Abstract
Poly(N-isopropylacrylamide) (PNIPAM) based electrically conductive hydrogels (PNIPAM-ECHs) have been extensively studied in recent decades due to their thermal-responsive (leading to the volume change of hydrogels) and electrically conductive performance. The incorporation of conductive components into the PNIPAM hydrogel network makes it become conductive hydrogel, and as a result, the PNIPAM hydrogel could become sensitive to an electrical signal, greatly expanding its application. In addition, conductive components usually bring new stimuli-responsive properties of PNIPAM-based hydrogels, such as near-infrared light and stress/strain responsive properties. PNIPAM-ECHs display a wide range of applications in human motion detection, actuators, controlled drug release, wound dressings, etc. To summarize recent research advances and achievements related to PNIPAM-ECHs, this manuscript first reviews the design and structure of representative PNIPAM-ECHs according to their conductive components. Then, the applications of PNIPAM-ECHs have been classified and discussed. Finally, the remaining problems related to PNIPAM-ECHs have been summarized and a future research direction is proposed which is to fabricate PNIPAM-ECHs with integrated multifunctionality.
Keywords: PNIPAM hydrogel, electrically conductive hydrogel, human motion detection, actuator, wound dressing
1. Introduction
Schild et al. [1] reported poly(N-isopropylacrylamide) (PNIPAM) for the first time in 1956, and Scarpa et al. [2] subsequently discovered that PNIPAM displayed a temperature-induced phase transition phenomenon in 1967. Since then, PNIPAM has become a commonly used polymer for the preparation of temperature-sensitive smart materials [3,4,5]. Many scientific researchers have carried out research on PNIPAM because of its temperature-sensitive property, and thousands of related papers have been reported [6,7].
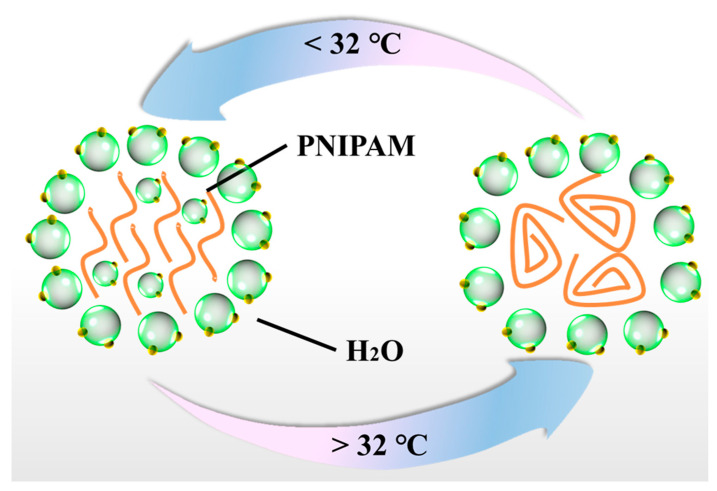
The PNIPAM possesses a typical amide bond (-CO-NH-) and isopropyl group (-CH(CH3)2) structure: the amide bond is hydrophilic, and the isopropyl group is hydrophobic [8]. The PNIPAM aqueous solution can exhibit phase transition behavior around 32 °C as shown in Figure 1. The hydrogen bond interaction between the amide bond and water is strong and the hydrophobic interaction between the isopropyl group and water is weak when the temperature is lower than 32 °C, and as a result, the PNIPAM chain can be dissolved in water. When the temperature is higher than 32 °C, the hydrogen bond interaction between the amide bond and water is weakened and the hydrophobic interaction between the isopropyl group and water is enhanced, and the PNIPAM chain starts to be agglomerated, leading to phase separation [9]. The phase transition temperature of PNIPAM can be adjusted by chemical or physical modification. Generally speaking, the introduction of hydrophilic components can increase its phase transition temperature, and the introduction of hydrophobic components can reduce its phase transition temperature. PNIPAM-based hydrogels (PNIPAM-Hs) can exhibit a significant volume transition behavior (swelling/shrinking) around 32 °C due to the thermal responsive characteristic of PNIPAM [10,11,12]. At the same time, the PNIPAM-Hs also displayed changes of transparency during the swelling/shrinking process [13,14,15]. Due to the specific thermal responsive property of PNIPAM-Hs, great efforts have been made by researchers to investigate PNIPAM-Hs, and they have been extensively reported as drug/cell delivery vehicles [16,17,18,19,20], tissue engineering scaffolds [21,22,23], soft actuators [24,25,26,27], etc.
Figure 1.
Scheme of phase transition behavior of PNIPAM.
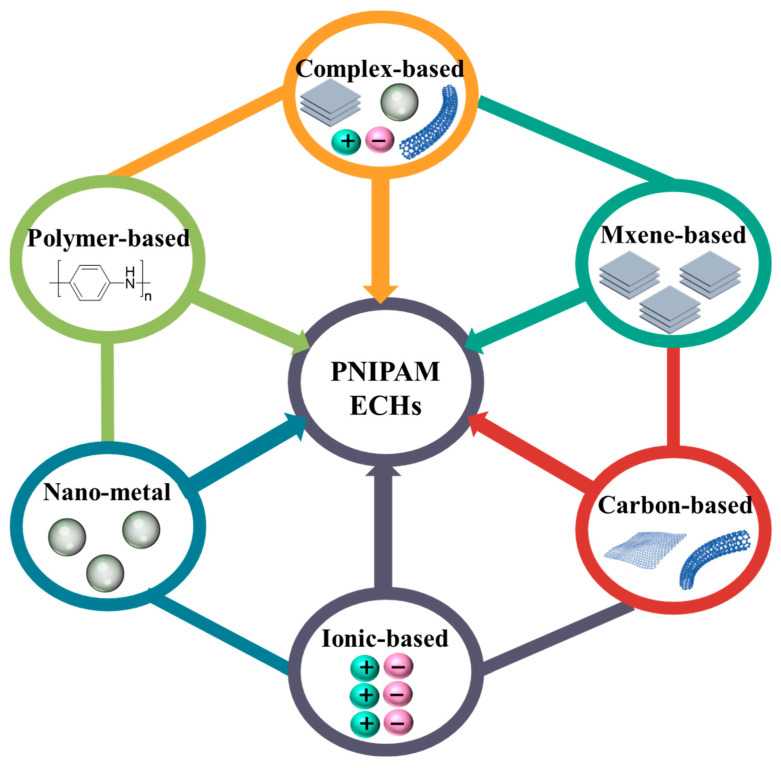
Electrically conductive hydrogels possess both flexibility (originating from hydrogel) and electrical conductivity (originating from conductive components) [28,29,30,31]. Electrically conductive hydrogels can be divided into metal-based (gold nanoparticles and silver nanowires, etc.) conductive hydrogel [32,33,34,35,36], polymer-based conductive hydrogel (PANI, PPY, and PT, etc.) [37,38,39,40,41], carbon materials based (carbon nanotubes and graphene, etc.) conductive hydrogel [42,43,44,45], MXene-based conductive hydrogel [46,47,48], ionic conductive hydrogel (lithium chloride and sulfonate, etc.) [49,50,51,52] or multiple conductive substrates conductive hydrogel [53,54,55] according to their conductive components shown in Figure 2.
Figure 2.
Scheme of 6 categories of PNIPAM-ECHs based on their conductive components.
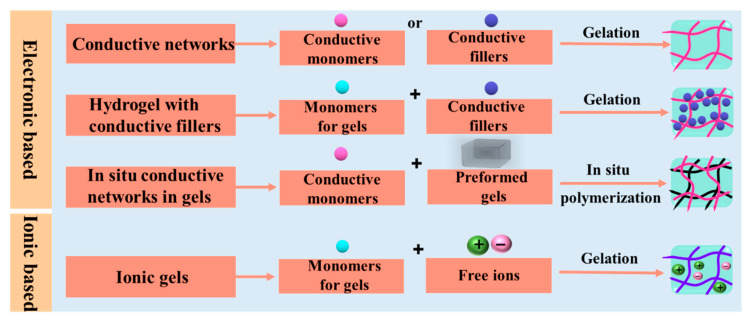
The preparation method of electrically-conductive hydrogel is shown in Figure 3. The basic idea is to introduce conductive components into the hydrogel matrix [56,57,58,59]. The first preparation method is that conductive components form a hydrogel by themselves. For example, Bao et al. [60] used phytic acid as the crosslinking agent and monomer aniline as the raw material to crosslink polyaniline resulting in the formation of a conductive hydrogel. The second method is that the conductive component and hydrogel precursor are first mixed uniformly, and then the hydrogel precursor crosslinks to form a three-dimensional hydrogel network structure within conductive components [61,62,63,64]. For example, carbon nanotubes (CNTs) are modified and grafted with hydrophilic groups or functional groups to improve their dispersibility, and then hydrogel precursors are crosslinked with CNTs to construct conductive hydrogels. The third method is that conductive monomers in situ polymerize to form conductive polymers in a non-conductive hydrogel matrix to form a binary conductive hydrogel. For example, Yu et al. [65] prepared PNIPAM/PANI and PNIPAM/PPY binary conductive hydrogel, where PANI or PPY is formed after the PNIPAM hydrogel adsorbs the aniline or pyrrole monomers and then conductive monomers polymerized. The last method is to prepare ionic conductive hydrogels, where conductive ions can originate from the solvent or raw materials of the hydrogel [66,67]. The conductive hydrogels prepared by the first three methods usually show an opaque dark color (black or dark green), and the ionic conductive hydrogels prepared by the last method usually demonstrate a transparent light color. For different occasions, there will be different requirements for the transparency of the hydrogel, and researchers can choose a suitable preparation method to meet their application needs.
Figure 3.
Scheme of preparation methods of conductive hydrogels. Copyright from Ref. [68] Royal Society of Chemistry.
To combine the advantages of PNIPAM-based hydrogels and electrically conductive hydrogels, researchers have designed and prepared PNIPAM-based electrically conductive hydrogels (PNIPAM-ECHs). Conventional PNIPAM hydrogels don’t show electrical conductivity which could only be sensitive to temperature change, while the incorporation of conductive components into the PNIPAM hydrogel network makes it become conductive hydrogel, as a result, PNIPAM hydrogel could be sensitive to an electrical signal, greatly expanding the application scenarios of them. In addition, the majority of conductive components usually demonstrate photothermal properties (absorb NIR light and turn it into heat). The incorporation of conductive components into PNIPAM hydrogels could endow their NIR-light responsive property. On the other hand, there will be an electrical conductivity change of hydrogels when mechanical stimuli such as strain/stress are applied to PNIPAM-ECHs. Therefore, PNIPAM-ECHs can respond to mechanical stimuli. The introduction of conductive components usually endows new stimuli-responsive characteristics such as near-infrared (NIR) light and stress/strain stimuli-responsive properties [69,70,71], which are favored for specific applications of PNIPAM-ECHs. Currently, the applications of PNIPAM-ECHs focus on wearable electronic devices for human activity detection [72,73,74,75,76,77], soft robots and actuators [78,79,80,81,82], on-off switch [65,70,83,84,85,86], controlled drug release [87], photothermal therapy [88], and wound healing and closure [89,90,91], etc. To summarize the research progress related to PNIPAM-ECHs in the past decades, the preparation and performance of PNIPAM-ECHs are first classified and depicted according to their conductive components. Then, the applications of PNIPAM-ECHs in human motion detection, soft actuators, wound healing and closure, and drug release are discussed. Finally, the remaining problems and future research direction of PNIPAM-ECHs are concluded and directed.
2. Preparation and Performance of PNIPAM-ECHs
This section is mainly focused on the preparation and performance of PNIPAM-ECHs. Based on the conductive components of PNIPAM-ECHs shown in Table 1, they can be divided into a conductive polymer, carbon material, MXene material, metal nanoparticles, ions, and multiple conductive components based PNIPAM-ECHs. The design, preparation method, mechanical property, and electrical conductivity were summarized comprehensively.
Table 1.
Representative PNIPAM-ECHs based on their conductive components. “-” means not determined.
| Gel Code | Conductive Components |
Electrical Conductivity or Resistance | Applications | Reference |
|---|---|---|---|---|
| 1 | PANI or PPY | 0.8 S/m | On-off switch | [65] |
| 2 | PANI or PPY | - | On-off switch, and pressure sensing | [101] |
| 3 | PANI | 0.64 S/m | Repaired electric circuit, on-off switch | [70] |
| 4 | PANI | 0.06 mS/cm | Actuator | [79] |
| 5 | PPY | - | Actuator | [78] |
| 6 | PPY | - | Actuator | [100] |
| 7 | MWCNTs | - | Scaffold | [104] |
| 8 | MWCNTs | 1.5 MΩ | Sensor and actuator | [72] |
| 9 | MWCNTs | - | Photothermal therapy | [88] |
| 10 | Graphene | 10 S/m | - | [106] |
| 11 | GO | - | On-off switch | [86] |
| 12 | rGO | - | Wound dressing | [90] |
| 13 | MXene | ~1.1 S/m | Pressure sensing | [120] |
| 14 | MXene | ~5.5 mS/cm | Human motion detection | [121] |
| 15 | MXene | - | Auto valve | [116] |
| 16 | Mxene | Actuator | [117] | |
| 17 | Ag nanoparticles | - | - | [125] |
| 18 | Au nanoparticles | - | - | [126] |
| 19 | Au nanorods | - | On-off switch | [122] |
| 20 | Au nanorods | - | Drug delivery | [123] |
| 21 | Ions | 4.40 S/m | Human motion detection | [128] |
| 22 | Ions | 0.064 S/m, | Touch sensors | [77] |
| 23 | Ions | ~0.5 S/m | Strain and pressure sensing | [127] |
| 24 | Ions | - | Human motion detection | [69] |
| 25 | Ions | - | Wireless human motion detection | [74] |
| 26 | MWCNTs and ions | 0.19 S/m | Human motion detection and 3D printing | [43] |
| 27 | MWCNTs and PPY | 35 S/m | Human motion detection and pressure sensing | [130] |
| 28 | Ions and rGO | 5.6 mS/cm | Wound dressing | [89] |
| 29 | PEDOT:PSS and GO | 0.08 S/m | Human motion detection | [75] |
| 30 | PEDOT:PSS and ions | - | Human motion detection | [73] |
2.1. PNIPAM-ECHs with Conductive Polymer Served as Conductive Components
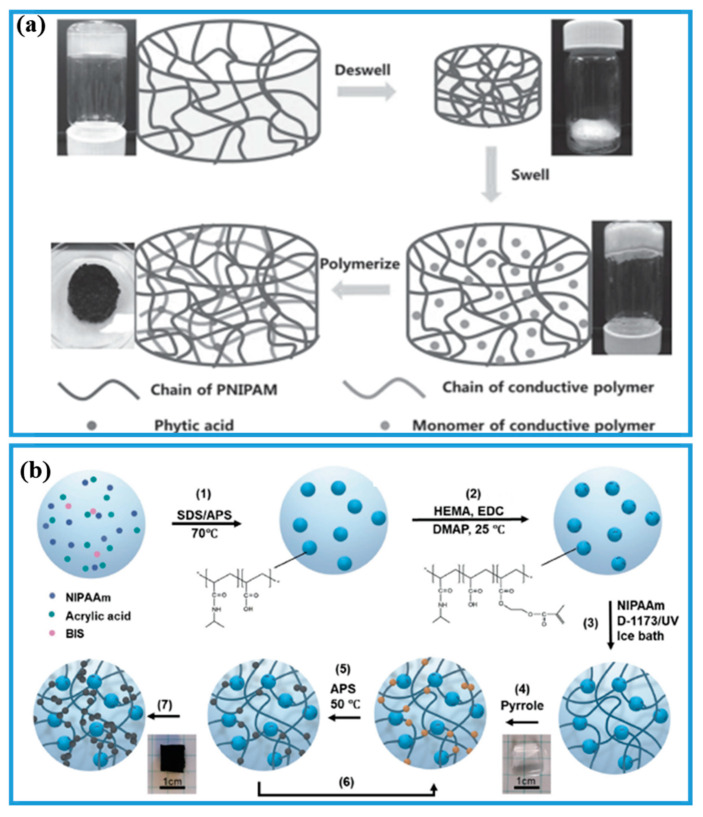
Since the discovery and development of conductive polymers, they have prompted ever-increasing interest in scientific research [92,93,94,95]. Conductive polymers usually exhibited brittle and rigid mechanical properties which might block their way in flexible sensors, energy storage, and tissue engineering scaffolds utilization [95,96]. To overcome those defects, significant advances by researchers have been made, and one of the ways is by introducing conductive polymers into a soft hydrogel matrix [97,98,99]. For example, Yu and coworkers [65] demonstrated double network PNIPAM-ECHs composed of PNIPAM-PANI and PNIPAM-PPY. As shown in Figure 4a, they first prepared PNIPAM hydrogel and then deswelled PNIPAM hydrogel was placed in conductive monomer aniline or pyrrole solution to absorb conductive monomer followed by polymerization of aniline or pyrrole monomer with phytic acid as crosslinker. The obtained PNIPAM-PANI and PNIPAM-PPY hydrogels displayed a compressive modulus of 66.1 and 54.5 Pa and conductivity of 0.2 S/m and 0.8 S/m, respectively. However, the mechanical tensile property of PNIPAM-PANI and PNIPAM-PPY hydrogels were not displayed. PNIPAM-PANI and PNIPAM-PPY hydrogels showed a slow deswelling/swelling speed, taking more than 10 h to complete a deswelling/swelling circle.
Figure 4.
(a) Illustration of preparation of PNIPAM-PANI and PNIPAM-PPY double network PNIPAM-ECHs. Copyright from Ref. [65] John Wiley and Sons. (b) Illustration of preparation of well-distributed PPY PNIPAM-ECHs. Copyright from Ref. [100] Elsevier.
To solve the slow temperature-responsive speed of PNIPAM-ECHs, the cryogel method was used to fabricate PNIPAM-ECHs with rapid temperature-responsive speed. The cryogel was prepared under the melting point of the solvent, while solute polymeric precursors crosslinked and interconnected large macropores formed to obtain cryogel. Guo and coworkers [101] first prepared an interconnected macro-porous PNIPAM cryogel at −20 °C with rapid deswelling/swelling speed, followed by introducing PANI or PPY nanoparticles into the PNIPAM cryogel. The prepared PNIPAM-ECHs could accomplish their deswelling/swelling behavior within 2 min, demonstrating a breakthrough for the preparation of PNIPAM-ECHs with fast temperature-responsive speed. But the above method might cause poor distribution of conductive polymers (rich shell and poor core of conductive polymers) in the hydrogel matrix. Therefore, He and coworkers [100] designed a new type of PNIPAM-ECHs to fabricate well distribution of conductive polymers shown in Figure 4b. They first synthesized poly(NIPAM-co-AA) nanogel and then 2-hydroxyethyl methacrylate (HEMA) were reacted with poly(NIPAM-co-AA) to obtain C=C bond functionalized poly(NIPAM-co-AA) nanogel. The NIPAM was further polymerized and crosslinked by HEMA functionalized poly(NIPAM-co-AA). Finally, the PNIPAM hydrogel was immersed in pyrrole DMSO solution to absorb pyrrole because DMSO solvent could reduce the polymerization rate of pyrrole and simultaneously PNIPAM would not shrink when the temperature surpassed 32 °C, and as a consequence, the well distributed PPY of PNIPAM-ECH was carefully fabricated.
Generally speaking, conductive polymers are good candidates for fabricating PNIPAM-ECHs but the electrical conductivity and morphology of conductive polymers are not easy to be regulated, which should be further ameliorated.
2.2. PNIPAM-ECHs with Carbon Materials Served as Conductive Components
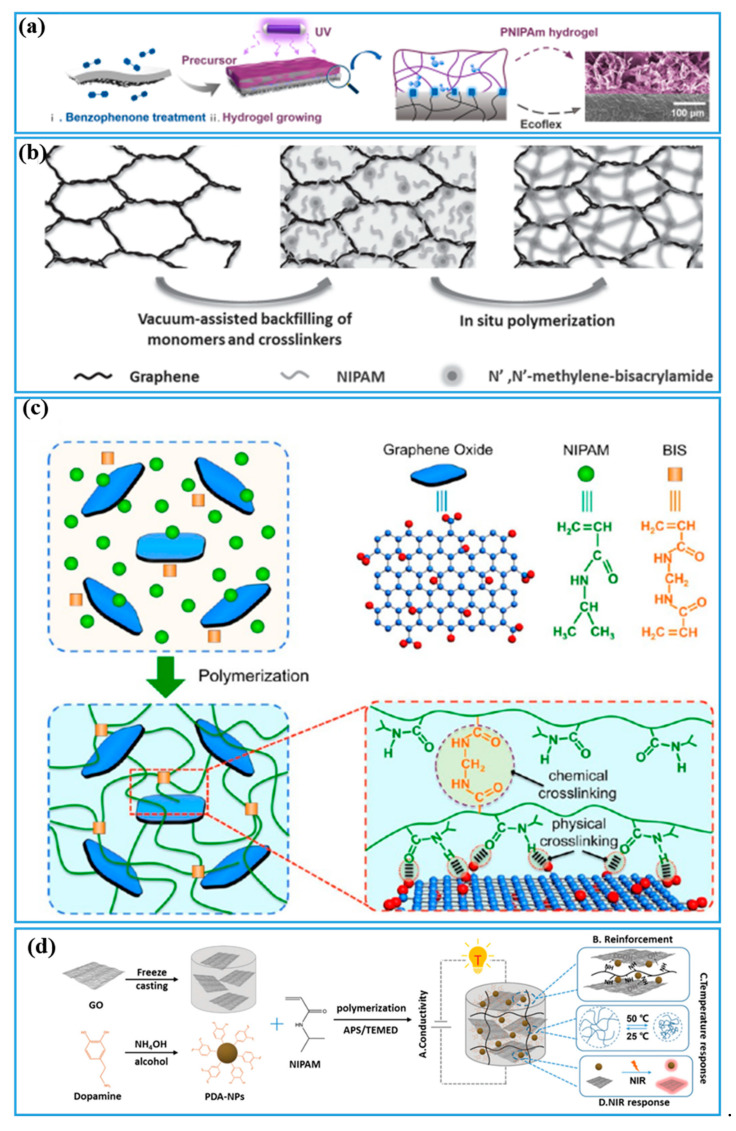
Carbon nanotubes (CNTs) and graphene oxide (GO) nanofillers are commonly used carbon materials for the preparation of ECHs. Among them, CNTs is one-dimensional nanotubes made of carbon that has a large aspect ratio and high electrical conductivity with high elastic modulus and stiffness [102,103]. The researchers consequently integrate CNTs into a soft flexible hydrogel network to construct conductive flexible hydrogel, including PNIPAM-ECHs. For instance, Hsiue and coworkers [104] fabricated PNIPAM-ECHs with multi-walled carbon nanotubes (MWCNTs) as conductive filler and polyethylene glycol dimethacrylate as crosslinker. However, the mechanical property and electrical conductivity were not comprehensively characterized. Apart from nanofillers, CNTs can also be used as a conductive substrate to construct PNIPAM-ECHs as demonstrated in Figure 5a [72]. They first prepared a CNTs film and Ecoflex/CNTs film, then afterward, PNIPAM hydrogel precursor was sprayed on Ecoflex/CNTs film and crosslinked. They obtained a composite hydrogel that demonstrated strong adhesiveness between film and hydrogel with a decent tensile strength of 12 kPa and tensile strain of 350%. But the composite hydrogel displayed high resistance of 1.5 MΩ, which is much higher than other PNIPAM-ECHs.
Figure 5.
(a) Illustration of preparation process of PNIPAM/Ecoflex/CNTs hydrogel. Copyright from Ref. [72] Elsevier. (b) Illustration of preparation of PNIPAM and graphene aerogel double network hydrogel. Copyright from Ref. [105] John Wiley and Sons. (c) Illustration of preparation of PNIPAM with GO as conductive component reinforcement hydrogel. Copyright from Ref. [86] ACS publications. (d) Illustration of preparation of PNIPAM with rGO as conductive component and reinforcement hydrogel. Copyright from Ref. [90] Royal Society of Chemistry.
Because CNTs are hydrophobic and insoluble in water, the dispersion of CNTs should be improved for preparing hydrophilic conductive hydrogel. Chemical modification of hydrophilic groups (-OH and –COOH, etc.) on CNTs surface are frequently applied to improve the dispersity of CNTs and interfacial physical interactions (hydrogen bonding and π-π interaction) between CNTs and hydrogels.
Graphene is a two-dimensional carbon material consisting of a single layer of carbon atoms with hexagonal lattice nanostructure [106]. It is the thinnest carbon material with high electrical conductivity and tensile strength. Scientists also prepared PNIPAM-ECHs with graphene as a conductive component and mechanical reinforcement. As a typical example, researchers prepared mechanically robust PNIPAM-ECHs with a double network structure shown in Figure 5b [105]. They prepared graphene aerogel as the first network and conductive component at the same time. Then, the second PNIPAM hydrogel network was fabricated after graphene aerogel absorb the NIPAM solution. Similar to CNTs, graphene is hydrophobic and scientists prepared graphene oxide (GO) with many –OH, -COOH, and epoxy groups on GO nanosheets which could be soluble in water uniformly due to their strong physical interactions with water [107,108]. Lots of PNIPAM/GO hydrogel were reported by in situ polymerization methods in past decades. The obtained PNIPAM/GO hydrogel demonstrated an enhanced mechanical property compared to pure PNIPAM hydrogel displayed in Figure 5c [86]. However, GO sacrificed the electrical conductivity of graphene because the oxidation process greatly destroyed its conjugated structure, resulting in unsatisfied electrical conductivity. Therefore, partially reduced GO (rGO) was designed with better electrical conductivity compared to GO because of a reshaped conjugated structure without losing solubility and hydrophilicity. As a typical example shown in Figure 5d [90], the polydopamine (PDA) nanoparticles (NPs), GO and NIPAM were polymerized to prepare PNIPAM-ECHs, GO could be partially reduced by PDA nanoparticles resulting in rGO during the preparation process. The rGO could not only endow electrical conductivity but also provide mechanical reinforcement of PNIPAM hydrogel. It should be noticed that PDA would consume free radicals so more of an initiator, such as APS, should be added [109].
Although numerous advances of GO and rGO-based PNIPAM-ECHs have been achieved, some of the drawbacks couldn’t be ignored. First, the electrical conductivity of GO and rGO couldn’t compete with CNTs, graphene, and other conductive fillers. What is more, the oxidation and reduction reaction of graphene is complicated and time-consuming. Preparation of GO with high electrical conductivity and efficacy is meaningful and challenging.
2.3. PNIPAM-ECHs with MXene Served as Conductive Components
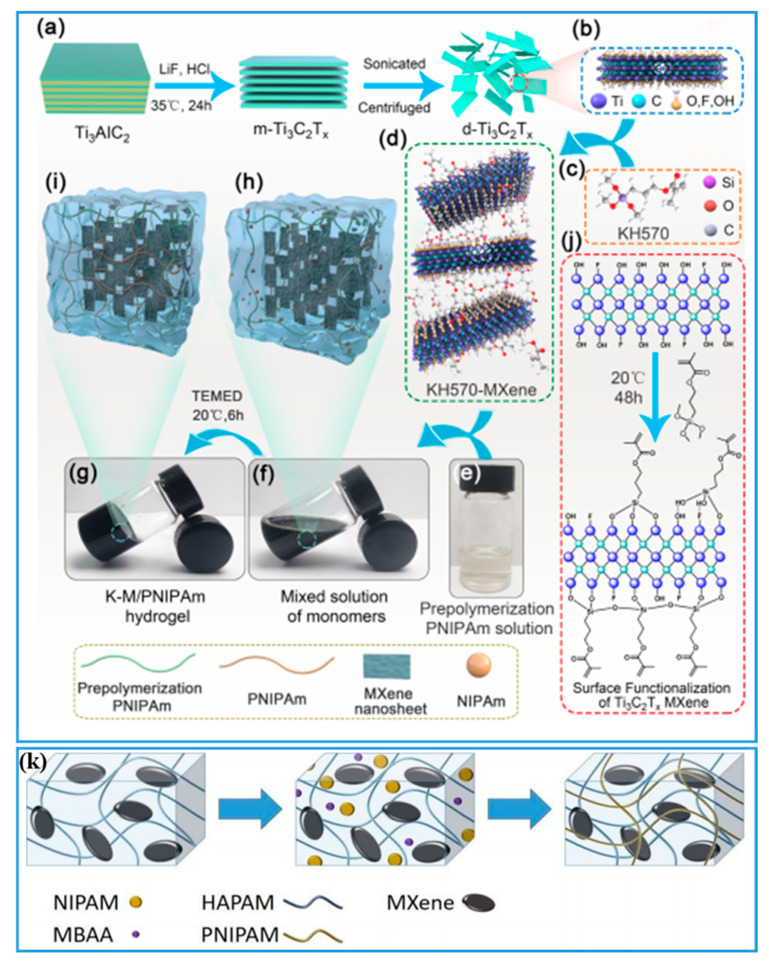
MXene is a two-dimensional material composed of transition metal carbide and/or nitride was first reported in 2011 [110]. There are lots of hydrophilic groups such as –OH groups on the MXene surface so that they can be soluble in water easily [111,112,113]. Besides, MXene also demonstrates metal-like electrical conductivity without disposing of its hydrophilicity [114,115]. It has been extensively used for building PNIPAM-ECHs in the past due to MXene’s fantastic characteristics [116,117]. First, MXene can have strong physical interactions with the hydrogel network thus it can provide enhanced mechanical properties of the hydrogel. Besides, the distribution of MXene in the hydrogel network is uniform and even, without aggregation, so that the conductive pathway is stable and sensitive. The general preparation process of the MXene nanosheet is described as follows [118,119]: a typical “MAX” (M represents the transition metal, A refers to IIIA and IVA elements, X is C and/or N) was chemically etched by solution containing F-, and consequently, MXene was built. During etching, the A in “MAX” was selectively removed resulting in the MXene nanosheet. As a typical example shown in Figure 6k, Ran and coworkers [120] first prepared supramolecular hydrogel using acrylamide (AM), OP-10 emulsifier, and lauryl methacrylate in Ti3C2 MXene water solution followed by lyophilization. Then, the lyophilized hydrogel was put in PNIPAM hydrogel precursor solution to take it in completely, followed by slow polymerization of PNIPAM at low temperature for 7 days to obtain a binary network of PNIPAM-ECHs. The double network PNIPAM-ECH displayed a stretchability of 1400%, a tensile strength of 0.4 MPa, and electrical conductivity of ~1.1 S/m with self-healing property. To further ameliorate the compatibility between MXene and PNIPAM, MXene could be surfaced modified by a silane coupler displayed in Figure 6a–j [121]. Researchers first prepared Ti3C2Tx MXene by HF acid etching followed by sonication and centrifugation, then MXene was functionalized with γ-methacryloxypropyltrimethoxysilane (KH570) to improve compatibility with PNIPAM. Second, NIPAM and AM were copolymerized to form a PNIPAM-PAM solution. Third, surface modified MXene solution was mixed with PNIPAM-PAM solution followed by adding NIPAM, initiator, and crosslinker to fabricate PNIPAM-ECHs. The prepared PNIPAM-ECHs displayed a desirable strain sensitivity with a gauge factor of ~4.5.
Figure 6.
(a) Illustration of preparation process of Ti3C2Tx Mxene. (b) Structure of Ti3C2Tx Mxene. (c) Structure of KH570. (d) Structure of KH570-Mxene. (e) Picture of PNIPAM-PAM solution. (f) Picture of mixed solution with MXene. (g) Picture of prepared PNIPAM-ECH. (h) Structure of mixed solution with MXene. (i) Structure of prepared PNIPAM-ECH. (j) Illustration of surface functionalization of MXene. Copyright from Ref. [121] ACS publications. (k) Illustration of preparation of double network PNIPAM-ECH. Copyright from Ref. [120] ACS publications.
Some limitations of MXene are unavoidable in the ever-increasing development of PNIPAM-ECHs. First of all, the preparation of MXene is complicated. Moreover, dangerous and harmful chemicals such as HF acid might be applied for etching. We should be very careful when we use HF acid because HF acid could not only harm humans but also the environment. Therefore, it is demanded and significant to come up with new ways to generate MXene safely and simply.
2.4. PNIPAM-ECHs with Metal Nanoparticles Served as Conductive Components
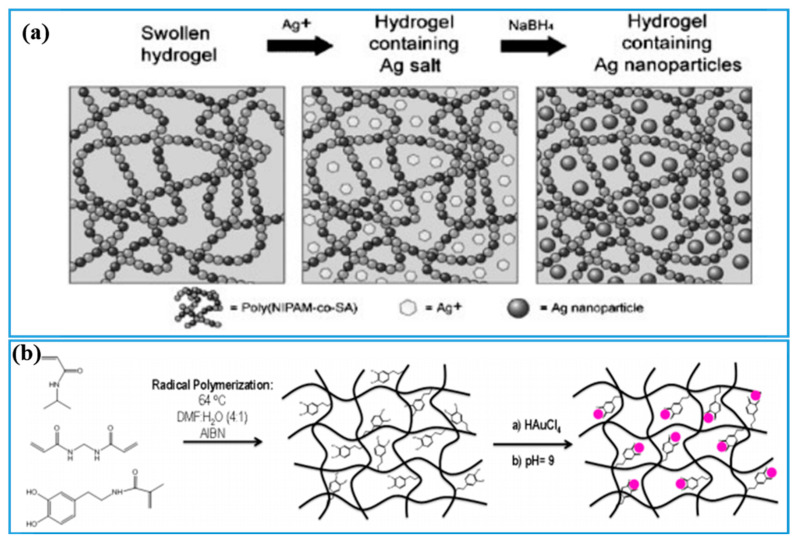
Metal is a highly conductive material and it is usually designed in nanoparticle (Ag or Au nanoparticle), nanosliver (Ag nanosliver), and nanorod (Au nanorod) forms to fabricate PNIPAM-ECHs [122,123]. The metal nanoparticles could be prepared by adding extracts to metal salt solutions and metal ions are reduced to metal nanoparticles [124]. Au and Ag nanomaterials are frequently used to fabricate PNIPAM-ECHs because they possess extra biological functions such as antibacterial and photodynamic therapy properties. As a proof of concept shown in Figure 7a [125], NIPAM and sodium acrylate (SA) were first copolymerized to obtain poly(NIPAM-co-SA) hydrogel, and then poly(NIPAM-co-SA) hydrogel was placed in Ag salt solution to swell Ag salt completely, followed by adding NaBH4 to reduce Ag+ to Ag nanoparticles. Au nanorods were incorporated in a similar way shown in Figure 7b, where Marcelo and coworkers [126] first copolymerized catechol-methacrylamide and NIPAM to obtain hydrogel and then hydrogel swelled the HAuCl4 solution quickly and Au+ reduced to Au gradually by catechol groups to obtain PNIPAM-ECH. Although nano metals demonstrated high electrical conductivity, their distribution in hydrogel still needs to be regulated, and precious metals such as Au and Ag are expensive which would increase the cost of PNIPAM-ECHs.
Figure 7.
(a) Illustration of preparation process of PNIPAM-ECH with Ag nanoparticles served as conductive filler. Copyright from Ref. [125] John Wiley and Sons. (b) Illustration of preparation process of PNIPAM-ECH with Au nanorods served as conductive filler. Copyright from Ref. [126] ACS publications.
2.5. PNIPAM-ECHs with Ion Served as Conductive Components
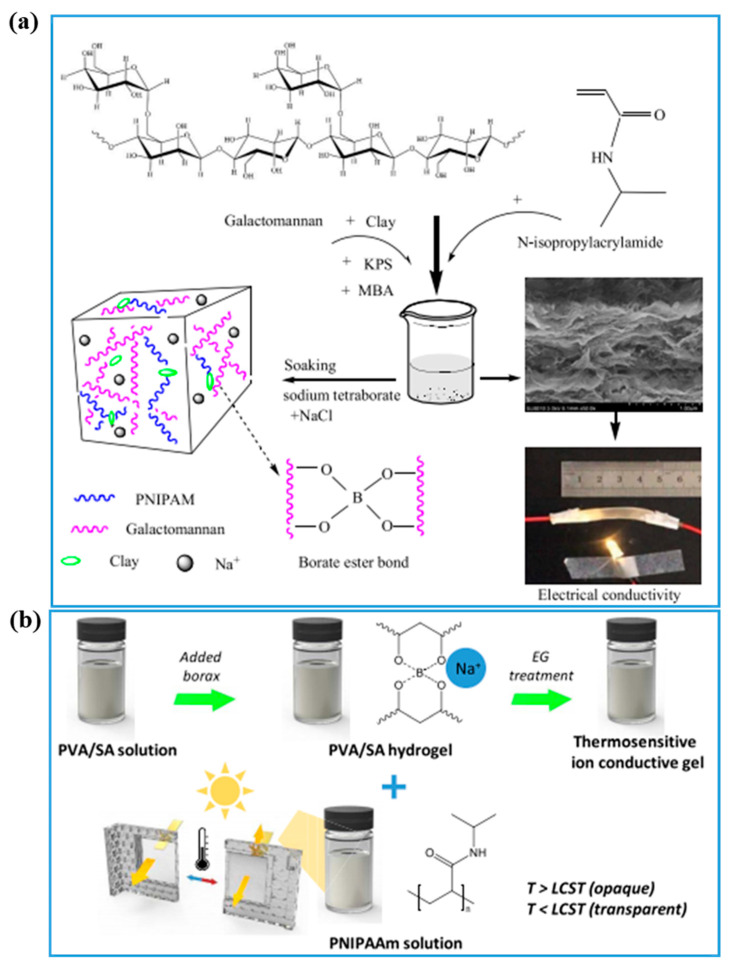
The above listed conductive components are electronically conductive and prepared ECHs which usually displayed a dark color. Apart from the electronic conductive mechanism, the ions [127] could also be used for the preparation of PNIPAM-ECHs with a light and transparent appearance. This kind of morphology and color are suitable for some utilizations such as wound dressing; thus the wound healing process is visible. The ions can come from the polyelectrolytes and/or salts solution. As shown in Figure 8a, Duan and coworkers [128] first fabricated a hydrogel by copolymerization of NIPAM and galactomannan with clay as mechanical reinforcement. Then, the hydrogel was placed in NaCl and sodium borate solution to obtain binary network ionic conductive PNIPAM-ECH. The obtained PNIPAM-ECHs displayed enhanced mechanical performance after being treated with NaCl and sodium borate solution with the highest modulus of ~60 MPa, elongation at break of 1370%, and electrical conductivity of 4.40 S/m. Free ions from salt solution might show poor retention in the hydrogel matrix resulting in a decrease in electrical conductivity. Therefore, ions from polymer are also involved in the preparation of PNIPAM-ECHs demonstrated in Figure 8b [77]. The sodium polyacrylate solution was mixed with PVA solution gradually, then PNIPAM was added to the above mixture solution followed by adding borax to crosslink and form PNIPAM-ECHs. The prepared PNIPAM-ECHs exhibited a modulus of 423 kPa, elongation at break of ~600%, and electrical conductivity of 6.64 × 10−2 S/m, which is much lower than clay reinforced hydrogel. What’s more, nanoclay of laponite could also be used to fabricate ionic PNIPAM-ECHs because it can release Na+. In addition to provide ionic electrical conductivity, they can be served as nano-reinforcement to enhance the mechanical property of hydrogels significantly whereby strong physical interactions (hydrogen bonding and electrostatic interaction) between hydrogel and laponite formed.
Figure 8.
(a) Illustration of preparation process of PNIPAM-ECH with Na+ served as conductive filler. Copyright from Ref. [128] ACS publications. (b) Illustration preparation process of PNIPAM-ECH with clay and Na+ served as conductive filler. Copyright from Ref. [77] ACS publications.
2.6. PNIPAM-ECHs with Multiple Components as Conductive Components
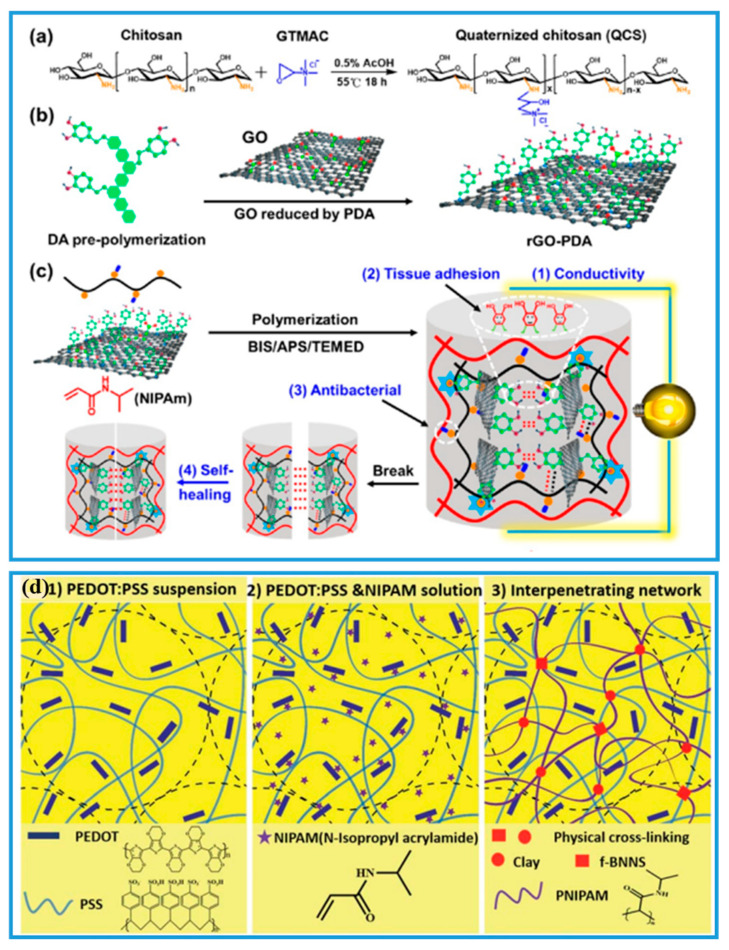
In addition to the single conductive component of PNIPAM-ECHs, the multiple conductive components were also designed by researchers because they might not only improve the electrical conductivity but also enhance the mechanical performance as well as bring new stimuli-responsive characteristics and functions of PNIPAM-ECHs [43,76,129,130]. As a typical example shown in Figure 9a–c, Guo and coworkers [89] prepared double networks of PNIPAM-ECHs using quaternized chitosan (QCS), rGO-PDA, and NIPAM. The PNIPAM could form the first network by BIS crosslinker, and the second network was built between rGO-PDA and quaternized chitosan through a Schiff-base reaction. The second network contributed to the antibacterial (inherent and photothermal) and self-healing properties of the hydrogel as well as tissue adhesiveness, which significantly broaden the application of PNIPAM-ECHs in the biomedical field. Apart from introducing a new function, the mechanical property of PNIPAM-ECHs could also be enhanced, for instance, as proof shown in Figure 9d, Xu and coworkers [73] prepared strong physical crosslinked PNIPAM-ECHs with self-healing, adhesive, and photothermal responsive properties by using NIPAM, PEDOT:PSS, functionalized boron nitride nanosheets (f-BNNS), and nanoclay. The constructed hydrogel displayed a maximum strain of ~2600% and it can even withstand 90% compressive strain without mechanical failure, demonstrating superior mechanical performances. These results were 2 times higher than their previously reported PNIPAM/f-BNNS/nanoclay hydrogel. Because PSS could form strong hydrogen bond interactions with PNIPAM and f-BNNS, and other physical interactions such as electrostatic interactions, π-π static interactions existed between PEDOT and PSS. Moreover, the hydrogel displayed enhanced electrical conductivity compared to pure PEDOT:PSS.
Figure 9.
(a) Illustration of preparation process of quaternized chitosan. (b) Illustration of preparation process of rGO-PDA. (c) Illustration of preparation process of PNIPAM-ECH with quaternized chitosan and rGO-PDA served as conductive components. Copyright from Ref. [89] ACS publications. (d) Illustration of preparation process with PEDOT:PSS and clay served as conductive components. Copyright from Ref. [73] Royal Society of Chemistry.
The six categories of PNIPAM-ECHs based on their conductive components are comprehensively summarized. The incorporation of conductive components made a huge difference in PNIPAM hydrogel function. First, the introduction of electrically conductivity ensures PNIPAM-ECHs could be applied in the electronic field such as conductive sensors and on-off switches. Second, the electronic conductive components usually exhibited photothermal behavior so that PNIPAM-ECHs have NIR-light responsive properties at the same time. PNIPAM-ECHs could be served as NIR-light-controlled actuators or soft robots after introducing NIR-light responsive conductive components. Third, conductive components can also be used as mechanical reinforcement as they greatly improve the mechanical performance of PNIPAM-ECHs. Other properties such as self-healing, adhesive and shape memory properties can also be achieved by PNIPAM-ECHs to develop integrated multifunctional PNIPAM-ECHs, greatly expanding their application scenarios.
3. Applications of PNIPAM-ECHs
PNIPAM-ECHs were extensively applied in a variety of fields due to their advantages, including human motion sensors, soft actuators and bionic robots, on-off switch, photo-thermal therapy, drug delivery vehicles, and wound dressing. This section is mainly highlighted the biomedical application of PNIPAM-ECHs.
3.1. Human Motion Detection Application of PNIPAM-ECHs
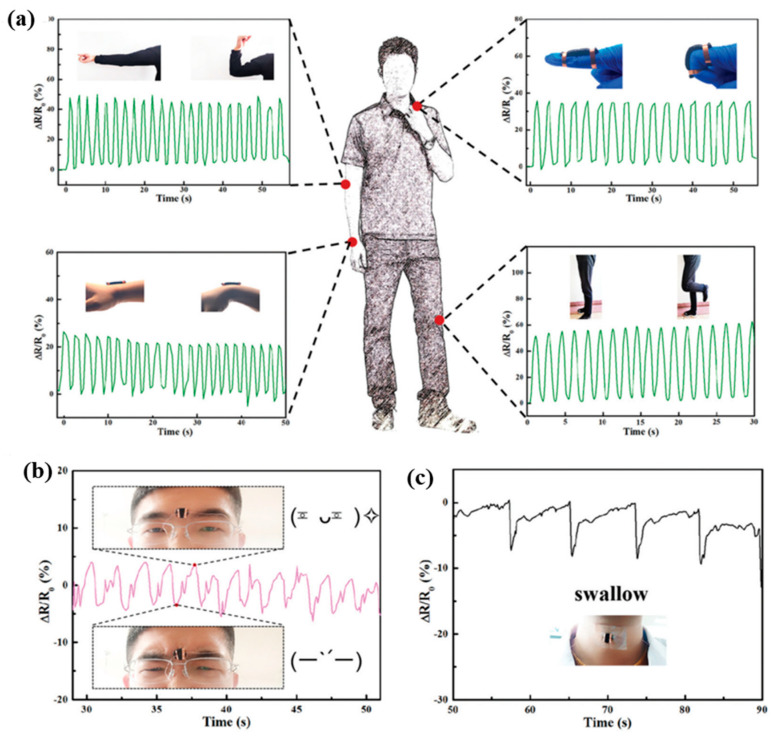
Similar to other conductive hydrogel sensors, the PNIPAM-ECHs could also be used for human motion sensing and detection because of their potential excellent mechanical property. On the other hand, the body temperature would increase after human motion, resulting in an electrical conductivity change in the PNIPAM-ECHs. The hydrogel sensor fabrication and sensing mechanism is described as follows: the hydrogel is mounted on the human body to be tested and then the wire is connected to the hydrogel and detector. The hydrogel resistance would change as long as the tested part is moving because the strain and stress of hydrogel would change simultaneously. As a typical example in Figure 10a,b [75], large scale motion (including finger bending, wrist bending, elbow and knee motion, etc.) and small scale motion (swallow, facial expression and subtle motion, etc.) can both be detected. They prepared a PNIPAM-ECH using NIPAM and conductive components PEDOT:PSS and rGO. The obtained hydrogel exhibited a high tensile strain (~2500%) and strength (29 kPa) as well as adhesive property. They first characterized the large-scale motion including elbow, finger bending, wrist, and knee, which displayed resistance changes of ~50%, ~28%, ~38%, and ~50%, respectively. The facial expression and swallow motion were subsequently tested, which exhibited much smaller resistance changes compared to large-scale motion.
Figure 10.
(a) PNIPAM-ECH for large-scale human motion detection (elbow motion, finger bending, wrist bending, and knee motion). (b) PNIPAM-ECH for facial expression detection. (c) PNIPAM-ECH for swallow motion detection. Copyright from Ref. [75] Royal Society of Chemistry.
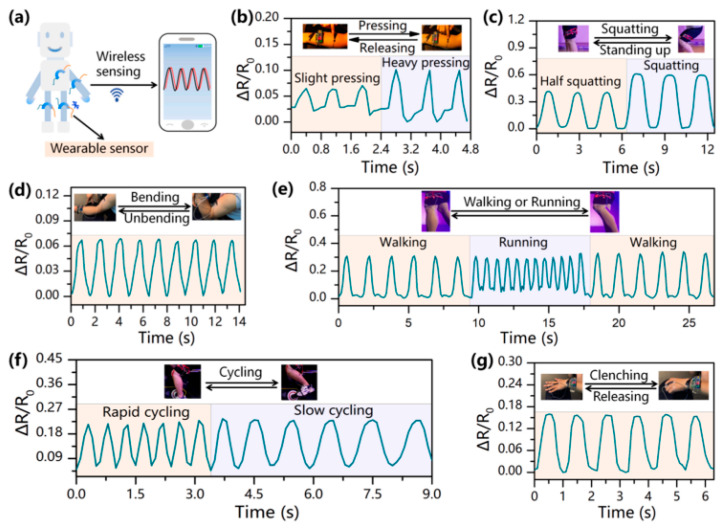
It is inconvenient for human motion detection sometimes because the wire is mounted on the human body. Therefore, researchers developed wireless sensors by using PNIPAM-ECH shown in Figure 11 [74]. The PNIPAM-ECH consists of PVA, Poly(NIPAM-co-AM), Fe3O4, and KCl, among them, PVA was introduced to improve the adhesiveness of hydrogel, Fe3O4 was the magnetic responsive component and KCl served as a conductive component. A Bluetooth transmission system was implanted in hydrogel so that it can transfer resistance change wirelessly. The pressing, squatting, bending, walking, or running, cycling, and clenching motions were characterized carefully. All of the motions demonstrated stable and regular resistance change.
Figure 11.
(a) Illustration of wearable sensor for wireless sensing. PNIPAM-ECH for (b) pressing-releasing detection. (c) squatting-standing up detection. (d) finger bending-unbending detection. (e) walking or running detection. (f) rapid cycling and slow cycling detection. (g) clenching and releasing detection. Copyright from Ref. [74] Elsevier.
Although there are lots of PNIPAM-ECHs applied in human motion detection, PNIPAM-ECHs applied in physiological signal monitoring (electrocardiogram (ECG) and electroencephalogram (EEG) signal) works have not been reported to the best of our knowledge. Human physiological signal monitoring is vital for human health so it is meaningful to design and prepare PNIPAM-ECHs for ECG and EEG signal detection. What is more, the lack of long-term stability and environmental tolerance would block their way on human motion detection, because PNIPAM-ECHs would lose water in a dry environment and freeze when the temperature is low. Environment tolerant PNIPAM-ECHs are required to be fabricated for human motion detection in special environments.
Currently, most of the PNIPAM-ECHs for sensors need an external energy supply such as batteries. Self-powered sensors based on PNIPAM-ECHs that maintain their sensing functions are designed and fabricated by the Park and In group [131]. They designed a solar cell system combined with a stress and strain sensor so that the self-powered system could detect human motion directly under sunlight.
3.2. Soft Actuators and Robotics Application of PNIPAM-ECHs
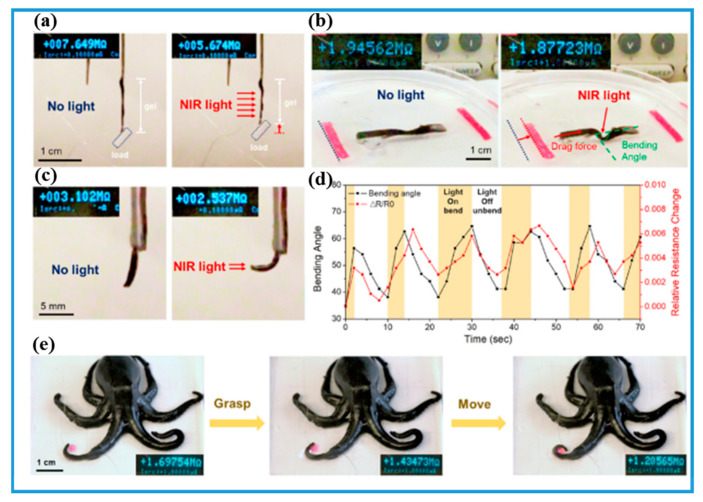
The actuator could convert stimuli signals or energy to motion. It requires an actuation force to allow for its movement and interaction with the external environment [132,133,134]. Soft actuator [135,136] is composed of flexible materials rather than rigid materials (metals, hard plastics, and ceramics), which is beneficial to compatibility with humans. Soft actuators can be applied as artificial muscles, medical devices, and soft grippers. PNIPAM-ECHs can convert heat stimuli to motion and electrical conductivity change at the same time, which is beneficial for real-time data recording for actuators and robotics applications. He and coworkers [79,100] recently designed and prepared two types of PNIPAM-ECHs for soft actuators and robotics. As shown in Figure 12a, actuation behavior induced by the NIR light of PNIPAM-ECH was first demonstrated. The PNIPAM-ECH would shrink after NIR light exposure and pull up the load at the same time. The bending behavior of PNIPAM-ECH was shown in Figure 12b where PNIPAM-ECH showed a maximum bending angle of ~65° when exposed to NIR light. The bending angle would turn to 40° after the light was switched off. What is more, the relative resistance change curve of PNIPAM-ECH corresponded to the bending angle curve displayed in Figure 12d, exhibiting stable and controllable actuation behavior induced by NIR-light. PNIPAM-ECH was further designed and fabricated into octopus-like hydrogel for soft robotics shown in Figure 12e. The octopus could grasp a pink ball after hydrogel exposure to NIR light. PNIPAM-ECH is a good candidate for soft actuator because of the thermal responsive property of PNIPAM. Moreover, the conductive components are usually NIR light-responsive so that PNIPAM-ECH could be manipulated by NIR light. However, the accuracy, responsive speed, and stability of PNIPAM-ECH soft actuators are not good enough to compare with conventional actuators due to the disadvantages of PNIPAM-ECHs. It is still a great challenge to fabricate PNIPAM-ECH soft actuators and robotics with rapid response speed and actuating stability.
Figure 12.
(a) Pictures of loading behavior of PNIPAM-ECH hydrogel triggered by NIR light. (b) Pictures of bending behavior of PNIPAM-ECH hydrogel triggered by NIR light. (c) Actuation behavior of octopus-like PNIPAM-ECH hydrogel. (d) Bending angle and resistance change curves of PNIPAM-ECH. (e) Actuation behavior of octopus-like PNIPAM-ECH hydrogel. Copyright from Ref. [100] Elsevier.
3.3. Wound Dressing and Wound Closure Application of PNIPAM-ECHs
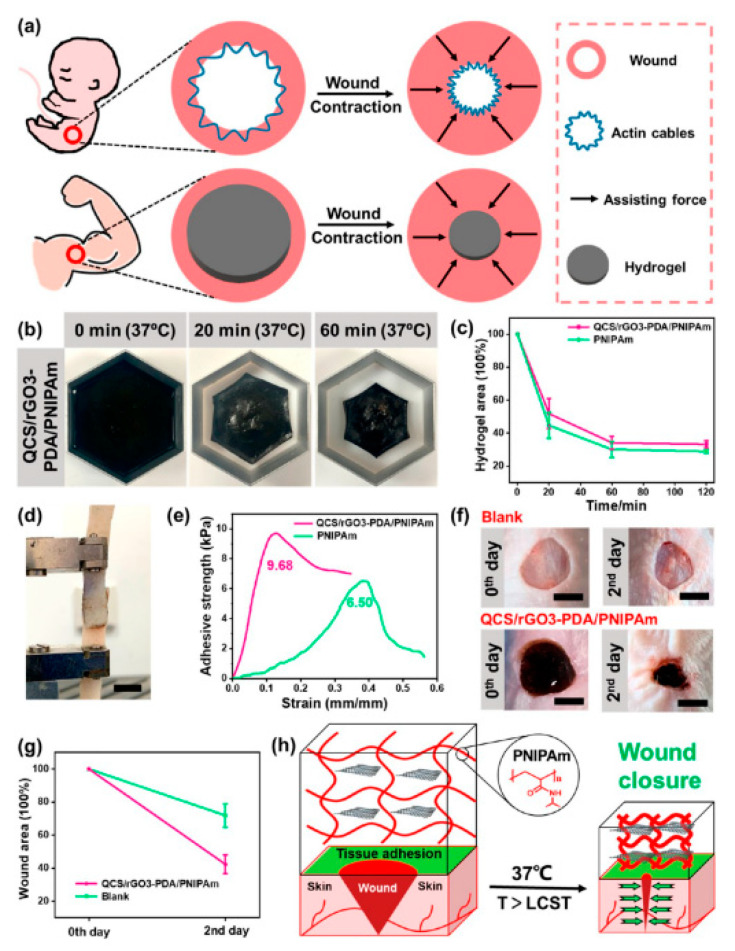
Wound dressings are used to clean and protect the wound from the external environment to promote wound healing [137,138,139]. The main purpose of wound closure is to stop wound bleeding, speed wound healing, and prevent wound infection [140,141,142]. Hydrogel dressing could keep moisture and absorb exudate to accelerate wound healing which was developed rapidly in recent years [143,144]. The PNIPAM-ECHs are also designed as wound dressings to promote wound healing and wound closure because PNIPAM-ECHs would shrink with body temperature which can induce wound contraction. In addition, electrical conductivity enables cellular signaling and function in many types of tissues such as human skin, cardiac tissue, muscle tissue, and nerve tissue. It plays a significant role in tissue function. On the other hand, some of the conductive components such as polyaniline possess good free radical scavenging capacity to reduce wound inflammation and enhance wound healing. As a typical example shown in Figure 13a by Guo and coworkers [89], the illustration of PNIPAM-ECH is used for wound dressing to promote wound healing and wound closure. Figure 13b,c demonstrated the shrinking behavior of PNIPAM-ECH at 37 °C. The adhesive property of PNIPAM-ECH is shown in Figure 13d,e. Wound closure results are shown in Figure 13f–h, the PNIPAM-ECH group displayed an obvious closure of the wound area compared to the blank group to promote wound healing. Other functions such as antibacterial and adhesive properties are usually integrated into PNIPAM-ECH to accelerate wound healing. However, the biosafety of PNIPAM-ECH dressing might be a problem, because PNIPAM and conductive components are not biodegradable, and NIPAM is toxic to humans. The biocompatibility of PNIPAM-ECH should be further evaluated before clinical utilization.
Figure 13.
(a) Illustration of wound closure process of PNIPAM-ECH wound dressing. (b) Pictures of shrunk behavior of PNIPAM-ECH at 37 °C. (c) Change of hydrogel area of PNIPAM-ECH at 37 °C. (d) Picture of PNIPAM-ECH for adhesive strength testing. (e) Adhesive strength of PNIPAM-ECH. (f) Wound closure pictures of PNIPAM-ECH hydrogel group and blank group. (g) Wound area curves of PNIPAM-ECH hydrogel group and blank group. (h) Illustration of wound closure process of wound closure. Copy right from Ref. [89] ACS publications.
3.4. Drug Delivery Application of PNIPAM-ECHs
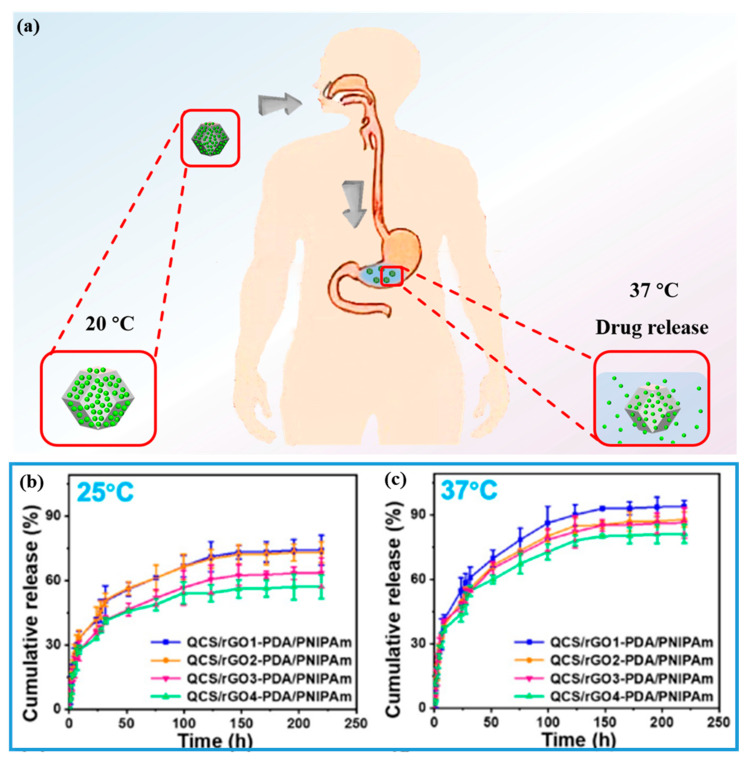
Hydrogel drug delivery means that hydrogel could improve its biosafety and efficacy by controlling release rate, quantity, and place of drugs. The hydrogel drug delivery system should be stable, and delivery could be controlled under various physiological changes, as a result, increasing the bioavailability of the drug. PNIPAM-ECHs could be used for temperature and electrical-stimuli-dependent drug delivery vehicles due to their thermal and electrical responsive property. Figure 14a demonstrates the drug release process of PNIPAM-ECHs in vivo. Figure 14b,c [89] shows the tetracycline of doxycycline drug release curve at 25 °C and 37 °C, respectively. 57~74% (from QCS/rGO4-PDA/PNIPAM to QCS/rGO1-PDA/PNIPAM) of drug released after 10 days at 25 °C, while 81~93% (from QCS/rGO4-PDA/PNIPAM to QCS/rGO1-PDA/PNIPAM) of drug released after 10 days at 37 °C, exhibited higher drug release amount at 37 °C. This is because PNIPAM-ECHs would shrink at 37 °C and more drugs could release from the hydrogel matrix.
Figure 14.
(a) Scheme of drug release process of PNIPAM-ECH in vivo. (b) Cumulative release of drug at (b) 25 °C and (c) 37 °C of PNIPAM-ECH. Copy right from Ref. [89] ACS publications.
Although PNIPAM-ECHs could be used as drug delivery vehicles, PNIPAM-ECHs drug delivery vehicles also have some disadvantages. First, the release process of the drug is temperature-dependent, but the on-demand release of the drug is still needs to be regulated. Second, the biodegradability of PNIPAM is a problem for drug release studies in vivo, which could cause immune reactions in creatures. Third, how to maintain drug levels in a suitable therapeutic range should be further investigated.
4. Conclusions and Outlooks
In this review, the recent progress related to PNIPAM-ECHs is comprehensively summarized. The preparation and properties are first demonstrated and followed by the applications of PNIPAM-ECHs. Great strides in the use of PNIPAM-ECHs have been made, and PNIPAM-ECHs were successfully used in various settings, especially in human motion sensing, wound dressing, and soft actuators, and controlled drug release. However, there are still some problems that need to be resolved according to our recent study related to PNIPAM-ECHs: (1) The research of PNIPAM-ECHs for human physiological signal monitoring (such as ECG signals and EEG signals) is not yet sufficient. Such applications require hydrogels to possess high sensitivity to external stimuli signals and how to recognize those stimuli signals and respond to them accurately still needs to be explored; (2) PNIPAM-ECHs have drawbacks in their long-term stability and environmental tolerance. These defects may limit the application of PNIPAM-ECHs in extreme environments. (3) The accurate actuation of PNIPAM-ECHs still needs to be improved for hydrogel machine application. (4) Some of the raw materials of PNIPAM-ECHs are toxic, PNIPAM and conductive components are usually not biodegradable which may cause harm to the organism. Given these issues, the following prospects for future research are proposed:
-
(1)
Preparation of PNIPAM-ECHs with high sensitivity and stability as flexible wearable sensing devices so that they can be better used for human ECG signal, EEG signal, and arterial pressure monitoring.
-
(2)
Because the hydrogel is prone to dehydration/freezing in a dry/frozen environment, moisturizing and anti-freezing PNIPAM-ECHs are required to improve the long-term stability and ability to withstand the extreme environment of PNIPAM-ECHs.
-
(3)
Accuracy and response speed of PNIPAM-ECHs need to be regulated for soft robots and actuators.
-
(4)
For biomedical medical applications, the biocompatibility of PNIPAM-ECHs needs to be further evaluated.
Abbreviations
| PNIPAM | Poly(N-isopropylacrylamide) |
| PNIPAM-ECHs | Poly(N-isopropylacrylamide) based electrically conductive hydrogels |
| ECHs | electrically conductive hydrogels |
| PNIPAM-Hs | Poly(N-isopropylacrylamide) based hydrogels |
| PANI | Polyaniline |
| PPY | Polypyrrole |
| PT | Polythiophene |
| CNTs | Carbon nanotubes |
| NIR | Near-infrared |
| HEMA | 2-Hydroxyethyl methacrylate |
| MWCNTs | Multi-walled carbon nanotubes |
| AA | Acrylic acid |
| DMSO | Dimethyl sulfoxide |
| GO | Graphene oxide |
| rGO | reduced Graphene oxide |
| SA | Sodium acrylate |
| PDA | Polydopamine |
| NPs | Nanoparticles |
| KH570 | γ-methacryloxypropyltrimethoxysilane |
| f-BNNS | functionalized boron nitride nanosheets |
| PVA | Polyvinyl alcohol |
| PEDOT:PSS | Poly(3:4-ethylenedioxythiophene):polystyrene sulfonate |
| BIS N: N’ | methylenebis(acrylamide) |
| QCS | Quaternized chitosan |
| APS | Ammonium persulphate |
| TEMED | Tetramethylethylenediamine |
| PAM | Polyacrylamide |
| AM | Acrylamide |
| ECG | Electrocardiogram |
| EEG | Electroencephalogram |
Author Contributions
Z.D.: Writing, Conceptualization and supervision of the manuscript; Y.G.: Writing and revision of the manuscript; X.Z.: Revision of the manuscript; T.D.: Revision of the manuscript; J.Z.: Revision of the manuscript; Y.X.: Revision of the manuscript; F.W.: Revision of the manuscript; Y.W.: Revision of the manuscript; M.G.: Conceptualization, revision and supervision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
We thank the financial support of High-level Talents Foundation for Scientific Research of Xi’ an University of Science and Technology (2050122015), Medical and Health Science and Technology Project of Zhejiang Province (2021KY153).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schild H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992;17:163–249. doi: 10.1016/0079-6700(92)90023-R. [DOI] [Google Scholar]
- 2.Scarpa J.S., Mueller D.D., Klotz I.M. Slow hydrogen-deuterium exchange in a non-.alpha.-helical polyamide. J. Am. Chem. Soc. 1967;89:6024–6030. doi: 10.1021/ja01000a006. [DOI] [Google Scholar]
- 3.Liu J., Jiang L., He S., Zhang J., Shao W. Recent progress in PNIPAM-based multi-responsive actuators: A mini-review. Chem. Eng. J. 2022;433:133496. doi: 10.1016/j.cej.2021.133496. [DOI] [Google Scholar]
- 4.Chen Y., Wang Z., Harn Y.W., Pan S., Li Z., Lin S., Peng J., Zhang G., Lin Z. Resolving Optical and Catalytic Activities in Thermoresponsive Nanoparticles by Permanent Ligation with Temperature-Sensitive Polymers. Angew. Chem. Int. Ed. Engl. 2019;58:11910–11917. doi: 10.1002/anie.201906329. [DOI] [PubMed] [Google Scholar]
- 5.Ge S., Li J., Geng J., Liu S., Xu H., Gu Z. Adjustable dual temperature-sensitive hydrogel based on a self-assembly cross-linking strategy with highly stretchable and healable properties. Mater. Horiz. 2021;8:1189–1198. doi: 10.1039/D0MH01762K. [DOI] [PubMed] [Google Scholar]
- 6.Haq M.A., Su Y., Wang D. Mechanical properties of PNIPAM based hydrogels: A review. Mat. Sci. Eng. C. 2017;70:842–855. doi: 10.1016/j.msec.2016.09.081. [DOI] [PubMed] [Google Scholar]
- 7.Tang L., Wang L., Yang X., Feng Y., Li Y., Feng W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021;115:100702. doi: 10.1016/j.pmatsci.2020.100702. [DOI] [Google Scholar]
- 8.Halperin A., Kröger M., Winnik F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. Engl. 2015;54:15342–15367. doi: 10.1002/anie.201506663. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Wu C. Light-Scattering Study of Coil-to-Globule Transition of a Poly(N-isopropylacrylamide) Chain in Deuterated Water. Macromolecules. 1999;32:4299–4301. doi: 10.1021/ma9902450. [DOI] [Google Scholar]
- 10.Han L., Zhang Y., Lu X., Wang K., Wang Z., Zhang H. Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces. 2016;8:29088–29100. doi: 10.1021/acsami.6b11043. [DOI] [PubMed] [Google Scholar]
- 11.Cao M., Wang Y., Hu X., Gong H., Li R., Cox H., Zhang J., Waigh T.A., Xu H., Lu J.R. Reversible Thermoresponsive Peptide–PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules. 2019;20:3601–3610. doi: 10.1021/acs.biomac.9b01009. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Lin L., Cheng Q., Jiang L. A Strong Bio-Inspired Layered PNIPAM–Clay Nanocomposite Hydrogel. Angew. Chem. Int. Ed. Engl. 2012;51:4676–4680. doi: 10.1002/anie.201200267. [DOI] [PubMed] [Google Scholar]
- 13.Ding H., Li B., Liu Z., Liu G., Pu S., Feng Y., Jia D., Zhou Y. Decoupled pH- and Thermo-Responsive Injectable Chitosan/PNIPAM Hydrogel via Thiol-Ene Click Chemistry for Potential Applications in Tissue Engineering. Adv. Healthc. Mater. 2020;9:2000454. doi: 10.1002/adhm.202000454. [DOI] [PubMed] [Google Scholar]
- 14.Zheng W.J., An N., Yang J.H., Zhou J., Chen Y.M. Tough Al-alginate/Poly(N-isopropylacrylamide) Hydrogel with Tunable LCST for Soft Robotics. ACS Appl. Mater. Interfaces. 2015;7:1758–1764. doi: 10.1021/am507339r. [DOI] [PubMed] [Google Scholar]
- 15.Tian J., Peng H., Du X., Wang H., Cheng X., Du Z. Hybrid thermochromic microgels based on UCNPs/PNIPAm hydrogel for smart window with enhanced solar modulation. J. Alloys Compd. 2021;858:157725. doi: 10.1016/j.jallcom.2020.157725. [DOI] [Google Scholar]
- 16.Ashraf S., Park H.-K., Park H., Lee S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016;24:297–304. doi: 10.1007/s13233-016-4052-2. [DOI] [Google Scholar]
- 17.Ayar Z., Shafieian M., Mahmoodi N., Sabzevari O., Hassannejad Z. A rechargeable drug delivery system based on pNIPAM hydrogel for the local release of curcumin. J. Appl. Polym. Sci. 2021;138:51167. doi: 10.1002/app.51167. [DOI] [Google Scholar]
- 18.Liu M., Song X., Wen Y., Zhu J.-L., Li J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces. 2017;9:35673–35682. doi: 10.1021/acsami.7b12849. [DOI] [PubMed] [Google Scholar]
- 19.Mou F., Chen C., Zhong Q., Yin Y., Ma H., Guan J. Autonomous Motion and Temperature-Controlled Drug Delivery of Mg/Pt-Poly(N-isopropylacrylamide) Janus Micromotors Driven by Simulated Body Fluid and Blood Plasma. ACS Appl. Mater. Interfaces. 2014;6:9897–9903. doi: 10.1021/am502729y. [DOI] [PubMed] [Google Scholar]
- 20.Hoang H.T., Jo S.-H., Phan Q.-T., Park H., Park S.-H., Oh C.-W., Lim K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using “click” chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021;260:117812. doi: 10.1016/j.carbpol.2021.117812. [DOI] [PubMed] [Google Scholar]
- 21.Rana M.M., De la Hoz Siegler H. Tuning the Properties of PNIPAm-Based Hydrogel Scaffolds for Cartilage Tissue Engineering. Polymers. 2021;13:3154. doi: 10.3390/polym13183154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atoufi Z., Kamrava S.K., Davachi S.M., Hassanabadi M., Saeedi Garakani S., Alizadeh R., Farhadi M., Tavakol S., Bagher Z., Hashemi Motlagh G. Injectable PNIPAM/Hyaluronic acid hydrogels containing multipurpose modified particles for cartilage tissue engineering: Synthesis, characterization, drug release and cell culture study. Int. J. Biol. Macromol. 2019;139:1168–1181. doi: 10.1016/j.ijbiomac.2019.08.101. [DOI] [PubMed] [Google Scholar]
- 23.Dosh R.H., Jordan-Mahy N., Sammon C., Le Maitre C.L. Use of l-pNIPAM hydrogel as a 3D-scaffold for intestinal crypts and stem cell tissue engineering. Biomater. Sci. 2019;7:4310–4324. doi: 10.1039/C9BM00541B. [DOI] [PubMed] [Google Scholar]
- 24.Ma C., Lu W., Yang X., He J., Le X., Wang L., Zhang J., Serpe M.J., Huang Y., Chen T. Bioinspired Anisotropic Hydrogel Actuators with On–Off Switchable and Color-Tunable Fluorescence Behaviors. Adv. Funct. Mater. 2018;28:1704568. doi: 10.1002/adfm.201704568. [DOI] [Google Scholar]
- 25.Warren H., Shepherd D.J., in het Panhuis M., Officer D.L., Spinks G.M. Porous PNIPAm hydrogels: Overcoming diffusion-governed hydrogel actuation. Sens. Actuators A. 2020;301:111784. doi: 10.1016/j.sna.2019.111784. [DOI] [Google Scholar]
- 26.Zhang E., Wang T., Hong W., Sun W., Liu X., Tong Z. Infrared-driving actuation based on bilayer graphene oxide-poly(N-isopropylacrylamide) nanocomposite hydrogels. J. Mater. Chem. A. 2014;2:15633–15639. doi: 10.1039/C4TA02866J. [DOI] [Google Scholar]
- 27.Yang Y., Tan Y., Wang X., An W., Xu S., Liao W., Wang Y. Photothermal Nanocomposite Hydrogel Actuator with Electric-Field-Induced Gradient and Oriented Structure. ACS Appl. Mater. Interfaces. 2018;10:7688–7692. doi: 10.1021/acsami.7b17907. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y., Wan C., Yang Y., Yang H., Wang S., Dai Z., Ji K., Jiang H., Chen X., Long Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019;29:1806220. doi: 10.1002/adfm.201806220. [DOI] [Google Scholar]
- 29.Deng Z., Wang H., Ma P.X., Guo B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale. 2020;12:1224–1246. doi: 10.1039/C9NR09283H. [DOI] [PubMed] [Google Scholar]
- 30.Green R. Elastic and conductive hydrogel electrodes. Nat. Biomed. Eng. 2019;3:9–10. doi: 10.1038/s41551-018-0342-7. [DOI] [PubMed] [Google Scholar]
- 31.Deng Z., Yu R., Guo B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021;5:2092–2123. doi: 10.1039/D0QM00868K. [DOI] [Google Scholar]
- 32.He X., Zhang C., Wang M., Zhang Y., Liu L., Yang W. An Electrically and Mechanically Autonomic Self-healing Hybrid Hydrogel with Tough and Thermoplastic Properties. ACS Appl. Mater. Interfaces. 2017;9:11134–11143. doi: 10.1021/acsami.7b00358. [DOI] [PubMed] [Google Scholar]
- 33.Cai J., Zhang X., Liu W., Huang J., Qiu X. Synthesis of highly conductive hydrogel with high strength and super toughness. Polymer. 2020;202:122643. doi: 10.1016/j.polymer.2020.122643. [DOI] [Google Scholar]
- 34.Wang L., Daoud W.A. Hybrid conductive hydrogels for washable human motion energy harvester and self-powered temperature-stress dual sensor. Nano Energy. 2019;66:104080. doi: 10.1016/j.nanoen.2019.104080. [DOI] [Google Scholar]
- 35.Zhang X., Liu W., Cai J., Huang J., Qiu X. Equip the hydrogel with armor: Strong and super tough biomass reinforced hydrogels with excellent conductivity and anti-bacterial performance. J. Mater. Chem. A. 2019;7:26917–26926. doi: 10.1039/C9TA10509C. [DOI] [Google Scholar]
- 36.Ohm Y., Pan C., Ford M.J., Huang X., Liao J., Majidi C. An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nat. Electron. 2021;4:185–192. doi: 10.1038/s41928-021-00545-5. [DOI] [Google Scholar]
- 37.Jin X., Jiang H., Li G., Fu B., Bao X., Wang Z., Hu Q. Stretchable, conductive PAni-PAAm-GOCS hydrogels with excellent mechanical strength, strain sensitivity and skin affinity. Chem. Eng. J. 2020;394:124901. doi: 10.1016/j.cej.2020.124901. [DOI] [Google Scholar]
- 38.Chen J., Peng Q., Thundat T., Zeng H. Stretchable, Injectable, and Self-Healing Conductive Hydrogel Enabled by Multiple Hydrogen Bonding toward Wearable Electronics. Chem. Mater. 2019;31:4553–4563. doi: 10.1021/acs.chemmater.9b01239. [DOI] [Google Scholar]
- 39.Wu T., Cui C., Huang Y., Liu Y., Fan C., Han X., Yang Y., Xu Z., Liu B., Fan G., et al. Coadministration of an Adhesive Conductive Hydrogel Patch and an Injectable Hydrogel to Treat Myocardial Infarction. ACS Appl. Mater. Interfaces. 2020;12:2039–2048. doi: 10.1021/acsami.9b17907. [DOI] [PubMed] [Google Scholar]
- 40.Ren K., Cheng Y., Huang C., Chen R., Wang Z., Wei J. Self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B. 2019;7:5704–5712. doi: 10.1039/C9TB01214A. [DOI] [PubMed] [Google Scholar]
- 41.Ting M.S., Narasimhan B.N., Travas-Sejdic J., Malmström J. Soft conducting polymer polypyrrole actuation based on poly(N-isopropylacrylamide) hydrogels. Sens. Actuators B. 2021;343:130167. doi: 10.1016/j.snb.2021.130167. [DOI] [Google Scholar]
- 42.Han L., Liu K., Wang M., Wang K., Fang L., Chen H., Zhou J., Lu X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018;28:1704195. doi: 10.1002/adfm.201704195. [DOI] [Google Scholar]
- 43.Deng Z., Hu T., Lei Q., He J., Ma P.X., Guo B. Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces. 2019;11:6796–6808. doi: 10.1021/acsami.8b20178. [DOI] [PubMed] [Google Scholar]
- 44.Park J., Jeon J., Kim B., Lee M.S., Park S., Lim J., Yi J., Lee H., Yang H.S., Lee J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020;30:2003759. doi: 10.1002/adfm.202003759. [DOI] [Google Scholar]
- 45.Jo H., Sim M., Kim S., Yang S., Yoo Y., Park J.-H., Yoon T.H., Kim M.-G., Lee J.Y. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 2017;48:100–109. doi: 10.1016/j.actbio.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 46.Li X., He L., Li Y., Chao M., Li M., Wan P., Zhang L. Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. ACS Nano. 2021;15:7765–7773. doi: 10.1021/acsnano.1c01751. [DOI] [PubMed] [Google Scholar]
- 47.Feng Y., Liu H., Zhu W., Guan L., Yang X., Zvyagin A.V., Zhao Y., Shen C., Yang B., Lin Q. Muscle-Inspired MXene Conductive Hydrogels with Anisotropy and Low-Temperature Tolerance for Wearable Flexible Sensors and Arrays. Adv. Funct. Mater. 2021;31:2105264. doi: 10.1002/adfm.202105264. [DOI] [Google Scholar]
- 48.Wang Q., Pan X., Lin C., Gao H., Cao S., Ni Y., Ma X. Modified Ti3C2TX (MXene) nanosheet-catalyzed self-assembled, anti-aggregated, ultra-stretchable, conductive hydrogels for wearable bioelectronics. Chem. Eng. J. 2020;401:126129. doi: 10.1016/j.cej.2020.126129. [DOI] [Google Scholar]
- 49.Huang H., Han L., Li J., Fu X., Wang Y., Yang Z., Xu X., Pan L., Xu M. Super-stretchable, elastic and recoverable ionic conductive hydrogel for wireless wearable, stretchable sensor. J. Mater. Chem. A. 2020;8:10291–10300. doi: 10.1039/D0TA02902E. [DOI] [Google Scholar]
- 50.Kong W., Wang C., Jia C., Kuang Y., Pastel G., Chen C., Chen G., He S., Huang H., Zhang J., et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018;30:1801934. doi: 10.1002/adma.201801934. [DOI] [PubMed] [Google Scholar]
- 51.Sui X., Guo H., Cai C., Li Q., Wen C., Zhang X., Wang X., Yang J., Zhang L. Ionic conductive hydrogels with long-lasting antifreezing, water retention and self-regeneration abilities. Chem. Eng. J. 2021;419:129478. doi: 10.1016/j.cej.2021.129478. [DOI] [Google Scholar]
- 52.Zhang H., Gao T., Zhang S., Zhang P., Li R., Ma N., Wei H., Zhang X. Conductive and Tough Smart Poly(N-isopropylacrylamide) Hydrogels Hybridized by Green Deep Eutectic Solvent. Macromol. Chem. Phys. 2021;222:2000301. doi: 10.1002/macp.202000301. [DOI] [Google Scholar]
- 53.Ye F., Li M., Ke D., Wang L., Lu Y. Ultrafast Self-Healing and Injectable Conductive Hydrogel for Strain and Pressure Sensors. Adv. Mater. Technol. 2019;4:1900346. doi: 10.1002/admt.201900346. [DOI] [Google Scholar]
- 54.Han S., Liu C., Lin X., Zheng J., Wu J., Liu C. Dual Conductive Network Hydrogel for a Highly Conductive, Self-Healing, Anti-Freezing, and Non-Drying Strain Sensor. ACS Appl. Mater. Interfaces. 2020;2:996–1005. doi: 10.1021/acsapm.9b01198. [DOI] [Google Scholar]
- 55.Tong X., Du L., Xu Q. Tough, adhesive and self-healing conductive 3D network hydrogel of physically linked functionalized-boron nitride/clay /poly(N-isopropylacrylamide) J. Mater. Chem. A. 2018;6:3091–3099. doi: 10.1039/C7TA10898B. [DOI] [Google Scholar]
- 56.Hu C., Zhang Y., Wang X., Xing L., Shi L., Ran R. Stable, Strain-Sensitive Conductive Hydrogel with Antifreezing Capability, Remoldability, and Reusability. ACS Appl. Mater. Interfaces. 2018;10:44000–44010. doi: 10.1021/acsami.8b15287. [DOI] [PubMed] [Google Scholar]
- 57.Han L., Yan L., Wang M., Wang K., Fang L., Zhou J., Fang J., Ren F., Lu X. Transparent, Adhesive, and Conductive Hydrogel for Soft Bioelectronics Based on Light-Transmitting Polydopamine-Doped Polypyrrole Nanofibrils. Chem. Mater. 2018;30:5561–5572. doi: 10.1021/acs.chemmater.8b01446. [DOI] [Google Scholar]
- 58.Peng Q., Chen J., Wang T., Peng X., Liu J., Wang X., Wang J., Zeng H. Recent advances in designing conductive hydrogels for flexible electronics. Infomat. 2020;2:843–865. doi: 10.1002/inf2.12113. [DOI] [Google Scholar]
- 59.Li L., Wang Y., Pan L., Shi Y., Cheng W., Shi Y., Yu G. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015;15:1146–1151. doi: 10.1021/nl504217p. [DOI] [PubMed] [Google Scholar]
- 60.Pan L., Yu G., Zhai D., Lee H.R., Zhao W., Liu N., Wang H., Tee B.C.-K., Shi Y., Cui Y., et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA. 2012;109:9287–9292. doi: 10.1073/pnas.1202636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao X., Guo B., Wu H., Liang Y., Ma P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018;9:2784. doi: 10.1038/s41467-018-04998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong R., Zhao X., Guo B., Ma P.X. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces. 2016;8:17138–17150. doi: 10.1021/acsami.6b04911. [DOI] [PubMed] [Google Scholar]
- 63.Zhao X., Wu H., Guo B., Dong R., Qiu Y., Ma P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47. doi: 10.1016/j.biomaterials.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Liang Y., Li Z., Huang Y., Yu R., Guo B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano. 2021;15:7078–7093. doi: 10.1021/acsnano.1c00204. [DOI] [PubMed] [Google Scholar]
- 65.Shi Y., Ma C., Peng L., Yu G. Conductive “Smart” Hybrid Hydrogels with PNIPAM and Nanostructured Conductive Polymers. Adv. Funct. Mater. 2015;25:1219–1225. doi: 10.1002/adfm.201404247. [DOI] [Google Scholar]
- 66.Liu H., Wang X., Cao Y., Yang Y., Yang Y., Gao Y., Ma Z., Wang J., Wang W., Wu D. Freezing-Tolerant, Highly Sensitive Strain and Pressure Sensors Assembled from Ionic Conductive Hydrogels with Dynamic Cross-Links. ACS Appl. Mater. Interfaces. 2020;12:25334–25344. doi: 10.1021/acsami.0c06067. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Tebyetekerwa M., Liu Y., Wang M., Zhu J., Xu J., Zhang C., Liu T. Extremely stretchable and healable ionic conductive hydrogels fabricated by surface competitive coordination for human-motion detection. Chem. Eng. J. 2021;420:127637. doi: 10.1016/j.cej.2020.127637. [DOI] [Google Scholar]
- 68.Wang Z., Cong Y., Fu J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B. 2020;8:3437–3459. doi: 10.1039/C9TB02570G. [DOI] [PubMed] [Google Scholar]
- 69.Di X., Hang C., Xu Y., Ma Q., Li F., Sun P., Wu G. Bioinspired tough, conductive hydrogels with thermally reversible adhesiveness based on nanoclay confined NIPAM polymerization and a dopamine modified polypeptide. Mater. Chem. Front. 2020;4:189–196. doi: 10.1039/C9QM00582J. [DOI] [Google Scholar]
- 70.Zhu Y., Liu S., Shi X., Han D., Liang F. A thermally responsive host–guest conductive hydrogel with self-healing properties. Mater. Chem. Front. 2018;2:2212–2219. doi: 10.1039/C8QM00324F. [DOI] [Google Scholar]
- 71.Bongiovanni Abel S., Riberi K., Rivarola C.R., Molina M., Barbero C.A. Synthesis of a Smart Conductive Block Copolymer Responsive to Heat and Near Infrared Light. Polymers. 2019;11:1744. doi: 10.3390/polym11111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H., Liang Y., Gao G., Wei S., Jian Y., Le X., Lu W., Liu Q., Zhang J., Chen T. Asymmetric bilayer CNTs-elastomer/hydrogel composite as soft actuators with sensing performance. Chem. Eng. J. 2021;415:128988. doi: 10.1016/j.cej.2021.128988. [DOI] [Google Scholar]
- 73.Cao S., Tong X., Dai K., Xu Q. A super-stretchable and tough functionalized boron nitride/PEDOT:PSS/poly(N-isopropylacrylamide) hydrogel with self-healing, adhesion, conductive and photothermal activity. J. Mater. Chem. A. 2019;7:8204–8209. doi: 10.1039/C9TA00618D. [DOI] [Google Scholar]
- 74.Zhou H., Jin Z., Gao Y., Wu P., Lai J., Li S., Jin X., Liu H., Chen W., Wu Y., et al. Thermoresponsive, magnetic, adhesive and conductive nanocomposite hydrogels for wireless and non-contact flexible sensors. Colloids Surf. A. 2022;636:128113. doi: 10.1016/j.colsurfa.2021.128113. [DOI] [Google Scholar]
- 75.Zhang H., Yue M.Q., Wang T.T., Wang J.Q., Wu X.Z., Yang S.R. Conductive hydrogel-based flexible strain sensors with superior chemical stability and stretchability for mechanical sensing in corrosive solvents. New J. Chem. 2021;45:4647–4657. doi: 10.1039/D0NJ05880G. [DOI] [Google Scholar]
- 76.Wang Z., Zhou H., Chen W., Li Q., Yan B., Jin X., Ma A., Liu H., Zhao W. Dually Synergetic Network Hydrogels with Integrated Mechanical Stretchability, Thermal Responsiveness, and Electrical Conductivity for Strain Sensors and Temperature Alertors. ACS Appl. Mater. Interfaces. 2018;10:14045–14054. doi: 10.1021/acsami.8b02060. [DOI] [PubMed] [Google Scholar]
- 77.Kweon O.Y., Samanta S.K., Won Y., Yoo J.H., Oh J.H. Stretchable and Self-Healable Conductive Hydrogels for Wearable Multimodal Touch Sensors with Thermoresponsive Behavior. ACS Appl. Mater. Interfaces. 2019;11:26134–26143. doi: 10.1021/acsami.9b04440. [DOI] [PubMed] [Google Scholar]
- 78.Luo R., Wu J., Dinh N.-D., Chen C.-H. Gradient Porous Elastic Hydrogels with Shape-Memory Property and Anisotropic Responses for Programmable Locomotion. Adv. Funct. Mater. 2015;25:7272–7279. doi: 10.1002/adfm.201503434. [DOI] [Google Scholar]
- 79.Zhao Y., Lo C.-Y., Ruan L., Pi C.-H., Kim C., Alsaid Y., Frenkel I., Rico R., Tsao T.-C., He X. Somatosensory actuator based on stretchable conductive photothermally responsive hydrogel. Sci. Robot. 2021;6:eabd5483. doi: 10.1126/scirobotics.abd5483. [DOI] [PubMed] [Google Scholar]
- 80.Cheng Y., Chan K.H., Wang X.Q., Ding T., Li T., Lu X., Ho G.W. Direct-Ink-Write 3D Printing of Hydrogels into Biomimetic Soft Robots. ACS Nano. 2019;13:13176–13184. doi: 10.1021/acsnano.9b06144. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y., Wu W., Yu J., Wang Y., Zhu J., Hu Z. Mechanical strong stretchable conductive multi-stimuli-responsive nanocomposite double network hydrogel as biosensor and actuator. J. Biomater. Sci. Polym. Ed. 2020;31:1770–1792. doi: 10.1080/09205063.2020.1775760. [DOI] [PubMed] [Google Scholar]
- 82.Sun Z., Wei C., Liu W., Liu H., Liu J., Hao R., Huang M., He S. Two-Dimensional MoO2 Nanosheet Composite Hydrogels with High Transmittance and Excellent Photothermal Property for Near-Infrared Responsive Actuators and Microvalves. ACS Appl. Mater. Interfaces. 2021;13:33404–33416. doi: 10.1021/acsami.1c04110. [DOI] [PubMed] [Google Scholar]
- 83.Liu W., Zhang X., Wei G., Su Z. Reduced Graphene Oxide-Based Double Network Polymeric Hydrogels for Pressure and Temperature Sensing. Sensors. 2018;18:3162. doi: 10.3390/s18093162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C.H., Dong W.B., Li P.Q., Wang Y.F., Tu H.Y., Tan S., Wu Y., Watanabe M. Reversible ion-conducting switch by azobenzene molecule with light-controlled sol-gel transitions of the PNIPAm ion gel. ACS Appl. Mater. Interfaces. 2020;12:42202–42209. doi: 10.1021/acsami.0c12910. [DOI] [PubMed] [Google Scholar]
- 85.Sun N., Sun P., Wu A., Qiao X., Lu F., Zheng L. Facile fabrication of thermo/redox responsive hydrogels based on a dual crosslinked matrix for a smart on-off switch. Soft Matter. 2018;14:4327–4334. doi: 10.1039/C8SM00504D. [DOI] [PubMed] [Google Scholar]
- 86.Shi K., Liu Z., Wei Y.Y., Wang W., Ju X.J., Xie R., Chu L.Y. Near-Infrared Light-Responsive Poly(N-isopropylacrylamide)/Graphene Oxide Nanocomposite Hydrogels with Ultrahigh Tensibility. ACS Appl. Mater. Interfaces. 2015;7:27289–27298. doi: 10.1021/acsami.5b08609. [DOI] [PubMed] [Google Scholar]
- 87.Strachota B., Strachota A., Slouf M., Brus J., Cimrova V. Monolithic intercalated PNIPAm/starch hydrogels with very fast and extensive one-way volume and swelling responses to temperature and pH: Prospective actuators and drug release systems. Soft Matter. 2019;15:752–769. doi: 10.1039/C8SM02153H. [DOI] [PubMed] [Google Scholar]
- 88.Zhou M., Liu S., Jiang Y., Ma H., Shi M., Wang Q., Zhong W., Liao W., Xing M.M.Q. Doxorubicin-Loaded Single Wall Nanotube Thermo-Sensitive Hydrogel for Gastric Cancer Chemo-Photothermal Therapy. Adv. Funct. Mater. 2015;25:4730–4739. doi: 10.1002/adfm.201501434. [DOI] [Google Scholar]
- 89.Li M., Liang Y., He J., Zhang H., Guo B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing To Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020;32:9937–9953. doi: 10.1021/acs.chemmater.0c02823. [DOI] [Google Scholar]
- 90.Zhu Y., Zeng Q., Zhang Q., Li K., Shi X., Liang F., Han D. Temperature/near-infrared light-responsive conductive hydrogels for controlled drug release and real-time monitoring. Nanoscale. 2020;12:8679–8686. doi: 10.1039/D0NR01736A. [DOI] [PubMed] [Google Scholar]
- 91.Yang X., Zhang C., Deng D., Gu Y., Wang H., Zhong Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small. 2022;18:2104368. doi: 10.1002/smll.202104368. [DOI] [PubMed] [Google Scholar]
- 92.Nezakati T., Seifalian A., Tan A., Seifalian A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018;118:6766–6843. doi: 10.1021/acs.chemrev.6b00275. [DOI] [PubMed] [Google Scholar]
- 93.Pang H., Xu L., Yan D.-X., Li Z.-M. Conductive polymer composites with segregated structures. Prog. Polym. Sci. 2014;39:1908–1933. doi: 10.1016/j.progpolymsci.2014.07.007. [DOI] [Google Scholar]
- 94.Zhan C., Yu G., Lu Y., Wang L., Wujcik E., Wei S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C. 2017;5:1569–1585. doi: 10.1039/C6TC04269D. [DOI] [Google Scholar]
- 95.Deng H., Lin L., Ji M., Zhang S., Yang M., Fu Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014;39:627–655. doi: 10.1016/j.progpolymsci.2013.07.007. [DOI] [Google Scholar]
- 96.Zhang F., Feng Y., Feng W. Three-dimensional interconnected networks for thermally conductive polymer composites: Design, preparation, properties, and mechanisms. Mat. Sci. Eng. R. 2020;142:100580. doi: 10.1016/j.mser.2020.100580. [DOI] [Google Scholar]
- 97.Ma Z., Shi W., Yan K., Pan L., Yu G. Doping engineering of conductive polymer hydrogels and their application in advanced sensor technologies. Chem. Sci. 2019;10:6232–6244. doi: 10.1039/C9SC02033K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li P., Jin Z., Peng L., Zhao F., Xiao D., Jin Y., Yu G. Stretchable All-Gel-State Fiber-Shaped Supercapacitors Enabled by Macromolecularly Interconnected 3D Graphene/Nanostructured Conductive Polymer Hydrogels. Adv. Mater. 2018;30:1800124. doi: 10.1002/adma.201800124. [DOI] [PubMed] [Google Scholar]
- 99.Zhang C., Hsieh M.-H., Wu S.-Y., Li S.-H., Wu J., Liu S.-M., Wei H.-J., Weisel R.D., Sung H.-W., Li R.-K. A self-doping conductive polymer hydrogel that can restore electrical impulse propagation at myocardial infarct to prevent cardiac arrhythmia and preserve ventricular function. Biomaterials. 2020;231:119672. doi: 10.1016/j.biomaterials.2019.119672. [DOI] [PubMed] [Google Scholar]
- 100.Lo C.-Y., Zhao Y., Kim C., Alsaid Y., Khodambashi R., Peet M., Fisher R., Marvi H., Berman S., Aukes D., et al. Highly stretchable self-sensing actuator based on conductive photothermally-responsive hydrogel. Mater. Today. 2021;50:35–43. doi: 10.1016/j.mattod.2021.05.008. [DOI] [Google Scholar]
- 101.Deng Z., Guo Y., Ma P.X., Guo B. Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J. Colloid Interface Sci. 2018;526:281–294. doi: 10.1016/j.jcis.2018.04.093. [DOI] [PubMed] [Google Scholar]
- 102.Soni S.K., Thomas B., Kar V.R. A Comprehensive Review on CNTs and CNT-Reinforced Composites: Syntheses, Characteristics and Applications. Mater. Today Commun. 2020;25:101546. doi: 10.1016/j.mtcomm.2020.101546. [DOI] [Google Scholar]
- 103.Liu Y.-L. Effective approaches for the preparation of organo-modified multi-walled carbon nanotubes and the corresponding MWCNT/polymer nanocomposites. Polym. J. 2016;48:351–358. doi: 10.1038/pj.2015.132. [DOI] [Google Scholar]
- 104.Chen Y.-S., Tsou P.-C., Lo J.-M., Tsai H.-C., Wang Y.-Z., Hsiue G.-H. Poly(N-isopropylacrylamide) hydrogels with interpenetrating multiwalled carbon nanotubes for cell sheet engineering. Biomaterials. 2013;34:7328–7334. doi: 10.1016/j.biomaterials.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 105.Qiu L., Liu D., Wang Y., Cheng C., Zhou K., Ding J., Truong V.-T., Li D. Mechanically Robust, Electrically Conductive and Stimuli-Responsive Binary Network Hydrogels Enabled by Superelastic Graphene Aerogels. Adv. Mater. 2014;26:3333–3337. doi: 10.1002/adma.201305359. [DOI] [PubMed] [Google Scholar]
- 106.Geim A.K. Graphene: Status and Prospects. Science. 2009;324:1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 107.Dreyer D.R., Park S., Bielawski C.W., Ruoff R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010;39:228–240. doi: 10.1039/B917103G. [DOI] [PubMed] [Google Scholar]
- 108.Zhu Y., Murali S., Cai W., Li X., Suk J.W., Potts J.R., Ruoff R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010;22:3906–3924. doi: 10.1002/adma.201001068. [DOI] [PubMed] [Google Scholar]
- 109.Han L., Lu X., Wang M., Gan D., Deng W., Wang K., Fang L., Liu K., Chan C.W., Tang Y., et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small. 2017;13:1601916. doi: 10.1002/smll.201601916. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y.-Z., El-Demellawi J.K., Jiang Q., Ge G., Liang H., Lee K., Dong X., Alshareef H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020;49:7229–7251. doi: 10.1039/D0CS00022A. [DOI] [PubMed] [Google Scholar]
- 111.Lei J.-C., Zhang X., Zhou Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015;10:276–286. doi: 10.1007/s11467-015-0493-x. [DOI] [Google Scholar]
- 112.Li X., Wang C., Cao Y., Wang G. Functional MXene Materials: Progress of Their Applications. Chem. Asian J. 2018;13:2742–2757. doi: 10.1002/asia.201800543. [DOI] [PubMed] [Google Scholar]
- 113.Gao L., Li C., Huang W., Mei S., Lin H., Ou Q., Zhang Y., Guo J., Zhang F., Xu S., et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020;32:1703–1747. doi: 10.1021/acs.chemmater.9b04408. [DOI] [Google Scholar]
- 114.Cai Y., Shen J., Yang C.-W., Wan Y., Tang H.-L., Aljarb A.A., Chen C., Fu J.-H., Wei X., Huang K.-W., et al. Mixed-dimensional MXene-hydrogel heterostructures for electronic skin sensors with ultrabroad working range. Sci. Adv. 2020;6:eabb5367. doi: 10.1126/sciadv.abb5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y., Gong M., Wan P. MXene hydrogel for wearable electronics. Matter-Us. 2021;4:2655–2658. doi: 10.1016/j.matt.2021.06.041. [DOI] [Google Scholar]
- 116.Yang C., Xu D., Peng W., Li Y., Zhang G., Zhang F., Fan X. Ti2C3Tx nanosheets as photothermal agents for near-infrared responsive hydrogels. Nanoscale. 2018;10:15387–15392. doi: 10.1039/C8NR05301D. [DOI] [PubMed] [Google Scholar]
- 117.Xue P., Bisoyi H.K., Chen Y., Zeng H., Yang J., Yang X., Lv P., Zhang X., Priimagi A., Wang L., et al. Near-Infrared Light-Driven Shape-Morphing of Programmable Anisotropic Hydrogels Enabled by MXene Nanosheets. Angew. Chem. Int. Ed. Engl. 2021;60:3390–3396. doi: 10.1002/anie.202014533. [DOI] [PubMed] [Google Scholar]
- 118.Shuck C.E., Sarycheva A., Anayee M., Levitt A., Zhu Y., Uzun S., Balitskiy V., Zahorodna V., Gogotsi O., Gogotsi Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater. 2020;22:1901241. doi: 10.1002/adem.201901241. [DOI] [Google Scholar]
- 119.Okubo M., Sugahara A., Kajiyama S., Yamada A. MXene as a Charge Storage Host. Acc. Chem. Res. 2018;51:591–599. doi: 10.1021/acs.accounts.7b00481. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y., Chen K., Li Y., Lan J., Yan B., Shi L., Ran R. High-Strength, Self-Healable, Temperature-Sensitive, MXene-Containing Composite Hydrogel as a Smart Compression Sensor. ACS Appl. Mater. Interfaces. 2019;11:47350–47357. doi: 10.1021/acsami.9b16078. [DOI] [PubMed] [Google Scholar]
- 121.Hao F., Wang L., Chen B., Qiu L., Nie J., Ma G. Bifunctional Smart Hydrogel Dressing with Strain Sensitivity and NIR-Responsive Performance. ACS Appl. Mater. Interfaces. 2021;13:46938–46950. doi: 10.1021/acsami.1c15312. [DOI] [PubMed] [Google Scholar]
- 122.Zhang C.-L., Cao F.-H., Wang J.-L., Yu Z.-L., Ge J., Lu Y., Wang Z.-H., Yu S.-H. Highly Stimuli-Responsive Au Nanorods/Poly(N-isopropylacrylamide) (PNIPAM) Composite Hydrogel for Smart Switch. ACS Appl. Mater. Interfaces. 2017;9:24857–24863. doi: 10.1021/acsami.7b05223. [DOI] [PubMed] [Google Scholar]
- 123.Jiang S., Wang K., Dai Y., Zhang X., Xia F. Near-Infrared Light-Triggered Dual Drug Release Using Gold Nanorod-Embedded Thermosensitive Nanogel-Crosslinked Hydrogels. Macromol. Mater. Eng. 2019;304:1900087. doi: 10.1002/mame.201900087. [DOI] [Google Scholar]
- 124.Thoniyot P., Tan M.J., Karim A.A., Young D.J., Loh X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015;2:1400010. doi: 10.1002/advs.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mohan Y.M., Premkumar T., Lee K., Geckeler K.E. Fabrication of Silver Nanoparticles in Hydrogel Networks. Macromol. Rapid Commun. 2006;27:1346–1354. doi: 10.1002/marc.200600297. [DOI] [Google Scholar]
- 126.Marcelo G., López-González M., Mendicuti F., Tarazona M.P., Valiente M. Poly(N-isopropylacrylamide)/Gold Hybrid Hydrogels Prepared by Catechol Redox Chemistry. Characterization and Smart Tunable Catalytic Activity. Macromolecules. 2014;47:6028–6036. doi: 10.1021/ma501214k. [DOI] [Google Scholar]
- 127.Di X., Kang Y., Li F., Yao R., Chen Q., Hang C., Xu Y., Wang Y., Sun P., Wu G. Poly(N-isopropylacrylamide)/polydopamine/clay nanocomposite hydrogels with stretchability, conductivity, and dual light- and thermo- responsive bending and adhesive properties. Colloids Surf. B. 2019;177:149–159. doi: 10.1016/j.colsurfb.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 128.Duan J., Wen H., Zong S., Li T., Lv H., Liu L. Soft/Hard Controllable Conversion Galactomannan Ionic Conductive Hydrogel as a Flexible Sensor. ACS Appl. Mater. Interfaces. 2021;3:5000–5014. doi: 10.1021/acsaelm.1c00795. [DOI] [Google Scholar]
- 129.Yu Q.J., Mao J., Wang S., Guo Z.Y. A simple multifunctional PNIPAM-GO/PANI hydrogel preparation strategy and its application in dye adsorption and infrared switching. J. Macmomol. Sci. A. 2020;57:751–760. doi: 10.1080/10601325.2020.1772672. [DOI] [Google Scholar]
- 130.Deng Z., Guo Y., Zhao X., Ma P.X., Guo B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host–Guest Interactions. Chem. Mater. 2018;30:1729–1742. doi: 10.1021/acs.chemmater.8b00008. [DOI] [Google Scholar]
- 131.Shit A., Kim S.G., In I., Park S.Y. Self-repairable and recyclable self-powered human motion sensor with NIR/pH-responsive amplified Stretchable, Conductive, and Self-Healable hydrogel. Chem. Eng. J. 2021;426:131846. doi: 10.1016/j.cej.2021.131846. [DOI] [Google Scholar]
- 132.Dong Y., Wang J., Guo X., Yang S., Ozen M.O., Chen P., Liu X., Du W., Xiao F., Demirci U., et al. Multi-stimuli-responsive programmable biomimetic actuator. Nat. Commun. 2019;10:4087. doi: 10.1038/s41467-019-12044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mu J., Wang G., Yan H., Li H., Wang X., Gao E., Hou C., Pham A.T.C., Wu L., Zhang Q., et al. Molecular-channel driven actuator with considerations for multiple configurations and color switching. Nat. Commun. 2018;9:590. doi: 10.1038/s41467-018-03032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cai G., Ciou J.-H., Liu Y., Jiang Y., Lee P.S. Leaf-inspired multiresponsive MXene-based actuator for programmable smart devices. Sci. Adv. 2019;5:eaaw7956. doi: 10.1126/sciadv.aaw7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim J., Kim J.W., Kim H.C., Zhai L., Ko H.-U., Muthoka R.M. Review of Soft Actuator Materials. Int. J. Precis. Eng. Man. 2019;20:2221–2241. doi: 10.1007/s12541-019-00255-1. [DOI] [Google Scholar]
- 136.Apsite I., Salehi S., Ionov L. Materials for Smart Soft Actuator Systems. Chem. Rev. 2022;122:1349–1415. doi: 10.1021/acs.chemrev.1c00453. [DOI] [PubMed] [Google Scholar]
- 137.Liang Y., He J., Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15:12687–12722. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 138.Simões D., Miguel S.P., Ribeiro M.P., Coutinho P., Mendonça A.G., Correia I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018;127:130–141. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 139.Yu R., Zhang H., Guo B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2021;14:1. doi: 10.1007/s40820-021-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang N., Zhang R., Zheng Y., Wang J., Khatib M., Jiang X., Zhou C., Omar R., Saliba W., Wu W., et al. Highly Efficient Self-Healing Multifunctional Dressing with Antibacterial Activity for Sutureless Wound Closure and Infected Wound Monitoring. Adv. Mater. 2022;34:2106842. doi: 10.1002/adma.202106842. [DOI] [PubMed] [Google Scholar]
- 141.Blacklow S.O., Li J., Freedman B.R., Zeidi M., Chen C., Mooney D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019;5:eaaw3963. doi: 10.1126/sciadv.aaw3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li S., Chen N., Li X., Li Y., Xie Z., Ma Z., Zhao J., Hou X., Yuan X. Bioinspired Double-Dynamic-Bond Crosslinked Bioadhesive Enables Post-Wound Closure Care. Adv. Funct. Mater. 2020;30:2000130. doi: 10.1002/adfm.202000130. [DOI] [Google Scholar]
- 143.Li M., Zhang Z., Liang Y., He J., Guo B. Multifunctional Tissue-Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces. 2020;12:35856–35872. doi: 10.1021/acsami.0c08285. [DOI] [PubMed] [Google Scholar]
- 144.Qu J., Zhao X., Liang Y., Xu Y., Ma P.X., Guo B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019;362:548–560. doi: 10.1016/j.cej.2019.01.028. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.