Abstract
Postmenopausal women tend to have worse cardiovascular outcomes in a manner that is associated with osteoporosis severity. In this study, we performed the first evaluation of the left ventricle and aortic valve phenotype of ovariectomized mice aged on Western diet to 1 yr. Disease was monitored in vivo using echocardiography and dual X-ray absorptiometry imaging and ex vivo using quantitative histological and immunostaining analysis. Mice had decreased bone mineral density in response to ovariectomy and increased fat mass in response to Western diet. Ovariectomized mice had a significantly increased left ventricle mass compared with control animals, absent of fibrosis. There was a slight increase in aortic valve peak velocity but no change in mean pressure gradient across the valve in the ovariectomy group. There was no evidence of leaflet hypertrophy, fibrosis, or calcification. This model of ovariectomy may present a novel method of studying left ventricle hypertrophy in female populations but does not have a phenotype for the study of aortic stenosis. This is particularly useful as it does not require genetic manipulation or drug treatment and more faithfully mimics aging, high-cholesterol diet, and postmenopausal osteoporosis that many female patients experience potentially resulting in a more translatable disease model.
NEW & NOTEWORTHY This article uses in vivo and ex vivo analysis to track the development of osteoporosis and left heart cardiovascular disease in an aged, high-cholesterol diet, mouse ovariectomy model. Mice develop early left ventricle hypertrophy without concurrent fibrosis or aortic valve stenosis. These findings allow for a new model of the study of left ventricle hypertrophy in postmenopausal osteoporosis that more closely mimics the natural progression of disease in female patients.
Keywords: female, hypertrophy, ovariectomy
INTRODUCTION
Cardiovascular disease is the most common cause of death in the United States (1). Diseases of the left heart are among the most deadly and are increasing in prevalence with left ventricle hypertrophy (LVH) and aortic valve stenosis (AVS) being among the top contributors (2). Younger female patients have long been known to develop cardiovascular disease at lower rates than males due to the protective effects of higher circulating estrogen levels (3). However, postmenopausal women (onset between 45 and 55 yr) experience diminishing estrogen levels and lose the protective effect, developing cardiovascular disease at much higher rates (4).
Despite this, there is significant understudy of female-specific cardiovascular disease in preclinical research. Meta-analysis suggests that only 10% of preclinical cardiovascular research focuses exclusively on female populations (5). This contributes to a fundamental disconnect in how female cardiovascular disease is understood. Female patients with AVS tend to have worse outcomes, lower referral rates, and more complications following valve replacement (6). This is potentially due to divergent disease mechanisms with female patients tending to have a lower calcium burden and higher prevalence of fibrosis compared with male counterparts (7, 8). Similarly, female sex has shown to be an important factor in LVH development secondary to AVS, resulting in greater wall thickness and ejection fraction (9).
The ovariectomy (OVX) procedure is commonly used to study the effects of menopause, particularly postmenopausal osteoporosis (PMO), in a preclinical setting. This model results in rapid loss of bone minerals via estrogen depletion (10). Women experiencing PMO are at a greater risk for cardiovascular disease that is proportional to the severity of their disease (11). Young women receiving bilateral oophorectomy have an increase in cardiovascular mortality (12).
There are no models that are specific to female cardiovascular disease. Female mice are often excluded from cardiovascular studies due to the protective effect of estrogen. The OVX procedure could fill this gap by providing a model for the long-term development of heart disease due to estrogen depletion. To address this, we performed OVX and sham surgeries on female mice and mice aged to 1 yr on a high-cholesterol diet. Mice developed LVH without significant fibrosis or AVS indicating an early heart failure phenotype. Therefore, OVX may be a useful model for early LVH.
MATERIALS AND METHODS
Animals
Mouse experiments were carried out under appropriate supervision and were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Twenty female wild-type mice of the C57BL6/J background were obtained from Jackson Laboratory. At 4 mo of age, mice were randomly selected for the sham or OVX groups (n = 10 for each group). Briefly, mice were lightly anesthetized using isoflurane induction [heart rate (HR), 517 ± 39 beats/min]. The lower back slightly above the hips was shaved and disinfected. A small incision was made, and the mouse uterus, fallopian tubes, and ovaries were removed. The ovaries were quickly excised using a cautery pen. Mice in the sham group underwent the same procedure; however, the ovaries were left intact at the final stage. This procedure was repeated for both ovaries. The uterus was returned to the body cavity and the skin was closed using surgical staples, local anesthetic was applied, and mice were allowed to recover on a heated pad. One mouse in the sham group died of unknown surgical complications resulting in a final sample size of nine in this group. After 1 wk of recovery, mice were transitioned to a 1% cholesterol Western diet (TestDiet 5TJT). Food and water were provided ad libitum at all stages of the study. Mice were aged to 12 mo of age and euthanized by carbon dioxide inhalation and cervical dislocation. Tissue was harvested for ex vivo analysis at the study endpoint (Fig. 1A).
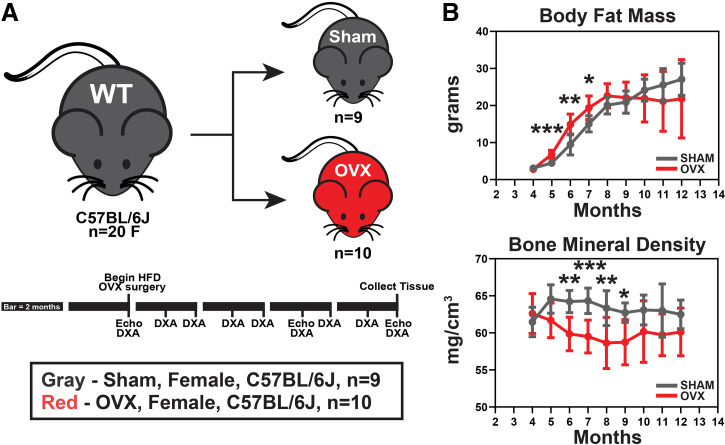
Figure 1.
The OVX surgical model of postmenopausal osteoporosis, combined with aging and high-cholesterol diet, was evaluated as a potential model for the preclinical study of AVS. A: at 4 mo of age, 20 female C57BL/6J mice received sham or OVX procedure. They were then aged to 12 mo receiving monthly DXA scanning and echocardiography at 4, 9, and 12 mo of age. B: DXA scans were used to measure body composition. High-cholesterol diet induced rapid fat mass gain in both groups (top) and OVX mice had reduced bone mineral density compared with sham mice (bottom) indicating successful development of estrogen-depletion-induced osteoporosis. Means ± SD, unpaired Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001. OVX, ovariectomy; WT, wild type.
Echocardiography
Echocardiographic imaging was performed at 4, 9, and 12 mo of age to track the hemodynamic development of aortic stenosis over time. All measurements were performed by skilled technicians at the Vanderbilt Cardiovascular Physiology Core using the Visual Sonics Vevo 2100 small animal imaging system.
Mice were lightly anesthetized using isoflurane and laid supine on a heated platform. Transthoracic AV pulsed-wave Doppler imaging was used to record blood velocity profiles. Parasternal short-axis M-mode imaging was used to visualize LV structure and function over the course of the heart cycle.
AV metrics were determined using a custom MATLAB script which automatically traced the Doppler waveforms to determine metrics such as AV velocity and pressure gradient across the leaflets (13). Three independent measurements were made for each mouse at each time point to account for operator variability. In total, between 50 and 100 heart cycles were considered to generate representative values for each sample at each time point. M-mode measurements were performed by hand by the study authors. Three cycles were measured across three repeated images per mouse per time point. Nine cycles were considered for each sample to generate representative metrics including LV mass and ejection fraction.
Dual X-Ray Absorptiometry
Bone metrics were assessed by the study authors using a Hologic UltraFocus Dual Energy X-Ray Absorptiometry Vision once per month from 4 to 12 mo of age. Mice were anesthetized using isoflurane induction and placed facing down on the capture stage. Images were acquired at ×2 magnification in a series of four captures at 40 kV, followed then by four additional captures at 80 kV. Manufacturer software (v. 3.1) was used to determine whole body fat mass content and bone mineral density over time.
Histological Staining
Whole hearts were excised from mice at 12 mo and embedded in optimal cutting temperature (OCT) compound and flash frozen. Serial sections (12 µm) were cut using a −20°C cryostat and affixed to histology slides. For all staining analyses, three sections across different regions of the heart were analyzed and averaged (in the case of quantitative analysis) to generate representative metrics.
Masson’s Trichrome (MTC) stain was used to assess LV and AV morphology and collagen density in both regions. Staining was performed by expert histological technicians at the Vanderbilt University Medical Center translational pathology shared resource. Alizarin Red S (ARS, Sigma-Aldrich) staining was used to study the prevalence of calcification on the valve leaflets. Sections were washed in phosphate-buffered saline without calcium or magnesium to remove OCT. Sections were then rinsed in deionized water for 2 min at room temperature and then covered in 14 mM ARS solution for 5 min at room temperature. The slides were then shaken dry, rinsed with deionized water, cleared, and dehydrated in sequential baths of acetone, 1:1 acetone/xylene mixture, then xylene. Slides were then cover-slipped in organic mounting media and dried overnight.
Bright-field images were captured for both stains at ×1 (LV) and ×4 (AV) using the Nikon Eclipse E800 microscope and an Olympus DP74 digital polychromatic camera. Representative images were chosen to match the trends determined from quantitative analysis.
Quantitative Image Analysis
Custom MATLAB scripts were used to measure tissue area and collagen composition as previously described (14–16). Images were all captured using identical exposure and background levels to accurately compare across samples. Whole heart images obtained at ×1 magnification were cropped to only include LV tissue. AV leaflets were similarly removed from ×4 images. Colorimetric segmentation was used to determine regions of collagen, stained blue (H = 150°–250°, S = 0.1–1.0, L = 0.1–0.93), and cytoplasm and myocardium, stained red (H = 250°–25°, S = 0.1–1.0, L = 0.1–0.93) where H is hue, S is saturation, and L is lightness. Whole tissue fraction as well as collagen and cytoplasm/myocardium fractions were calculated as the ratio of positive pixels to nonbackground pixels in the specified region of interest.
Statistical Analysis
All data are displayed as means ± SE with raw data overlaid as defined in the figure captions. Direct comparisons between sham and OVX data used the Student’s unpaired t test. Comparing groups in time course data were analyzed using the two-way analysis of variance (ANOVA) test with multiple comparisons corrected by the Šidák method. Each data set was analyzed for statistical outliers using the ROUT method. A P value of <0.05 was considered statistically significant for all tests.
RESULTS
OVX Results in Diminished Bone Mineral Density and Increased Fat Mass
OVX is a common procedure used to simulate PMO in a small-animal model. DXA scans were used to assess body composition over time to confirm the expected phenotype. Both groups rapidly gained fat mass with the OVX initially accruing more rapidly before both groups reached a steady state around 20–25 g (Fig. 1B, top). As expected, mice that underwent the OVX procedure had reduced bone mineral density. Bone mineral density was significantly lowered at 6–9 mo of age but a leveling of the OVX group and a gradual decrease in the sham group resulted in groups no longer being significantly different from 10 to 12 mo of age (Fig. 1B, bottom).
OVX Results in Increased LV Mass
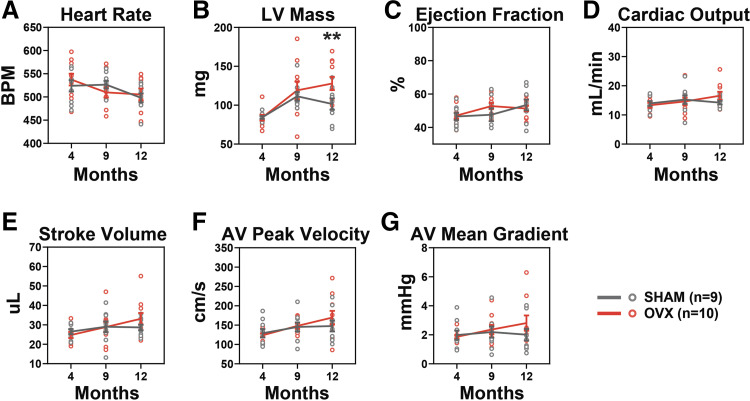
Hemodynamics, structure, and function of the LV and AV were tracked over time using echocardiography. Heart rate decreased over time at similar rates in both groups (Fig. 2A). Both groups had increased LV mass at 9 mo but the OVX continued to increase at 12 mo (Fig. 2B). LV function as measured by ejection fraction did not change between the two groups (Fig. 2C). Cardiac output (Fig. 2D) and stroke volume (Fig. 2E) both increased in OVX mice over time but did not significantly differ from the sham group. OVX mice had a slight increase in AV peak velocity over time but did not significantly differ from the sham group (Fig. 2F). Mean pressure gradient across the AV did not change between groups (Fig. 2G).
Figure 2.
OVX results in increased LV mass. M-mode and pulsed wave Doppler echocardiography were used to compare AV and LV metrics at 4, 9, and 12 mo of age. A: heart rate decreased over time but did not differ between groups. B: LV mass was significantly increased in OVX compared with sham at 12 mo. C: there was no functional change noted in either group as measured by an ejection fraction of the LV. Cardiac output (D) and stroke volume (E) did not differ between groups. F: peak velocity was increased in OVX over time but there was no group difference. G: there was no change in either group in the mean pressure gradient across the AV. Means ± SE, two-way ANOVA. Significance markers indicate group (sham vs. OVX) changes at each time point. **P < 0.01. AV, aortic valve; LV, left ventricle; OVX, ovariectomy.
Histological Analysis Shows Increased LV Area
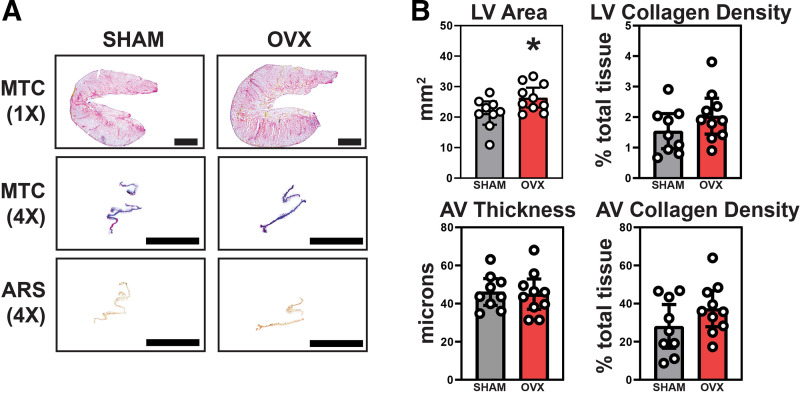
LV and AV morphology and collagen composition were measured using Masson’s Trichrome stain, whereas AV leaflet calcification was assessed using Alizarin Red S staining (Fig. 3A). The average area of the LV was increased in OVX mice as expected based on results from echocardiographic measurements (95% CI [17.54, 25.15] vs. [23.15, 29.63]; P = 0.033) (Fig. 3B, top left). There was no evidence of significant fibrosis in the LVs at 12 mo in the sham or OVX mice (95% CI [0.96, 2.11] vs. [1.45, 2.62]; P = 0.19) (Fig. 3B, top right). AV thickening (Fig. 3B, bottom left) was not detected in sham nor OVX groups (95% CI [39.06, 53.13] vs. [36.97, 52.91]; P = 0.81) and no significant change in collagen leaflet density (95% CI [16.6, 39.51] vs. [27.83, 46.74]; P = 0.17) (Fig. 3B, bottom right). There was no evidence of calcification on AV leaflets in either group (quantification data not shown).
Figure 3.
LV hypertrophy was noted in OVX hearts via ex vivo analysis, but valves were absent of any significant thickening, collagen alteration, or calcification. A: Masson’s Trichrome staining was used to study gross and collagen-specific morphology in the LV (top row) and AV (middle row). Alizarin Red S staining was used to identify potential regions of calcification on the AV leaflets (bottom row). B: the LV area (top left) was significantly greater in OVX mice bolstering the LV hypertrophic phenotype noted in echocardiography. There was no significant difference in LV fibrosis (top right). Measurement of AV fibrosa-ventricularis thickness (bottom left) and collagen density (bottom right) showed no difference between groups further proving a significant AV disease phenotype secondary to OVX unlikely. A: scale bar magnitudes: 1× = 10 mm, 4× = 1 mm. B: means ± 95% CI, unpaired Student’s t test, *P < 0.05. AV, aortic valve; LV, left ventricle; OVX, ovariectomy.
DISCUSSION
In this study, we characterized the utility of the OVX procedure combined with a high-cholesterol diet and 1 yr of aging on the development of left heart disease in a mouse model. This could potentially lead to an easily repeatable preclinical model in an understudied patient population. To our knowledge, this is the first study to undertake this specific analysis.
In vivo imaging (echo and DXA) was used to monitor mouse body composition and AV/LV hemodynamics over time. Both groups of mice underwent substantial weight gain in response to high-cholesterol diet and diminished bone mineral density for the majority of the study, indicating a successful recapitulation of the PMO phenotype (Fig. 1). Mice in the OVX group developed a slight increase in AV peak velocity from baseline but not in mean gradient. There was a marked increase in LV mass in the OVX group at 12 mo, which may indicate early LVH, however, there was no change in ejection fraction which may point to no difference in LV performance at this early disease stage (Fig. 2). Regardless of changes in LV function, LVH is a risk factor on its own and could point to OVX mice having increased hypertension or another hypertrophic cardiomyopathy.
Blood pressure measurements were unable to be performed for this study. Future studies could monitor blood pressure to determine whether OVX mice are more prone to the development of hypertension than sham which could result in LVH. Tail cuff or telemetry measurements are both measurement options, although telemetry may prove difficult to the high levels of fat mass accrued by mice on the high-fat diet (17). Menopause is known to cause concentric LV thickening in human patients, which is recapitulated in this study (18). The OVX procedure combined with a high-cholesterol diet may be a useful tool in understanding the mechanisms of LVH development in postmenopausal women and determining optimal treatments.
A similar analysis was performed in aortic regurgitation in rats, however, there was no change between OVX and sham animals (19). This may indicate the need for Western diet and aging to produce the phenotype. Myocardial hypertrophy commonly occurs in women with PMO at greater rates than in male counterparts (20). The most common methods of inducing myocardial hypertrophy in mice include surgical intervention (transverse aortic constriction, aortic banding) or genetic modifications (collagens, matrix metalloproteinases) (21). Using OVX and aging may present a more generalized model and specifically focus on an understudied patient group at risk for the disease.
To better understand the observed in vivo phenotype, quantitative histology was performed to characterize LV and AV disease progression (Fig. 3). There was no noted increase in AV thickness or collagen morphology as determined by Masson’s Trichrome staining and no evidence of calcium deposition based on Alizarin Red S staining. Similarly, there was no evidence of widespread LV fibrosis. However, the LV of OVX mice was significantly enlarged that bolstered the increase in LV mass noted from echocardiography. The C57BL6/J mouse is notoriously resistant to the development of hemodynamic alterations although some studies note gross histological disease hallmarks (22, 23). Combining the OVX procedure with mutant mouse lines known to develop atherosclerosis and AVS such as the Notch1 or Apo models may prove fruitful (22, 24, 25). In addition, using a high-salt diet would amplify hypertension and potentially lead to a stronger LV phenotype that could provide further clarification of the observations from this study (26).
LV hypertrophy is often a compensatory mechanism to normalize cardiac output as the leaflet orifice becomes smaller and less compliant. Fibrosis typically accompanies hypertrophic tissue during progression to heart failure. It is possible that pressure overload is occurring due to distal causes such as hypertension, which is also shown to present along with osteoporosis (27).
Limitations and Future Studies
Twelve months of aging is relatively low and aging to 18 or 24 mo may have more accurately mimicked the advanced age patients generally develop AVS. Monthly blood pressure measurements and echocardiographs would provide more information on hypertension development as well as left heart function and morphology over the course of the study. Combining the OVX procedure with mutant mice with a propensity for atherosclerosis or AVS as well as a high salt-supplemented diet may provide larger effect sizes at early time points. Assessment of stiffness of peripheral vasculature should be performed to characterize the hypertensive phenotype. Inclusion of a standard diet group would help in determining the precise effects of OVX on the baseline C57BL6/J mouse.
Conclusions
The focus of this study was to determine the feasibility of using the OVX procedure as a preclinical model to study left heart diseases in female patients. Our findings suggest that mice do not develop a strong AV phenotype after OVX, a high-cholesterol diet and 12 mo of aging. However, there does appear to be a significant increase in LV hypertrophy, which may indicate potential utility as a model for LV disease in female mice.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL151115 (to J.E.J., II), HL146951 (to M.R.B.), and HL135790 (to W.D.M.); Innovative Engineering Research in Surgery and Intervention T32 Training Program Grant EB021937 (to J.E.J., II); Veterans Affairs Merit Grant I01BX004297 (to J.S.N.); and the Leducq Foundation (to W.D.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.J. and W.D.M. conceived and designed research; J.E.J. performed experiments; J.E.J., M.R.B., and J.S.N. analyzed data; J.E.J., M.R.B., and J.S.N. interpreted results of experiments; J.E.J. prepared figures; J.E.J. drafted manuscript; J.E.J., M.R.B., J.S.N., and W.D.M. edited and revised manuscript; J.E.J., M.R.B., J.S.N., and W.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alison Richards and Dan Perrien for technical assistance with DXA and OVX, respectively.
REFERENCES
- 1.Murphy S, Kochanek K, Xu J, Arias E. Mortality in the United States, 2020. Natl Cent Heal Stat 427: 1–8, 2021. https://www.cdc.gov/nchs/data/databriefs/db427.pdf. [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2021 Update. Circulation 143: e254–e743, 2021. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 3.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease. Ann Intern Med 85: 447–452, 1976. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez FD, Motazedian P, Jung RG, Di Santo P, MacDonald Z, Simard T, Clancy AA, Russo JJ, Welch V, Wells GA, Hibbert B. Sex bias is increasingly prevalent in preclinical cardiovascular research: implications for translational medicine and health equity for women. Circulation 135: 625–626, 2017. doi: 10.1161/CIRCULATIONAHA.116.026668. [DOI] [PubMed] [Google Scholar]
- 6.Goliasch G, Lang IM. Impact of sex on the management and outcome of aortic stenosis patients: a female aortic valve stenosis paradox, and a call for personalized treatments? Eur Heart J 42: 2692–2694, 2021. doi: 10.1093/eurheartj/ehab331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal SR, Clavel M-A, Messika-Zeitoun D, Cueff C, Malouf J, Araoz PA, Mankad R, Michelena H, Vahanian A, Enriquez-Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging 6: 40–47, 2013. doi: 10.1161/CIRCIMAGING.112.980052. [DOI] [PubMed] [Google Scholar]
- 8.Simard L, Côté N, Dagenais F, Mathieu P, Couture C, Trahan S, Bossé Y, Mohammadi S, Pagé S, Joubert P, Clavel M-A. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis. Circ Res 120: 681–691, 2017. doi: 10.1161/CIRCRESAHA.116.309306. [DOI] [PubMed] [Google Scholar]
- 9.Kostkiewicz M, Tracz W, Olszowska M, Podolec P, Drop D. Left ventricular geometry and function in patients with aortic stenosis: gender differences. Int J Cardiol 71: 57–61, 1999. doi: 10.1016/S0167-5273(99)00114-X. [DOI] [PubMed] [Google Scholar]
- 10.Komori T. Animal models for osteoporosis. Eur J Pharmacol 759: 287–294, 2015. doi: 10.1016/j.ejphar.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res 20: 1912–1920, 2005. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 12.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Roger VL, Melton LJ, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 16: 15–23, 2009. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferruzzi J, Di Achille P, Tellides G, Humphrey JD. Combining in vivo and in vitro biomechanical data reveals key roles of perivascular tethering in central artery function. PLoS One 13: e0201379, 2018. doi: 10.1371/journal.pone.0201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joll IIJ, Clark CR, Peters CS, Raddatz MA, Bersi MR, Merryman WD. Genetic ablation of serotonin receptor 2B improves aortic valve hemodynamics of Notch1 heterozygous mice in a high-cholesterol diet model. PLoS One 15: e0238407, 2020. doi: 10.1371/journal.pone.0238407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroer AK, Bersi MR, Clark CR, Zhang Q, Sanders LH, Hatzopoulos AK, Force TL, Majka SM, Lal H, Merryman WD. Cadherin-11 blockade reduces inflammation-driven fibrotic remodeling and improves outcomes after myocardial infarction. JCI Insight 4, 2019. doi: 10.1172/jci.insight.131545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bersi MR, Khosravi R, Wujciak AJ, Harrison DG, Humphrey JD. Differential cell-matrix mechanoadaptations and inflammation drive regional propensities to aortic fibrosis, aneurysm or dissection in hypertension. J R Soc Interface 14: 20170327, 2017. doi: 10.1098/rsif.2017.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X, Ho D, Gao S, Hong C, Vatner DE, Vatner SF. Arterial pressure monitoring in mice. Curr Protoc Mouse Biol 1: 105–122, 2011. doi: 10.1002/9780470942390.mo100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Porcellati C. Early cardiac changes after menopause. Hypertension 32: 764–769, 1998. doi: 10.1161/01.hyp.32.4.764. [DOI] [PubMed] [Google Scholar]
- 19.Drolet M-C, Lachance D, Plante E, Roussel E, Couet J, Arsenault M. Gender-related differences in left ventricular remodeling in chronic severe aortic valve regurgitation in rats. J Heart Valve Dis 15: 345–351, 2006. [PubMed] [Google Scholar]
- 20.Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham study). Am J Cardiol 64: 1066–1068, 1989. doi: 10.1016/0002-9149(89)90816-3. [DOI] [PubMed] [Google Scholar]
- 21.Rai V, Sharma P, Agrawal S, Agrawal DK. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol Cell Biochem 424: 123–145, 2017. doi: 10.1007/s11010-016-2849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JD, Weiss RM, Heistad DD, Towler DA. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res 108: 1392–1412, 2011. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marie-Claude D, Elise R, Yves D, Jacques C, Marie A. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J Am Coll Cardiol 47: 850–855, 2006. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 24.Clark CR, Bowler MA, Snider JC, Merryman WD. Targeting cadherin-11 prevents notch1-mediated calcific aortic valve disease. Circulation 135: 2448–2450, 2017. doi: 10.1161/CIRCULATIONAHA.117.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274, 2005. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 26.Yu Q, Larson DF, Slayback D, Lundeen TF, Baxter JH, Watson RR. Characterization of high-salt and high-fat diets on cardiac and vascular function in mice. Cardiovasc Toxicol 4: 37–46, 2004. doi: 10.1385/CT:4:1:37. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Castrillon JL, Justo I, Sanz-Cantalapiedra A, Pueyo C, Hernandez G, Dueñas A. Effect of the antihypertensive treatment on the bone mineral density and osteoporotic fracture. Curr Hypertension Rev 1: 61–66, 2005. doi: 10.2174/1573402052952843. [DOI] [Google Scholar]