Abstract
Acarbose inhibits starch digestion in the human small intestine. This increases the amount of starch available for microbial fermentation to acetate, propionate, and butyrate in the colon. Relatively large amounts of butyrate are produced from starch by colonic microbes. Colonic epithelial cells use butyrate as an energy source, and butyrate causes the differentiation of colon cancer cells. In this study we investigated whether colonic fermentation pathways changed during treatment with acarbose. We examined fermentations by fecal suspensions obtained from subjects who participated in an acarbose-placebo crossover trial. After incubation with [1-13C]glucose and 12CO2 or with unlabeled glucose and 13CO2, the distribution of 13C in product C atoms was determined by nuclear magnetic resonance spectrometry and gas chromatography-mass spectrometry. Regardless of the treatment, acetate, propionate, and butyrate were produced from pyruvate formed by the Embden-Meyerhof-Parnas pathway. Considerable amounts of acetate were also formed by the reduction of CO2. Butyrate formation from glucose increased and propionate formation decreased with acarbose treatment. Concomitantly, the amounts of CO2 reduced to acetate were 30% of the total acetate in untreated subjects and 17% of the total acetate in the treated subjects. The acetate, propionate, and butyrate concentrations were 57, 20, and 23% of the total final concentrations, respectively, for the untreated subjects and 57, 13, and 30% of the total final concentrations, respectively, for the treated subjects.
Acarbose, an oligosaccharide formed by strains of the genus Actinoplanes (23), is an α-glucosidase inhibitor that is used to treat non-insulin-dependent diabetes mellitus (2). This compound inhibits starch digestion in the small intestine (2, 5, 6). The increased amount of colonic starch selects for growth of starch-using bacteria (22). The ratio of viable starch-hydrolyzing bacteria to total viable anaerobic bacteria in feces increases (22), and fecal suspensions produce more butyrate from starch (22). Starch fermentation by colonic bacteria produces large amounts of butyrate (3, 4, 10, 21). Enhancement of colonic starch fermentation by acarbose treatment increases the butyrate concentration and the proportion of butyrate in the short-chain fatty acid (SCFA) component of feces (18, 22). Butyrate is an energy source for colonic epithelial cells (17), and this compound promotes differentiation and inhibits growth of colon cancer cells (1, 19).
Analysis of the labeling of fermentation products obtained from isotopically labeled substrates can reveal the metabolic pathways used by colonic microbes to form products. Using radioactive glucose and CO2 as substrates, Miller and Wolin showed previously that the colonic flora of two adults used the Embden-Meyerhof-Parnas (EMP) pathway as the primary metabolic route for SCFA production (15). The flora also formed considerable amounts of acetate by reducing CO2 rather than by direct formation from glucose (15). Wolin et al. used 13C-labeled glucose and CO2 to study fermentations by the fecal flora of breast-fed infants (25, 26). Although the products differed from the products found in adults, the flora of two breast-fed infants less than 1 month old used the EMP pathway to metabolize glucose. After 5 months, reexamination of one infant showed that a totally different fermentation pathway, used only by bifidobacteria, had replaced the EMP pathway. In contrast to the adult flora, the flora of the infants was incapable of reducing CO2 to acetate.
We examined these pathways in a larger group of adults to investigate possible changes caused by acarbose treatment. The isotopic composition of products of fermentations by the fecal bacteria of subjects who participated in an acarbose-placebo crossover trial with a 3- to 4-month rest period between treatments was determined. We incubated fecal suspensions with [1-13C]glucose and 12CO2 or with unlabeled glucose and 13CO2 and examined the distribution of 13C in the product C atoms. The data showed that acarbose treatment resulted in large decreases in the reduction of CO2 to acetate, as well as the formation of propionate from glucose. Butyrate formation from glucose increased considerably with acarbose treatment. The EMP pathway was the major pathway used for fermentation with or without acarbose treatment.
MATERIALS AND METHODS
Subjects and fecal suspensions.
Fermentations were examined by using a fecal suspension from each of 40 patients who participated in an acarbose-placebo crossover trial (unpublished data). Baseline samples were taken from 21 subjects before they were given either acarbose (100-mg tablet daily) or a placebo (100-mg tablet daily). Samples were then taken from 10 subjects after they received acarbose for 4 months and from 6 subjects who received the placebo for 4 months. A 3- to 4-month rest period followed the 4 months of acarbose or placebo regimen before a crossover of each subject’s treatment was begun. We examined baseline samples from three subjects after the rest period. Statistical analysis showed that the data obtained during the baseline and placebo regimens were not different from each other. The results obtained from the baseline and placebo samples were combined into one data set which represented subjects that did not receive acarbose (designated the Aneg group). These results were compared with results obtained with samples from acarbose-treated subjects (designated the Apos group).
Suspensions of feces were prepared in anaerobic dilution solutions under CO2 as described previously (14, 20). The suspensions were kept at 4°C and were used within 24 h of collection. Anaerobic conditions were maintained by using the serum bottle modification of the Hungate technique (13). Duplicate (5-ml) portions were dried to constant weight to determine the fecal dry matter content. The human fecal fermentation protocols were reviewed and approved by the New York State Department of Health Institutional Review Board. The protocols used for the acarbose-placebo crossover trial were reviewed and approved by the Mary Imogene Bassett Hospital Institutional Review Board.
Fermentations.
Fermentations of glucose were performed in anaerobic culture tubes (18 by 150 mm; Bellco Glass Inc., Vineland, N.J.). Glucose (50 mg) was added as a dry powder prior to insertion of butyl rubber stoppers and sealing with aluminum seals. The tubes were gassed with 80% N2–20% CO2. After 5-ml portions of 76 mM NaHCO3 from a serum bottle with a 100% N2 atmosphere were injected, the tubes were cooled to 0°C in ice water. Formate was added to some fermentations by adding 0.2 ml of a 0.5 M sodium formate solution from a serum bottle with a 100% N2 atmosphere. Fermentation was started by injecting 5.0 ml of a suspension. After incubation with rotation for 24 h at 37°C, the tubes were boiled for 10 min. The contents were either analyzed immediately or frozen at −20°C and thawed before gas samples were removed for gas chromatography and mass spectrometric analyses. Suspensions were then acidified by adding 0.5 ml of 5 N H2SO4 and were centrifuged at 16,000 × g for 15 min. Supernatants were analyzed to determine their fermentation product and residual glucose contents.
Fermentation analyses.
Soluble fermentation product and glucose contents were determined by high-performance liquid chromatography procedures (9) as described by Wolin et al. (26). H2 and CH4 were quantified by using previously described gas chromatographic procedures (15). Mass spectral analyses to determine 13CO2 contents were carried out with a model 5890A gas chromatograph (Hewlett-Packard, Palo Alto, Calif.) equipped with a model 5970 Series mass selective detector (Hewlett-Packard) as previously described by Wolin et al. (26).
NMR.
Nuclear magnetic resonance (NMR) spectra were acquired with a model XL-300 spectrometer (Varian Associates, Walnut Creek, Calif.) operating at 75.43 MHz. Pulses of 36° were used, and the delay time was 1 s. The number of transients ranged from 5,000 to 40,000. The NMR locking material was deuterium oxide. All spectra were recorded at 25°C with a spectral width of 16,000 Hz.
Chemical shifts for SCFA were assigned directly by using previously published values and samples of labeled acetate. To measure the percentage of enrichment of 13C, we used natural abundance dioxane (reagent grade and distilled from lithium aluminum hydride; purity, >99.9%) as a standard. Dioxane gives a strong singlet at 67.4 ppm that is separate from the signals of the species studied under the conditions used for the NMR analyses. Pure unlabeled dioxane (5 μl) was sealed in a capillary tube with D2O, and this capillary tube was inserted into the NMR sample tubes as a reference each time that a spectrum was acquired. We prepared three standard samples of labeled acetate with the same concentration, 53.63 mM. Two of these samples were singly labeled with 13C at either C-1 or C-2; the third was doubly labeled. All three samples had an enrichment of 31.01%. Their spectra were acquired under identical conditions with the reference capillary tube mentioned above in the NMR tube. All three samples exhibited agreement in the ratio of peak intensity of C-1 or C-2 to dioxane intensity, which was determined to be 2.1 or 3.1 (designated Ro). The spectrum of a fermentation sample was acquired with the same capillary tube, and the ratio of its peak intensity to dioxane intensity (R) was obtained. To convert this ratio to the millimolar concentration of 13C carbon atom, C, the following equation was used: C = R/R0 × 0.3101 × 53.63. Concentrations of 13C-labeled methyl, 13C-labeled methylene, and 13C-labeled carboxyl of propionate and butyrate were calculated similarly by using Ro = 2.1 for the methyl and methylene groups and Ro = 3.1 for the carboxyl groups. This method was verified by determining the concentrations 13C in the carbons of propionate and butyrate with known concentrations of 13C in the respective C atoms.
NMR analysis of fermentation samples.
Acidified fermentation supernatant was added to the NMR tube without any solvent. The same dioxane reference capillary tube was also put into the tube each time. The products were identified and the enrichment of 13C was determined by the methods described above.
Materials.
13C-labeled C-1, C-2, and doubly labeled sodium acetate were obtained from MSD Isotopes (Montreal, Canada) and were 99% enriched. 13C-labeled glucose, sodium bicarbonate, and sodium formate (99% enriched) were purchased from Cambridge Isotope Laboratories (Woburn, Mass.). All other chemicals were reagent grade or better.
Data analysis.
Unless stated otherwise, the means obtained for the treatments are presented below. Excel (Microsoft Corporation, Redmond, Wash.) was used to perform Student’s t test in order to determine the significance of differences between means.
RESULTS
Fermentation of glucose and endogenous substrates.
Table 1 shows the SCFA formed from glucose and endogenous substrates in the fecal suspensions and the mean concentrations obtained for the Aneg and Apos groups. The sum of the C atoms in the products was 19.4% greater than the number of C atoms in the added glucose for the Aneg group and 32.7% greater for the Apos group. Additional products formed by fermentation of endogenous substrates were the probable sources of the additional C. The starch concentration in feces of healthy volunteers increased from 68.5 to 241.2 μmol per g (dry weight) of feces when the volunteers received 200 mg of acarbose per day (22). Table 1 shows that the amount of butyrate formed was greater in the Apos group and that butyrate accounted for 23% of the SCFA for the Aneg group and 30% of the SCFA for the Apos group. Although the amount of propionate formed was smaller in the Apos group, the difference between the two groups was not statistically significant; however, the difference in the percentages of the SCFA (20% for the Aneg group and 13% for the Apos group) was significant (Table 1).
TABLE 1.
Amounts of SCFA produced in 24 h from [1-13C]glucose and endogenous substrates
| Acid | Subjectsa
|
|||
|---|---|---|---|---|
| Aneg group
|
Apos group
|
|||
| Concn (mM) | % | Concn (mM) | % | |
| Acetate | 42.4 (7.8)b | 56.8 | 46.1 (9.1) | 56.9 |
| Propionate | 14.9 (5.9) | 20.0c | 10.8 (5.2) | 13.3c |
| Butyrate | 17.4 (5.9)d | 23.2e | 24.1 (10.3)d | 29.8e |
The values are the averages of the values from two or three fermentations for each sample. The percentages are percentages of the total concentration of the three SCFA.
The values in parentheses are standard deviations.
The difference between the Aneg group value and the Apos group value is significant (P < 0.002), as determined by Student’s t test.
The difference between the Aneg group value and the Apos group value is significant (P < 0.02), as determined by Student’s t test.
The difference between the Aneg group value and the Apos group value is significant (P < 0.02), as determined by Student’s t test.
Incorporation of 13CO2.
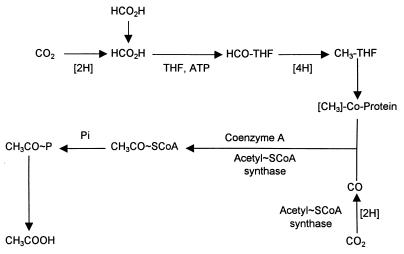
The flora in the fecal suspensions incorporated 13CO2 mainly into the methyl and carboxyl C atoms of acetate and the carboxyl C atoms of propionate after incubation with 13CO2 and unlabeled glucose (Table 2). The other C atoms of propionate and the C atoms of butyrate contained much smaller amounts of 13C. Incorporation into both C atoms of acetate resulted from the reduction of CO2 to both the methyl and carboxyl C atoms that is characteristic of the Wood-Ljungdahl pathway of formation of acetate from CO2 (Fig. 1). More 13CO2 was incorporated into the carboxyl group than into the methyl group of acetate (Table 2). Exchange of 13CO2 with the unlabeled carboxyl of acetate formed from glucose or endogenous substrates could explain the differential labeling of the two C atoms. Acetyl S-coenzyme A (acetyl-SCoA) synthase catalyzes the exchange of CO2 with the carboxyl group of acetate (16).
TABLE 2.
Enrichment of SCFA and CO2 after incubation with 13CO2 and unlabeled glucose
| Incorporation of 13CO2
|
Aneg group
|
Apos group
|
P valueb | |||
|---|---|---|---|---|---|---|
| SCFA | Position | % 13C enrichment of C atom | 13C concn (mM)a | % 13C enrichment of C atom | 13C concn (mM)a | |
| Acetate | CH3 | 3.60 | 1.51c | 2.08 | 0.93d | <0.0004 |
| COOH | 5.79 | 2.38c | 3.02 | 1.32d | <0.00002 | |
| CH3 | 1.92 | 0.33 | 1.10 | 0.27 | ||
| Butyrate | CH2 | 2.48 | 0.43 | 1.44 | 0.36 | |
| CH2 | 1.90 | 0.32 | 1.09 | 0.27 | ||
| COOH | 2.79 | 0.47 | 1.60 | 0.40 | ||
| Propionate | CH3 | 1.19 | 0.17 | 0.63 | 0.08 | <0.0002 |
| CH2 | 0.94 | 0.14 | 0.46 | 0.05 | <0.0007 | |
| COOH | 17.98 | 2.58 | 13.86 | 1.42 | <0.002 | |
| CO2 | 21.70 | 26.20 | <0.00002 | |||
Calculated by multiplying the SCFA concentration shown in Table 1 by the percent 13C enrichment divided by 100.
Probability that the Aneg group and Apos group values are different, as determined by Student’s t test.
The methyl and carboxyl group values are significantly different (P < 9 × 10−13), as determined by Student’s t test.
The methyl and carboxyl group values are significantly different (P < 0.0002), as determined by Student’s t test.
FIG. 1.
Wood-Ljungdahl pathway for reduction of CO2 to acetate. THF, tetrahydrofolic acid; Co-Protein, corrinoid enzyme; Pi, inorganic phosphate (adapted from reference 8 with permission from the publisher).
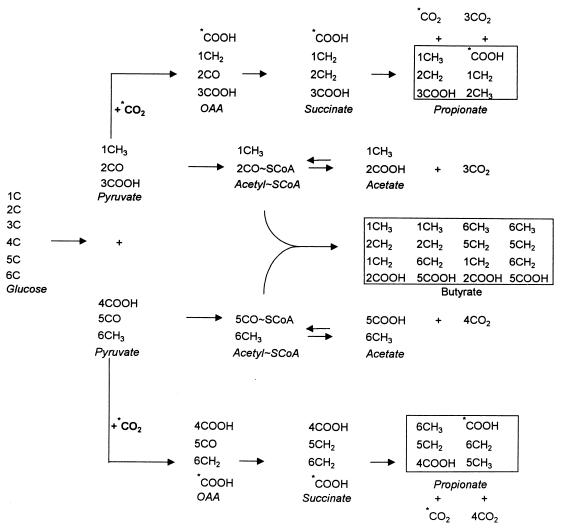
Incorporation of 13CO2 into butyrate resulted from the conversion of 13C-labeled acetate to acetyl-SCoA. Interconversion of acetate and acetyl-SCoA is a common process in bacteria and is facilitated by the enzymes acetate kinase (acetate + ATP → acetyl-phosphate + ADP) and phosphotransacetylase (acetyl-phosphate → acetyl-SCoA + inorganic phosphate). Butyrate is usually formed from acetyl-SCoA units produced from the oxidative decarboxylation of pyruvate (Fig. 2). Exchange occurs between the acetyl-SCoA units formed from labeled acetate produced from 13CO2 and the acetyl-SCoA units formed from unlabeled pyruvate. The extents of exchange of 13C-labeled acetyl-SCoA into butyric acid were 16 and 21% for the Aneg and Apos groups, respectively.
FIG. 2.
EMP pathway for glucose decomposition and production of acetate, propionate, and butyrate from pyruvate. The numbers of the C atoms of glucose that are found in the various C atoms of products formed by the pathway are indicated, as are the carbons of propionate that are formed from carbon dioxide (indicated by asterisks). Details of the enzyme reactions between glucose and pyruvate and between pyruvate and acetate, propionate, and butyrate are not shown. For details of enzyme reactions between glucose and pyruvate and between pyruvate and acetate, propionate, and butyrate, see reference 11.
Amount of acetyl units formed by CO2 reduction.
The total amount of acetyl units formed from CO2 was equal to the amount of labeled acetyl units in butyrate and in free acetate after incubation with 13CO2. We calculated the amount of acetate and butyrate acetyl units formed from 13CO2 from the amount of labeled methyl groups because of possible exchange of 13CO2 with the carboxyl carbon of acetate. Incorporation of labeled acetyl units into butyrate was evaluated by determining the 13C content of the butyrate C atoms. The amount of the methyl C of the acetyl units incorporated was determined from the 13C contents of the C-2 and C-4 atoms of butyrate. The 13C concentrations in the different C atoms were almost identical, and the mean was used to calculate the amount of labeled acetyl units in butyrate. This amount was added to the amount in free acetate calculated from the amount of acetate methyl C formed from 13CO2.
Since 13CO2 and 12CO2 were present, four molecular species of acetate were formed. The enzymes of the pathway can use either 12CO2 or 13CO2 as a source of the methyl and carboxyl carbons of acetate. Table 3 shows the four species of acetate formed from CO2 and the formulas used to calculate the proportion of each molecular species from the percentage of 13C enrichment of CO2. The method used to calculate the total concentration of the four species from the concentration of 13CH312COOH is also shown. Table 4 shows the concentrations of acetate produced by the CO2 reduction pathway. The amount of acetate formed from CO2 by the fecal fermentations of the Aneg group was 30% of the total amount of acetate produced. This compares with the much lower amount obtained for the Apos group (17% of the total amount of acetate produced).
TABLE 3.
Calculation of the proportions of acetate species formed from CO2a
| CO2 isotopes | Acetate species | % of species |
|---|---|---|
| 12CO2, 12CO2 | 12CH312COOH | (% 12CO2/100) × (% 12CO2/100) |
| 13CO2, 13CO2 | 13CH313COOH | (% 13CO2/100) × (% 13CO2/100) |
| 12CO2, 13CO2 | 12CH313COOH | (% 12CO2/100) × (% 13CO2/100) |
| 13CO2, 12CO2 | 13CH312COOH | (% 12CO2/100) × (% 13CO2/100) |
For example, with 75% 12CO2 and 25% 13CO2, 12CH3 12COOH = 0.75 × 0.75 = 0.56 and 13CH3 13COOH = 0.25 × 0.25 = 0.06. 12CH3 13COOH and 13CH3 12COOH each accounted for 0.75 × 0.25 or 0.19 of the total acetate with methyl and carboxyl carbons from CO2. The total number of micromoles obtained with methyl and carboxyl C from 12CO2 13CO2 was calculated from the concentration of the 13C methyl group of acetate and was equal to 1/[(% 12CO2/100) × (% 13CO2/100) × (mM 13CH3)].
TABLE 4.
Calculated amounts of acetate formed from the CO2 reduction pathway in 24 h
| Group | Acetate concn (mM) with the following sources of acetate:
|
% of total from CO2 | |
|---|---|---|---|
| CO2 | CO2 + substrate Ca | ||
| Aneg | 12.87b | 42.38 | 30.36 |
| Apos | 7.76b | 46.10 | 16.83 |
See Table 1 for the total amounts of acetate formed by fermentation.
Values are significantly different (P < 0.002), as determined by Student’s t test.
Relationship between acetate formation from CO2 and CH4 production.
CH4 formation in the colon is a CO2 reduction process, as is the formation of acetate from CO2. We compared the two processes by studying 21 samples. Ten of the samples (all baseline samples) produced less than 0.26 μmol of CH4 per ml of suspension, and 11 samples (10 baseline samples and 1 Apos sample) produced more than 4.17 μmol of CH4 per ml of suspension incubated with glucose and bicarbonate. H2 was not detected as a final fermentation product in any of the fecal suspensions. The means (and standard deviations) were 0.04 (0.08) and 8.37 (3.17) μmol of CH4 per ml of suspension for the low- and high-methane-content samples, respectively. The corresponding means (and standard deviations) for production of acetate from CO2 were 16.40 (4.50) and 11.17 (2.75) μmol per ml of suspension for the low- and high-methane-content samples, respectively, and these values were significantly different (P = 0.002), as determined by Student’s t test. The differences between the other fermentation measurements (i.e., SCFA formation or formation of labeled products from [1-13C]glucose or 13CO2) were not significant.
Carboxylation and CO2 exchange and propionate production.
Incorporation of 13CO2 into the carboxyl C of propionate can result from the carboxylation of pyruvate to oxaloacetate in the succinate pathway shown in Fig. 2. After reduction of oxaloacetate to succinate, decarboxylation yields propionate. One carboxyl of succinate is from the carboxyl of pyruvate, and the other is from CO2. Decarboxylation produces propionate in which one-half of the propionate molecules have a carboxyl C from CO2 and one-half have a carboxyl C from the carboxyl of pyruvate. CO2 can also enter the carboxyl C of pyruvate by exchange reactions of enzyme systems that form acetyl-SCoA units and CO2 from pyruvate (24). Of the 14.95 mM propionate formed by the Aneg group (Table 1), 82% of the carboxyl groups contained 13C, whereas 52% of the 10.81 mM propionate formed by the Apos group (Table 1) contained 13C. Since the level of labeling of the carboxyl by the flora of the Aneg group was significantly greater than 50%, the propionate-forming flora apparently produced substantial exchange of CO2 into the carboxyl of pyruvate.
Production of SCFA from [1-13C]glucose.
Table 5 shows the 13C enrichments of C atoms of the SCFA and CO2 produced by the fecal fermentations of [1-13C]glucose. The 13C was incorporated primarily into C-2 of acetate, C-2 and C-3 of propionate, and C-2 and C-4 of butyrate. Acetyl-SCoA units produced from [1-13C]glucose by the EMP pathway (Fig. 2) and labeled in the methyl but not the carboxyl C are converted to free acetate. An equivalent amount of unlabeled acetyl-SCoA units and acetate are formed from C-5 and C-6 of glucose. Butyrate formation requires the condensation of two acetyl-SCoA units (Fig. 2). Only one-half of the acetyl groups in butyrate are labeled because of the synthesis of unlabeled acetyl-SCoA units from C-6 and C-5 of [1-13C]glucose. As discussed above, propionate is produced after carboxylation of pyruvate and formation of succinate (Fig. 2). After decarboxylation of succinate, 50% of the 13C from [1-13C]glucose is in the methyl C and 50% is in the C-2 atom of propionate (Fig. 2). The concentrations of 13CH312CH2COOH and 12CH3 13CH2COOH are the concentrations in the respective 13C-labeled C atoms (Table 5). An equivalent amount of unlabeled propionate is formed from the pyruvate made from the C-6 end of glucose (Fig. 2). The amounts of SCFA calculated from the data in Table 5 were as follows: 20 mM acetate, 7 mM propionate, and 5 mM butyrate for the Aneg group and 19 mM acetate, 4 mM propionate, and 7 mM butyrate for the Apos group. The propionate and butyrate concentrations and the percentages of the three SCFA of the two groups were significantly different (P < 0.05), as determined by Student’s t test.
TABLE 5.
Concentrations of 13C in C atoms of SCFA after incubation with [1-13C]glucose
| Incorporation of 13C
|
Aneg group
|
Apos group
|
P valueb | |||
|---|---|---|---|---|---|---|
| SCFA | Position | % 13C enrichment of C atom | 13C concn (mM)a | % 13C enrichment of C atom | 13C concn (mM)a | |
| Acetate | CH3 | 24.09 | 9.80 | 21.18 | 9.59 | |
| COOH | 2.61 | 1.09 | 1.40 | 0.64 | <0.0004 | |
| CH3 | 15.56 | 2.57 | 14.07 | 3.40 | <0.0002 | |
| Butyrate | CH2 | 0.47 | 0.11 | 0.32 | 0.10 | <0.0003 |
| CH2 | 15.00 | 2.49 | 13.77 | 3.34 | ||
| COOH | 0.13 | 0.02 | 0.00 | 0.00 | <0.04 | |
| Propionate | CH3 | 13.06 | 1.87 | 7.29 | 0.90 | |
| CH2 | 12.27 | 1.78 | 6.78 | 0.85 | <0.03 | |
| COOH | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CO2 | 2.19 | 2.44 | ||||
Calculated by multiplying the SCFA concentration shown in Table 1 by the percent 13C enrichment divided by 100.
Probability that the Aneg group and Apos group values are different, as determined by Student’s t test.
Production of CO2 from [1-13C]glucose.
The amount of CO2 formed from the C-1 atom of [1-13C]glucose was calculated from the percentage of 13CO2 in the CO2 found after incubation with [1-13C]glucose or [13C]bicarbonate. After incubation with [13C]bicarbonate, the percentage of the 13CO2 was 22.6%, and after incubation with [1-13C]glucose the percentage of 13CO2 was 2.3%. The amount of CO2 formed from the C-1 atom of glucose was 38.8 μmol. The mean percentage of conversion of the C-1 atom of glucose to CO2 for all of the samples was 10.54% (standard deviation, 0.51%) of the added [1-13C]glucose.
DISCUSSION
NMR analysis of fermentations of samples from 40 subjects provided additional evidence (15) that the Wood-Ljungdahl pathway plays a major role in the production of acetate in the human colon. Bacteria that uses this pathway couple the oxidation of carbohydrates to acetate and CO2 to the reduction of CO2 to acetate. They also use other electron sources, such as H2 produced by other bacteria, to reduce CO2 to acetate (7). Their contribution to the overall fermentation decreases during acarbose treatment. The acetate formed from CO2 reduction was 30% of the total acetate formed by the Aneg group and only 17% of the total acetate formed by the Apos group. The contribution of bacteria that produce propionate also diminishes. At the same time, the contribution of bacteria that form butyrate increases. Selection for starch-using, butyrate-forming bacteria probably occurs during acarbose treatment because of the increased amounts of starch available for growth and fermentation in the colon.
The incorporation of less 13CO2 into the methyl C than into the carboxyl C of acetate is probably due to the CO2 exchange reaction catalyzed by the acetyl-SCoA synthase of the CO2 reduction pathway (Fig. 1). Unlabeled formate is a possible product of carbohydrate fermentation by some of the microorganisms in the microbial community. This compound might be directly incorporated into the methyl group of acetate (Fig. 1). This would decrease the ratio of 13CH3 to 13COOH. We examined the incorporation of [13C]formate into the methyl group of acetate in the presence of unlabeled glucose and CO2 by using specimens from 21 subjects (data not shown). The [13C]formate was incorporated into the methyl group of acetate. However, since most of the formate was converted to 13CO2, it was not possible to distinguish between direct incorporation and formation of the methyl group by reduction of CO2.
Product labeling allowed us to compare the relative importance of the reduction of CO2 to acetate or methane. Reduction of CO2 to acetate diminished when methane was formed. The mean difference in the concentration of acetate formed from CO2 between the methane-negative and methane-positive groups was 5 mM, and the mean concentration of methane formed by the positive group was 8 mM. This suggests that methanogens, when present, preferentially use electrons for reduction of CO2 that are otherwise used by bacteria that reduce CO2 to acetate. However, more acetate than methane was produced by CO2 reduction by the flora of the methane-positive samples. The mean level of acetate formed from CO2 by the methane-positive samples was 1.4 times greater than the mean level of methane formed from CO2.
The labeling of propionate that occurred when [1-13C]glucose was fermented showed that propionate was formed by the succinate pathway during all treatment periods. The labeling of both the C-2 and C-3 atoms of propionate by the C-1 atom of glucose is consistent with formation of the symmetrical succinate molecule after carboxylation of pyruvate and subsequent decarboxylation of succinate to propionate (Fig. 2). CO2 incorporation into the carboxyl group of propionate (Table 2) is also consistent with the succinate pathway. However, the amount of CO2 incorporated should be only 50% of the total amount of propionate carboxyl groups produced during fermentation. Since this value was greater than 50% for the Aneg group, the results suggest that the enzymes of the colonic propionate-forming bacteria of this group catalyze the exchange of the C atoms of CO2 and the carboxyl C of pyruvate (24).
The 13C isotope distribution in all of the products is consistent with the use of the EMP pathway of glucose fermentation by the Aneg and Apos groups. CO2 is not produced from C-1 of glucose by the EMP pathway (Fig. 2). The conversion of about 10% of the C-1 atoms to CO2 may be due to a small amount of metabolism by other pathways in the colonic microbes. Formation of small amounts of CO2 from C-1 of glucose may result from minor catabolic pathways that produce CO2 from the C-1 position. This CO2 may also result from anabolic pathways that generate pentose for biosynthetic reactions, as was concluded from observations made in a previous study in which a 14C radioisotope analysis of the fecal fermentations of two healthy adults was performed (15).
Our results show that acarbose treatment results in decreases in the activities of colonic bacteria that use the Wood-Ljungdahl pathway and bacteria that form propionate and an increase in the activity of bacteria that produce butyrate. This is presumably caused by preferential growth of butyrate-forming bacteria after acarbose allows more starch to reach the colon. Measurements of overall fermentations and pathways by NMR or other isotope procedures can detect changes in the colonic fermentation that can be important to the host and are extremely difficult to ascertain by enumeration of colonic microbial species. For example, a twofold increase in the relative concentration of butyrate-forming species could increase the relative production of butyrate per day twofold. The magnitude of the bacterial concentration changes would be difficult to measure. However, doubling the supply of a major source of energy for colonic epithelial cells may be important to the host. The difficulty of applying microbial enumeration procedures to population changes in the colon may obscure important changes caused by drugs, diet, or large-bowel diseases that can influence host physiology and health. Fermentation product analyses and fermentation pathway investigations provide another approach for determining if significant changes in populations and fermentations are caused by specific agents or conditions.
ACKNOWLEDGMENTS
We thank Colette T. Tangel, Jean A. Krause, and Margaret M. Parfitt for assistance.
Portions of this study were supported by the National Institutes of Health, by National Cancer Institute grant CA56432, and by the Irving A. Hansen Memorial Foundation. Bayer Corporation provided acarbose and the placebo.
REFERENCES
- 1.Archer S Y, Meng S, Shei A, Hodin R A. p21 WAF1 is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiasson J L, Josse R G, Hunt J A, Palmason C, Rodger N W, Ross S A, Ryan E A, Tan M H, Wolever T M. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus: a multicenter controlled clinical trial. Ann Intern Med. 1994;121:928–935. doi: 10.7326/0003-4819-121-12-199412150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Christl S U, Katzenmaier U, Hylla S, Kasper H, Scheppach W. In vitro fermentation of high-amylose cornstarch by a mixed population of colonic bacteria. J Parenter Enter Nutr. 1997;21:290–295. doi: 10.1177/0148607197021005290. [DOI] [PubMed] [Google Scholar]
- 4.Clausen M R, Bonnen H, Mortensen P B. Colonic fermentation of dietary fibre to short chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut. 1991;32:923–928. doi: 10.1136/gut.32.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coniff R F, Shapiro J A, Seaton T B. Long-term efficacy and safety of acarbose in the treatment of obese subjects with non-insulin-dependent diabetes mellitus. Arch Intern Med. 1994;154:2442–2448. [PubMed] [Google Scholar]
- 6.Coniff R F, Shapiro J A, Seaton T B, Bray G A. Multicenter, placebo-controlled trial comparing acarbose BAY g 5421 with placebo, tolbutamide, and tolbutamide-plus-acarbose in non-insulin-dependent diabetes mellitus. Am J Med. 1995;98:443–451. doi: 10.1016/S0002-9343(99)80343-X. [DOI] [PubMed] [Google Scholar]
- 7.Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. [Google Scholar]
- 8.Drake H L. Acetogenesis, acetogenic bacteria, and the acetyl-CoA “Wood-Ljungdahl pathway”: past and current perspectives. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 1–60. [Google Scholar]
- 9.Ehrlich G G, Goerlitz D F, Bourell J H, Eisen G V, Godsy E M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981;42:878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englyst H N, Hay S, Macfarlane G T. Polysaccharide break-down by mixed populations of human fecal bacteria. FEMS Microbiol Ecol. 1987;95:163–171. [Google Scholar]
- 11.Gottschalk G. Bacterial metabolism. 2nd ed. New York, N.Y: Springer-Verlag; 1985. [Google Scholar]
- 12.Holt P R, Atillasoy E, Lindenbaum J, Ho S B, Lupton J R, McMahon D, Moss S F. Effects of acarbose on fecal nutrients, colonic pH, and short-chain fatty acids and rectal proliferative indices. Metab Clin Exp. 1996;45:1179–1187. doi: 10.1016/s0026-0495(96)90020-7. [DOI] [PubMed] [Google Scholar]
- 13.Miller T L, Wolin M J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Environ Microbiol. 1974;27:985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller T L, Wolin M J. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol. 1982;131:14–18. doi: 10.1007/BF00451492. [DOI] [PubMed] [Google Scholar]
- 15.Miller T L, Wolin M J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62:1589–1592. doi: 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ragsdale S W. CO dehydrogenase and the central role of this enzyme in the fixation of carbon dioxide by anaerobic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 88–126. [Google Scholar]
- 17.Roediger W E W. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheppach W, Fabian M, Sach M, Kasper H J. The effect of starch malabsorption on fecal short chain acid excretion in man. Scand J Gastroenterol. 1988;23:755–759. doi: 10.3109/00365528809093945. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Bush K, Eguchi T, Ikekawa N, Takaguchi T, Kobayashi Y, Higgins P J. Effects of 1,25-dihydroxyvitamin D3 and its analogs on butyrate-induced differentiation of HT-29 human colonic carcinoma cells and on the reversal of the differentiated phenotype. Arch Biochem Biophys. 1990;276:415–423. doi: 10.1016/0003-9861(90)90740-p. [DOI] [PubMed] [Google Scholar]
- 20.Weaver G A, Krause J, Miller T L, Wolin M J. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986;27:698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver G A, Krause J A, Miller T L, Wolin M J. Cornstarch fermentation by the colonic microbial community yields more butyrate than does cabbage fiber fermentation: cornstarch fermentation rates correlate negatively with methanogenesis. Am J Clin Nutr. 1992;55:70–77. doi: 10.1093/ajcn/55.1.70. [DOI] [PubMed] [Google Scholar]
- 22.Weaver G A, Tangel C T, Krause J A, Parfitt M M, Jenkins P L, Rader J M, Lewis B A, Miller T L, Wolin M J. Acarbose enhances human butyrate fermentation. J Nutr. 1997;127:717–723. doi: 10.1093/jn/127.5.717. [DOI] [PubMed] [Google Scholar]
- 23.Windholz M, editor. The Merck index. An encyclopedia of chemicals, drugs, and biologicals. 10th ed. Rahway, N.J: Merck and Co., Inc.; 1983. [Google Scholar]
- 24.Wolfe R S, O’Kane D J. Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J Biol Chem. 1955;215:637–643. [PubMed] [Google Scholar]
- 25.Wolin M J, Yerry S, Miller T L, Zhang Y, Bank S. Changes in production of ethanol, acids and H2 from glucose by the fecal flora of a 16- to 158-d-old breast-fed infant. J Nutr. 1998;128:85–90. doi: 10.1093/jn/128.1.85. [DOI] [PubMed] [Google Scholar]
- 26.Wolin M J, Zhang Y, Bank S, Yerry S, Miller T L. NMR detection of 13CH313COOH from 3-13C-glucose: a signature for Bifidobacterium fermentation in the intestinal tract. J Nutr. 1998;128:81–86. doi: 10.1093/jn/128.1.91. [DOI] [PubMed] [Google Scholar]