Abstract
Donors of Agrobacterium tumefaciens harboring a transfer-constitutive derivative of the nopaline-type Ti plasmid pTiC58 transferred this element at frequencies 3 to 4 orders of magnitude higher in matings conducted on solid surfaces than in those conducted in liquid medium. However, as measured with a lacZ reporter fusion, the tra genes of the wild-type Ti plasmid were inducible by opines to indistinguishable levels on solid and in liquid medium. Donors induced in liquid transferred the Ti plasmid at high frequency when mated with recipients on solid medium. We conclude that while formation of stable mating pairs and subsequent transfer of the Ti plasmid is dependent on a solid stratum, the regulatory system can activate tra gene expression to equivalent levels in liquid and on solid surfaces.
Many, if not all Ti plasmids of Agrobacterium tumefaciens are self-conjugal (8, 20) and can transfer in situ on crown gall tumors (19, 20). In addition to disseminating the Ti plasmids, transfer certainly is involved in the evolution of these elements (reviewed in reference 9). Conjugation is strongly regulated at the transcriptional level by a hierarchical system involving induction by opines (8), followed by activation by TraR in concert with a self-produced signal called Agrobacterium autoinducer (AAI) (12, 23). This latter level of regulation, called autoinduction or quorum-sensing (15), requires that AAI accumulate to some critical extracellular level before TraR can induce expression of the tra genes (28). Induction by opines is required for expression of traR and also for high-level production of AAI by the donor cells (13, 24, 28). The tra system itself is composed of three operons organized into two clusters. Two of these operons, traAFB and traCDG, are divergently expressed from a central intergenic promoter region that also contains the oriT site (1, 7, 10). The third operon, trb, contains 12 genes and is located between 75 and 100 kb from the tra locus, depending upon the Ti plasmid (1, 21). This operon codes for production of AAI (12, 17) as well as for the mating pair formation system including the conjugal pilus (1, 21).
Some conjugal plasmids transfer with similar efficiency when matings are conducted in liquid or on solid surfaces (11, 18, 26). Donors harboring other types of plasmids mate best on solid surfaces (3, 26). To our knowledge, the effect of stratum on the efficiency of Ti plasmid transfer has not been determined. This is of particular interest because stratum preferences give clues as to the nature of the habitat in which conjugation of these plasmids occurs. Moreover, because transfer is controlled by the availability of opines and the accumulation of AAI in the environment, it is conceivable that the regulatory system is influenced by whether the bacteria are colonizing solid surfaces or interacting in a liquid environment. Thus, we designed a series of experiments to determine the influence of a liquid or a solid environment on the efficiency of Ti plasmid conjugation as well as on the induction of expression of the tra regulon.
Optimum conditions for Ti plasmid transfer.
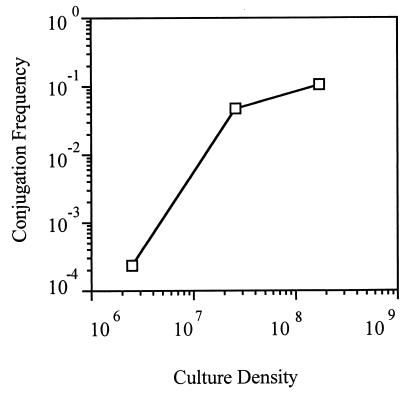
To maximize conjugal transfer efficiency, we first determined the influence of bacterial density on transfer frequency. Late-exponential-phase cultures of NT1 harboring the transfer-constitutive nopaline-agrocinopine A+B-type Ti plasmid pTiC58ΔaccR (2) were mated with C58C1RS (2) on sterile nitrocellulose filters (7) at various cell densities, with the input ratios of donor to recipient kept uniform at approximately 1:1. Filters were incubated on the surfaces of AB-mannitol (ABM) minimal agar medium (6) for 2 h, and the cells were resuspended by vortexing the filters in 1-ml volumes of 0.9% NaCl in water. The mating mixtures were diluted, and transconjugants were selected for by plating 0.1-ml volumes on AB minimal medium containing 9 mM arginine and 1 mM nopaline as the sole carbon source (2). Rifampin (50 μg per ml) and streptomycin (200 μg per ml) were included to select against the donors. Under these conditions, frequencies of transfer were maximal at total cell densities above 2 × 107 CFU per cm2 (Fig. 1). Transfer frequency dropped by 3 orders of magnitude at a density of 2 × 106 CFU per cm2 and was undetectable at densities of 2 × 105 CFU per cm2 or lower.
FIG. 1.
Minimum cell densities are required for mating. Donor and recipient bacteria at ratios of 1:1 were mated on filters at increasing total cell densities as described in the text. Transfer frequencies are expressed as transconjugants recovered per input donor, and culture densities are expressed as CFU per square centimeter. Each mating was quantified in triplicate, with less-than-10% differences in colony counts. The experiment was repeated two times. Although the actual frequencies of transfer differed between the three experiments, the patterns were identical. The results from a representative experiment are presented.
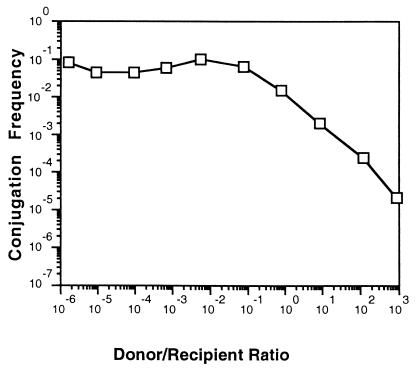
We also determined the optimum ratio of donors to recipients by mating late-exponential-phase cultures of NT1(pTiC58ΔaccR) at input densities ranging from 2 × 102 to 2 × 108 CFU per cm2 with cultures of C58C1RS at densities ranging from 2 × 108 to 2 × 102 per cm2. The resulting donor-to-recipient ratios on the filters ranged from 10−6 to 106, while total cell density was maintained above 2 × 107 CFU per cm2. Transfer frequencies, measured as transconjugants per input donor, were maximum and remained constant when the recipient was present in 10-fold or greater excess over the donor (Fig. 2). However, frequencies dropped by 10-fold increments as the donor-to-recipient ratio was increased by 10-fold increments. Thus, we performed subsequent matings by using donor-to-recipient ratios of 1:10 and total cell densities greater than 107 unless otherwise noted.
FIG. 2.
Ratio between donor and recipient cell densities influences conjugation. Donors harboring pTiC58ΔaccR were mated with recipients on nitrocellulose filters at various ratios as described in the text. The total cell density in each mating was greater than 107 CFU/cm2. Frequencies of transfer are expressed as transconjugants recovered per input donor. Each mating was quantified in triplicate, with less-than-10% differences between colony counts at each dilution. The experiment was repeated three times. As in Fig. 1, results from a representative experiment are presented.
Conjugation requires a solid surface.
We determined whether Ti plasmid transfer is surface dependent by comparing the transfer frequency of pTiC58ΔaccR in matings conducted in liquid and on solid media. Donor and recipient cultures were grown to late exponential phase in ABM liquid medium. Samples of a series of 10-fold dilutions of the donor culture, prepared in ABM liquid medium, were incubated with an equal volume of the recipient culture for 2 h. Concurrently, samples of the donor and recipient cultures were mated for 2 h on filters placed on ABM agar medium to determine the transfer frequency on a solid surface. Following the matings, samples of each mixture were collected and diluted, and 0.1-ml volumes from these dilutions were spread onto AB plates supplemented as described above to select for transconjugants. In addition, samples of donor and recipient cultures incubated separately in liquid medium were spread together onto selection plates to assess for matings that occurred after plating on the surface of the selection medium.
While transconjugants appeared on plates from both matings, cells mated on solid medium yielded progeny at a frequency 3 to 4 orders of magnitude higher than cells mated in liquid medium (Table 1). Moreover, the efficiency of transfer on solid medium remained constant, even with decreasing numbers of donors. Transconjugant colonies also arose on the control plates, indicating that at least some of the progeny appearing on plates spread with the liquid mixtures arose from matings that occurred on the selection plates. However, transfer frequencies from matings in liquid consistently were nearly double the transfer frequencies from matings that occurred on the selection plate, suggesting that donors harboring Ti plasmids can form productive mating pairs in liquid, albeit at a very low frequency.
TABLE 1.
Conjugal transfer of pTiC58 is most efficient on a solid surfacea
| Donor density in mating mixb | Transfer frequency when mated inc:
|
|
|---|---|---|
| Liquid | Solid | |
| 1.4 × 108 | 9.3 × 10−5 | 5.8 × 10−2 |
| 1.4 × 107 | 1.7 × 10−4 | 9.1 × 10−1 |
| 2.2 × 106 | 2.4 × 10−4 | 6.5 × 10−2 |
| 1.4 × 108d | 6.5 × 10−5 | NAe |
NT1(pTiC58ΔaccR) was mated with C58C1RS on solid and in liquid medium as described in the text.
Expressed as CFU per square centimeter for matings on solid medium or per milliliter for matings in liquid medium.
Expressed as transconjugants recovered per input donor. Values represent the averages of triplicate determinations for each mating. Colony counts at each dilution were within 10% of each other. The experiment was repeated twice, and the results of a representative experiment are presented.
The donor culture was mixed with the recipient culture immediately prior to dilution and plating on the selection medium all as described in the text.
NA, not applicable.
Preference for solid versus liquid strata correlates with the type of sex pilus encoded by the plasmid. Those that code for short, rigid pili transfer best on solid surfaces, while plasmids encoding flexible pili transfer equally well in liquid and on solid surfaces (3, 4). By analogy, our results suggest that the sex pilus encoded by pTiC58 is the rigid type. Consistent with this hypothesis, the trb genes of RP4, which are closely related to those of pTiC58 (21), direct the synthesis of a rigid-type pilus (16). More importantly, the results suggest that Ti plasmid transfer occurs on the surfaces of soil particles or crown gall tumor cells rather than free in the interstitial aqueous environment formed by films on the surface of these strata or bridging these strata. This preference for solid surfaces also is consistent with the fact that opines produced by the tumors and exuded to the cell surfaces and rhizosphere serve as specific inducers of the conjugal transfer apparatus for many Ti and opine-catabolic plasmids (8, 20). Presumably, opine concentrations are at their highest close to the surface of the crown gall tumor cells.
Conjugation is inducible in liquid medium.
While productive matings occurred at highest frequencies on solid surfaces, previous results with lacZ fusions indicated that the tra genes of pTiC58ΔaccR are expressed at high levels in liquid media (10, 17, 23, 24), prompting us to determine whether the tra regulon is inducible in a liquid environment. Strain C58(pJM749), which harbors a wild-type Ti plasmid as well as a recombinant plasmid containing a traA::lacZ reporter fusion (10, 23), was incubated with a preparation of agrocinopines A+B (25) on solid and in liquid media. For the former, the donor was grown overnight on sterile filters (13-mm diameter) placed on small towers of ABM agar medium (1 cm ht by 0.5 cm in diameter) impregnated with agrocinopines A and B (25) at a concentration of 2 mM. After induction, the cells were resuspended in 500 μl of 0.9% NaCl. For the latter, cells were grown overnight in 800 μl (a volume corresponding to that of the agar towers) of ABM medium containing the agrocinopines. The cells were harvested by centrifugation and resuspended in 500 μl of 0.9% NaCl. In each case the donors were spot mated (2) at various dilutions directly on selection plates previously spread with a culture of C58C1RS. In addition, samples of the donor cultures were assayed for β-galactosidase activity (17). Both induction conditions yielded transconjugants, with the cells induced in liquid medium transferring at a slightly higher frequency than those induced by growth on solid medium (Table 2). Moreover, the traA::lacZ reporter fusion was induced to similar levels in the two donor cultures (Table 2). Thus, the hierarchical regulatory system can function with equal efficiency in liquid and on solid strata.
TABLE 2.
Agrocinopines induce tra gene expression and conjugal transfer on solid and in liquid culture
| Induction mediuma | Donor densityb | Transfer frequencyc
|
Expression of traA::lacZd
|
|||
|---|---|---|---|---|---|---|
| −ACP | +ACPe | −ACP | +ACPe | Fold induction | ||
| Solid | 2.6 × 108 | <10−7 | 1.4 × 10−4 | 1 | 28 | 28 |
| Liquid | 3.1 × 108 | <10−7 | 4.0 × 10−4 | 1 | 31 | 31 |
C58(pJM749) was grown in ABM liquid or ABM solid medium for 28 h prior to spot mating with C58C1RS as described in the text.
Expressed as CFU per square centimeter for filter matings or per milliliter of medium for liquid matings.
Expressed as transconjugants recovered per input donor. Values represent the average of three replicate determinations at each dilution within the experiment. Replicate colony counts for each dilution were within 10% of each other. The experiment was repeated once, with a similar pattern of results.
Expressed as units of β-galactosidase activity per 109 CFU. Values represent the average of three replicate determinations within the experiment. The experiment was repeated once, with a similar pattern of results.
ABM medium was supplemented with a mixture of agrocinopines A+B (ACP) at a final concentration of 2 mM.
Fuqua and Winans (12) reported that the closely related tra genes of the octopine-mannityl opine-type Ti plasmid pTiR10 are not strongly expressed in liquid medium when induced by octopine alone but are expressed in cultures to which AAI also is added. They proposed that the low expression levels result from the lack of production of AAI by cells induced for transfer in liquid and concluded that tra gene activation requires factors specific for growth on solid surfaces. In contrast, we recently showed that another virtually identical Ti plasmid, pTi15955, is inducible for transfer and produces high levels of AAI in liquid medium containing octopine (22). These latter results are consistent with our observation that the tra genes of pTiC58 are expressed and AAI is produced at high levels during growth under inducing conditions in liquid medium (17, 24). Furthermore, strains harboring Trac Ti plasmids produce large amounts of AAI when grown in liquid medium, indicating that production of the signal is not dependent on solid surfaces (5, 17, 23, 27, 29, 30). It should be noted that while we measured conjugal transfer frequencies as well as gene induction, Fuqua and Winans (12) measured only the expression levels of the tra genes. Thus, while the tra regulon may not be strongly induced in liquid medium, the levels of expression are sufficient for conjugation to occur at normal frequency. In support of this hypothesis, the data of Fuqua and Winans (14) demonstrate that modest increases in tra gene expression result in maximal plasmid transfer and that induction of the tra regulon to higher levels does not necessarily result in elevated transfer rates. These results are consistent with our observations with pTiC58 and suggest that the low expression levels which may be seen in liquid environments do not necessarily limit conjugal transfer.
We conclude that the tra genes of both types of Ti plasmids are inducible and that the conjugation apparatus can be expressed in liquid environments. However, the formation of stable mating pairs requires a solid surface. We think it likely that given a regulatory system dependent upon the accumulation of AAI and a conjugation apparatus reliant on short rigid pili, that the majority of the Ti plasmid conjugal transfer events occur on the surface of the crown gall tumor or on root surfaces and soil particles sufficiently close to be influenced by opines produced by these plant neoplasias.
Acknowledgments
We thank Ingyu Hwang, Pei-Li Li, David M. Cook, Susanne Beck von Bodman, Clay Fuqua, and Stephen Winans for helpful discussions.
This work was supported by grant no. GM52465 from the NIH and grant no. AG93-337301-8943 from the USDA to S.K.F.
REFERENCES
- 1.Alt-Mörbe J, Stryker J L, Fuqua C, Li P-L, Farrand S K, Winans S C. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J Bacteriol. 1996;178:4248–4257. doi: 10.1128/jb.178.14.4248-4257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck von Bodman S, Hayman G T, Farrand S K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci USA. 1992;89:643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley D E. Conjugative pili of plasmids in Escherichia coli K12 and Pseudomonas species. In: Levy S B, Clowes R C, Koenig E L, editors. Molecular biology, pathogenicity and ecology of bacterial plasmids. New York, N.Y: Plenum Press; 1981. pp. 217–226. [Google Scholar]
- 4.Bradley D E, Taylor D E, Cohen D R. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J Bacteriol. 1980;143:1466–1470. doi: 10.1128/jb.143.3.1466-1470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha C, Gao P, Chen Y-C, Shaw P D, Farrand S K. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 6.Chilton M-D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Agrobacterium tumefaciens and PS8 bacteriophage DNA not detected in crown gall tumor DNA. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook D M, Farrand S K. The oriT region of the Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with T-region borders. J Bacteriol. 1992;174:6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis J G, Kerr A, Petit A, Tempé J. Conjugal transfer of nopaline and agropine Ti-plasmids—the role of agrocinopines. Mol Gen Genet. 1982;186:269–273. [Google Scholar]
- 9.Farrand S K. Conjugation in the Rhizobiaceae. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae: molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 199–233. [Google Scholar]
- 10.Farrand S K, Hwang I, Cook D M. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J Bacteriol. 1996;178:4233–4247. doi: 10.1128/jb.178.14.4233-4247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost L S, Ippen-Ihler K, Skurray R A. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuqua W C, Winans S C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuqua C, Winans S C. Localization of OccR-activated and TraR-activated promoters that express two ABC-type permeases and the traR gene of Ti plasmid pTiR10. Mol Microbiol. 1996;20:1199–1210. doi: 10.1111/j.1365-2958.1996.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 14.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua W C, Winans S C, Greenberg E P. Quorum-sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haase J, Lurz R, Grahn A M, Bamford D H, Lanka E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J Bacteriol. 1995;177:4779–4791. doi: 10.1128/jb.177.16.4779-4791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ippen-Ihler K A, Minkley J E G. The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- 19.Kerr A. Transfer of virulence between isolates of Agrobacterium. Nature (London) 1969;223:1175–1176. [Google Scholar]
- 20.Kerr A, Manigault P, Tempé J. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature (London) 1977;265:560–561. doi: 10.1038/265560a0. [DOI] [PubMed] [Google Scholar]
- 21.Li P-L, Everhart D M, Farrand S K. Genetic and sequence analysis of the pTiC58 trb locus encoding a mating-pair formation system related to members of the type IV secretion family. J Bacteriol. 1998;180:6164–6172. doi: 10.1128/jb.180.23.6164-6172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oger P, Kim K-S, Sackett R L, Piper K R, Farrand S K. Octopine-type Ti plasmids code for a mannopine-inducible dominant-negative allele of traR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol Microbiol. 1998;27:277–288. doi: 10.1046/j.1365-2958.1998.00671.x. [DOI] [PubMed] [Google Scholar]
- 23.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London) 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 24.Piper, K. R., S. Beck von Bodman, I. Hwang, and S. K. Farrand. Hierarchical gene regulatory systems arising from fortuitous gene associations: controlling quorum-sensing by the opine regulon in Agrobacterium. Mol. Microbiol., in press. [DOI] [PubMed]
- 25.Ryder M H, Tate M E, Jones G P. Agrocinopine A, a tumor-inducing plasmid-coded enzyme product, is a phosphodiester of sucrose and l-arabinose. J Biol Chem. 1984;259:9704–9710. [PubMed] [Google Scholar]
- 26.Willets N. Sites and systems for conjugal DNA transfer in bacteria. In: Levy S B, Clowes R C, Koenig E L, editors. Molecular biology, pathogenicity, and ecology of bacterial plasmids. New York, N.Y: Plenum Press; 1981. pp. 207–215. [Google Scholar]
- 27.Zhang L. Molecular biology and biochemistry of a novel conjugation factor in Agrobacterium. Ph.D. dissertation. Australia: University of Adelaide; 1993. [Google Scholar]
- 28.Zhang L, Kerr A. A diffusible compound can enhance conjugal transfer of the Ti plasmid in Agrobacterium tumefaciens. J Bacteriol. 1991;173:1867–1872. doi: 10.1128/jb.173.6.1867-1872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature (London) 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Beaber J W, Moré M I, Fuqua C, Eberhard A, Winans S C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]