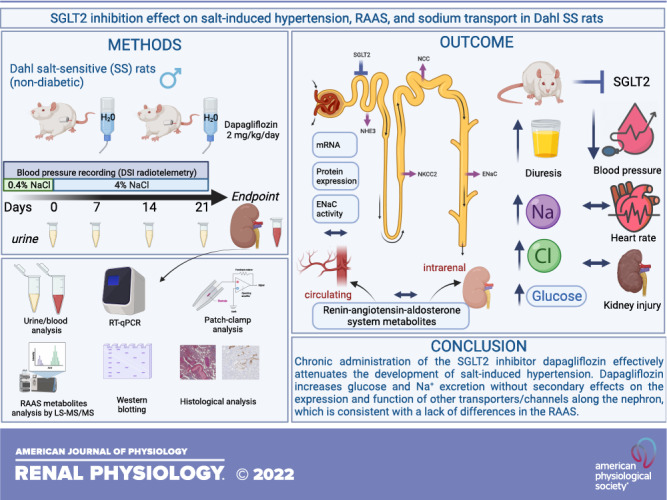
Keywords: Dahl salt-sensitive rats, epithelial Na+ channel, Na+-glucose cotransporter-2 inhibitors, renin-angiotensin-aldosterone system, salt-sensitive hypertension
Abstract
Na+-glucose cotransporter-2 (SGLT2) inhibitors are the new mainstay of treatment for diabetes mellitus and cardiovascular diseases. Despite the remarkable benefits, the molecular mechanisms mediating the effects of SGLT2 inhibitors on water and electrolyte balance are incompletely understood. The goal of this study was to determine whether SGLT2 inhibition alters blood pressure and kidney function via affecting the renin-angiotensin-aldosterone system (RAAS) and Na+ channels/transporters along the nephron in Dahl salt-sensitive rats, a model of salt-induced hypertension. Administration of dapagliflozin (Dapa) at 2 mg/kg/day via drinking water for 3 wk blunted the development of salt-induced hypertension as evidenced by lower blood pressure and a left shift of the pressure natriuresis curve. Urinary flow rate, glucose excretion, and Na+- and Cl−-to-creatinine ratios increased in Dapa-treated compared with vehicle-treated rats. To define the contribution of the RAAS, we measured various hormones. Despite apparent effects on Na+- and Cl−-to-creatinine ratios, Dapa treatment did not affect RAAS metabolites. Subsequently, we assessed the effects of Dapa on renal Na+ channels and transporters using RT-PCR, Western blot analysis, and patch clamp. Neither mRNA nor protein expression levels of renal transporters (SGLT2, Na+/H+ exchanger isoform 3, Na+-K+-2Cl− cotransporter 2, Na+-Cl− cotransporter, and α-, β-, and γ-epithelial Na+ channel subunits) changed significantly between groups. Furthermore, electrophysiological experiments did not reveal any difference in Dapa treatment on the conductance and activity of epithelial Na+ channels. Our data suggest that SGLT2 inhibition in a nondiabetic model of salt-sensitive hypertension blunts the development of salt-induced hypertension by causing glucosuria and natriuresis without changes in the RAAS or the expression or activity of the main Na+ channels and transporters.
NEW & NOTEWORTHY The present study indicates that Na+-glucose cotransporter-2 (SGLT2) inhibition in a nondiabetic model of salt-sensitive hypertension blunts the development and magnitude of salt-induced hypertension. Chronic inhibition of SGLT2 increases glucose and Na+ excretion without secondary effects on the expression and function of other Na+ transporters and channels along the nephron and hormone levels in the renin-angiotensin-aldosterone system. These data provide novel insights into the effects of SGLT2 inhibitors and their potential use in hypertension.
INTRODUCTION
As summarized by the Centers for Disease Control and Prevention, diabetes mellitus (DM) is the leading cause of chronic kidney disease (CKD), with roughly one in three people with type 2 DM developing kidney pathologies. Hypertension is widespread among patients with DM and is also a major contributor to CKD. The combination of diabetes and hypertension is associated with high morbidity and mortality because of an increased risk of cardiovascular and renal complications (1). Effective blood glucose and blood pressure management are essential to prevent renal and cardiovascular disease progression in patients with DM. Current therapies involve lowering glucose via restoring β-cell activity, insulin sensitivity, or tissue glucose uptake to normalize plasma glucose levels (2).
A novel mainstay in the treatment of DM uses inhibition of Na+-glucose cotransporter-2 (SGLT2), which is selectively expressed in the S1 and S2 segments of proximal tubule in the kidney. SGLT2 inhibition enhances urinary glucose and Na+ excretion by preventing their reabsorption (2, 3). Several SGLT2 inhibitors [canagliflozin, dapagliflozin (Dapa), empagliflozin, and ertugliflozin] (4, 5) have been approved by the United States Food and Drug Administration and are now widely used for the treatment of type 2 DM. Clinical outcome trials (CANVAS, Empa-REG, Declare-Timi 58, and Credence) have demonstrated that SGLT2 inhibitors reduce cardiovascular risk events, blood pressure levels, and the risk of developing end-stage kidney disease in patients with type 2 DM (6–9) with and without CKD (10). Numerous studies have shown that SGLT2 inhibitors prevent the decline in glomerular filtration rate (GFR) normally observed in CKD by reducing hyperfiltration, glomerular capillary pressure, renal hypertrophy, and albuminuria (11–17). As an example, a recent study (18) has demonstrated that in a nondiabetic model of CKD, the SGLT2 inhibitor Dapa provides glomerular protection in mice with protein overload proteinuria (induced by BSA) and limits glomerular lesions and podocyte dysfunction and loss. Typically, the blood pressure-lowering effect in these studies is reported as a secondary treatment effect after the onset of the hypoglycemic effect. However, the precise mechanisms of how SGLT2 inhibitors lower blood pressure are incompletely understood.
The reported benefits from SGLT2 inhibitor treatment include blood pressure reduction, osmotic diuresis, natriuresis, weight loss (6, 12), inhibition of sympathetic nervous system activity (19), lowering of uric acid levels, and improvement of arterial stiffness (20). For example, it has been reported that in patients with type 2 DM, the reduction in blood pressure was associated with a decrease in plasma volume, suggesting that SGLT2 inhibitors may have a diuretic-like effect (21). The transport of glucose and Na+ is coupled (1:1) in the proximal tubule, and inhibition of SGLT2 reduces both glucose and Na+ reabsorption. It leads to an increasing tubular load of glucose, Na+, and fluid to downstream nephron segments, which can be associated with changes in electrolyte balance. It has also been shown that patients with DM exhibit an elevated sensitivity to a salt load (22). Early studies in Dahl salt-sensitive (SS) rats treated with a high-salt (HS) diet (1% and 8% NaCl) under nondiabetic conditions (treated with Dapa) and type 1 streptozotocin-induced DM (treated with luseogliflozin) (11, 23) showed no significant effect on blood pressure. However, other reports have indicated that SGLT2 inhibitors have pleiotropic effects on renal transporters, such as Na+/H+ exchanger isoform 3 (NHE3), Na+-phosphate cotransporter 2a (NaPi-2a), α-epithelial Na+ channel (ENaC), and urate transporter 1 (URAT1) (12, 24, 25).
In this study, we used Dahl SS rats, a nondiabetic model of salt-induced hypertension and CKD (26), to test the relationship between inhibition of SGLT2, blood pressure, and possible modulation of the renin-angiotensin-aldosterone system (RAAS) and/or Na+ transport along the nephron. Interestingly, our results demonstrated that Dapa treatment blunts the development of salt-induced hypertension but does not affect kidney injury. The latter is consistent with the findings that the RAAS as well as expression levels of Na+ transporters and channels are not significantly different between groups. This study provides valuable new insights into the understanding of mechanisms of action of SGLT2 inhibitors and the potential use of these drugs in patients with hypertension without DM.
MATERIALS AND METHODS
Experimental Protocol and Animals
The animal use and welfare procedures adhered to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, following protocols reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Eight-week-old male Dahl SS rats (SS/JrHsdMcwi; RRID: RGD_61499) provided with a normal-salt diet (0.4% NaCl, No. 113755, Diets) represented the normotensive control. A HS diet (4% NaCl, No. D113756, Diets) was used to induce hypertension. Rats were maintained on the HS diet for 3 wk and treated with SGLT2 inhibitor or corresponding vehicle (water). Water and food were provided ad libitum. Rats were randomized to application of the SGLT2 inhibitor Dapa (No. 11574, Cayman Chemical, Ann Arbor, MI) added to the drinking water at ∼2 mg/kg/day. The concentration of the drug in water was adjusted with the increased water consumption to keep a constant dose during the experiment. The dose of Dapa was similar to previously published studies (8, 18, 27). The pharmacological treatment was initiated on the first day of the HS challenge, as shown in the experimental timeline in Fig. 1A. Further analysis of all samples was performed and assessed in a blinded fashion.
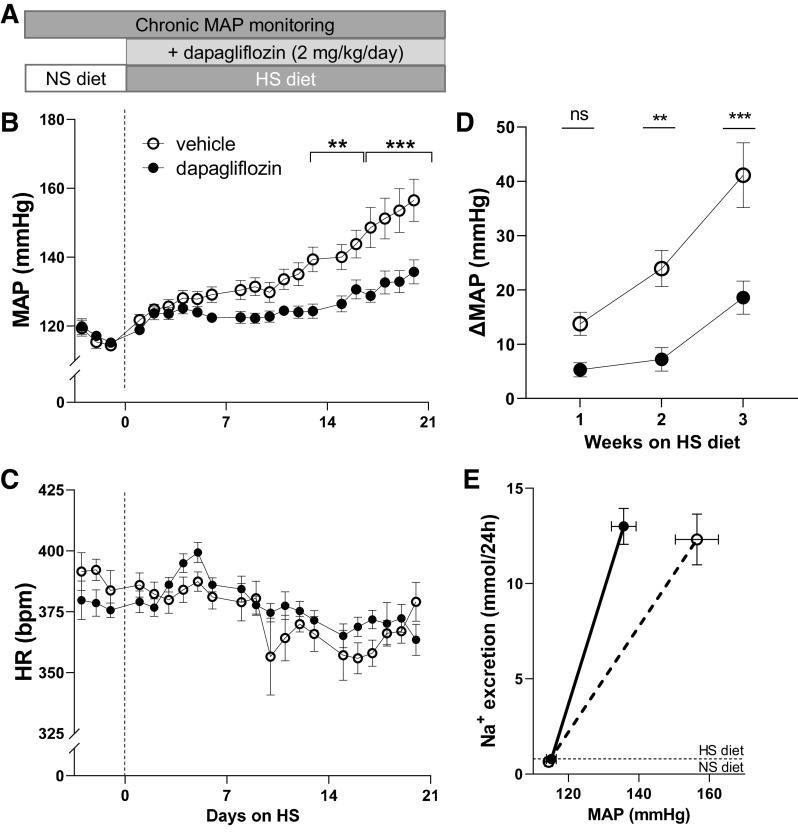
Figure 1.
Effect of chronic dapagliflozin (Dapa) administration on salt-induced hypertension in male Dahl salt-sensitive rats. A: schematic representation of the experimental protocol. B and C: the development of mean arterial blood pressure (MAP; B) and heart rate [HR; in beats/min (bpm); C] in Dahl salt-sensitive rats after a diet change from a normal-salt (NS; 0.4% NaCl, day 0) diet to a high-salt (HS; 4% NaCl) diet and chronic treatment with Na+-glucose cotransporter-2 inhibitor (Dapa) or vehicle. D: changes in MAP (Δ) in weeks 1, 2, and 3 of Dapa treatment on the HS diet (for baseline MAP on the NS diet was used). E: pressure-natriuresis curve from the NS diet (baseline) to 3 wk on the HS diet between vehicle- and Dapa-treated rats. n = 10–11 rats per group. **P < 0.01 and ***P < 0.001 (ANOVA).
Surgical Procedures
At the age of 7.5 wk, rats were anesthetized on a temperature-controlled platform via inhalation of 2.5% isoflurane in 0.5 L/min [O2/N2 (30%/70%)]. Subcutaneous implantation of a blood pressure transmitter (PA-C40, Data Sciences, New Brighton, MN), with the catheter tip secured in the abdominal aorta via the femoral artery, was performed as previously described (28, 29). Blood pressure and heart rate were recorded starting 4 days after recovery. At the end of the experimental period, rats were anesthetized and the kidneys were flushed with PBS via aortic catheterization as previously described (30–32). These terminal experiments were performed around 13:00 h. The right kidney was either snap frozen or used for patch-clamp analysis, and the left kidney was placed in 10% formalin for histological experiments.
Circadian Rhythm Analysis
As shown in the graphs, changes in blood pressure were sampled every 2 h. The variation in circadian blood pressure patterns was compared by a Student’s t test between individual time points. Data were fitted and collected using the single-cosinor method (33, 34). The sampled blood pressure data for Dapa- and vehicle-treated groups were fitted using the “least squares” method by a cosine curve with a period of 24 h. The mezor (24-h mean value of the data), amplitude of the cosine curve on either side of the mezor, and acrophase of the curve (the time at which the highest value encountered in the cycle occurs) were used to evaluate changes between the groups (33). The circadian change in nocturnal blood pressure decrease, known as dipping, was defined as the difference between active and inactive mean systolic blood pressure values (35).
Electrolyte Measurements and Albuminuria Assay
Urine was collected for 24 h in metabolic cages (No. 40615, Laboratory Product) at baseline and every 7 days of the HS protocol. Nonfasting blood was collected from the tail vein, and glucose levels were determined with a glucometer (Contour NEXT EZ). Before euthanasia, blood samples were collected using aortic catheterization in anesthetized animals. Glucose, creatinine, and electrolytes (Na+, K+, Ca2+, and Cl−) in plasma and urine were measured with a blood gas analyzer (ABL system 800 Flex, Radiometer, Copenhagen, Denmark). Phosphate levels in urine and plasma were determined using an inorganic phosphorus reagent (Pointe Scientific, Canton, MI) (36). Mg2+ levels were determined photometrically by a Stanbio LiquiColor Magnesium Test (Thermo Fisher Scientific, Middletown, VA). Plasma and urine samples were sent to IDEXX BioAnalytics (North Grafton, MA) to measure uric acid levels. Urine albumin was determined by a fluorescent assay (Albumin Blue 580 dye, Molecular Probes, Eugene, OR) using a fluorescent plate reader (FL600, Bio-Tek, Winooski, VT) (32).
Histological Analysis of Kidney Injury
Rat kidneys were formalin fixed, paraffin embedded, sectioned, and mounted on slides as previously described (32, 37). Slides were stained with Masson’s trichrome stain and used to detect medullary protein casts and fibrosis. Quantification of renal injury markers was performed by observers blinded to sample identity. Protein cast analysis was accomplished using color thresholding in Metamorph software (Molecular Devices, Sunnyvale, CA). Fibrosis was assessed using color deconvolution and thresholding in Fiji image software (ImageJ 1.51 u, NIH). For the renal tubule injury analysis, kidney tissue sections were immunohistochemically stained with kidney injury molecule-1 (KIM-1) an anti-T cell Ig- and mucin-domain-containing molecule antibody (A-12, No. sc-518008, Santa Cruz Biotechnology, 1:300). KIM-1 protein abundance was quantified using Fiji image analysis software (ImageJ 1.51 u, NIH) using standard deconvolution and color thresholding as previously described (32, 37).
Quantification of the RAAS
RAAS metabolite quantification was performed on snap-frozen plasma and kidney tissue collected at the end of the experimental period. Analysis and quantification of steady-state levels of ANG I, ANG II, ANG (1−7), ANG (2−8), ANG (3−8), ANG (2−10), ANG (2−7), ANG (1−9), ANG (3−7), and aldosterone in equilibrated heparin plasma samples and angiotensin metabolites [ANG I (1−10), ANG II (1−8), ANG III (2−8), ANG (1−7), and ANG (1−5)] in renal tissue were performed by Attoquant Diagnostics (Vienna, Austria) according to the company’s protocol (32, 38, 39). Briefly, ANG peptide levels were measured following 30 min of equilibration in conditioned lithium-heparin plasma at 37°C and subsequent stabilization of equilibrium peptide levels. Stable isotope-labeled internal standards for each ANG metabolite as well as the deuterated internal standard for aldosterone (aldosterone D4) were added to stabilized plasma samples at a concentration of 200 pg/mL and subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based ANG and steroid quantification by Attoquant Diagnostics. ANG metabolites in renal tissue were measured by LC-MS/MS analysis as previously described (32, 38, 39). Briefly, tissue samples were homogenized under liquid nitrogen and extracted with guanidinium-based extraction buffer. Stabilized tissue extracts were spiked with stable isotope-labeled internal standards for each individual target analyte (Sigma-Aldrich, St. Louis, MO) before being subjected to C18-based solid phase extraction and subsequent LC-MS/MS.
Quantitative RT-PCR Analysis
For real-time quantitative RT-PCR analysis, total RNA was extracted with TRIzol reagent (ThermoFisher Scientific) from renal cortical tissue. The quality and quantity of the individual samples were determined by spectrophotometry (multimode microplate reader BioTek Synergy Neo2). Primers for Slc5a2, Slc12a1, Scnn1a, Scnn1b, and Scnn1g genes were designed using National Center for Biotechnology Information Primer3 and BLAST and purchased from Invitrogen (Waltham, MA). Slc12a3 primers were obtained from Real Time Primers (Melrose Park, PA). cDNA from Dahl SS rat kidneys were used as a control for testing primers. Primer sets were tested using control cDNA in different cDNA concentrations, and the optimal set for each gene was selected. Primer sequences are shown in Table 1. One microgram of total RNA was reverse transcribed by random hexamer primers into cDNA (RevertAid First-Strand cDNA synthesis kit, ThermoFisher Scientific). RT-PCR analysis was performed using 4 ng cDNA with SYBR green chemistry on a Quant Studio 6 Pro (Applied Biosystems, Waltham, MA) (28, 40). Each experiment was performed in triplicate. Primers were assessed for specificity by sequencing the PCR products (GENEWIZ, Azenta Life Sciences, South Plainfield, NJ). Sequence results were verified using National Center for Biotechnology Information BLAST. Negative controls for the reverse transcription reaction were included to confirm the absence of genomic DNA. Quantification of Slc5a2, Slc12a1, Slc12a3, Scnn1a, Scnn1b, and Scnn1g mRNA was determined by normalizing to 18S (primers previously published) (41).
Table 1.
Primer sequences used for RT-PCR analysis
| Target Gene | Protein | Strand | Sequence (5′−3′) | Product Size, bp |
|---|---|---|---|---|
| Slc5a2 | SGLT2 | Forward | GGTGTTGGCTTGTGGTCTATGT | 134 |
| Reverse | ACAAAATGACCGCTGCCGAT | |||
| Slc12a1 | NKCC2 | Forward | AAAGGTGTGCTGGTGAGGTG | 131 |
| Reverse | GAGGTTACCATGGTGGAAAGAAG | |||
| Slc12a3 | NCC | Forward | GTGGCTGAACAAGAGGAAGA | 212 |
| Reverse | AGTTGAAGTCAAAGGCATCG | |||
| Scnn1a | α-ENaC | Forward | CCCTGCAACCAGGCGAATTA | 209 |
| Reverse | TCCTGACCATGCACCATCAC | |||
| Scnn1b | β-ENaC | Forward | CAGCTTTCTAAACAGGTGCCA | 114 |
| Reverse | TGCAGTACCACACTAGCAGC | |||
| Scnn1g | γ-ENaC | Forward | TCACGCTTTTCCACCATCCA | 113 |
| Reverse | GATGACTTGCAGCCCGTACT |
SGLT2, Na+-glucose cotransporter-2; NKCC2, Na+-K+-2Cl− cotransporter; NCC, Na+-Cl− cotransporter; ENaC, epithelial Na+ channel.
Western Blot Analysis
Kidney cortical lysates were prepared as previously described (28, 42). Kidney tissue samples (10–20 mg) were pulse sonicated in Laemmli buffer in the presence of protease and phosphatase inhibitor cocktail (Roche, Mannheim, Germany) to achieve a final protein concentration of 20 mg/mL and then spin cleared at 10,000 g for 10 min. The resulting supernatant was subjected to SDS-PAGE and transferred onto nitrocellulose membrane (Millipore, Bedford, MA) for antibody hybridization. Changes in protein expression were assessed using primary antibodies against α-ENaC (1:1,000, No. SPC-403D, RRID: AB_10640131, StressMarq Biosciences, Victoria, BC, Canada), β-ENaC (1:1,000, No. SPC-404D, RRID: AB_10644173, StressMarq Biosciences), γ-ENaC (1:1,000, No. SPC-405D, RRID: AB_10640369, StressMarq Biosciences), NHE3 (1:1,000, No. SPC-400D, RRID: AB_10643557, StressMarq Biosciences), SGLT2 (1:1,000, No. AGT-032, RRID: AB_2756649, Alomone Labs), NKCC2 (1:1,000, No. SPC-401D, RRID: AB_10640877, StressMarq Biosciences), phosphorylated (p)NKCC2 (Ser126) [1:1,000, kindly provided by Dr. Mark Knepper, National Heart, Lung, and Blood Institute (43)], NCC (1:1,000, No. AB3553, RRID:AB_571116, Millipore Sigma, Burlington, MA), and pNCC (Thr53) (1:1,000, No. p1311-53, RRID: AB_2650477, PhosphoSolutions, Aurora, CO). Secondary antibody was peroxidase-conjugated AffiniPure donkey anti-rabbit IgG (H + L) antibody (1:10,000, No. 711-035-152, RRID: AB_10015282, Jackson ImmunoResearch Laboratories, West Grove, PA). Immunoreactive proteins were detected by a ChemiDoc imaging system (Bio-Rad, Hercules, CA). Quantification of Western blot bands was performed by densitometry using Image Lab 6.1 Software (Bio-Rad) and normalized to loading controls [actin (I-19), sc-1616, RRID: AB_630836, 1:10,000, No. G1316, Santa Cruz Biotechnology; GAPDH (0411), 1:5,000, sc-47724, RRID: AB_627678, Santa Cruz Biotechnology; and β-tubulin, 1:10,000, No. AC030, RRID: AB_2769870, ABclonal, Woburn, MA).
Patch-Clamp Analysis
Patch-clamp electrophysiology was used to assess ENaC activity in isolated, split-open cortical collecting duct (CCD) tubules. CCDs were isolated using a vibrodissociation protocol (44). Freshly isolated renal tubules were transferred to microscopy slides coated with poly-l-lysine. Patch-clamp recordings were performed in a cell-attached voltage-clamp configuration using an Axopatch 200B amplifier and Digidata 1440 A analog-to-digital converter (Molecular Devices, San Jose, CA) to a PC running pClamp 10.7 suite software (Molecular Devices). All electrophysiological recordings were performed using physiological saline solution as the extracellular bath. The solution composition was (in mM) 150 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 HEPES (pH 7.35). ENaC activity was recorded on the apical membrane of split-open CCD tubules using patch pipettes filled with a solution of the following composition (in mM): 140 LiCl, 2 MgCl2, and 10 HEPES (pH 7.35). The resistance of the patch pipettes ranged from 8 to 10 MΩ (44). Gap-free single-channel current data from gigaohm seals in principal cells were acquired and subsequently analyzed with Clampfit 10.7 software (Molecular Devices). NPo, the product of the number of channels (N) and open probability (Po), was used to measure channel activity. Single-channel unitary current (i) was determined from the best-fit Gaussian distribution of amplitude histograms. Where appropriate, Po was calculated by normalizing NPo for the total number of estimated channels in the patch.
Statistics
Data are presented as means ± SE. In the box-plot graphs, the box represents ± SE. Data were tested for normality (Shapiro-Wilk) and equal variance (Levene’s homogeneity test). Statistical analysis consisted of one-way ANOVA or a Student’s t test (OriginPro 9.0 of GraphPad Prism 9.0) with a P value of <0.05 considered significant. In addition, when an ANOVA test was significant, a post hoc Holm-Sidak’s multiple comparison was performed.
RESULTS
Dapa Treatment Attenuates the Development and Magnitude of Salt-Induced Hypertension and Increases Urinary Flow Rate and Glucose Excretion
Mean arterial blood pressure (MAP) was similar between the groups at the start of the experiment (114 ± 1 vs. 115 ± 1 mmHg for the normal salt group). Blood pressure significantly increased after exposure to the HS diet for 21 days to 156 ± 6 and 135 ± 3 mmHg for vehicle- and Dapa-treated rats, correspondingly (P < 0.05). The Dapa-treated group exhibited significantly lower MAP over the last week of the protocol (days 13−21) and remained ∼20 mmHg lower at the end of the experiment (day 21, P < 0.001; Fig. 1B). This was also significant for both systolic and diastolic blood pressure in Dapa- versus vehicle-treated rats (P < 0.05; data not shown). Heart rate was not significantly different between groups (Fig. 1C). The change in MAP (Δ) compared with baseline was significantly lower after week 1 of the HS diet (Fig. 1D). The relationship between urinary Na+ excretion and MAP was shifted to the left in response to Dapa treatment (Fig. 1E).
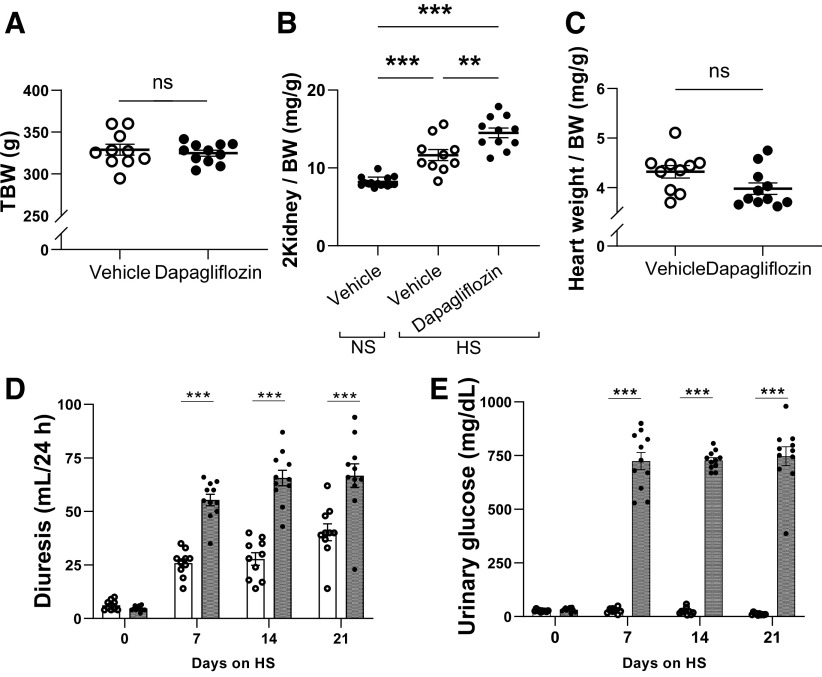
Total body weight was not significantly different between the control and experimental groups (Fig. 2A). However, the kidney weight-to-body weight (two kidney/body weight) ratio increased in the Dapa-treated group compared with the vehicle-treated group (Fig. 2B). Heart weight was lower in the Dapa-treated group; however, the heart weight-to-body weight ratio was not significantly different (Fig. 2C).
Figure 2.
Effect of chronic dapagliflozin administration on body composition and diuresis in Dahl salt-sensitive (SS) rats. A–C: total body weight (TBW; A), two-kidney weight normalized to body weight (2Kidney/BW; B), and heart weight normalized to body weight (heart weight/BW; C) in Dahl SS rats following 21 days of the high-salt (HS) diet. Also shown is the 2 kidney-to-body weight ratio in Dahl salt-sensitive rats fed a normal-salt (NS) diet. D: 24-h urine volumes of Dahl SS rats at days 0, 7, 14, and 21 following the HS challenge. E: urinary glucose concentrations at days 0, 7, 14, and 21 of the HS diet. n = 10–11. **P < 0.01 and ***P < 0.001. ns, not significant.
Dapa treatment significantly increased urinary flow rate and glucose excretion. Dapa treatment caused a significant diuretic effect compared with vehicle (55 ± 3 vs. 26 ± 2, 66 ± 4 vs. 28 ± 3, and 67 ± 6 vs. 40 ± 4 mL/day on day 7, day 14, and day 21, respectively, n = 10, P < 0.05; Fig. 2D). Urinary glucose concentration was significantly higher in Dapa-treated rats compared with vehicle-treated rats (747 ± 44 vs. 12 ± 2 mg/dL on day 21, n = 10, P < 0.001; Fig. 2E), and, consequently, glucose excretion was significantly different (500 ± 53 vs. 5 ± 1 mg/day on day 21, n = 10, P < 0.001). Blood glucose levels were not affected by Dapa treatment (Supplemental Fig. S1).
We further examined the impact of Dapa treatment on circadian rhythmicity during the development of hypertension. Dahl SS rats exhibited the dipper-type circadian rhythm pattern. That is, the baseline of the dark (active) period of MAP was higher compared with the light (inactive) period (Fig. 3A). Chronic Dapa treatment did not change the dipping of blood pressure in Dahl SS rats (Fig. 3B). Cosinor analysis of circadian rhythms showed a significantly lower mezor in most of the studied days of HS in Dapa-treated rats, but changes in the amplitude and acrophase were not significant in most of the analyzed days (Fig. 3, C–E).
Figure 3.
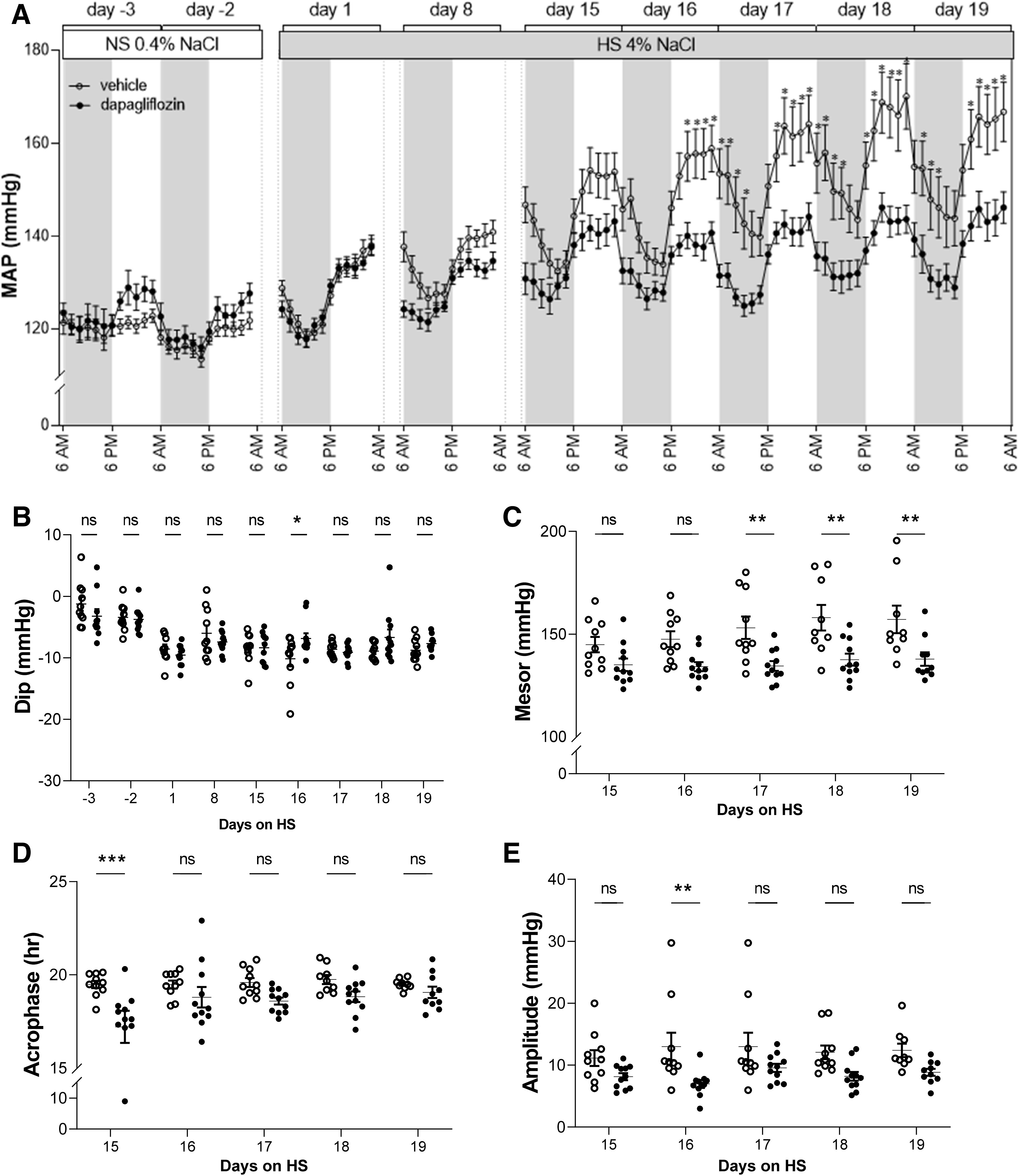
Circadian rhythm of blood pressure in Dahl salt-sensitive rats during dapagliflozin treatment. A: comparison of mean arterial blood pressure (MAP) of dapagliflozin- and vehicle-treated rats at 2-h intervals. B: calculated fall (dip) of systolic blood pressure in the inactive period. C–E: cosinor analysis of circadian rhythmicity of blood pressure showing the mezor (C), acrophase (D), and amplitude (E) in dapagliflozin- and vehicle-treated rats. Each point in the graphs in B−E represents parameters of average 1-h blood pressure. *P < 0.05, **P < 0.01, and ***P < 0.001. NS, normal-salt diet.
Dapa Treatment Did Not Affect GFR or Prevent Renal Damage in Hypertensive Rats
Next, we addressed if the beneficial effect of Dapa treatment on blood pressure has secondary consequences on renal function and structure. GFR was estimated by creatinine clearance and was not significantly different between the groups (Table 2). Urinary albumin excretion, urinary albumin-to-creatinine ratio (Table 2), and plasma creatinine (Table 3) were also not significantly different between groups.
Table 2.
Albuminuria and creatinine clearance
| Vehicle | Dapagliflozin | |
|---|---|---|
| Urinary albumin, mg/24 h | 117 ± 21 | 181 ± 27 |
| Creatinine clearance, mL/min | 1.8 ± 0.3 | 2.1 ± 0.2 |
| Urinary albumin-to-creatinine ratio | 11.0 ± 2.3 | 16.1 ± 2.1 |
Table 3.
Blood parameters in Dahl salt-sensitive rats after 21 days of the chronic experiment with Na+-glucose cotransporter-2 inhibitor treatment on a high-salt diet
| Vehicle | Dapagliflozin | |
|---|---|---|
| K+, mM | 3.7 ± 0.2 | 3.8 ± 0.1 |
| Na+, mM | 140 ± 10.9 | 140 ± 10.7 |
| Ca2+, mM | 1.3 ± 0.03 | 1.3 ± 0.01 |
| Mg2+, mM | 2.4 ± 0.2 | 2.4 ± 0.1 |
| Cl−, mM | 101.8 ± 1.6 | 102.7 ± 0.7 |
| , mM | 2.4 ± 0.2 | 2.4 ± 0.3 |
| Uric acid, mg/dL | 0.3 ± 0.1 | 0.3 ± 0.04 |
| Creatinine, mg/dL | 0.6 ± 0.1 | 0.5 ± 0.1 |
| Hematocrit, % | 43.5 ± 0.3 | 46.7 ± 2.0* |
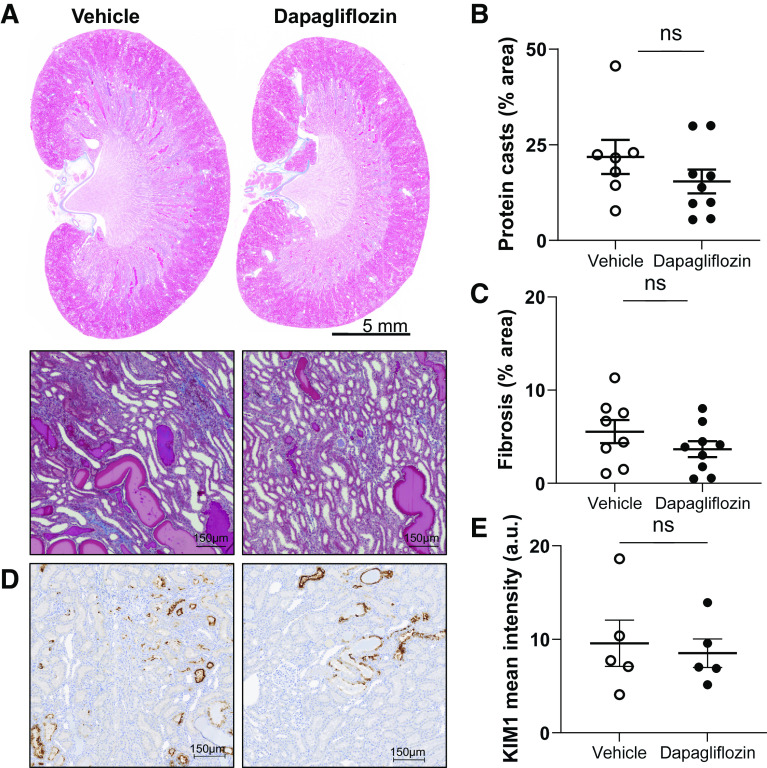
Subsequently, we determined the effects of SGLT2 inhibition on kidney injury. Masson trichrome-stained kidney sections showed that protein casts and fibrosis were not significantly different between groups (Fig. 4, A–C). We also measured the abundance of KIM-1, which is expressed in the apical membrane of the proximal tubule in response to injury and has proven to be a reliable indicator of kidney damage in the rat. Immunohistochemical quantification of KIM-1 did not show significant differences between groups (Fig. 4, D and E).
Figure 4.
Kidney injury in Dahl salt-sensitive rats. A: representative images of kidney tissue stained with Masson’s trichrome and expanded areas at ×10 magnification. Scale bars = 5 mm and 150 µm, respectively. B and C: summary graphs of the medullary protein cast area (percent total kidney area; B) and whole kidney fibrosis (C). D: representative immunohistochemical staining of kidney cortical sections for detection of kidney injury molecule-1 (KIM-1) at a magnification of ×10. Scale bars are shown. E: summary graph of tubular kidney injury by KIM-1 intensity. n ≥ 5. ns, not significant.
Dapa Increases Natriuresis but Does Not Affect Electrolyte and Mineral Homeostasis
Dapa treatment significantly increased urinary Na+-to-creatinine and Cl−-to-creatinine ratios on days 7, 14, and 21 compared with vehicle-treated rats (Fig. 5, A and B). No differences were observed in urinary K+-to-creatinine, Ca2+-to-creatinine, Mg2+-to-creatinine, -to-creatinine, and uric acid-to-creatinine ratios between groups (Fig. 5, C–G). Fractional excretion analyses demonstrated that Dapa treatment did not lead to changes in electrolytes, minerals (Na+, K+, Cl−, Ca2+, Mg2+, and ), or uric acid (Fig. 5H). Plasma electrolytes and other analyzed parameters, collected at the end of the experimental period, were not significantly different between groups (Table 3).
Figure 5.
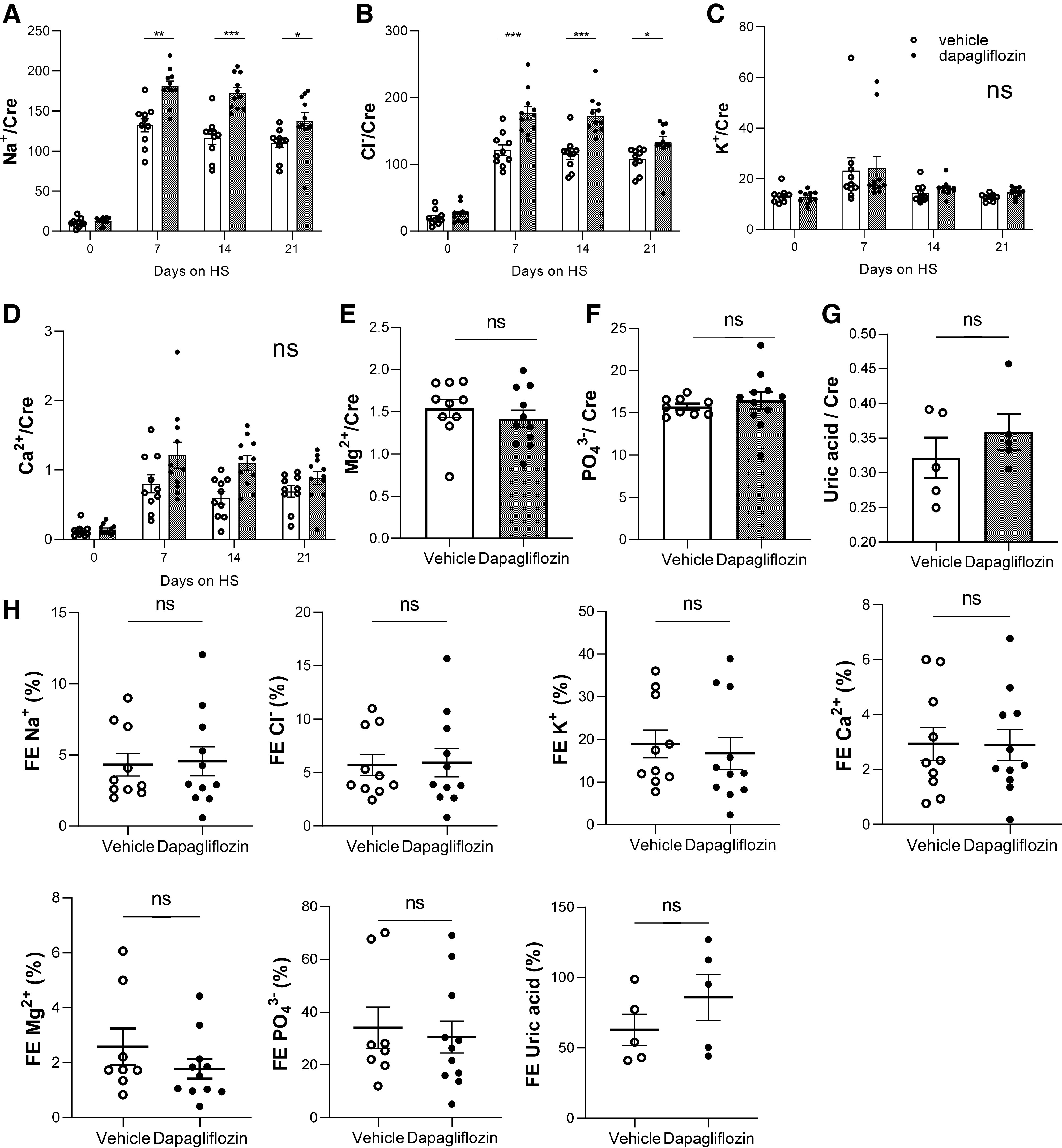
Urine electrolytes normalized to creatinine in Dahl salt-sensitive rats under Na+-glucose cotransporter-2 inhibitor treatment. Electrolytes were measured at days 0, 7, 14, and 21 of the high-salt (HS) diet. A: Na+. B: Cl−. C: K. D: Ca2+. Also shown are values for Mg2+ (E), (F), and uric acid (G) on day 21 of the HS diet. H: fractional excretion (FE) of Na+, Cl−, K+, Ca2+, Mg2+, , and uric acid over a 24-h period in vehicle- and dapagliflozin-treated Dahl salt-sensitive rats. n ≥ 5. Cre, creatinine; ns, not significant.
SGLT2 Inhibition Does Not Impact the Systemic or Intrarenal RAAS in Hypertensive Conditions
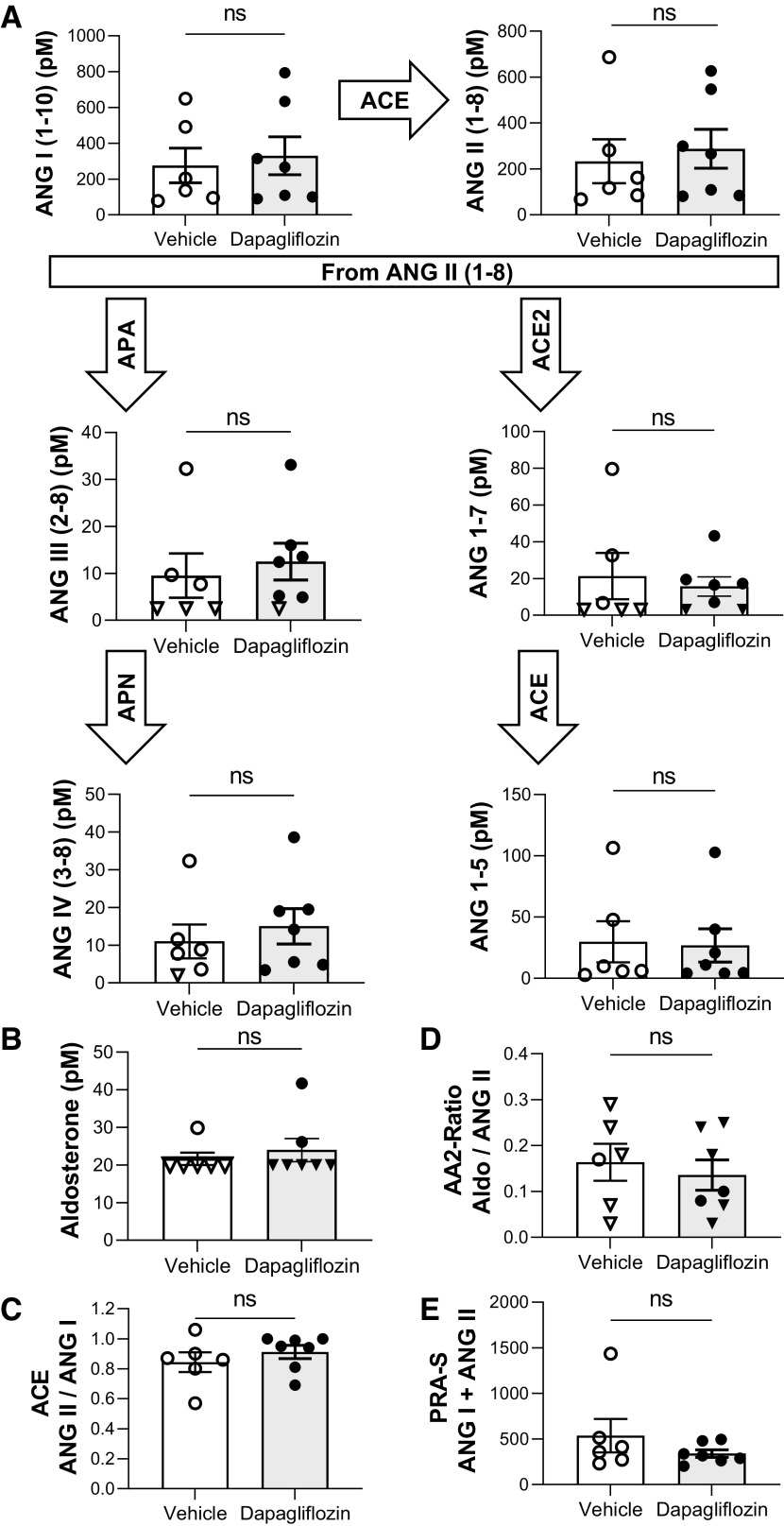
We examined the relation between SGLT2 inhibition-mediated changes in blood pressure and the RAAS in salt-induced hypertension. The groups displayed no significant differences between circulating ANG peptides (Fig. 6A) or aldosterone (Fig. 6B). Along those lines, no significant differences in angiotensin-converting enzyme activity (ANG II/ANG I; Fig. 6C), the adrenal response to ANG II (Fig. 6D), and plasma renin activity (ANG I + ANG II; Fig. 6E) were observed between groups. Intrarenal ANG I, ANG II, ANG III, ANG (1–7), and ANG (1–5) levels were not significantly different between groups (Fig. 7).
Figure 6.
Renin-angiotensin-aldosterone system metabolites in Dahl salt-sensitive rats treated with dapagliflozin or vehicle on day 21 of the high-salt diet. A: equilibrium levels of ANG I (1−10), ANG II, ANG III (2−8), ANG (1–7), ANG IV (3–8), and ANG (1−5). B: summary graph of plasma aldosterone levels in Dahl salt-sensitive rats. C: ratio of ANG II to ANG I representing ACE activity. D: adrenal response to ANG II (AA2 ratio) of aldosterone and ANG II. E: sum of ANG I and ANG I demonstrating the plasma renin activity (PRA). Triangles on the graphs indicate that levels were at or below lower limits of quantification [<3.0 pM for ANG (1−7), <2.5 pM for ANG II (2−8), <2.0 pM for ANG IV (3−8), and <20.0 pM for aldosterone]. n ≥ 6. ACE, angiotensin-converting enzyme; APA, aminopeptidase A; APN, aminopeptidase N; ns, not significant.
Figure 7.
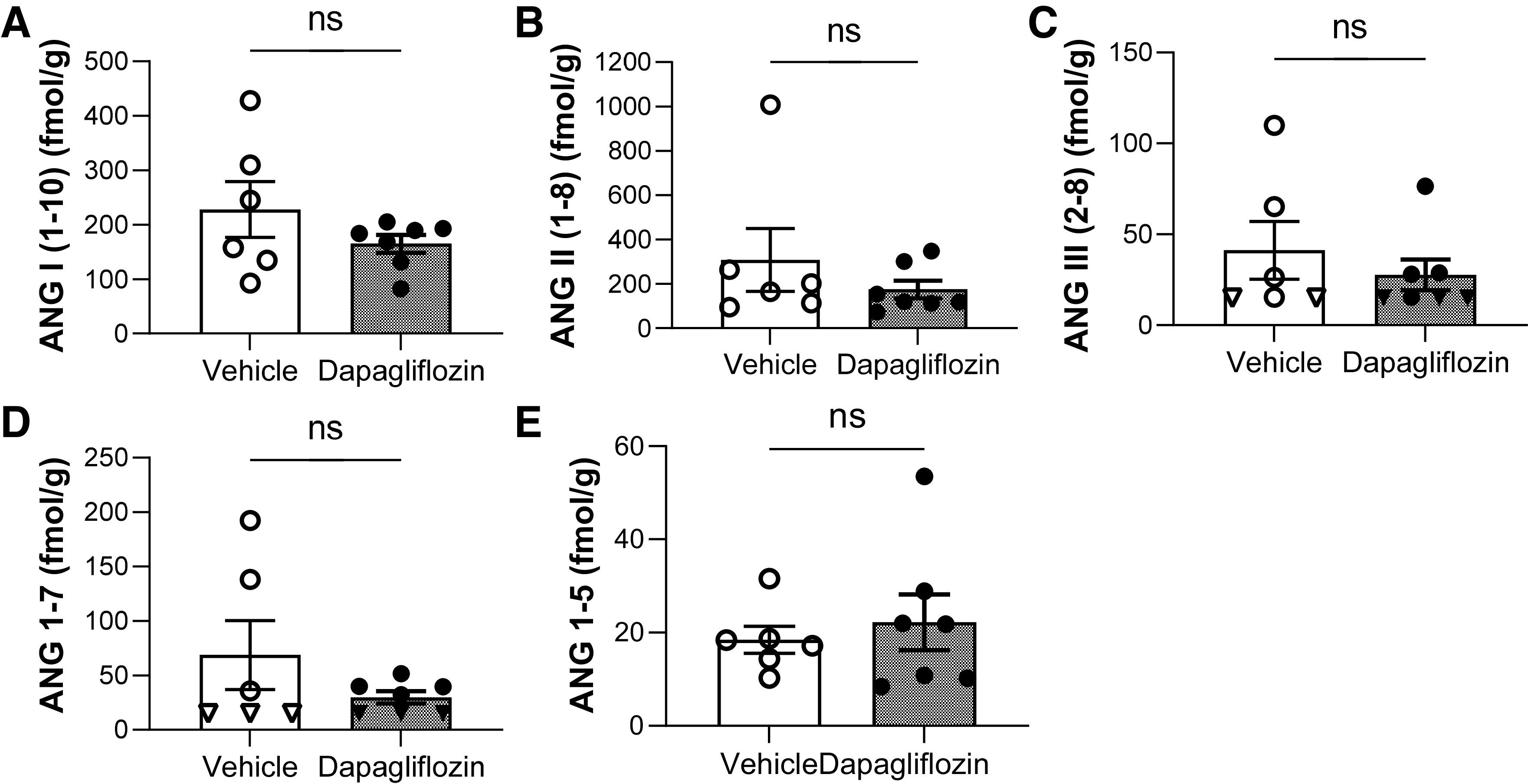
ANG peptide kidney tissue expression levels in Dahl salt-sensitive rats after the chronic experiment with Na+-glucose cotransporter-2 inhibitor treatment and vehicle. A–E: equilibrium levels of ANG I (1−10; A), ANG II (1−8; B), ANG III (2−8; C), ANG (1−7; D), and ANG (1–5; E). Triangles on the graphs indicate that levels were at or below lower limits of quantification [<15.0 fmol/g for ANG (1−7) and ANG III (2−8)]. ANG IV (3−8) levels were below the measurement point (<5 fmol/g) in all rats. n ≥ 5. ns, not significant.
Dapa Does Not Alter mRNA or Protein Expression of Na+ Transporters and ENaC Activity
To determine if Dapa affects Na+ transport during the development of hypertension, we compared mRNA expression levels of SGLT2, NKCC2, NCC, and α-, β-, and γ-subunits of ENaC in the cortical tissue of Dapa- and vehicle-treated rats at the end of the experimental protocol. No differences in gene expression levels were observed between groups (Fig. 8). Protein expression of NHE3, SGLT2, NKCC, pNKCC2, NCC, pNCC, and α-, β-, and γ-ENaC subunits confirmed the lack of differences seen between groups on the mRNA expression level (Fig. 9 and Supplemental Fig. S2). The ratios of pNKCC2 to NKCC2 and pNCC to NCC were also not different between groups.
Figure 8.
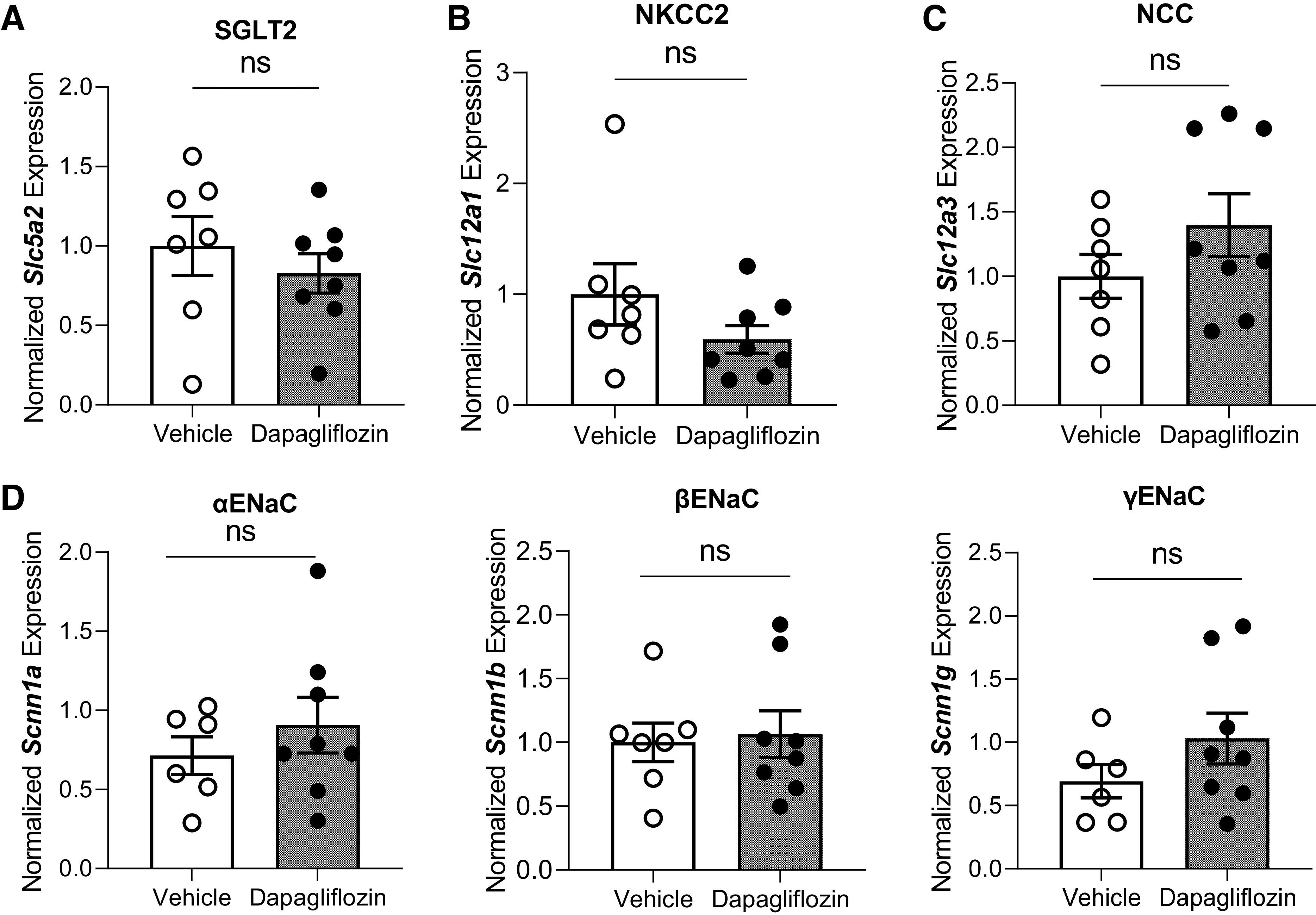
mRNA expression of Na+ transporters and channels estimated by RT-PCR in Dahl salt-sensitive rats after the chronic experiment with dapagliflozin or vehicle treatment. A–D: normalized mRNA expression of Na+-glucose cotransporter-2 (SGLT2; A), Na+-K+-2Cl− cotransporter (NKCC2; B), Na+-Cl− cotransporter (NCC; C), and α-, β- and γ-subunits of the epithelial Na+ channel (ENaC; D). Quantification of mRNA was determined by normalizing to 18S. Results obtained from untreated rats were designated as 1. n ≥ 7. ns, not significant.
Figure 9.
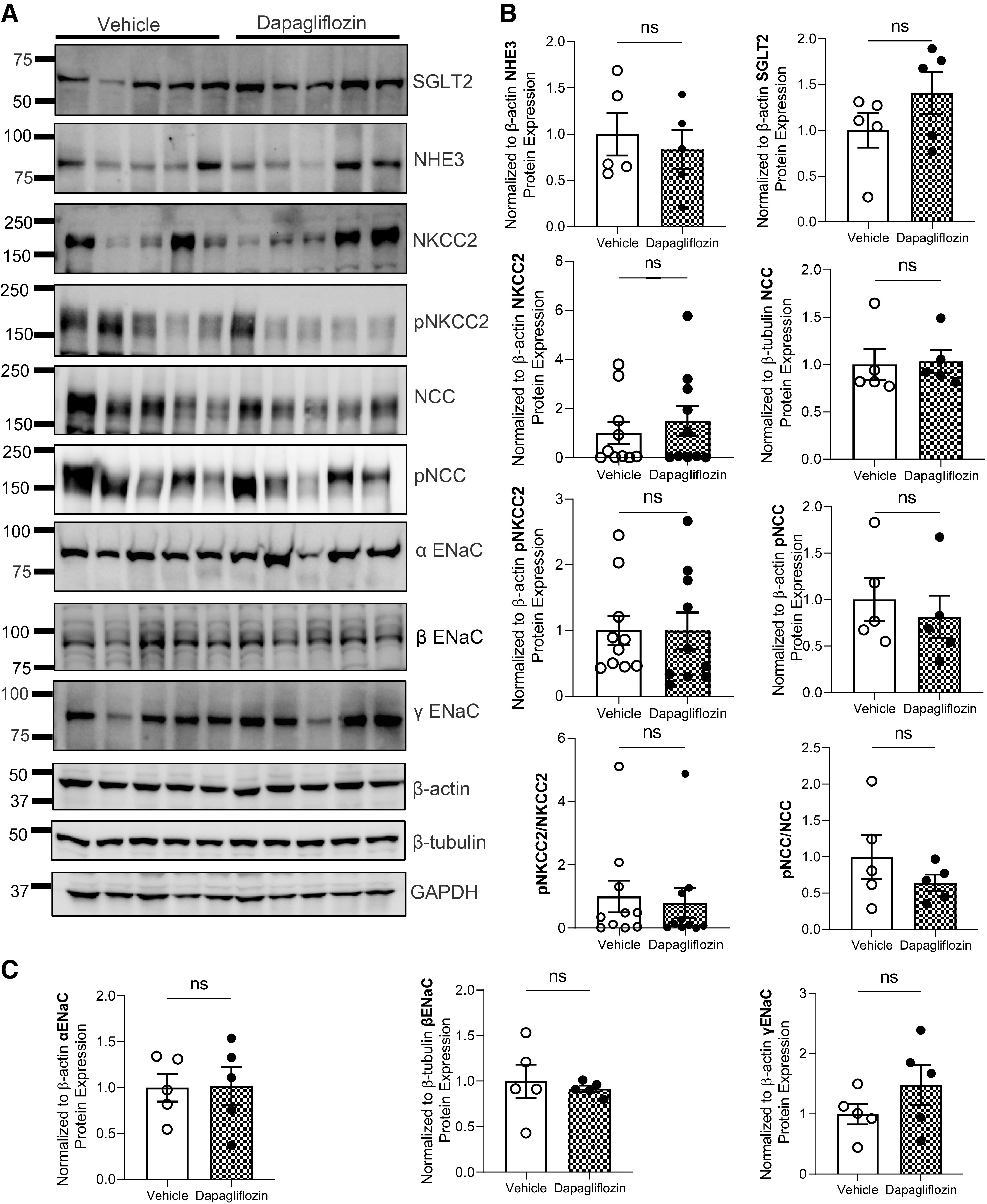
Protein expression of Na+ transporters in Dahl salt-sensitive rats after chronic dapagliflozin administration. A: Western blot analysis of Na+/H+ exchanger isoform 3 (NHE3), Na+-glucose cotransporter-2 (SGLT2), Na+-K+-2Cl− cotransporter (NKCC2), phosphorylated (p)NKCC2, Na+-Cl− cotransporter (NCC), pNCC, and α-, β-, and γ-subunits of the epithelial Na+ channel (ENaC). Each line represents 1 rat. B and C: summary graphs showing the average relative density of the bands (normalized to loading controls) in the studied groups. Quantification of protein expression was performed by normalizing to the corresponding loading control. Shown in Supplemental Fig. S2 are other blots for NKCC2 and additional loading controls. Results obtained from untreated rats were designated as 1. n ≥ 5. ns, not significant.
In addition to changes in expression levels, Na+ reabsorption via ENaC could be regulated by changes in channel gating properties. To test ENaC activity in the kidney, we performed single-channel analysis in freshly isolated CCDs using cell-attached patch-clamp recordings. Our data did not show differences in ENaC conductance, Po, or NPo between Dapa- and vehicle-treated rats (Fig. 10).
Figure 10.
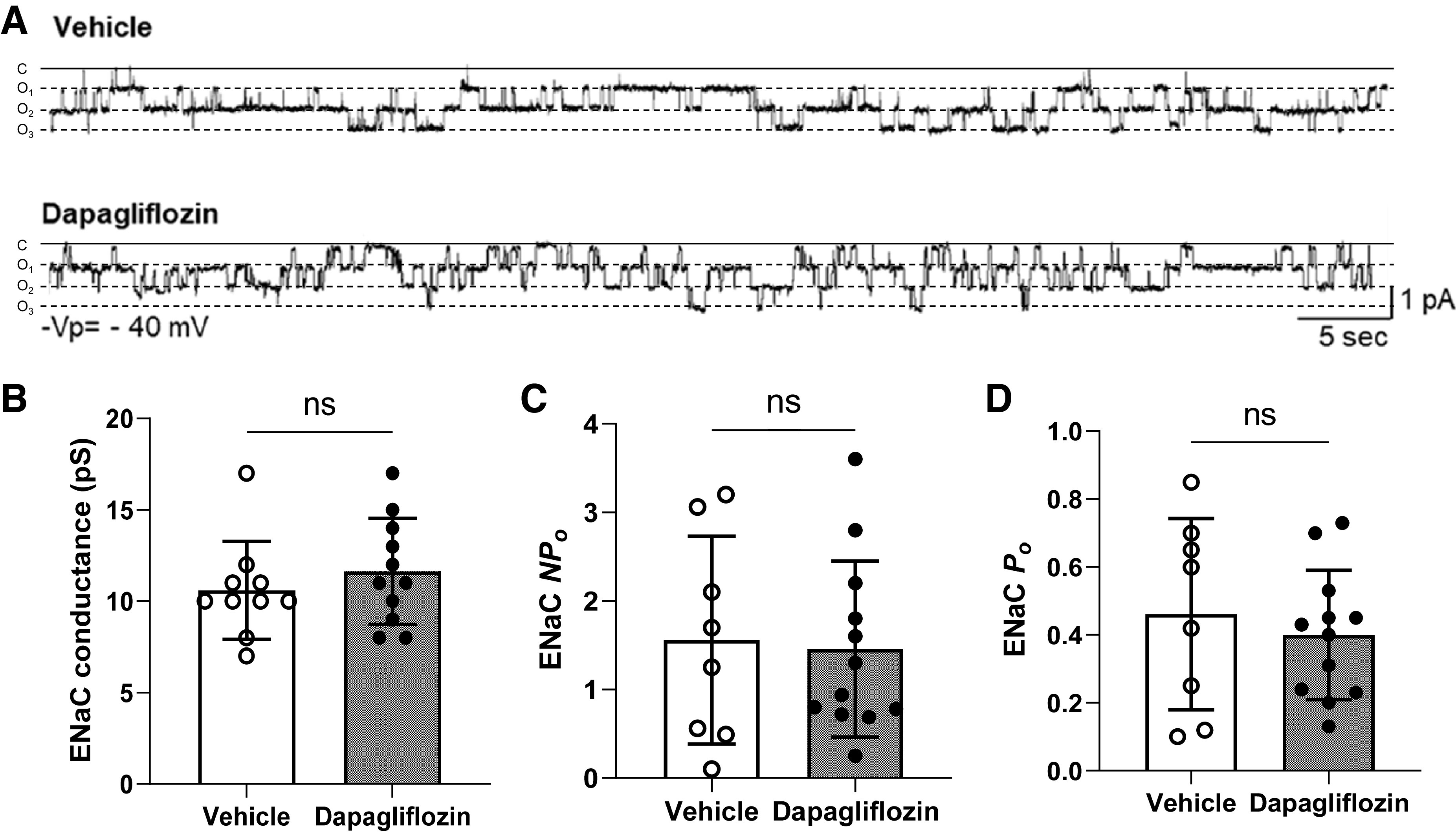
Epithelial Na+ channel (ENaC) activity in freshly isolated cortical collecting duct tubules. A: representative current traces of cell-attached patches containing ENaC recorded from the apical membrane of split-open cortical collecting duct cells of dapagliflozin- and vehicle-treated Dahl salt-sensitive rats on a high-salt diet. B–D: summary graphs of ENaC conductance (B), channel activity (NPo; C), and channel open probability (Po; D). P values are given for each graph. n ≥ 8. ns, not significant.
DISCUSSION
The main findings of the present study are that SGLT2 inhibition lowers blood pressure while maintaining a circadian rhythm in Dahl SS rats fed a high-NaCl diet in the absence of DM. We used this model to eliminate the influence of other variables linked to disturbances caused by diabetic disease. Our data further show that no pleiotropic and compensatory effects of Dapa were observed on other transporters and channels expressed along the nephron. The blood pressure-lowering effect of SGLT2 inhibitors is complex, and several, possibly coexisting, mechanisms contribute to this. Experimental evidence suggests that natriuresis, osmotic diuresis, sympathetic nerve activity, and body weight are possibly contributing factors (45, 46). However, most of these studies were performed on animals and patients with DM, and it is hard to delineate the contribution of general metabolic pathology from the changes in renal function.
A recent systematic review and meta-analysis of randomized controlled trials that included patients without DM (47) demonstrated a reduction of cardiovascular and metabolic outcomes in patients treated with SGLT2 inhibitors. Also, the randomized DAPA-CKD trial (10) showed improved cardiovascular causes and the risk of end-stage disease in the Dapa-treated group of patients without diabetes. There have been only a limited number of studies conducted to investigate the effect of Dapa in non-DM animals. In one of these studies (23), the authors did not observe significant changes in hypertension by weekly monitoring blood pressure with the tail-cuff method in Dahl SS rats. Also, acute administration of luseogliflozin, another SGLT2 inhibitor, via intraperitoneal injection in non-DM Sprague-Dawley rats (48) resulted in substantial glycosuria and natriuresis without changing renal hemodynamics. In another study (49), tofogliflozin decreased a rise of systolic blood pressure measured by tail-cuff plethysmography in Dahl SS rats fed a HS and high-fat diet after 9 wk of treatment. The development of salt-induced hypertension was prevented by empagliflozin administration in obese Otsuka Long–Evans Tokushima fatty (OLETF) rats (50). Experiments in nondiabetic adenine-induced CKD rats (19) showed that luseogliflozin administration attenuated the HS-induced blood pressure. Undoubtedly, animal (51, 52) and clinical (53–55) studies provide evidence of the blood pressure-lowering effect of SGLT2 inhibitors in DM. Of note, effects of Dapa on MAP were still observed despite the higher NaCl intake in this group, which speaks about the efficacy of the treatment.
In this study, we showed that administration of Dapa significantly attenuated salt-induced hypertension. However, the reductions in blood pressure found in our study were not associated with changes in heart rate. Previous reports (54, 56) have connected the lack of a compensatory increase in heart rate with a commensurate sympathetic nervous system activity blunting. The latter may also contribute to the beneficial effects of SGLT2 inhibitors compared with other diuretics.
Circadian rhythmicity is an important factor in most physiological processes including cardiovascular and renal function (57). For example, renal Na+ excretion has a circadian rhythm (35, 58, 59). There is evidence that the salt sensitivity of blood pressure and its nondipping pattern are connected (60). In our study, inhibition of Na+ reabsorption did not provoke blood pressure circadian dysfunctions in a SS model of hypertension.
We aimed to examine the effects of Dapa on the systemic and intrarenal RAAS, because intrarenal metabolites could be activated to compensate for Na+ and fluid waste. Patients with hypertension treated with diuretics experience activation of the RAAS (61). Disturbance of the RAAS was also shown in animal models with Slc5a2 mutation mimicking the glucosuric phenotype mediated by SGLT2 deficiency (62). Moreover, animal and clinical studies in type 2 DM have shown that SGLT2 inhibition might have opposing effects on systemic and intrarenal RAAS components (21, 63, 64). Clinical reports on the effect of SGLT2 inhibitors on plasma aldosterone and plasma renin activity could be conflicting due to the combination therapy with the high doses of angiotensin-converting enzyme and angiotensin receptor blockers. For example, it has been previously reported that Dapa increased aldosterone and plasma renin activity in patients with type 2 DM after 12 wk of treatment (21); however, another study suggested that overall plasma renin activity did not change after chronic inhibition of SGLT2 (64). An animal study (63) in OLETF rats demonstrated that treatment for 12 wk with Dapa did not change plasma aldosterone levels or plasma renin activity compared with vehicle treatment. The results of our study confirm that Dapa treatment did not activate circulating RAAS components in a model of salt-induced hypertension.
Dahl SS rats are a low renin strain (65–67), and a HS diet decreases not only plasma renin activity but also kidney angiotensinogen (AGT) levels and urinary excretion of AGT (66). SGLT2 inhibitor treatment might affect intrarenal AGT production through changes in glucose levels (68). The high glucose levels are expected to induce AGT synthesis in renal proximal tubular cells and further activate the intrarenal RAS (69). In type 2 DM mice, canagliflozin prevents intrarenal AGT upregulation (70), and administration of a SGLT2 inhibitor (TA-1887) did not activate the systemic and intrarenal RAS in nephrectomized rats (71). In our study, RAAS metabolites were not altered by Dapa during the development of hypertension; therefore, the observed diuretic and blood pressure-lowering effects are likely independent of RAAS signaling and might be due to a reduction in plasma volume (higher hematocrit). Here, we can speculate that inhibition of Na+ reabsorption in the proximal tubule might increase Na+ delivery to the macula densa that attenuates the intrarenal RAAS because of volume depletion. Comparing other diuretics with effects of SGLT2 inhibitors on RAAS metabolite activity will be important to better understand the role of reduced plasma volume in RAAS pathway activation in SS hypertension. SGLT2 inhibitors have a well-known diuretic effect due to increased urinary glucose and Na+ excretion (11, 48, 72). It has been shown that under Dapa treatment, SGLT2 expression was unchanged in high-fat diabetic mice, whereas NHE3, NaPi-2a, α-ENaC, and Na+-K+-ATPase (ATP1b1) were upregulated (24). A recent study (73) in DM rats identified that Dapa treatment did not affect NKCC2 protein expression. Another study (74) on the diabetic model of OLETF rats showed that empagliflozin decreased expression of NHE3 and NKCC2, but NCC expression was unaltered. A study in NHE3 knockout mice showed that acute natriuretic and chronic volume effects of empagliflozin depended on NHE3 (25). In addition, empagliflozin increased the expression of aquaporin 7, while it did not affect aquaporin 1 and 3 protein expression (74). Surprisingly, we did not observe any changes in Na+ channels and transporters in our experiments, suggesting that inhibition of SGLT2 does not mediate compensatory activation of downstream Na+ transport in the nephron.
Other effects of SGLT2 inhibitors are related to renal Mg2+ and handling (45). Recent clinical trials have found that patients with hypomagnesemia and type 2 DM increase serum Mg2+ levels after treatment with SGLT2 inhibitors (75, 76). Also, SGLT2 inhibition has been shown to increase tubular reabsorption, and numerous studies have demonstrated the adverse role of hyperphosphatemia on blood pressure and cardiovascular mortality (77). However, our study did not show significant differences in plasma and urinary Mg2+ or between control and Dapa-treated groups.
Surprisingly, Dapa treatment in our study was associated with a significant increase in the kidney-to-body weight ratio in Dahl SS rats after 3 wk of treatment. Tubule lumen enlargement may cause an increase in renal size due to the diuresis following SGLT2 treatment and upregulation of glucose reabsorption in the proximal tubule (78). A previous study (79) showed that a HS diet increased kidney weight. Diabetic hypertrophy (80) can be prevented with empagliflozin treatment; however, the growth induced by high NaCl intake in Dahl SS rats was unaffected. This is supported by our results in age-matched rats on a low-salt diet (Fig. 2B), which had a lower kidney-to-body weight ratio compared with vehicle- and Dapa-treated rats on a HS diet. Future studies are needed to address the effect of SGLT2 inhibition on kidney growth phenomenon in SS hypertension.
It is known that inflammatory and profibrotic effects in proximal tubule cells are caused by high glucose or by hyperplasic and profibrotic cytokine transforming growth factor-β, which leads to the development of tubulointerstitial fibrosis and then to diabetic nephropathy (81). A previous study has reported that treatment of type 1 diabetic Dahl-STZ rats with luseogliflozin alleviates glomerular injury, outer medullary fibrosis, and the formation of protein casts but does not reduce proteinuria (11). However, Dapa did not prevent fibrosis or protein cast formation in Dahl SS rats in our study. Consistently, Dapa did not reduce albuminuria in our model. We understand that our study has limitations and greater injury induced by 8% NaCl intake might have been able to unravel a beneficial effect of SGLT2 inhibition on kidney damage.
In summary, our data suggest that SGLT2 inhibitors are a potential therapeutic option for the treatment of hypertension. The present study indicates that chronic administration of the SGLT2 inhibitor Dapa effectively attenuates the development of salt-induced hypertension. Dapa increases glucose and Na+ excretion without secondary effects on the expression and function of other transporters/channels along the nephron, which is consistent with a lack of activation of the RAAS. Further research is required to fully understand the blood pressure-lowering mechanism(s) of SGLT2 inhibitors in nondiabetic models.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.19544059.v2.
GRANTS
This work was supported by National Institutes of Health Grants R35HL135749 (to A.S.), R01DK110621 (to T.R.), R01DK126720 (to O.P.), and K99HL153686 (to C.A.K.), National Institute of Diabetes and Digestive and Kidney Diseases Diabetic Complications Consortium (RRID: SCR_001415, www.diacomp.org), Grants DK076169 and DK115255 (to A.S. and T.R.), Department of Veteran Affairs Grants I01 BX004024 (to A.S.) and I01 BX004968 (to T.R.), American Heart Association Transformational Research Award 19TPA34850116 (to T.R.), and an American Physiological Society Postdoctoral Fellowship (to R.B.).
DISCLOSURES
Timo Rieg and Alexander Staruschenko are editors of American Journal of Physiology-Renal Physiology and were not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article.
AUTHOR CONTRIBUTIONS
O.K., O.P., and A.S. conceived and designed research; O.K., R.B., V.L., and T.R. performed experiments; O.K., R.B., V.L., C.A.K., and T.R. analyzed data; O.P., T.R., and A.S. interpreted results of experiments; O.K. and R.B. prepared figures; O.K. and A.S. drafted manuscript; O.K., R.B., O.P., C.A.K., T.R., and A.S. edited and revised manuscript; O.K., R.B., V.L., O.P., C.A.K., T.R., and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the colleagues at the Children’s Research Institute (Medical College of Wisconsin), Christine Duris and Tanya Bufford (Histology Core), for assistance with immunohistochemistry experiments as well as Dr. Suresh Kumar (Imaging Core), for the help with image scanning. BioRender was used to create the graphical abstract.
REFERENCES
- 1.Staruschenko A. Hypertension and diabetes mellitus: the chicken and egg problem. Hypertension 69: 787–788, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 3.Perry RJ, Shulman GI. Sodium-glucose cotransporter-2 inhibitors: understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem 295: 14379–14390, 2020. doi: 10.1074/jbc.REV120.008387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangaswami J, Bhalla V, de Boer IH, Staruschenko A, Sharp JA, Singh RR, Lo KB, Tuttle K, Vaduganathan M, Ventura H, McCullough PA; American Heart Association Council on the Kidney in Cardiovascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Lifestyle and Cardiometabolic Health. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American Heart Association. Circulation 142: e265–e286, 2020. doi: 10.1161/CIR.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 5.Staruschenko A, Bhalla V, Rangaswami J. SGLT2 inhibitors: diabetic kidney disease and beyond. Am J Physiol Renal Physiol 319: F780–F781, 2020. doi: 10.1152/ajprenal.00518.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu P-L, Dd Z, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez DE, Foresto RD, Ribeiro AB. SGLT-2 inhibitors in diabetes: a focus on renoprotection. Rev Assoc Med Bras 66: s17–s24, 2020. doi: 10.1590/1806-9282.66.S1.17. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Wahab AF, Bamagous GA, Al-Harizy RM, ElSawy NA, Shahzad N, Ibrahim IA, Ghamdi SSA. Renal protective effect of SGLT2 inhibitor dapagliflozin alone and in combination with irbesartan in a rat model of diabetic nephropathy. Biomed Pharmacother 103: 59–66, 2018. doi: 10.1016/j.biopha.2018.03.176. [DOI] [PubMed] [Google Scholar]
- 9.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 8: 262–275.e9, 2014. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde A-M, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 11.Kojima N, Williams JM, Slaughter TN, Kato S, Takahashi T, Miyata N, Roman RJ. Renoprotective effects of combined SGLT2 and ACE inhibitor therapy in diabetic Dahl S rats. Physiol Rep 3: e12436, 2015. doi: 10.14814/phy2.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 83: 503–528, 2021. doi: 10.1146/annurev-physiol-031620-095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson SC, Vallon V. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am J Physiol Renal Physiol 320: F761–F771, 2021. doi: 10.1152/ajprenal.00552.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 61: 2079–2086, 2018. doi: 10.1007/s00125-018-4654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Østergaard MV, Secher T, Christensen M, Salinas CG, Roostalu U, Skytte JL, Rune I, Hansen HH, Jelsing J, Vrang N, Fink LN. Therapeutic effects of lisinopril and empagliflozin in a mouse model of hypertension-accelerated diabetic kidney disease. Am J Physiol Renal Physiol 321: F149–F161, 2021. doi: 10.1152/ajprenal.00154.2021. [DOI] [PubMed] [Google Scholar]
- 17.Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 18.Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C, Villa S, Morigi M, Remuzzi G, Benigni A, Zoja C. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 3: e98720, 2018. doi: 10.1172/jci.insight.98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan N, Fujisawa Y, Kobara H, Masaki T, Nakano D, Rahman A, Nishiyama A. Effects of an SGLT2 inhibitor on the salt sensitivity of blood pressure and sympathetic nerve activity in a nondiabetic rat model of chronic kidney disease. Hypertens Res 43: 492–499, 2020. doi: 10.1038/s41440-020-0410-8. [DOI] [PubMed] [Google Scholar]
- 20.Filippatos TD, Tsimihodimos V, Elisaf MS. Mechanisms of blood pressure reduction with sodium-glucose co-transporter 2 (SGLT2) inhibitors. Expert Opin Pharmacother 17: 1581–1583, 2016. doi: 10.1080/14656566.2016.1201073. [DOI] [PubMed] [Google Scholar]
- 21.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853–862, 2013. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzu T. Salt and hypertension in diabetes. Diabetol Int 8: 154–159, 2017. doi: 10.1007/s13340-017-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappetta D, Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Miccichè A, Dell'Aversana C, D'Amario D, Cianflone E, Scavone C, Santini L, Palandri C, Naviglio S, Crea F, Rota M, Altucci L, Rossi F, Capuano A, Urbanek K, Berrino L. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res 157: 104781, 2020. doi: 10.1016/j.phrs.2020.104781. [DOI] [PubMed] [Google Scholar]
- 24.Ma C, de Baaij JHF, Millar PJ, Gault VA, de Galan BE, Bindels RJM, Hoenderop JGJ. Effect of dapagliflozin treatment on the expression of renal sodium transporters/channels on high-fat diet diabetic mice. Nephron 142: 51–60, 2019. doi: 10.1159/000496617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, Song P, Freeman B, Kim YC, Soleimani M, Sharma K, Thomson SC, Vallon V. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol 319: F712–F728, 2020. doi: 10.1152/ajprenal.00264.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, Reckelhoff JF, Iadecola C, Coffman TM. Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 73: e87–e120, 2019. doi: 10.1161/HYP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajasekeran H, Reich HN, Hladunewich MA, Cattran D, Lovshin JA, Lytvyn Y, Bjornstad P, Lai V, Tse J, Cham L, Majumder S, Bowskill BB, Kabir MG, Advani SL, Gibson IW, Sood MM, Advani A, Cherney DZI. Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Renal Physiol 314: F412–F422, 2018. doi: 10.1152/ajprenal.00445.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2: e92331, 2017. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlov TS, Levchenko V, Ilatovskaya DV, Li H, Palygin O, Pastor-Soler NM, Hallows KR, Staruschenko A. Lack of effects of metformin and AICAR chronic infusion on the development of hypertension in Dahl salt-sensitive rats. Front Physiol 8: 227, 2017. doi: 10.3389/fphys.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golosova D, Palygin O, Bohovyk R, Klemens CA, Levchenko V, Spires DR, Isaeva E, El-Meanawy A, Staruschenko A. Role of opioid signaling in kidney damage during the development of salt-induced hypertension. Life Sci Alliance 3: e202000853, 2020. doi: 10.26508/lsa.202000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlov TS, Levchenko V, Ilatovskaya DV, Moreno C, Staruschenko A. Renal sodium transport in renin-deficient Dahl salt-sensitive rats. J Renin Angiotensin Aldosterone Syst. 17: 147032031665385, 2016. doi: 10.1177/1470320316653858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palygin O, Spires D, Levchenko V, Bohovyk R, Fedoriuk M, Klemens CA, Sykes O, Bukowy JD, Cowley AW Jr, Lazar J, Ilatovskaya DV, Staruschenko A. Progression of diabetic kidney disease in T2DN rats. Am J Physiol Renal Physiol 317: F1450–F1461, 2019. doi: 10.1152/ajprenal.00246.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourdon L, Buguet A, Cucherat M, Radomski MW. Use of a spreadsheet program for circadian analysis of biological/physiological data. Aviat Space Environ Med 66: 787–791, 1995. [PubMed] [Google Scholar]
- 34.Munakata M, Imai Y, Minami N, Sasaki S, Ichijyo T, Yoshizawa M, Sekino H, Abe K, Yoshinaga K. Cosinor analysis of changes in circadian blood pressure rhythm with aging in spontaneously hypertensive rats. Tohoku J Exp Med 161: 55–64, 1990. doi: 10.1620/tjem.161.55. [DOI] [PubMed] [Google Scholar]
- 35.Crislip GR, Douma LG, Masten SH, Cheng KY, Lynch IJ, Johnston JG, Barral D, Glasford KB, Holzworth MR, Verlander JW, Wingo CS, Gumz ML. Differences in renal BMAL1 contribution to Na+ homeostasis and blood pressure control in male and female mice. Am J Physiol Renal Physiol 318: F1463–F1477, 2020. doi: 10.1152/ajprenal.00014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue J, Thomas L, Murali SK, Levi M, Fenton RA, Dominguez Rieg JA, Rieg T. Enhanced phosphate absorption in intestinal epithelial cell-specific NHE3 knockout mice. Acta Physiol (Oxf) 234: e13756, 2022. doi: 10.1111/apha.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spires DR, Palygin O, Levchenko V, Isaeva E, Klemens CA, Khedr S, Nikolaienko O, Kriegel A, Cheng X, Yeo JY, Joe B, Staruschenko A. Sexual dimorphism in the progression of type 2 diabetic kidney disease in T2DN rats. Physiol Genomics 53: 223–234, 2021. doi: 10.1152/physiolgenomics.00009.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blass G, Klemens CA, Brands MW, Palygin O, Staruschenko A. Postprandial effects on ENaC-mediated sodium absorption. Sci Rep 9: 4296, 2019. doi: 10.1038/s41598-019-40639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roksnoer LCW, Van Veghel R, De Vries R, Garrelds IM, Bhaggoe UM, Friesema ECH, Leijten FPJ, Poglitsch M, Domenig O, Clahsen-Van Groningen MC, Hoorn EJ, Jan Danser AH, Batenburg WW. Optimum AT1 receptor-neprilysin inhibition has superior cardioprotective effects compared with AT1 receptor blockade alone in hypertensive rats. Kidney Int 88: 109–120, 2015. doi: 10.1038/ki.2015.107. [DOI] [PubMed] [Google Scholar]
- 40.Cowley AW Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the importance of Nox4 in production of hypertension in dahl salt-sensitive rats. Hypertension 67: 440–450, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palygin O, Ilatovskaya DV, Levchenko V, Klemens CA, Dissanayake L, Williams AM, Pavlov TS, Staruschenko A. Characterization of purinergic receptor expression in ARPKD cystic epithelia. Purinergic Signal 14: 485–497, 2018. doi: 10.1007/s11302-018-9632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JH, Cowley AW Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol 24: 1053–1062, 2013. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feric M, Zhao B, Hoffert JD, Pisitkun T, Knepper MA. Large-scale phosphoproteomic analysis of membrane proteins in renal proximal and distal tubule. Am J Physiol Cell Physiol 300: C755–C770, 2011. doi: 10.1152/ajpcell.00360.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isaeva E, Fedoriuk M, Bohovyk R, Klemens CA, Khedr S, Golosova D, Levchenko V, El-Meanawy A, Palygin O, Staruschenko A. Vibrodissociation method for isolation of defined nephron segments from human and rodent kidneys. Am J Physiol Renal Physiol 317: F1398–F1403, 2019. doi: 10.1152/ajprenal.00448.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominguez Rieg JA, Xue J, Rieg T. Tubular effects of sodium-glucose cotransporter 2 inhibitors: intended and unintended consequences. Curr Opin Nephrol Hypertens 29: 523–530, 2020. doi: 10.1097/MNH.0000000000000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kario K, Ferdinand KC, Vongpatanasin W. Are SGLT2 inhibitors new hypertension drugs? Circulation 143: 1750–1753, 2021. doi: 10.1161/CIRCULATIONAHA.121.053709. [DOI] [PubMed] [Google Scholar]
- 47.Teo YH, Teo YN, Syn NL, Kow CS, Yoong CSY, Tan BYQ, Yeo TC, Lee CH, Lin W, Sia CH. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc 10: e019463, 2021. doi: 10.1161/JAHA.120.019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ansary TM, Fujisawa Y, Rahman A, Nakano D, Hitomi H, Kobara H, Masaki T, Titze JM, Kitada K, Nishiyama A. Responses of renal hemodynamics and tubular functions to acute sodium-glucose cotransporter 2 inhibitor administration in non-diabetic anesthetized rats. Sci Rep 7: 9555, 2017. doi: 10.1038/s41598-017-09352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura T, Nakamura K, Miyoshi T, Yoshida M, Akazawa K, Saito Y, Akagi S, Ohno Y, Kondo M, Miura D, Wada J, Ito H. Inhibitory effects of tofogliflozin on cardiac hypertrophy in Dahl salt-sensitive and salt-resistant rats fed a high-fat diet. Int Heart J 60: 728–735, 2019. doi: 10.1536/ihj.18-392. [DOI] [PubMed] [Google Scholar]
- 50.Choi JB, Yoo JM, Lee Y-J, Kim JW, Lee S-J, Kim HY, Lee DS, Ko S-H, Choe H-S. Effect of the sodium-glucose cotransporter 2 inhibitor, dapagliflozin, on genitourinary infection in an animal model of type 2 diabetes. Int Neurourol J 24: 21–28, 2020. doi: 10.5213/inj.1938220.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saleh S, Hanna G, El-Nabi SH, El-Domiaty H, Shabaan A, Ewida SF. Dapagliflozin, a sodium glucose cotransporter 2 inhibitors, protects cardiovascular function in type-2 diabetic murine model. J Genet 99: 46, 2020. doi: 10.1007/s12041-020-01196-9. [DOI] [PubMed] [Google Scholar]
- 52.Arow M, Waldman M, Yadin D, Nudelman V, Shainberg A, Abraham NG, Freimark D, Kornowski R, Aravot D, Hochhauser E, Arad M. Sodium-glucose cotransporter 2 inhibitor dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol 19: 7, 2020. doi: 10.1186/s12933-019-0980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, Uchiyama K, Niijima Y, Katsuya T, Urata H, Osuga J-I, Fujiwara T, Yamazaki S, Tomitani N, Kanegae H, Hoshide S, Yamamoto M, Eguchi K, Mizuno A, Nakano S, Kemi Y, Iwashita C, Wada K, Kaneko A, Ryan N. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation 139: 2089–2097, 2019. doi: 10.1161/CIRCULATIONAHA.118.037076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheen AJ. Effect of SGLT2 inhibitors on the sympathetic nervous system and blood pressure. Curr Cardiol Rep 21: 70, 2019. doi: 10.1007/s11886-019-1165-1. [DOI] [PubMed] [Google Scholar]
- 55.Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 17: 1180–1193, 2015. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuttle KR, Brosius FC 3rd, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, Manley T, McGuire DK, Molitch ME, Mottl AK, Perreault L, Rosas SE, Rossing P, Sola L, Vallon V, Wanner C, Perkovic V. SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a Scientific Workshop sponsored by the National Kidney Foundation. Am J Kidney Dis 77: 94–109, 2021. doi: 10.1053/j.ajkd.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Solocinski K, Gumz ML. The circadian clock in the regulation of renal rhythms. J Biol Rhythms 30: 470–486, 2015. doi: 10.1177/0748730415610879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Douma LG, Holzworth MR, Solocinski K, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Renal Na-handling defect associated with PER1-dependent nondipping hypertension in male mice. Am J Physiol Renal Physiol 314: F1138–F1144, 2018. doi: 10.1152/ajprenal.00546.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alli A, Yu L, Holzworth M, Richards J, Cheng KY, Lynch IJ, Wingo CS, Gumz ML. Direct and indirect inhibition of the circadian clock protein Per1: effects on ENaC and blood pressure. Am J Physiol Renal Physiol 316: F807–F813, 2019. doi: 10.1152/ajprenal.00408.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura G. Sodium, kidney, and circadian rhythm of blood pressure. Clin Exp Nephrol 5: 13–18, 2001. doi: 10.1007/PL00012172. [DOI] [Google Scholar]
- 61.McNally RJ, Farukh B, Chowienczyk PJ, Faconti L. Effect of diuretics on plasma aldosterone and potassium in primary hypertension: a systematic review and meta-analysis. Br J Clin Pharmacol 88: 1964–1977, 2021. doi: 10.1111/bcp.15156. [DOI] [PubMed] [Google Scholar]
- 62.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH, Kim JW, Ahn YB, Kim ES, Moon SD, Kim MJ, Ko SH. Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One 11: e0165703, 2016. doi: 10.1371/journal.pone.0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mori I, Ishizuka T. Effects of SGLT2 inhibitors on renin-aldosterone system for one month and six months in type 2 diabetes. Diabetes 67: 1196, 2018. doi: 10.2337/db18-1196-P. [DOI] [Google Scholar]
- 65.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW Jr.. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol 295: F837–F842, 2008. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manis AD, Palygin O, Khedr S, Levchenko V, Hodges MR, Staruschenko A. Relationship between the renin-angiotensin-aldosterone system and renal Kir5.1 channels. Clin Sci (Lond) 133: 2449–2461, 2019. doi: 10.1042/CS20190876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci 20: 629, 2019. doi: 10.3390/ijms20030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Shibayama Y, Kobori H, Liu Y, Kobara H, Masaki T, Wang Z, Nishiyama A. High glucose augments angiotensinogen in human renal proximal tubular cells through hepatocyte nuclear factor-5. PLoS One 12: e0185600, 2017. doi: 10.1371/journal.pone.0185600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woods TC, Satou R, Miyata K, Katsurada A, Dugas CM, Klingenberg NC, Fonseca VA, Navar LG. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol 49: 331–342, 2019. doi: 10.1159/000499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L, Konishi Y, Morikawa T, Zhang Y, Kitabayashi C, Kobara H, Masaki T, Nakano D, Hitomi H, Kobori H, Nishiyama A. Effect of a SGLT2 inhibitor on the systemic and intrarenal renin-angiotensin system in subtotally nephrectomized rats. J Pharmacol Sci 137: 220–223, 2018. doi: 10.1016/j.jphs.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeshige Y, Fujisawa Y, Rahman A, Kittikulsuth W, Nakano D, Mori H, Masaki T, Ohmori K, Kohno M, Ogata H, Nishiyama A. A sodium-glucose co-transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt-treated obese rats. Hypertens Res 39: 415–422, 2016. doi: 10.1038/hr.2016.2. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, LaRocque LM, Efe O, Wang J, Sands JM, Klein JD. Effect of dapagliflozin treatment on fluid and electrolyte balance in diabetic rats. Am J Med Sci 352: 517–523, 2016. doi: 10.1016/j.amjms.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung S, Kim S, Son M, Kim M, Koh ES, Shin SJ, Ko SH, Kim HS. Empagliflozin contributes to polyuria via regulation of sodium transporters and water channels in diabetic rat kidneys. Front Physiol 10: 271, 2019. doi: 10.3389/fphys.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ray EC, Boyd-Shiwarski CR, Liu P, Novacic D, Cassiman D. SGLT2 inhibitors for treatment of refractory hypomagnesemia: a case report of 3 patients. Kidney Med 2: 359–364, 2020. doi: 10.1016/j.xkme.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32: 650–657, 2009. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim HK, Mizuno M, Vongpatanasin W. Phosphate, the forgotten mineral in hypertension. Curr Opin Nephrol Hypertens 28: 345–351, 2019. doi: 10.1097/MNH.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez D, Kapoor S, Edenhofer I, Segerer S, Riwanto M, Kipar A, Yang M, Mei C, Wüthrich RP. Inhibition of sodium-glucose cotransporter 2 with dapagliflozin in han: SPRD rats with polycystic kidney disease. Kidney Blood Press Res 40: 638–647, 2015. doi: 10.1159/000368540. [DOI] [PubMed] [Google Scholar]
- 79.McCormick CP, Rauch AL, Buckalew VM Jr.. Differential effect of dietary salt on renal growth in Dahl salt-sensitive and salt-resistant rats. Hypertension 13: 122–127, 1989. doi: 10.1161/01.hyp.13.2.122. [DOI] [PubMed] [Google Scholar]
- 80.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panchapakesan U, Pegg K, Gross S, Komala MG, Mudaliar H, Forbes J, Pollock C, Mather A. Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLoS One 8: e54442, 2013. doi: 10.1371/journal.pone.0054442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.19544059.v2.