mutation can occur early in lymphopoiesis in WM, before the expansion of the malignant B-cell clone, and that it is not restricted to the clonal population. They also reinforce the idea that MYD88L265P alone does not conduce a malignant transformation. Other genetic changes (e.g., del(6q), CXCR4, CD79B, ARID1A, TNFAIP3, TP53, and BCL2) are required to cooperate with MYD88L265P, providing an advantage for B-cell clonal selection and leading to the different types of B-cell malignancies [27,127,128]. In fact, most somatic mutations detected in progenitor cells are undetectable in mature B lymphocytes, suggesting continuous B-cell clonal selection during lymphopoiesis. However, since not all of the tumor cells harbor other genetic changes, it is unknown what makes the difference between a normal cell and a tumor cell when both are MyD88-mutated or, in other words, what makes ‘normal’ cells acquire the tumor phenotype. It has been shown that the acquisition of the MYD88 mutation in hematopoietic progenitors is associated with changes in the immune microenvironment that lead to progressive growth and evolution of the B-cell clone [118]. In addition, studies have suggested that B cells harboring the MYD88L265P mutation still require TLR signals to maintain their proliferation and continued survival [30,129,130]. Therefore, it could be hypothesized that lymphomagenesis is driven by the interaction of microbial or viral ligands with the TLR and the subsequent changes in the upstream pathway of MyD88-mutated lymphomas, which may be different in tumor cells and ‘normal’ cells. Moreover, as not all mutated IgM-MGUS progress to the symptomatic disease, it must be discussed whether MYD88L265P should always be considered a preneoplastic event and, therefore, a biomarker for the early detection of B-cell lymphomas. The presence of mutations in earlier progenitors opens up new lines of investigation, which should be extended to other MYD88-mutated lymphomas.
Another important consequence derived from these findings is whether the presence of MYD88L265P in ‘normal’ cells of patients could lead to false-positive results in MRD monitoring. An analysis of MYD88 has shown a limited prognostic value for evaluating the treatment efficacy when compared to multiparametric flow cytometry (MFC) [127]. As MYD88L265P is present in phenotypically normal B cells, a positive PCR result does not imply the persistence of clonal tumor cells in the B-cell compartment. Nevertheless, further studies are required to confirm these results, increasing the sensitivity of MFC to ensure that real residual clonal cells are detected and assessing both B-lymphocyte and plasma cell populations [131,132,133].
5. Summary
MYD88 is an important oncogene that can be recurrently mutated in several types of lymphoma. An assessment of the MYD88L265P alteration has been shown to improve lymphoma diagnosis and treatment and is becoming increasingly requested for certain entities, such as IgM monoclonal disorders, among all B-cell lymphoproliferative disorders. Its role in disease pathogenesis is not yet well-established, since recent findings have reported the presence of the mutation in earlier progenitors and mature B lymphocytes, suggesting that further alterations or changes in the tumor immune microenvironment are needed to drive the oncogenic transformation. Finally, MyD88-mutant progenitors may be an interesting target for therapy to improve WM curability.
Acknowledgments
The authors thank the Hematology Department of the University Hospital of Salamanca for its support during the writing of this manuscript. The figures were created using BioRender.com.
Author Contributions
Conceptualization, methodology, and investigation, C.J., M.A., R.M., A.M. and M.G.-Á.; writing—original draft preparation, C.J.; writing—review and editing, R.G.-S. and M.A.; supervision, M.C.C., M.E.S., M.G. and V.G.-C.; and funding acquisition, M.A. and R.G.-S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the Instituto de Salud Carlos III through the projects PI18/01866 and PI22/00568 (co-funded by the European Union: European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future”), the Spanish Ministry of Economy and Competitiveness CIBERONC-CB16/12/00233, and “Una manera de hacer Europa” (Innocampus; CEI-2010-1-0010)”. C.J. was supported by the Instituto de Salud Carlos III (Contrato Sara Borrell CD19/00030).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C.A. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 1998;2:253–258. doi: 10.1016/S1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 2.Burns K., Janssens S., Brissoni B., Olivos N., Beyaert R., Tschopp J. Inhibition of interleukin 1 receptor/toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardiman G., Jenkins N.A., Copeland N.G., Gilbert D.J., Garcia D.K., Naylor S.L., Kastelein R.A., Bazan J.F. Genetic structure and chromosomal mapping of MyD88. Genomics. 1997;45:332–339. doi: 10.1006/geno.1997.4940. [DOI] [PubMed] [Google Scholar]
- 4.Muzio M., Ni J., Feng P., Dixit V.M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 5.Wesche H., Henzel W.J., Shillinglaw W., Li S., Cao Z. MyD88: An adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/S1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 6.Baeuerle P.A., Baltimore D. Nf-κB: Ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 7.Grilli M., Chiu J.J.S., Lenardo M.J. IMF-κB and Rel: Participants in a Multiform Transcriptional Regulatory System. Int. Rev. Cytol. 1993;143:1–62. doi: 10.1016/S0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 8.Deguine J., Barton G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen P. The TLR and IL-1 signalling network at a glance. J. Cell Sci. 2014;127:2383–2390. doi: 10.1242/jcs.149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balka K.R., De Nardo D. Understanding early TLR signaling through the Myddosome. J. Leukoc. Biol. 2019;105:339–351. doi: 10.1002/JLB.MR0318-096R. [DOI] [PubMed] [Google Scholar]
- 11.Treon S.P., Xu L., Yang G., Zhou Y., Liu X., Cao Y., Sheehy P., Manning R.J., Patterson C.J., Tripsas C., et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N. Engl. J. Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 12.Varettoni M., Arcaini L., Zibellini S., Boveri E., Rattotti S., Riboni R., Corso A., Orlandi E., Bonfichi M., Gotti M., et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenström’s macroglobulinemia and related lymphoid neoplasms. Blood. 2013;88:2522–2528. doi: 10.1182/blood-2012-09-457101. [DOI] [PubMed] [Google Scholar]
- 13.Xu L., Hunter Z.R., Yang G., Zhou Y., Cao Y., Liu X., Morra E., Trojani A., Greco A., Arcaini L., et al. MYD88 L265P in Waldenstrom’s Macroglobulinemia, IgM Monoclonal Gammopathy, and other B-cell Lymphoproliferative Disorders using Conventional and Quantitative Allele-Specific PCR. Blood. 2013;121:2051–2058. doi: 10.1182/blood-2012-09-454355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulain S., Roumier C., Decambron A., Renneville A., Herbaux C., Bertrand E., Tricot S., Daudignon A., Galiègue-Zouitina S., Soenen V., et al. MYD88 L265P mutation in Waldenstrom macroglobulinemia. Blood. 2013;121:4504–4511. doi: 10.1182/blood-2012-06-436329. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez C., Sebastián E., Chillón M.C., Giraldo P., Mariano Hernández J., Escalante F., González-López T.J., Aguilera C., García de Coca A., Murillo I., et al. MYD88 L265P is a marker highly characteristic of, but not restricted to, waldenström’s macroglobulinemia. Leukemia. 2013;27:1722–1728. doi: 10.1038/leu.2013.62. [DOI] [PubMed] [Google Scholar]
- 16.Castillo J.J., Advani R.H., Branagan A.R., Buske C., Dimopoulos M.A., D’Sa S., Kersten M.J., Leblond V., Minnema M.C., Owen R.G., et al. Consensus treatment recommendations from the tenth International Workshop for Waldenström Macroglobulinaemia. Lancet. Haematol. 2020;7:e827–e837. doi: 10.1016/S2352-3026(20)30224-6. [DOI] [PubMed] [Google Scholar]
- 17.Von Bernuth H., Picard C., Jin Z., Pankla R., Xiao H., Ku C.L., Chrabieh M., Ben Mustapha I., Ghandil P., Camcioglu Y., et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loiarro M., Gallo G., Fantò N., De Santis R., Carminati P., Ruggiero V., Sette C. Identification of critical residues of the MyD88 death domain involved in the recruitment of downstream kinases. J. Biol. Chem. 2009;284:28093–28103. doi: 10.1074/jbc.M109.004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin S.C., Lo Y.C., Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen R.G., Treon S.P., Al-Katib A., Fonseca R., Greipp P.R., McMaster M.L., Morra E., Pangalis G.A., San Miguel J.F., Branagan A.R., et al. Clinicopathological definition of Waldenström’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 21.Kyle R.A., Benson J.T., Larson D.R., Therneau T.M., Dispenzieri A., Kumar S., Melton L.J., Rajkumar S.V. Progression in smoldering Waldenström’s macroglobulinemia: Long-term results. Blood. 2012;119:4462–4466. doi: 10.1182/blood-2011-10-384768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morel P., Duhamel A., Gobbi P., Dimopoulos M.A., Dhodapkar M.V., McCoy J., Crowley J., Ocio E.M., Garcia-Sanz R., Treon S.P., et al. International prognostic scoring system for Waldenström macroglobulinemia. Blood. 2009;113:4163–4170. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 23.Ngo V.N., Young R.M., Schmitz R., Jhavar S., Xiao W., Lim K.H., Kohlhammer H., Xu W., Yang Y., Zhao H., et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puente X.S., Pinyol M., Quesada V., Conde L., Ordóñez G.R., Villamor N., Escaramis G., Jares P., Beà S., González-Díaz M., et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousseau S., Martel G. Gain-of-function mutations in the toll-like receptor pathway: TPL2-mediated ERK1/ERK2 MAPK activation, a path to tumorigenesis in lymphoid neoplasms? Front. Cell Dev. Biol. 2016;4:50. doi: 10.3389/fcell.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang G., Zhou Y., Liu X., Xu L., Cao Y., Manning R.J., Patterson C.J., Buhrlage S.J., Gray N., Tai Y.-T., et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood. 2013;122:1222–1232. doi: 10.1182/blood-2012-12-475111. [DOI] [PubMed] [Google Scholar]
- 27.Wang J.Q., Jeelall Y.S., Humburg P., Batchelor E.L., Kaya S.M., Yoo H.M., Goodnow C.C., Horikawa K. Synergistic cooperation and crosstalk between MYD88L265P and mutations that dysregulate CD79B and surface IgM. J. Exp. Med. 2017;214:2759–2776. doi: 10.1084/jem.20161454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson W.H., Young R.M., Schmitz R., Yang Y., Pittaluga S., Wright G., Lih C.-J.J., Williams P.M., Shaffer A.L., Gerecitano J., et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez C., Prieto-Conde M.I., García-Álvarez M., Alcoceba M., Escalante F., Chillón M.D.C., García de Coca A., Balanzategui A., Cantalapiedra A., Aguilar C., et al. Unraveling the heterogeneity of IgM monoclonal gammopathies: A gene mutational and gene expression study. Ann. Hematol. 2018;97:475–484. doi: 10.1007/s00277-017-3207-3. [DOI] [PubMed] [Google Scholar]
- 30.Phelan J.D., Young R.M., Webster D.E., Roulland S., Wright G.W., Kasbekar M., Shaffer A.L., Ceribelli M., Wang J.Q., Schmitz R., et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560:387–391. doi: 10.1038/s41586-018-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munshi M., Liu X., Chen J.G., Xu L., Tsakmaklis N., Demos M.G., Kofides A., Guerrera M.L., Jimenez C., Chan G.G., et al. SYK is activated by mutated MYD88 and drives pro-survival signaling in MYD88 driven B-cell lymphomas. Blood Cancer J. 2020;10:12. doi: 10.1038/s41408-020-0277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argyropoulos K., Vogel R., Ziegler C., Altan-Bonnet G., Velardi E., Calafiore M., Dogan A., Arcila M., Patel M., Knapp K., et al. Clonal B cells in Waldenström’s macroglobulinemia exhibit functional features of chronic active B-cell receptor signaling. Leukemia. 2016;30:1116–1125. doi: 10.1038/leu.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang G., Buhrlage S., Tan L., Liu X., Chen J., Xu L., Tsakmaklis N., Chen J.G., Patterson C.J., Brown J.R., et al. HCK is a survival determinant transactivated by mutated MYD88, and a direct target of ibrutinib. Blood. 2016;127:3237–3253. doi: 10.1182/blood-2016-01-695098. [DOI] [PubMed] [Google Scholar]
- 34.Lam L.T., Wright G., Davis R.E., Lenz G., Farinha P., Dang L., Chan J.W., Rosenwald A., Gascoyne R.D., Staudt L.M. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-kκB pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111:3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukumura K., Kawazu M., Kojima S., Ueno T., Sai E., Soda M., Ueda H., Yasuda T., Yamaguchi H., Lee J., et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol. 2016;131:865–875. doi: 10.1007/s00401-016-1536-2. [DOI] [PubMed] [Google Scholar]
- 36.Kraan W., Van Keimpema M., Horlings H.M., Schilder-Tol E.J.M., Oud M.E.C.M., Noorduyn L.A., Kluin P.M., Kersten M.J., Spaargaren M., Pals S.T. High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B-cell lymphoma. Leukemia. 2014;28:719–720. doi: 10.1038/leu.2013.348. [DOI] [PubMed] [Google Scholar]
- 37.Kraan W., Horlings H.M., van Keimpema M., Schilder-Tol E.J.M.M., Oud M.E.C.M.C.M., Scheepstra C., Kluin P.M., Kersten M.J., Spaargaren M., Pals S.T. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J. 2013;3:e139. doi: 10.1038/bcj.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J.H., Jeong H., Choi J.W., Oh H.E., Kim Y.S. Clinicopathologic significance of MYD88 L265P mutation in diffuse large B-cell lymphoma: A meta-analysis. Sci. Rep. 2017;7:1785. doi: 10.1038/s41598-017-01998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz R., Wright G.W., Huang D.W., Johnson C.A., Phelan J.D., Wang J.Q., Roulland S., Kasbekar M., Young R.M., Shaffer A.L., et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treon S.P., Cao Y., Xu L., Yang G., Liu X., Hunter Z.R. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood. 2014;123:2791–2796. doi: 10.1182/blood-2014-01-550905. [DOI] [PubMed] [Google Scholar]
- 41.Treon S.P., Gustine J., Xu L., Manning R.J., Tsakmaklis N., Demos M., Meid K., Guerrera M.L., Munshi M., Chan G., et al. MYD88 wild-type Waldenstrom Macroglobulinaemia: Differential diagnosis, risk of histological transformation, and overall survival. Br. J. Haematol. 2018;180:374–380. doi: 10.1111/bjh.15049. [DOI] [PubMed] [Google Scholar]
- 42.Zanwar S., Abeykoon J.P., Durot E., King R., Perez Burbano G.E., Kumar S., Gertz M.A., Quinquenel A., Delmer A., Gonsalves W., et al. Impact of MYD88L265P mutation status on histological transformation of Waldenström Macroglobulinemia. Am. J. Hematol. 2020;95:274–281. doi: 10.1002/ajh.25697. [DOI] [PubMed] [Google Scholar]
- 43.Hunter Z.R., Xu L., Tsakmaklis N., Demos M.G., Kofides A., Jimenez C., Chan G.G., Chen J., Liu X., Munshi M., et al. Insights into the genomic landscape of MYD88 wild-type Waldenström macroglobulinemia. Blood Adv. 2018;2:2937–2946. doi: 10.1182/bloodadvances.2018022962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abeykoon J.P., Paludo J., King R.L., Ansell S.M., Gertz M.A., LaPlant B.R., Halvorson A.E., Gonsalves W.I., Dingli D., Fang H., et al. MYD88 mutation status does not impact overall survival in Waldenström macroglobulinemia. Am. J. Hematol. 2018;93:187–194. doi: 10.1002/ajh.24955. [DOI] [PubMed] [Google Scholar]
- 45.García-Sanz R. WM, MYD88, and CXCR4: Following the thread. Blood. 2016;128:746–748. doi: 10.1182/blood-2016-06-723924. [DOI] [PubMed] [Google Scholar]
- 46.Landgren O., Staudt L. MYD88 L265P Somatic Mutation in IgM MGUS. N. Engl. J. Med. 2012;367:2255–2257. doi: 10.1056/NEJMc1212050. [DOI] [PubMed] [Google Scholar]
- 47.Fonseca R., Braggio E. The MYDas touch of next-gen sequencing. Blood. 2013;121:2373–2374. doi: 10.1182/blood-2013-02-481986. [DOI] [PubMed] [Google Scholar]
- 48.Knittel G., Liedgens P., Korovkina D., Seeger J.M., Al-Baldawi Y., Al-Maarri M., Fritz C., Vlantis K., Bezhanova S., Scheel A.H., et al. B-cell-specific conditional expression of Myd88p.L252P leads to the development of diffuse large B-cell lymphoma in mice. Blood. 2016;127:2732–2741. doi: 10.1182/blood-2015-11-684183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sewastianik T., Guerrera M.L., Adler K., Dennis P.S., Wright K., Shanmugam V., Huang Y., Tanton H., Jiang M., Kofides A., et al. Human MYD88L265P is insufficient by itself to drive neoplastic transformation in mature mouse B cells. Blood Adv. 2019;3:3360–3374. doi: 10.1182/bloodadvances.2019000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gachard N., Parrens M., Soubeyran I., Petit B., Marfak A., Rizzo D., Devesa M., Delage-Corre M., Coste V., Laforêt M.P., et al. IGHV gene features and MYD88 L265P mutation separate the three marginal zone lymphoma entities and Waldenström macroglobulinemia/lymphoplasmacytic lymphomas. Leukemia. 2013;27:183–189. doi: 10.1038/leu.2012.257. [DOI] [PubMed] [Google Scholar]
- 51.Willenbacher W., Willenbacher E., Brunner A., Manzl C. Improved accuracy of discrimination between IgM Multiple Myeloma and Waldenström Macroglobulinaemia by testing for MYD88 L265P mutations. Br. J. Haematol. 2013;161:902–904. doi: 10.1111/bjh.12313. [DOI] [PubMed] [Google Scholar]
- 52.Argentou N., Vassilopoulos G., Ioannou M., Germenis A.E., Speletas M. Rapid detection of MYD88-L265P mutation by PCR-RFLP in B-cell lymphoproliferative disorders. Leukemia. 2014;28:447–449. doi: 10.1038/leu.2013.294. [DOI] [PubMed] [Google Scholar]
- 53.Ondrejka S.L., Lin J.J., Warden D.W., Durkin L., Cook J.R., Hsi E.D. MYD88 L265P somatic mutation: Its usefulness in the differential diagnosis of bone marrow involvement by B-cell lymphoproliferative disorders. Am. J. Clin. Pathol. 2013;140:387–394. doi: 10.1309/AJCP10ZCLFZGYZIP. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Lopez A., Curiel-Olmo S., Mollejo M., Cereceda L., Martinez N., Montes-Moreno S., Almaraz C., Revert J.B., Piris M.A. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am. J. Surg. Pathol. 2015;39:644–651. doi: 10.1097/PAS.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 55.Je E.M., Yoo N.J., Lee S.H. Absence of MYD88 gene mutation in acute leukemias and multiple myelomas. Eur. J. Haematol. 2012;88:273–274. doi: 10.1111/j.1600-0609.2011.01720.x. [DOI] [PubMed] [Google Scholar]
- 56.Willenbacher E., Willenbacher W., Wolf D.G., Zelger B., Peschel I., Manzl C., Haun M., Brunner A. Digital PCR in bone marrow trephine biopsies is highly sensitive for MYD88 L265P detection in lymphomas with plasmacytic/plasmacytoid differentiation. Br. J. Haematol. 2019;186:189–191. doi: 10.1111/bjh.15792. [DOI] [PubMed] [Google Scholar]
- 57.Xu L., Hunter Z.R., Yang G., Cao Y., Liu X., Manning R., Tripsas C., Chen J., Patterson C.J., Kluk M., et al. Detection of MYD88 L265P in peripheral blood of patients with Waldenström’s Macroglobulinemia and IgM monoclonal gammopathy of undetermined significance. Leukemia. 2014;28:1698–1704. doi: 10.1038/leu.2014.65. [DOI] [PubMed] [Google Scholar]
- 58.Ferrante M., Furlan D., Zibellini S., Borriero M., Candido C., Sahnane N., Uccella S., Genuardi E., Alessandria B., Bianchi B., et al. MYD88 L265P detection in IgM monoclonal gammopathies: Methodological considerations for routine implementation. Diagnostics. 2021;11:779. doi: 10.3390/diagnostics11050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura A., Ohwada C., Takeuchi M., Takeda Y., Tsukamoto S., Mimura N., Nagisa O.H., Sugita Y., Tanaka H., Wakita H., et al. Detection of MYD88 L265P mutation by next-generation deep sequencing in peripheral blood mononuclear cells of Waldenström’s macroglobulinemia and IgM monoclonal gammopathy of undetermined significance. PLoS ONE. 2019;14:e0221941. doi: 10.1371/journal.pone.0221941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagratuni T., Ntanasis-Stathopoulos I., Gavriatopoulou M., Mavrianou-Koutsoukou N., Liacos C., Patseas D., Kanellias N., Migkou M., Ziogas D.C., Eleutherakis-Papaiakovou E., et al. Detection of MYD88 and CXCR4 mutations in cell-free DNA of patients with IgM monoclonal gammopathies. Leukemia. 2018;32:2617–2625. doi: 10.1038/s41375-018-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ntanasis-Stathopoulos I., Bagratuni T., Gavriatopoulou M., Patseas D., Liacos C., Kanellias N., Fotiou D., Tsiligkeridou E., Andreatou A., Mavrianou-Koutsoukou N., et al. Cell-free DNA analysis for the detection of MYD88 and CXCR4 mutations in IgM monoclonal gammopathies; an update with clinicopathological correlations. Am. J. Hematol. 2020;95:E148–E150. doi: 10.1002/ajh.25802. [DOI] [PubMed] [Google Scholar]
- 62.Kiss T.L., Xu W.M., Jamal N., Messner H.A. Comparative testing of peripheral blood and bone marrow for BCR-ABL transcripts in patients post allogeneic bone marrow transplantation and during interferon treatment for chronic myeloid leukemia. Leuk. Lymphoma. 1999;34:493–500. doi: 10.3109/10428199909058476. [DOI] [PubMed] [Google Scholar]
- 63.Passamonti F. How I treat polycythemia vera. Blood. 2012;120:275–284. doi: 10.1182/blood-2012-02-366054. [DOI] [PubMed] [Google Scholar]
- 64.Tiacci E., Schiavoni G., Forconi F., Santi A., Trentin L., Ambrosetti A., Cecchini D., Sozzi E., Francia di Celle P., Di Bello C., et al. Simple genetic diagnosis of hairy cell leukemia by sensitive detection of the BRAF-V600E mutation. Blood. 2012;119:192–195. doi: 10.1182/blood-2011-08-371179. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe J., Natsumeda M., Okada M., Kobayashi D., Kanemaru Y., Tsukamoto Y., Oishi M., Kakita A., Fujii Y. High Detection Rate of MYD88 Mutations in Cerebrospinal Fluid From Patients with CNS Lymphomas. JCO Precis. Oncol. 2019;3:1–13. doi: 10.1200/PO.18.00308. [DOI] [PubMed] [Google Scholar]
- 66.Hiemcke-Jiwa L.S., Minnema M.C., Radersma-van Loon J.H., Jiwa N.M., de Boer M., Leguit R.J., de Weger R.A., Huibers M.M.H. The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol. Oncol. 2018;36:429–435. doi: 10.1002/hon.2489. [DOI] [PubMed] [Google Scholar]
- 67.Hattori K., Sakata-Yanagimoto M., Okoshi Y., Goshima Y., Yanagimoto S., Nakamoto-Matsubara R., Sato T., Noguchi M., Takano S., Ishikawa E., et al. MYD88 (L265P) mutation is associated with an unfavourable outcome of primary central nervous system lymphoma. Br. J. Haematol. 2017;177:492–494. doi: 10.1111/bjh.14080. [DOI] [PubMed] [Google Scholar]
- 68.Drandi D., Genuardi E., Dogliotti I., Ferrante M., Jiménez C., Guerrini F., Lo Schirico M., Mantoan B., Muccio V., Lia G., et al. Highly sensitive MYD88L265P mutation detection by droplet digital polymerase chain reaction in Waldenström macroglobulinemia. Haematologica. 2018;103:1029–1037. doi: 10.3324/haematol.2017.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dimopoulos M.A., Kastritis E. How I treat Waldenström macroglobulinemia. Blood. 2019;134:2022–2035. doi: 10.1182/blood.2019000725. [DOI] [PubMed] [Google Scholar]
- 70.Varettoni M., Zibellini S., Arcaini L., Boveri E., Rattotti S., Pascutto C., Mangiacavalli S., Gotti M., Pochintesta L., Paulli M., et al. MYD88 (L265P) mutation is an independent risk factor for progression in patients with IgM monoclonal gammopathy of undetermined significance. Blood. 2013;122:2284–2285. doi: 10.1182/blood-2013-07-513366. [DOI] [PubMed] [Google Scholar]
- 71.Varettoni M., Zibellini S., Boveri E., Klersy C., Candido C., Rattotti S., Ferretti V.V., Defrancesco I., Mangiacavalli S., Nizzoli M.E., et al. A risk-stratification model based on the initial concentration of the serum monoclonal protein and MYD88 mutation status identifies a subset of patients with IgM monoclonal gammopathy of undetermined significance at high risk of progression to Waldenström. Br. J. Haematol. 2019;187:441–446. doi: 10.1111/bjh.16086. [DOI] [PubMed] [Google Scholar]
- 72.Bustoros M., Sklavenitis-Pistofidis R., Kapoor P., Liu C.J., Kastritis E., Zanwar S., Fell G., Abeykoon J.P., Hornburg K., Neuse C.J., et al. Progression risk stratification of asymptomatic Waldenström macroglobulinemia. J. Clin. Oncol. 2019;37:1403–1411. doi: 10.1200/JCO.19.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zanwar S., Abeykoon J.P., Ansell S.M., Gertz M.A., Colby C., Larson D., Paludo J., He R., Warsame R., Greipp P.T., et al. Disease outcomes and biomarkers of progression in smouldering Waldenström macroglobulinaemia. Br. J. Haematol. 2021;195:210–216. doi: 10.1111/bjh.17691. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Gali V.L., Xu-Monette Z.Y., Sano D., Thomas S.K., Weber D.M., Zhu F., Fang X., Deng M., Zhang M., et al. Molecular and genetic biomarkers implemented from next-generation sequencing provide treatment insights in clinical practice for Waldenström macroglobulinemia. Neoplasia. 2021;23:361–374. doi: 10.1016/j.neo.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chakraborty R., Novak A.J., Ansell S.M., Muchtar E., Kapoor P., Hayman S.R., Dispenzieri A., Buadi F.K., Lacy M.Q., King R.L., et al. First report of MYD88 L265P somatic mutation in IgM-associated light-chain amyloidosis. Blood. 2016;127:2936–2938. doi: 10.1182/blood-2016-02-702035. [DOI] [PubMed] [Google Scholar]
- 76.Varettoni M., Zibellini S., Defrancesco I., Ferretti V.V., Rizzo E., Malcovati L., Gallì A., Giovanni M., Porta D., Boveri E., et al. Pattern of somatic mutations in patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance. Haematologica. 2017;102:2077–2085. doi: 10.3324/haematol.2017.172718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L., Lawrence M.S., Wan Y., Stojanov P., Sougnez C., Stevenson K., Werner L., Sivachenko A., DeLuca D.S., Zhang L., et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N. Engl. J. Med. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martínez-Trillos A., Pinyol M., Navarro A., Aymerich M., Jares P., Juan M., Rozman M.M., Colomer D., Delgado J., Giné E., et al. Mutations in TLR/MYD88 pathway identify a subset of young chronic lymphocytic leukemia patients with favorable outcome. Blood. 2014;123:3790–3796. doi: 10.1182/blood-2013-12-543306. [DOI] [PubMed] [Google Scholar]
- 79.Baliakas P., Hadzidimitriou A., Agathangelidis A., Rossi D., Sutton L.A., Kminkova J., Scarfo L., Pospisilova S., Gaidano G., Stamatopoulos K., et al. Prognostic relevance of MYD88 mutations in CLL: The jury is still out. Blood. 2015;126:1043–1044. doi: 10.1182/blood-2015-05-648634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qin S.C., Xia Y., Miao Y., Zhu H.Y., Wu J.Z., Fan L., Qiao C., Xu W., Li J.Y. MYD88 mutations predict unfavorable prognosis in chronic lymphocytic leukemia patients with mutated IGHV gene. Blood Cancer J. 2017;7:651. doi: 10.1038/s41408-017-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernández-Rodríguez C., Bellosillo B., García-García M., Sánchez-González B., Gimeno E., Vela M.C., Serrano S., Besses C., Salar A. MYD88 (L265P) mutation is an independent prognostic factor for outcome in patients with diffuse large B-cell lymphoma. Leukemia. 2014;28:2104–2106. doi: 10.1038/leu.2014.184. [DOI] [PubMed] [Google Scholar]
- 82.Rovira J., Karube K., Valera A., Colomer D., Enjuanes A., Colomo L., Martínez-Trillos A., Giné E., Dlouhy I., Magnano L., et al. MYD88 L265P mutations, but no other variants, identify a subpopulation of DLBCL patients of activated B-cell origin, extranodal involvement, and poor outcome. Clin. Cancer Res. 2016;22:2755–2764. doi: 10.1158/1078-0432.CCR-15-1525. [DOI] [PubMed] [Google Scholar]
- 83.Vermaat J.S., Somers S.F., De Wreede L.C., Kraan W., De Groen R.A.L., Schrader A.M.R., Kerver E.D., Scheepstra C.G., Berenschot H., Deenik W., et al. MYD88 mutations identify a molecular subgroup of diffuse large B-cell lymphoma with an unfavorable prognosis. Haematologica. 2020;105:424–434. doi: 10.3324/haematol.2018.214122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Treon S.P., Xu L., Hunter Z. MYD88 Mutations and Response to Ibrutinib in Waldenström’s Macroglobulinemia. N. Engl. J. Med. 2015;373:584–586. doi: 10.1056/NEJMc1506192. [DOI] [PubMed] [Google Scholar]
- 85.Treon S.P., Meid K., Gustine J., Yang G., Xu L., Liu X., Patterson C.J., Hunter Z.R., Branagan A.R., Laubach J.P., et al. Long-Term Follow-Up of Ibrutinib Monotherapy in Symptomatic, Previously Treated Patients with Waldenström Macroglobulinemia. J. Clin. Oncol. 2021;39:565–575. doi: 10.1200/JCO.20.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Treon S.P., Gustine J., Meid K., Yang G., Xu L., Liu X., Demos M., Kofides A., Tsakmaklis N., Chen J.G., et al. Ibrutinib Monotherapy in Symptomatic, Treatment-Naïve Patients with Waldenström Macroglobulinemia. J. Clin. Oncol. 2018;36:2755–2761. doi: 10.1200/JCO.2018.78.6426. [DOI] [PubMed] [Google Scholar]
- 87.Buske C., Tedeschi A., Trotman J., García-Sanz R., MacDonald D., Leblond V., Mahe B., Herbaux C., Matous J.V., Tam C.S., et al. Ibrutinib Plus Rituximab Versus Placebo Plus Rituximab for Waldenström’s Macroglobulinemia: Final Analysis from the Randomized Phase III iNNOVATE Study. J. Clin. Oncol. 2022;40:52–62. doi: 10.1200/JCO.21.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dimopoulos M., Sanz R.G., Lee H.P., Trneny M., Varettoni M., Opat S., D’Sa S., Owen R.G., Cull G., Mulligan S., et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenström macroglobulinemia: A substudy of the phase 3 ASPEN trial. Blood Adv. 2020;4:6009–6018. doi: 10.1182/bloodadvances.2020003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trotman J., Opat S., Gottlieb D., Simpson D., Marlton P., Cull G., Munoz J., Tedeschi A., Roberts A.W., Seymour J.F., et al. Zanubrutinib for the treatment of patients with Waldenstrom macroglobulinemia: 3 years of follow-up. Blood. 2020;136:2027–2037. doi: 10.1182/blood.2020006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owen R.G., McCarthy H., Rule S., D’Sa S., Thomas S.K., Tournilhac O., Forconi F., Kersten M.J., Zinzani P.L., Iyengar S., et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: A single-arm, multicentre, phase 2 study. Lancet Haematol. 2020;7:e112–e121. doi: 10.1016/S2352-3026(19)30210-8. [DOI] [PubMed] [Google Scholar]
- 91.Treon S.P., Xu L., Guerrera M.L., Jimenez C., Hunter Z.R., Liu X., Demos M., Gustine J., Chan G., Munshi M., et al. Genomic landscape of Waldenström macroglobulinemia and its impact on treatment strategies. J. Clin. Oncol. 2020;38:1198–1208. doi: 10.1200/JCO.19.02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang S.Q., Smith S.M., Zhang S.Y., Lynn Wang Y. Mechanisms of ibrutinib resistance in chronic lymphocytic leukaemia and non-Hodgkin lymphoma. Br. J. Haematol. 2015;170:445–456. doi: 10.1111/bjh.13427. [DOI] [PubMed] [Google Scholar]
- 93.Jimenez C., Chan G.G., Xu L., Tsakmaklis N., Kofides A., Demos M.G., Chen J., Liu X., Munshi M., Yang G., et al. Genomic evolution of ibrutinib-resistant clones in Waldenström macroglobulinaemia. Br. J. Haematol. 2020;189:1165–1170. doi: 10.1111/bjh.16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.George B., Chowdhury S.M., Hart A., Sircar A., Singh S.K., Nath U.K., Mamgain M., Singhal N.K., Sehgal L., Jain N. Ibrutinib resistance mechanisms and treatment strategies for B-cell lymphomas. Cancers. 2020;12:1328. doi: 10.3390/cancers12051328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelly P.N., Romero D.L., Yang Y., Shaffer A.L., Chaudhary D., Robinson S., Miao W., Rui L., Westlin W.F., Kapeller R., et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J. Exp. Med. 2015;212:2189–2201. doi: 10.1084/jem.20151074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loiarro M., Sette C., Gallo G., Ciacci A., Fantó N., Mastroianni D., Carminati P., Ruggiero V. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-κB. J. Biol. Chem. 2005;280:15809–15814. doi: 10.1074/jbc.C400613200. [DOI] [PubMed] [Google Scholar]
- 97.Scott J.S., Degorce S.L., Anjum R., Culshaw J., Davies R.D.M., Davies N.L., Dillman K.S., Dowling J.E., Drew L., Ferguson A.D., et al. Discovery and Optimization of Pyrrolopyrimidine Inhibitors of Interleukin-1 Receptor Associated Kinase 4 (IRAK4) for the Treatment of Mutant MYD88 L265P Diffuse Large B-Cell Lymphoma. J. Med. Chem. 2017;60:10071–10091. doi: 10.1021/acs.jmedchem.7b01290. [DOI] [PubMed] [Google Scholar]
- 98.Wang J.Q., Beutler B., Goodnow C.C., Horikawa K. Inhibiting TLR9 and other UNC93B1-dependent TLRs paradoxically increases accumulation of MYD88L265P plasmablasts in vivo. Blood. 2016;128:1604–1608. doi: 10.1182/blood-2016-03-708065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhagat L., Wang D., Jiang W., Agrawal S. Abstract 2570: IMO-8400, a selective antagonist of TLRs 7, 8 and 9, inhibits MYD88 L265P mutation-driven signaling and cell survival: A potential novel approach for treatment of B-cell lymphomas harboring MYD88 L265P mutation. Cancer Res. 2014;74:2570. doi: 10.1158/1538-7445.am2014-2570. [DOI] [Google Scholar]
- 100.Olson M.A., Lee M.S., Kissner T.L., Alam S., Waugh D.S., Saikh K.U. Discovery of small molecule inhibitors of MyD88-dependent signaling pathways using a computational screen. Sci. Rep. 2015;5:14246. doi: 10.1038/srep14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ansell S.M., Hodge L.S., Secreto F.J., Manske M., Braggio E., Price-Troska T., Ziesmer S., Li Y., Johnson S.H., Hart S.N., et al. Activation of TAK1 by MYD88 L265P drives malignant B-cell Growth in non-Hodgkin lymphoma. Blood Cancer J. 2014;4:e183. doi: 10.1038/bcj.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brenner L., Arbeit R.D., Sullivan T. IMO-8400, an Antagonist of Toll-like Receptors 7, 8, and 9, in Development for Genetically Defined B-Cell Lymphomas: Safety and Activity in Phase 1 and Phase 2 Clinical Trials. Blood. 2014;124:3101. doi: 10.1182/blood.V124.21.3101.3101. [DOI] [Google Scholar]
- 103.Thomas S.K., Harb W.A., Beck J.T., Nashat G., Palomba M.L., Ansell S.M., Eradat H., Libby E.N., Advani R.H., Hajdenberg J., et al. Preliminary Results from a Phase 1/2, Open-Label, Dose-Escalation Clinical Trial of IMO-8400 in Patients with Relapsed or Refractory Waldenstrom’s Macroglobulinemia. Blood. 2015;126:1540. doi: 10.1182/blood.V126.23.1540.1540. [DOI] [Google Scholar]
- 104.Chen Y., Bai G., Ning Y., Cai S., Zhang T., Song P., Zhou J., Duan W., Ding J., Xie H., et al. Design and synthesis of Imidazo[1,2-b]pyridazine IRAK4 inhibitors for the treatment of mutant MYD88 L265P diffuse large B-cell lymphoma. Eur. J. Med. Chem. 2020;190:112092. doi: 10.1016/j.ejmech.2020.112092. [DOI] [PubMed] [Google Scholar]
- 105.Delvecchio V.S., Sana I., Mantione M.E., Vilia M.G., Ranghetti P., Rovida A., Angelillo P., Scarfò L., Ghia P., Muzio M. Interleukin-1 receptor-associated kinase 4 inhibitor interrupts toll-like receptor signalling and sensitizes chronic lymphocytic leukaemia cells to apoptosis. Br. J. Haematol. 2020;189:475–488. doi: 10.1111/bjh.16386. [DOI] [PubMed] [Google Scholar]
- 106.Dadashian E.L., McAuley E.M., Liu D., Shaffer A.L., Young R.M., Iyer J.R., Kruhlak M.J., Staudt L.M., Wiestner A., Herman S.E.M. TLR signaling is activated in lymph node–resident CLL cells and is only partially inhibited by ibrutinib. Cancer Res. 2019;79:360–371. doi: 10.1158/0008-5472.CAN-18-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giménez N., Schulz R., Higashi M., Aymerich M., Villamor N., Delgado J., Juan M., López-Guerra M., Campo E., Rosich L., et al. Targeting IRAK4 disrupts inflammatory pathways and delays tumor development in chronic lymphocytic leukemia. Leukemia. 2020;34:100–114. doi: 10.1038/s41375-019-0507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ni H., Shirazi F., Baladandayuthapani V., Lin H., Kuiatse I., Wang H., Jones R.J., Berkova Z., Hitoshi Y., Ansell S.M., et al. Targeting myddosome signaling in Waldenstrom’s macroglobulinemia with the interleukin-1 receptor-associated kinase 1/4 inhibitor R191. Clin. Cancer Res. 2018;24:6408–6420. doi: 10.1158/1078-0432.CCR-17-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu X., Hunter Z.R., Xu L., Chen J.G.J., Chen J.G.J., Tsakmaklis N., Patterson C.J., Castillo J.J., Buhrlage S., Gray N., et al. Targeting Myddosome Assembly in Waldenstrom Macroglobulinaemia. Br. J. Haematol. 2017;177:808–813. doi: 10.1111/bjh.14103. [DOI] [PubMed] [Google Scholar]
- 110.Wang X., Tan Y., Huang Z., Huang N., Gao M., Zhou F., Hu J., Feng W. Disrupting myddosome assembly in diffuse large B-cell lymphoma cells using the MYD88 dimerization inhibitor ST2825. Oncol. Rep. 2019;42:1755–1766. doi: 10.3892/or.2019.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shiratori E., Itoh M., Tohda S. MYD88 inhibitor ST2825 suppresses the growth of lymphoma and leukaemia cells. Anticancer Res. 2017;37:6203–6209. doi: 10.21873/anticanres.12070. [DOI] [PubMed] [Google Scholar]
- 112.Avbelj M., Wolz O.O., Fekonja O., Benčina M., Repič M., Mavri J., Krüger J., Schärfe C., Garcia M.D., Panter G., et al. Activation of lymphoma-associated myd88 mutations via allostery-induced tir-domain oligomerization. Blood. 2014;124:3896–3904. doi: 10.1182/blood-2014-05-573188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang G., Wang J., Tan L., Munshi M., Liu X., Kofides A., Chen J.G., Tsakmaklis N., Demos M.G., Guerrera M.L., et al. The HCK/BTK inhibitor KIN-8194 is active in MYD88-driven lymphomas and overcomes mutated BTKCys481 ibrutinib resistance. Blood. 2021;138:1966–1979. doi: 10.1182/blood.2021011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Munshi M., Liu X., Kofides A., Tsakmaklis N., Gustine J., Sarosiek S., Flynn C.A., Meid K., White T.P., Leventoff C., et al. Pirtobrutinib (LOXO-305) Is Active and Overcomes ERK Related Pro-Survival Signaling in Ibrutinib Resistant, BTK Cys481 Mutant Expressing WM and ABC DLBCL Lymphoma Cells Driven By Activating MYD88 Mutations. Blood. 2021;138:2261. doi: 10.1182/blood-2021-153856. [DOI] [Google Scholar]
- 115.Mato A.R., Shah N.N., Jurczak W., Cheah C.Y., Pagel J.M., Woyach J.A., Fakhri B., Eyre T.A., Lamanna N., Patel M.R., et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet. 2021;397:892–901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PubMed] [Google Scholar]
- 116.Nelde A., Walz J.S., Kowalewski D.J., Schuster H., Wolz O.O., Peper J.K., Cardona Gloria Y., Langerak A.W., Muggen A.F., Claus R., et al. HLA class I-restricted MYD88 L265P-derived peptides as specific targets for lymphoma immunotherapy. Oncoimmunology. 2017;6:e1219825. doi: 10.1080/2162402X.2016.1219825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nielsen J.S., Chang A.R., Wick D.A., Sedgwick C.G., Zong Z., Mungall A.J., Martin S.D., Kinloch N.N., Ott-Langer S., Brumme Z.L., et al. Mapping the human T cell repertoire to recurrent driver mutations in MYD88 and EZH2 in lymphoma. Oncoimmunology. 2017;6:e1321184. doi: 10.1080/2162402X.2017.1321184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaushal A., Nooka A.K., Carr A.R., Pendleton K.E., Barwick B.G., Manalo J., McCachren S.S., Gupta V.A., Joseph N.S., Hofmeister C.C., et al. Aberrant Extrafollicular B Cells, Immune Dysfunction, Myeloid Inflammation, and MyD88-Mutant Progenitors Precede Waldenstrom Macroglobulinemia. Blood Cancer Discov. 2021;2:600–615. doi: 10.1158/2643-3230.BCD-21-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Çlnar Ö., Brzezicha B., Grunert C., Kloetzel P.M., Beier C., Peuker C.A., Keller U., Pezzutto A., Busse A. High-affinity T-cell receptor specific for MyD88 L265P mutation for adoptive T-cell therapy of B-cell malignancies. J. Immunother. Cancer. 2021;9:e002410. doi: 10.1136/jitc-2021-002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lai Y., Weng J., Wei X., Qin L., Lai P., Zhao R., Jiang Z., Li B., Lin S., Wang S., et al. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia. 2018;32:801–808. doi: 10.1038/leu.2017.249. [DOI] [PubMed] [Google Scholar]
- 121.George P., Dasyam N., Giunti G., Mester B., Bauer E., Andrews B., Perera T., Ostapowicz T., Frampton C., Li P., et al. Third-generation anti-CD19 chimeric antigen receptor T-cells incorporating a TLR2 domain for relapsed or refractory B-cell lymphoma: A phase i clinical trial protocol (ENABLE) BMJ Open. 2020;10:e034629. doi: 10.1136/bmjopen-2019-034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Collinson-Pautz M.R., Chang W.C., Lu A., Khalil M., Crisostomo J.W., Lin P.Y., Mahendravada A., Shinners N.P., Brandt M.E., Zhang M., et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia. 2019;33:2195–2207. doi: 10.1038/s41375-019-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weng J., Lai P., Qin L., Lai Y., Jiang Z., Luo C., Huang X., Wu S., Shao D., Deng C., et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018;11:25. doi: 10.1186/s13045-018-0572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith E.L., Palomba M.L., Park J.H., Brentjens R.J. A Systemic Xenograft Model of Waldenström’s Macroglobulinemia Demonstrates the Potent Anti-Tumor Effect of Second Generation CD19 Directed Chimeric Antigen Receptor Modified T Cells in This Disease. Blood. 2014;124:4484. doi: 10.1182/blood.V124.21.4484.4484. [DOI] [Google Scholar]
- 125.Mata M., Gerken C., Nguyen P., Krenciute G., Spencer D.M., Gottschalk S. Inducible activation of myD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov. 2017;7:1306–1319. doi: 10.1158/2159-8290.CD-17-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaczanowska S., Joseph A.M., Guo J., Tsai A.K., Lasola J.J., Younger K., Zhang Y., Gonzales C.V., Davila E. A synthetic CD8α: MyD88 coreceptor enhances CD8+ T-cell responses to weakly immunogenic and lowly expressed tumor antigens. Cancer Res. 2017;77:7049–7058. doi: 10.1158/0008-5472.CAN-17-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodriguez S., Celay J., Goicoechea I., Jimenez C., Botta C., Garcia-Barchino M.-J., Garces J.-J., Larrayoz M., Santos S., Alignani D., et al. Preneoplastic somatic mutations including MYD88L265P in lymphoplasmacytic lymphoma. Sci. Adv. 2022;8:eabl4644. doi: 10.1126/sciadv.abl4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hunter Z.R., Xu L., Yang G., Zhou Y., Liu X., Cao Y., Manning R.J., Tripsas C., Patterson C.J., Sheehy P., et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014;123:1637–1646. doi: 10.1182/blood-2013-09-525808. [DOI] [PubMed] [Google Scholar]
- 129.Wang J.Q., Jeelall Y.S., Beutler B., Horikawa K., Goodnow C.C. Consequences of the recurrent MYD88L265P somatic mutation for B cell tolerance. J. Exp. Med. 2014;211:413–426. doi: 10.1084/jem.20131424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lim K.H., Staudt L.M. Toll-Like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013;5:a011247. doi: 10.1101/cshperspect.a011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Theunissen P., Mejstrikova E., Sedek L., Van Der Sluijs-Gelling A.J., Gaipa G., Bartels M., Sobral da Costa E., Kotrová M., Novakova M., Sonneveld E., et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. 2017;129:347–357. doi: 10.1182/blood-2016-07-726307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flores-Montero J., Sanoja-Flores L., Paiva B., Puig N., García-Sánchez O., Böttcher S., Van Der Velden V.H.J., Pérez-Morán J.J., Vidriales M.B., García-Sanz R., et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094–2103. doi: 10.1038/leu.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barakat F.H., Medeiros L.J., Wei E.X., Konoplev S., Lin P., Jorgensen J.L. Residual monotypic plasma cells in patients with waldenström macroglobulinemia after therapy. Am. J. Clin. Pathol. 2011;135:365–373. doi: 10.1309/AJCP15YFULCZHZVH. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.