Keywords: central pattern generator, feeding, modulation, mollusk, neuropeptide
Abstract
These experiments focus on an interneuron (B63) that is part of the feeding central pattern generator (CPG) in Aplysia californica. Previous work has established that B63 is critical for program initiation regardless of the type of evoked activity. B63 receives input from a number of different elements of the feeding circuit. Program initiation occurs reliably when some are activated, but we show that it does not occur reliably with activation of others. When program initiation is reliable, modulatory neuropeptides are released. For example, previous work has established that an ingestive input to the feeding CPG, cerebral buccal interneuron 2 (CBI-2), releases feeding circuit activating peptide (FCAP) and cerebral peptide 2 (CP-2). Afferents with processes in the esophageal nerve (EN) that trigger egestive motor programs release small cardioactive peptide (SCP). Previous studies have described divergent cellular and molecular effects of FCAP/CP-2 and SCP on the feeding circuit that specify motor activity. Here, we show that FCAP/CP-2 and SCP additionally increase the B63 excitability. Thus, we show that peptides that have well-characterized divergent effects on the feeding circuit additionally act convergently at the level of a single neuron. Since convergent effects of FCAP/CP-2 and SCP are not necessary for specifying the type of network output, we ask why they might be important. Our data suggest that they have an impact during a task switch, i.e., when there is a switch from egestive to ingestive activity.
NEW & NOTEWORTHY The activity of multifunctional central pattern generators (CPGs) is often configured by neuromodulators that exert divergent effects that are necessary to specify motor output. We demonstrate that ingestive and egestive inputs to the feeding CPG in Aplysia act convergently (as well as divergently). We ask why this convergence may be important and suggest that it may be a mechanism for a type of arousal that occurs during task switching.
INTRODUCTION
Many central pattern generators (CPGs) are multifunctional. For example, CPGs that mediate feeding are often capable of generating both ingestive and egestive motor programs (1–4). Multifunctional CPGs often receive modulatory input (5, 6). Each modulator, or set of modulators, impacts the CPG in a particular manner, i.e., effects on the CPG are at least to some extent divergent (yellow and red in Fig. 1A). As a result, each modulator configures activity in a particular behaviorally appropriate manner. A question we address in this study is whether modulators that evoke different motor programs exert convergent as well as divergent effects (orange in Fig. 1A). Furthermore, since convergent neuromodulatory effects are not necessary for specifying network output, we ask why they might be important.
Figure 1.

A: schematic showing divergent and convergent effects of input activation on a feeding central pattern generator (CPG). CPGs often receive multiple inputs. For example, a stimulus that triggers food ingestion activates one input (input 1, yellow) that modifies the properties of neurons in the CPG so that an ingestive motor program is generated (output 1). A stimulus that triggers food egestion activates a second input (input 2, red) that generates egestive activity (output 2). Input 1 and input 2 trigger 2 different types of motor programs because at least to some extent effects on the CPG are divergent (yellow and red circles). A question we address in this study is, can input activation also exert convergent effects on some CPG elements (orange circles)? B: circuit diagrams showing neurons activated with stimulation of an ingestive CPG input [cerebral buccal interneuron 2 (CBI-2)] (B2) and neurons activated with stimulation of an egestive input to the feeding CPG [the posterior branch of the esophageal nerve (EN)] (B1). Symbols used to indicate synaptic connections: filled triangles, fast excitatory connections; open triangles, slow excitatory connections; filled circles, fast inhibitory connections; resistor, electrical coupling. Dashed lines with arrows show modulatory effects of neuropeptides. The gray shading is used to align neural elements with a classification, e.g., CBI-2 and the EN are inputs to the CPG. At bottom, dark gray shading is used for the B31/B32 neurons to indicate that they belong to >1 category, i.e., they are motor neurons that are also CPG elements. CP-2, cerebral peptide 2; FCAP, feeding circuit activating peptide; RINs, retraction interneurons; RMNs, retraction motor neurons; SCP, small cardioactive peptide.
Our experiments are conducted in the feeding system of the mollusk Aplysia californica. Aplysia generate ingestive behaviors that pull food into the buccal cavity and egestive behaviors that push food out (7, 8). During ingestive behaviors the structure used to grasp food, the radula, is closed as it retracts. During egestive behaviors the radula is closed as it protracts. The feeding CPG in Aplysia is well characterized and has been studied in vivo and in various in vitro preparations (9, 10). In vitro, ingestive motor programs can be triggered by stimulating the command-like neuron cerebral buccal interneuron 2 (CBI-2) (11–14). Egestive motor programs can be triggered by stimulating the posterior branch of a peripheral nerve that contains gut afferents [the posterior branch of the esophageal nerve (EN)] (15, 16). Stimulation of both inputs releases modulatory neuropeptides that configure motor activity (17–20). Peptides released by CBI-2 differ from those released by EN stimulation. Previous studies have shown that EN and CBI-2 peptides exert divergent effects on the feeding circuity (e.g., Refs. 18, 19, 21–23). In this study we show that additionally these peptides act convergently on a different target interneuron and exert an effect that could contribute to a type of arousal that occurs during task switching.
METHODS
Animals/Cell Identification
Adult sea slugs (Aplysia californica) were purchased from Marinus Scientific (Long Beach, CA) and maintained in artificial seawater (ASW) (Instant Ocean, Cincinnati, OH). Aplysia are hermaphrodites and are therefore both male and female. Animals were anesthetized by injection of isotonic MgCl2. Cerebral and buccal ganglia were dissected out with the cerebral-buccal connectives and esophageal and buccal nerves intact. The connective tissue was removed while ganglia were bathed in a solution containing 50% isotonic MgCl2 and 50% ASW (in mM: 460 NaCl, 10 KCl, 55 MgCl2, 11 CaCl2, and 10 HEPES buffer, pH 7.6).
Neurons were identified on the basis of their location, size, and electrophysiological and morphological characteristics as has previously been described (12, 24–28).
Electrophysiological Recordings
Intracellular recordings were obtained with glass micropipettes filled with 2 M potassium acetate or, in the occlusion experiments, a solution containing 0.6 M K2SO4 and 60 mM KCl. Electrodes were fabricated with a Flaming/Brown micropipette puller (Sutter Instrument Co., Novato, CA) and beveled to yield a final resistance of 4–10 MΩ. Recordings were obtained from electrodes held in HS-2A headstages (Molecular Devices, San Jose, CA) connected to AxoClamp 2B amplifiers (Molecular Devices) or Getting amplifiers (Getting Instruments, Iowa City, IA). Extracellular nerve recordings were obtained with polyethylene suction electrodes connected to a model 1700 differential AC amplifier (A-M Systems, San Diego, CA). Both intracellular and extracellular signals were digitized with a Digidata 1320A (Molecular Devices).
Induction of Motor Activity
Motor programs were triggered with the posterior branch of the EN, CBI-2, B34, or B65. The EN branch was stimulated extracellularly with a suction electrode. Stimulation was continuous at 2 Hz with 3-ms, 2- to 10-V pulses for an amount of time that was determined by the number of cycles of activity that were required for a particular experiment. When programs were triggered by CBI-2, B34, or B65, neurons were activated by intracellular injections of direct-current pulses (20–30 ms) that were generated by a model S48 stimulator (Grass Instruments, Quincy, MA). The frequency of stimulation was 10 Hz. Because neuron B34 is a high-threshold neuron, it was predepolarized with a DC current injection just before injection of the brief current pulses. Current pulses were adjusted so that each pulse elicited a single action potential. Program-activating neurons were stimulated until the end of the protraction phase of the motor program or for a maximum of 30 s, whichever came first. After the end of stimulation, there was a 30-s rest period before the next cycle of activity was generated.
The protraction phase of motor programs was monitored via extracellular recordings from the I2 nerve. This nerve contains the axons of protraction motor neurons (24, 29). Retraction was monitored via extracellular recordings from buccal nerve 2 (Bn2) (7, 8) or by depolarization in B8.
Excitability Experiments
To determine effects of neural stimulation and peptides on B63 excitability, action potentials were elicited by injecting 2- to 4-nA, 3-s constant-current depolarizing pulses every 30 s. The specific amount of current injected in an individual experiment was selected to generate a relatively consistent number of spikes under control conditions. The number of spikes triggered under control conditions was then compared to the number of spikes triggered after either peptide application or stimulation of a CPG input. In all cases only the spikes triggered during the current injection were included in analyses. Spikes triggered after current injections ended were not included in box plots or in statistical analyses. In occlusion experiments excitability was measured at least 30 min after washout of SCP and 45 min of washout of FCAP/CP-2.
Exogenous Peptides
Lyophilized aliquots of peptides were dissolved in 50–100 µL of deionized H2O and then diluted to working concentrations in the carrier solution for bath perfusion. Carrier solutions were either ASW (in mM: 460 NaCl, 10 KCl, 55 MgCl2, 11 CaCl2, and 10 HEPES buffer, pH = 7.6), or high-divalent ASW (Hi Di; in mM: 368 NaCl, 10 KCl, 101 MgCl2, 13.8 CaCl2, and 10 HEPES, pH = 7.6). FCAP and CP-2 were obtained from SynPep (Dublin, CA), and SCP was obtained from Anaspec (Fremont, CA).
Statistical Analyses
Data were analyzed in Clampfit (Molecular Devices) or Spike2 (Cambridge Electronic Design) and organized in Excel. Data were plotted and analyzed in Prism (GraphPad Software, San Diego, CA). Error bars indicate SEs, and the significance level was set at P < 0.05. Where applicable, measurements under different conditions were treated as repeated measures as indicated in the text. In experiments where repeated measures were made we did not assume sphericity, i.e., Prism used the Geisser and Greenhouse method to reduce the number of degrees of freedom when necessary. Throughout the results, n refers to the number of preparations.
RESULTS
Convergence in the Feeding CPG: EN and CBI-2 Stimulation Both Modify B63 Activity
In a previous study (23), the feeding network was studied by triggering motor programs from within the CPG, i.e., without release of the modulators endogenous to the CPG inputs. Programs triggered in this manner were then used as “controls” and compared to programs triggered when CPG inputs were activated and modulators were released (23). This approach successfully characterized divergent effects of neuromodulators, particularly neuropeptides. Programs were triggered with the protraction interneuron B63 (Fig. 1B). Protraction is the first phase of the motor program, and protraction interneurons are capable of inducing a complete cycle of activity since they provide slow excitatory input to neurons active during the subsequent phase, retraction (27, 30). B63 is active during both ingestive and egestive motor programs and therefore was a potential site for convergent modulation (3, 19, 23, 31). We therefore triggered motor activity with two different protraction interneurons that are “input specific.” One neuron, B65, is activated when programs are triggered by EN stimulation, but it generally does not fire when programs are triggered by CBI-2 stimulation (14, 30, 32, 33). The second neuron, B34, is primarily associated with activity triggered by CBI-2 (14, 34–37).
Initial experiments focused on activity triggered by B65. A previous report demonstrated that although B65 can trigger complete cycles of motor programs, with repeated B65 stimulation patterned activity is not sustained (28). We confirmed that this is the case and quantified the change in the probability of triggering a complete cycle of a motor program when a series of 10 successive attempts were made (Fig. 2A) (n = 30). Observed effects were progressive, suggesting that a persistent effect was becoming increasingly larger.
Figure 2.

A: program induction fails when there is an attempt to trigger a series of motor programs with B65. B65 was stimulated 10 times with brief current pulses at 10 Hz (A1). Stimulation was terminated either after 30 s or at the onset of retraction. The intertrial interval was 30 s. The top trace in A1 and A2 is an intracellular recording from B65, the middle trace is an extracellular recording from the I2 nerve (I2N), and the bottom trace is an extracellular recording from buccal nerve 2 (Bn2). The open bars under the traces in A1 and A2 mark the protraction phase of the motor program; the filled bars mark retraction. Trials 1, 6, and 10 are enlarged in A2. A3 plots the probability that a complete cycle was triggered (n = 30). Note that the probability progressively decreases. B: effects are cumulative when there is >1 series of bursts in B65. B65 was stimulated as indicated in A (series 1), the preparation was allowed to rest for 10 min, and the process was repeated (series 2 and 3). After series 3 there was a 1-h rest period and B65 was stimulated again to test for recovery (B1, top) (n = 6). B2 plots the mean number of complete cycles in the first 5 attempts. Note the reduction in the number of complete cycles in series 3. Only significant differences between means are indicated. *P < 0.05; **P < 0.01.
To develop a paradigm that could be used in subsequent experiments, we generated three series of 10 attempts to trigger motor activity, with an intervening 10-min rest between series 1 and 2 and between series 2 and 3 (Fig. 2B1, top). We then waited 1 h and triggered a final series to test for recovery. Cumulative effects were reliably observed from the first to the third series (Fig. 2B1, bottom). To quantify these effects, we measured the number of complete cycles in the first five trials (where effects appeared to be most pronounced) and found that there were differences (Fig. 2B2) (1-way repeated-measures ANOVA with Tukey’s post hoc comparisons; F1.7,8.3 = 18.73; P = 0.0011; n = 6). The number of complete cycles in series 3 was lower than the number in series 1 (control) (P = 0.015). After 60 min, recovery was observed (for the series 1 vs. recovery comparison P = 0.4630). These data indicate that there is a cumulative decrease in the occurrence of motor programs following B65 stimulation.
When programs are repeatedly triggered “upstream” of B65, i.e., with the posterior branch of the EN (Fig. 1B1), cycles of motor activity are complete (e.g., Refs. 16, 21). This suggests that when the EN is stimulated something is released that promotes motor program induction. Consistent with this idea, we found that when the EN was stimulated during the 10-min rest period between series 2 and 3 program induction during the first five trials was altered (Fig. 3A). Thus, as expected, there was a decrease in the number of cycles observed during series 2 (before EN stimulation) (Fig. 3B) (1-way repeated-measures ANOVA with Tukey’s post hoc comparisons; F1.82,9.1 = 6.412; P = 0.0199; n = 6; for the series 1 vs. series 2 comparison P = 0.0312). After EN stimulation the number of cycles increased and was not different from the number of cycles observed during series 1 (for the series 1 vs. series 3 comparison P = 0.1351). These data indicate that EN stimulation can impact B65’s ability to trigger motor programs. With EN stimulation, the cumulative decrease in motor programs elicited by repeated bouts of B65 stimulation was not observed.
Figure 3.

Stimulation of the posterior branch of the esophageal nerve (EN) enhances B65’s ability to trigger motor programs. A: B65 was stimulated as indicated in Fig. 2A (series 1), except that during the last 3 min of the second 10-min rest period the EN was stimulated so that it generated ∼3 motor programs just prior to B65 activation (n = 6). This modified B65’s ability to trigger complete motor programs during the first 5 attempts of series 3, as indicated in the group data in B. Only significant differences between means are indicated. *P < 0.05.
The fact that EN stimulation modified B65’s ability to trigger programs was not surprising in that both are part of the egestive input pathway to the feeding CPG (Fig. 1B1). An additional question we asked was, will program initiation be impacted by stimulation of CBI-2, a cell in the ingestive input pathway? To address this question, we triggered four series of motor programs using B65. As usual, when we measured the mean number of complete cycles in the first five trials there were cumulative effects, i.e., the number of complete cycles in series 2 was lower than the mean number in series 1 (Fig. 4) (1-way repeated-measures ANOVA with Tukey’s post hoc comparisons; F2.005,10.02 = 10.74; P = 0.0032; n = 6; for the series 1 vs. series 2 comparison P = 0.0004). CBI-2 stimulation in the rest period between series 2 and series 3 reversed cumulative effects, i.e., the number of complete cycles in series 3 was greater than the number in series 2 (P = 0.0122) and was not different from the number in series 1 (P = 0.713). This suggests that activation of the ingestive input neuron (CBI-2) modifies the ability of an egestive interneuron (B65) to trigger motor programs.
Figure 4.

A: cerebral buccal interneuron 2 (CBI-2) stimulation modifies B65’s ability to trigger motor programs. A: B65 was stimulated as indicated in Fig. 3A except that CBI-2 was stimulated just prior to B65 activation. CBI-2 was stimulated during the last 5 min of the rest period so that ∼5 cycles of motor activity were generated. In B, group data are plotted showing the effect of CBI-2 stimulation on the mean number of complete cycles during the first 5 attempts (n = 6). Note that the CBI-2 stimulation was effective, e.g., there were more complete cycles in series 3 (after CBI-2 stimulation). Only significant differences between means are indicated. *P < 0.05; ***P < 0.001.
To determine whether the opposite is also true, i.e., whether an egestive neuron can modify the ability of an ingestive input to trigger motor activity, we took advantage of the fact that there is a protraction interneuron in the ingestive pathway, B34, that is like B65 in that it does not reliably trigger motor programs (Fig. 1B2) (31). When a series of attempts was made to trigger complete cycles with B34, we found that it was unlike B65 in that there was no clear progressive change in program induction. The probability of triggering a program was seldom >50% even for the first cycle (e.g., Fig. 5, B1 and C1). We initially sought to determine whether CBI-2 (also in the ingestive input pathway) would impact B34-induced activity. We found that interleaving B34 and CBI-2 stimulation (Fig. 5A) produced a significant increase in the mean number of complete cycles [Fig. 5B; 1-way repeated-measures ANOVA with Tukey’s post hoc comparisons; F1.19,4.76 = 34.29; P = 0.0021; n = 5; for the series 1 (control) vs. series 2 (CBI-2 stimulation) comparison P = 0.0004]. To test for an effect of an egestive input, we then did similar experiments but interleaved B34 and EN stimulation (Fig. 5A). EN stimulation also produced a significant increase in the mean number of complete cycles (Fig. 5C) [1-way repeated-measures ANOVA with Tukey’s post hoc comparisons; F1.799,8.997 = 51.79; P < 0.0001; n = 6; for the series 1 (control) vs. series 2 (EN stimulation) comparison P = 0.0017].
Figure 5.

A: paradigm used to determine the effect of input activation on B34-induced activity. A series of 10 cycles of activity were induced by stimulating B34 at 10 Hz with a 30-s interstimulus interval (ISI) (series 1). After a 10-min rest period, input activation and B34 stimulation were interleaved (series 2). Inputs were stimulated so that a single cycle of activity was triggered. After a second 10-min rest period, B34 was again stimulated (series 3). B: cerebral buccal interneuron 2 (CBI-2) stimulation impacts B34-induced activity (n = 5). Group data show that the probability that a complete cycle will be triggered is increased (B1 and B2). Sample recordings from series 2 are shown in the inset. Bn2, buccal nerve 2; I2N, I2 nerve. C: stimulation of the posterior branch of the esophageal nerve (EN) also impacts B34-induced activity (n = 6). Group data show that the probability that a complete cycle will be triggered is increased (C1) and that there is an increase in the mean number of complete cycles triggered (C2). Sample recordings from series 2 are shown in the inset. Only significant differences between means are indicated. *P < 0.05; **P < 0.01; ***P < 0.001.
The fact that an egestive CPG input can modify the ability of an ingestive interneuron to induce motor activity (and vice versa) suggests that input activation modifies the properties of a neuron within the CPG that is active during all types of motor programs. As discussed above, a neuron of particular interest was B63 (Fig. 1B). Previous work had established that B63 can be essential for program generation under other circumstances (e.g., Refs. 31, 35, 38). We found that B63 is also essential for generating a complete cycle of activity when programs are triggered by B65 (Fig. 6A). In these experiments we attempted to trigger a cycle of motor activity every 5 min. This is a modification of a protocol developed in an earlier study (28) that prevents the decrement from occurring. Programs were triggered under control conditions (before B63 hyperpolarization), with bilateral hyperpolarization of B63, and after B63 hyperpolarization (Fig. 6A). In all preparations tested B63 hyperpolarization resulted in an incomplete motor program, i.e., there was no retraction phase (n = 3).
Figure 6.

A: impact of bilateral hyperpolarization of B63 on a B65-induced motor program (n = 3). Programs were triggered by stimulating B65 for 30 s every 4 min so that decrement would not occur. The gray bars indicate when B63 was hyperpolarized. Note that when B63 was hyperpolarized the cycle of activity was incomplete, i.e., there was no retraction. B: changes in the activity of protraction interneurons when programs are induced via repeated stimulation of B65 (n = 9). B65 was stimulated as indicated in Fig. 2A so that 10 attempts were made to trigger motor programs. Recordings during a typical experiment are shown in B1. With repeated stimulation there was a decrease in the probability that a complete cycle would be generated (B2, top) and a progressive decrease in the firing frequency of B63, an essential protraction interneuron (B2, bottom). Bn2, buccal nerve 2; c-B63, contralateral B63; I2N, I2 nerve.
To determine whether there are decrements in B63 activity when programs are triggered by repeated activation of B65, we attempted to repeatedly trigger activity using the protocol developed in earlier experiments (e.g., Fig. 2A1). We found that there was a progressive decrease in the B63 firing frequency that occurred in parallel with the progressive decrease in the probability that a complete cycle would be generated (Fig. 6B). Taken together, these data show that as program initiation fails the firing frequency of B63, an essential protraction interneuron, decreases to the point where it is virtually inactive.
Since input activation restores motor activity, an obvious hypothesis would be that it does so by exerting effects on B63. To test this idea, we conducted experiments in which we attempted to trigger a series of 10 cycles of activity with B65 and then after firing frequency decrements were observed we immediately stimulated either the EN (Fig. 7A1) or CBI-2 (Fig. 7B1). Immediately after input activation we then reattempted to trigger a cycle of activity with B65. We compared the B63 firing frequency after input stimulation to both the 1st cycle before input stimulation (when there was no decrement) and the 10th cycle before input stimulation (after decrement had occurred). There were significant changes in B63 activity with both EN (Fig. 7A) and CBI-2 (Fig. 7B) stimulation (1-way repeated-measures ANOVAs with Tukey’s post hoc comparisons; for EN F1.624,6.497 = 16.86, P = 0.0033, n = 5; for the 1st cycle vs. the 10th cycle comparison P = 0.0345, for the 10th cycle vs. Post comparison P = 0.0177, and for the 1st cycle vs. Post comparison P = 0.4192; for CBI-2 F1.384,64.152 = 35.78, P = 0.0029, n = 4; for the 1st cycle vs. the 10th cycle comparison P = 0.0030, for the 10th cycle vs. Post comparison P = 0.0155, and for the 1st cycle vs. Post comparison P = 0.6549). These data indicate that when B65 is used to trigger motor programs input activation that restores motor activity also produces parallel changes in B63 activity.
Figure 7.
Input activation that modifies B65-induced activity changes the B63 firing frequency. A: impact of esophageal nerve (EN) stimulation on programs induced by stimulating B65 10 times with the paradigm described in Fig. 2A1. After the 10th attempt to trigger motor activity the posterior branch of the EN was stimulated for 1 min. An 11th cycle was then induced to determine the impact of input stimulation (Post). Group data are plotted in A2 (n = 5). Note that the B63 firing frequency decreased during repeated cycle induction and then recovered after EN stimulation. B: impact of cerebral buccal interneuron 2 (CBI-2) stimulation on programs induced by stimulating B65. A typical experiment is shown in B1, and group data are plotted in B2 (n = 4). Note that CBI-2 stimulation also impacts program generation and produces an increase in the B63 firing frequency. Bn2, buccal nerve 2; I2N, I2 nerve. *P < 0.05; **P < 0.01.
To determine whether the same is true when cycles of activity are triggered with B34 we conducted a series of experiments using a similar protocol. However, with B34 stimulation the cycle after input stimulation was compared to either a “complete” control cycle or a “failed” cycle (Fig. 8, A1 and B1). Complete and failed programs were not necessarily the 1st and 10th cycles because with B34 there is no progressive, predictable change in program induction (e.g., Fig. 5B1). With both EN and CBI-2 stimulation there were significant changes in B63 activity (Fig. 8) (1-way repeated-measures ANOVAs with Tukey’s post hoc comparisons; for EN F1.134,3.401 = 75.75, P = 0.0018, n = 3; for the 1st cycle vs. the 10th cycle comparison P = 0.0044, for the 10th cycle vs. Post comparison P = 0.0068, and for the 1st cycle vs. Post comparison P = 0.0646; for CBI-2 F1.358,2.715 = 53.42, P = 0.007, n = 3; for the 1st cycle vs. the 10th cycle comparison P = 0.0149, for the 10th cycle vs. Post comparison P = 0.0389, and for the 1st cycle vs. Post comparison P = 0.8597). These data indicate that when B34 is used to trigger motor programs input activation that restores motor activity also impacts B63 activity. Taken together, these data indicate that there is a site of convergence in the feeding CPG in that both EN and CBI-2 stimulation alter B63 activity to impact program induction.
Figure 8.
Input activation that modifies B34-induced activity changes the B63 firing frequency. A: impact of input activation on programs induced by stimulating B34 10 times with the paradigm described in Fig. 5A. After the 10th attempt to trigger motor activity the esophageal nerve (EN) was stimulated for 1 min. An 11th cycle was then induced to determine the impact of input stimulation (Post). Group data are plotted in A2 (n = 3). Note that the B63 firing frequency decreased during repeated cycle induction and then recovered after stimulation of the posterior branch of the EN. B: impact of cerebral buccal interneuron 2 (CBI-2) stimulation on programs induced by stimulating B34. A typical experiment is shown in B1, and group data are plotted in B2 (n = 3). Note that the B63 did not fire when B34 failed to trigger a complete program but was active after CBI-2 stimulation. Bn2, buccal nerve 2; I2N, I2 nerve. *P < 0.05; **P < 0.01.
EN and CBI-2 Stimulation Both Increase B63 Excitability
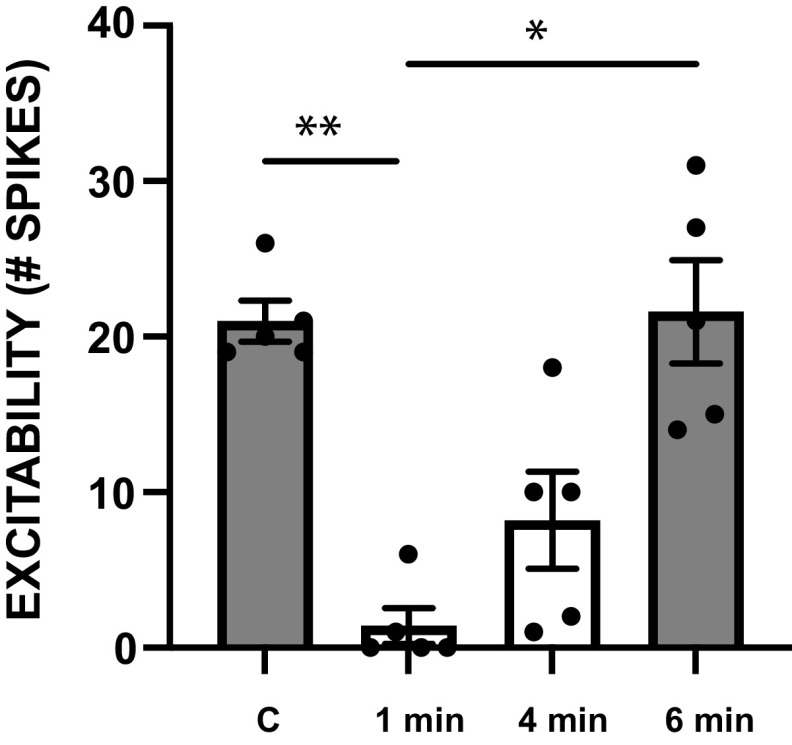
A further question is, how does input activation modify B63 activity? A hypothesis we tested is that it impacts excitability. Thus, we postulated that when program initiation fails the B63 firing frequency decreases, at least in part because of an excitability decrease. Input activation “counteracts” this effect by increasing excitability. As a first test of this hypothesis, we measured the B63 excitability under control conditions, 1 min after repeated stimulation of B65, and then at subsequent time points to test for recovery (Fig. 9). We did in fact observe an excitability decrease (1-way repeated-measures ANOVAs with Tukey’s post hoc comparisons; F1.828,7.313 = 13.27, P = 0.0041, n = 5; for control vs. 1 min post P = 0.0026, for control vs. 4 min post P = 0.0814, for control vs. 6 min post P = 0.9982, for 1 min post vs. 4 min post P = 0.2967, for 1 min post vs. 6 min post P = 0.0237, and for 4 min post vs. 6 min post P = 0.2179). Repeated B65 stimulation does, therefore, decrease B63 excitability.
Figure 9.
Effect of repeated stimulation of B65 on B63 excitability. B63 excitability was measured by injecting a 3-s constant-current pulse every 30 s under control conditions and then after stimulation of B65 so that 5 cycles of motor activity were induced. Excitability was measured until it returned to control levels (recovery). Group data are plotted under control conditions (C) and at 1, 4, and 6 min after B65 stimulation (n = 5). Note that B65 stimulation reduced B63 excitability. Only significant differences between means are indicated. *P < 0.05; **P < 0.01.
To determine whether input activation has the opposite effect (i.e., increases B63 excitability), we measured it under control conditions and after stimulating either the EN (Fig. 10) or CBI-2 (Fig. 11). Stimulation of both inputs increased excitability (1-way repeated-measures ANOVAs with Tukey’s post hoc comparisons; for EN stimulation F2,15 = 34.62, P < 0.0001, n = 6; for control vs. EN P < 0.0001, for control vs. recovery P = 0.6322, and for EN vs. recovery P < 0.0001; for CBI-2 stimulation F2,12 = 136.8, P < 0.001, n = 6; for control vs. post CBI-2 P < 0.0001, for post CBI-2 vs. recovery P < 0.0001, and for control vs. recovery P = 0.8779). Thus, EN and CBI-2 stimulation both increase B63 excitability.
Figure 10.

Effect of stimulation of the posterior branch of the esophageal nerve (EN) on the B63 excitability and occlusion experiments conducted in the presence of small cardioactive peptide (SCP). Excitability was measured by injecting constant-current pulses into B63 so that ∼10 spikes were triggered. Effects of EN stimulation were initially determined in normal artificial seawater (ASW) (A1, B1, C, left). EN stimulation produced an excitability increase (B1, C, left). Subsequently, effects of EN stimulation were determined in 1 µM SCP with the protocol shown in A2. SCP increased excitability (B2, C, center). Consequently, the size of the current pulse was adjusted so that ∼10 spikes were triggered. EN stimulation had no further effect on excitability (B2, C, center). After occlusion experiments, preparations were retested to verify that EN stimulation still increased excitability (C, right) (n = 6). Only significant differences between means are indicated. ****P < 0.0001.
Figure 11.

Effect of cerebral buccal interneuron 2 (CBI-2) stimulation on the B63 excitability and occlusion experiments conducted in the presence of feeding circuit activating peptide (FCAP)/cerebral peptide 2 (CP-2). Excitability was measured by injecting constant-current pulses into B63 so that ∼10 spikes were triggered. Effects of CBI-2 stimulation were initially determined in normal artificial seawater (ASW) (A1, B1, C, left). CBI-2 stimulation produced an excitability increase (B1, C, left). Subsequently, effects of CBI-2 stimulation were determined in 1 µM FCAP/CP-2 with the protocol shown in A2. Peptides increased excitability (B2, C, center). Consequently, the size of the current pulse was adjusted so that ∼10 spikes were triggered. CBI-2 stimulation had no further effect (B2, C, center). After occlusion experiments, preparations were retested to verify that CBI-2 stimulation was still effective (C, right) (n = 6). Only significant differences between means are indicated. ***P < 0.001; ****P < 0.0001.
Effects of Input Stimulation on B63 Excitability Are Peptide Mediated
Both inputs stimulated in these experiments contain modulatory neuropeptides. In particular, when the EN is stimulated the small cardioactive peptides (SCPs) are released (Fig. 1B1) (18), and when CBI-2 is stimulated feeding circuit activating peptide (FCAP) and cerebral peptide 2 (CP-2) are released (Fig. 1B2) (17). To determine whether exogenous application of these peptides impacts B63 excitability, we measured it in a high-divalent solution before peptide application, after 5 min in 10−6 M peptide, and after peptide washout. Excitability was increased by both SCP (Fig. 10) and a mixture of FCAP and CP-2 (FCAP/CP-2, Fig. 11) (1-way repeated-measures ANOVAs with Tukey’s post hoc comparisons; for SCP F1.035,10.35 = 77.65, P < 0.0001, n = 11; for control vs. SCP P < 0.001, for SCP vs. recovery P < 0.001, and for control vs. recovery P = 0.7422; for FCAP/CP-2 F1.382,19.35 = 134.5, P < 0.001, n = 15; for control vs. FCAP/CP-2 P < 0.001, for FCAP/CP-2 vs. recovery P < 0.001, and for control vs. recovery P = 0.8860). In a separate set of experiments we tested effects of peptides on resting membrane potential and did not observe a significant effect (for SCP 1-way repeated-measures ANOVA, F1.313,5.254 = 0.2783, P = 0.6803, n = 5; for FCAP/CP-2 1-way repeated-measures ANOVA, F1.105,4.420, P = 0.7106, n = 50). These data indicate that exogenous peptide application mimics and might mediate effects of input activation on B63 excitability.
To confirm this, we conducted a set of occlusion experiments in which we measured EN-induced excitability changes in B63 in the presence of 1 µM SCP (Fig. 10). Excitability was measured by injecting constant-current pulses that induced ∼10 spikes. As expected, peptide application increased excitability (1-way repeated-measures ANOVAs with Tukey’s post hoc comparisons; F3,20 = 67.24, n = 6; for the control vs. SCP comparison P < 0.001). The size of the current pulse was therefore reduced to decrease the number of spikes triggered to control levels and prevent a ceiling effect [for the SCP vs. SCP minus current (SCP/−I) comparison P < 0.001 and for the control vs. SCP/−I comparison P = 0.9937). In the presence of peptide, EN stimulation did not produce an excitability increase (for the SCP/−I vs. SCP/−I/post EN comparison P = 0.9926 and for the control vs. SCP/−I/post EN comparison P = 0.9498). These data indicate that SCP plays a major role in mediating effects of EN stimulation on B63.
In other occlusion experiments, we followed a similar protocol and measured B63 excitability after CBI-2 stimulation under control conditions, in the presence of 1 µM FCAP/CP-2, and after peptide washout (recovery) (Fig. 11). CBI-2 stimulation increased excitability under control conditions (1-way repeated-measures ANOVAs with Tukey’s post hoc comparisons; F3,20 = 61.4, n = 6; for the control vs. FCAP-CP-2 comparison P < 0.001. The size of the current pulse was reduced to decrease the number of spikes triggered [for the FCAP/CP-2 vs. FCAP/CP-2 minus current (FCAP/CP-2/−I) comparison P < 0.001 and for the control vs. FCAP/CP-2/−I comparison P = 0.9349). In the presence of peptide, CBI-2 stimulation did not produce a further excitability increase (for the FCAP/CP-2/−I vs. FCAP/CP-2/−I/post CBI-2 comparison P = 0.9812 and for the control vs. FCAP/CP-2/−I/post CBI-2 comparison P = 0.7708). These data indicate that FCAP/CP-2 plays a major role in mediating effects of CBI-2 stimulation on B63.
Protraction Duration Is Decreased during a Switch from Egestive to Ingestive Activity
Our results indicate that after a bout of feeding behavior the B63 excitability will be relatively high. This will be true regardless of whether activity was ingestive or egestive. Previous work has established that a form of priming occurs when one type of feeding behavior is repeated (39). Results of the present study have potential implications for what may occur during task switching, e.g., when an ingestive response is triggered after a bout of egestive activity. In this situation the B63 excitability will be increased during the initial egestive activity and B63 will fire at a higher frequency during the subsequent ingestive response. Previous work suggested that an increase in B63 activity would decrease protraction duration (35). To determine whether a modification of protraction is more apt to impact ingestive or egestive activity, we measured protraction duration during CBI-2- and EN-induced programs. We found that protraction duration was significantly longer when programs were triggered by CBI-2 (Fig. 12A). On average, it was 36.1 ± 8.1 s when programs were triggered by CBI-2 and it was 3.7 ± 0.8 s when programs were triggered by the EN (unpaired t test; n = 7 for CBI-2-induced programs and n = 5 for EN-induced programs; P = 0.0079). These data suggest that a modification that decreases protraction duration is most likely to impact ingestive activity.
Figure 12.

A: protraction duration during cerebral buccal interneuron 2 (CBI-2)- and esophageal nerve (EN)-induced cycles of activity triggered in a previously quiescent preparation (n = 7 for CBI-2; n = 5 for EN). Note that protraction duration is longer when programs are triggered by CBI-2. B: protraction duration is decreased when there is a switch from egestive to ingestive activity. Protraction duration was measured when cycles of motor activity were triggered by stimulating CBI-2 alone (no switch, black) and when CBI-2 induced activity was triggered after repeated stimulation of the EN (i.e., after egestive/ingestive switch, red). Group data are plotted in B1 (n = 7). In B2, individual values are shown for the first 2 cycles. The lines connect matched data points. Note that in all cases protraction duration was shorter after the switch. The inset above B2 is sample data. *P < 0.05; **P < 0.01.
To determine whether a switch from egestive to ingestive activity does impact protraction duration, we measured the mean protraction duration when a series of motor programs was triggered by CBI-2 under control conditions (no prior egestive activity) and after repeated stimulation of the EN. We found that there was a decrease in protraction duration during the first two cycles of CBI-2-induced activity following EN stimulation (Fig. 12B1). After that, effects of prior EN stimulation apparently wore off. Individual values for cycle 1 and 2 data are plotted in Fig. 12B2, which shows that protraction duration was shorter after the task switch in all preparations tested (paired t tests; n = 7; P = 0.0187 for the cycle 1 comparison and P = 0.0071 for the cycle 2 comparison).
DISCUSSION
Many CPGs are capable of generating multiple functionally distinct outputs (40, 41). Which output is generated at a given time is determined by multiple factors, one being the nature of the pathway that triggers activity (40). For example, projection or sensory neurons can trigger distinct motor programs. Additionally, multifunctional networks are often subject to neuromodulation (6). Modulators, such as neuropeptides, exert “divergent” and “convergent” effects on central pattern generating networks to determine motor output (e.g., Refs. 42–44).
The terms “divergent” and “convergent” can be used to refer to a cellular effect (2 or more modulators impact the same neuron) or a molecular effect (2 or more modulators induce the same current or synaptic modification). A number of studies that have sought to determine how output is specified have focused on identifying sites where there is at least one type of divergence. This work has demonstrated that two modulators can act on the same current but can target distinct sets of neurons to produce a unique network output (e.g., Ref. 45). Alternatively, two modulators can act on the same neuron but can specify network output by virtue of the fact that they each produce a different molecular modification [e.g., induce a different current (22)].
Cellular and molecular convergence has also been demonstrated, and interestingly it does not preclude motor program specification. For example, in the stomatogastric nervous system a situation has been described in which two peptides activate the same current in the same neuron (46). One neuropeptide is released as a neurotransmitter, the other neuropeptide as a hormone. Release of the neurotransmitter is impacted by presynaptic inhibition. Consequently, there are phasic dynamics in the maximal conductance it induces. In contrast, the hormone retains the same maximal conductance in both phases of the motor program. As a result, the two modulators produce distinct effects on the network (46).
Our results differ in that the convergence that we demonstrate results in a similar network effect. Comparable motor activity triggered by different modulatory inputs has been described in other systems (e.g., Refs. 47–49). For example, the respiratory network is modulated by multiple substances that activate the same second messenger pathway and exert similar effects on respiratory frequency (49). In this situation, blocking the effect of each modulator produces a frequency decrease. This suggests that effects of modulators are cumulative and a single modulator is not sufficient to maximally activate a given second messenger system. It is not clear, however, why control is mediated via the release of multiple substances.
In this report, we demonstrate convergence in that we show that the excitability of an interneuron in the Aplysia feeding network (the cell B63) is increased by both FCAP/CP-2 and SCP. FCAP/CP-2 and SCP are peptides that are present in two different inputs to the feeding CPG. FCAP and CP-2 are coreleased from the commandlike neuron CBI-2, which is an ingestive CPG input (17). SCP is released from afferents in the esophageal nerve (EN), which is an egestive CPG input (Fig. 1B) (18). Thus, we show that “ingestive” and “egestive” peptides both have the same cellular effect: they increase the excitability of B63.
Further experiments will be needed to determine whether convergence at the molecular (or at least current) level is also observed. Elsewhere in the feeding circuit FCAP/CP-2 exert cAMP-mediated effects and induce an inward current that is presumably directly gated by cAMP itself (22, 50). Possibly this current is present in B63 and can be induced by SCP (as well as FCAP/CP-2). Consistent with this idea, cAMP-mediated effects of SCP have been reported in other neurons and muscles in Aplysia (51, 52) and in other species (53–55). Thus, it is possible that FCAP/CP-2 and SCP induce the same inward current.
The functional consequences of the cellular convergence that we observe are likely to be influenced by how activity of the feeding CPG is induced. Under physiological conditions feeding responses can be triggered by stimulus (food) presentation. In intact head preparations, food contact excites cerebral buccal interneurons (e.g., CBI-2), and these neurons fire at a frequency that triggers motor programs (12, 14). Thus, when food is present motor programs are presumably triggered by input to the CPG from the cerebral ganglion. Additionally, animals make exploratory bites when there is no food contact (56). How this activity is triggered is not entirely clear, but it is likely that it is generated within the buccal CPG itself. Cerebral buccal interneurons are not likely to be activated under these conditions, and in vitro experiments have demonstrated that spontaneous, low-frequency CPG activity can be recorded in the isolated buccal ganglion (e.g., Refs. 57–61).
Experiments that have sought to determine how activity is initiated within the CPG have demonstrated that B63 plays a pivotal role (57, 58, 60, 61). Thus, spontaneous, endogenously generated, subthreshold depolarizations are recorded from B63 in otherwise quiescent preparations. Although most of these depolarizations are low amplitude, at irregular intervals plateau potentials are triggered and full-blown motor programs are recorded in the buccal ganglion. Although B63-induced spontaneous programs are infrequent under “control” conditions, there are long-term increases in their frequency and regularity after operant conditioning (57–60).
In this report we describe manipulations that can produce an increase in the excitability of B63. Presumably these manipulations would modify “spontaneous” program induction. For example, if the B63 excitability is increased via the induction of an inward current, current induction is likely to increase the likelihood that intrinsically generated subthreshold depolarizations will trigger plateau depolarizations. This in turn is likely to increase the number of full-blown motor programs. Our data suggest that the excitability of B63 is likely to be elevated after bouts of activity explicitly triggered by stimulus presentation. One consequence of this circuit modification is that it may increase the likelihood that stimulus-driven activity will be followed by a period of semiautonomous CPG activity. Our data suggest that this would be the case after a series of either ingestive or egestive responses.
In the situation where feeding is explicitly linked to stimulus presentation, we demonstrate that the convergence that we identify is likely to lead to a form of arousal that is manifested as an impact on feeding rate. Feeding rate is obviously determined by both the amount of time each response takes and the interresponse interval. Our data show an effect of prior activity on response duration. More specifically, we demonstrate a decrease in the longer of the two phases of the motor program, radula protraction.
The decrease in radula protraction that we observe presumably results from the increase in B63 excitability that we demonstrate is a consequence of modulator release. Since the B63 excitability is increased by both ingestive and egestive peptides, decreases in radula protraction are observed during task switching, namely when there is a switch from egestive to ingestive activity. Previous research has demonstrated that egestive/ingestive switching in Aplysia occurs when animals have difficulty ingesting a piece of seaweed during a feeding bout. For example, in a laboratory setting, switching is observed when animals are given strips of seaweed that are attached to a substrate (62). “Clearance” reflexes are also triggered in mollusks when the esophagus is stretched (63). These reflexes ensure the egestion of objects that are too large to swallow. In either type of context, a rapid return to a functional ingestive behavior is likely to be advantageous. A quick return to ingestion would mean a shorter behavioral interruption and more time spent taking food in.
In conclusion, in this report we study two different inputs to a feeding central pattern generator. The two inputs trigger fundamentally different types of motor activity, i.e., ingestive versus egestive motor programs. Previous research has demonstrated that the inputs exert divergent effects on the CPG. We now show that, additionally, inputs produce convergent effects, i.e., both increase the excitability of a key interneuron. We ask why this might occur given the fact that it does not play a role in specifying motor activity. We show that at least in part it may facilitate relatively rapid task switching.
GRANTS
This research was supported by the National Institutes of Health (Grants NS066587 and NS118606) and the National Natural Science Foundation of China (Grants 32171011, 31861143036, 31671097, 31371104).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R.D., J.J., K.R.W., and E.C.C. conceived and designed research; M.R.D. and Y.W. performed experiments; M.R.D. and Y. W. analyzed data; M.R.D., Y.W., M.A.B., J.J., K.R.W., and E.C.C. interpreted results of experiments; Y.W. and E.C.C. prepared figures; M.R.D. and E.C.C. drafted manuscript; Y.W., M.A.B., J.J., K.R.W., and E.C.C. edited and revised manuscript; M.R.D., Y.W., M.A.B., J.J., C.N.R., K.R.W., and E.C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to acknowledge the late Michael R. Due.
REFERENCES
- 1.Croll RP, Davis WJ, Kovac MP. Neural mechanisms of motor program switching in the mollusc Pleurobranchaea. I. Central motor programs underlying ingestion, egestion, and the “neutral” rhythm(s). J Neurosci 5: 48–55, 1985. doi: 10.1523/JNEUROSCI.05-01-00048.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travers JB, DiNardo LA, Karimnamazi H. Medullary reticular formation activity during ingestion and rejection in the awake rat. Exp Brain Res 130: 78–92, 2000. doi: 10.1007/s002219900223. [DOI] [PubMed] [Google Scholar]
- 3.Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci 21: 7349–7362, 2001. doi: 10.1523/JNEUROSCI.21-18-07349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crossley M, Staras K, Kemenes G. A central control circuit for encoding perceived food value. Sci Adv 4: eaau9180, 2018. doi: 10.1126/sciadv.aau9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays 34: 458–465, 2012. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 6.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A 173: 519–536, 1993. doi: 10.1007/BF00197761. [DOI] [PubMed] [Google Scholar]
- 8.Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol A 172: 17–32, 1993. doi: 10.1007/BF00214712. [DOI] [PubMed] [Google Scholar]
- 9.Cropper EC, Jing J, Weiss KR. The feeding network of Aplysia; features that are distinctive and shared with other molluscs. In: The Oxford Handbook of Invertebrate Neurobiology, edited by Byrne JH. New York: Oxford University Press, 2019, p. 401–421. [Google Scholar]
- 10.Elliott CJ, Susswein AJ. Comparative neuroethology of feeding control in molluscs. J Exp Biol 205: 877–896, 2002. doi: 10.1242/jeb.205.7.877. [DOI] [PubMed] [Google Scholar]
- 11.Church PJ, Lloyd PE. Activity of multiple identified motor neurons recorded intracellularly during evoked feedinglike motor programs in Aplysia. J Neurophysiol 72: 1794–1809, 1994. doi: 10.1152/jn.1994.72.4.1794. [DOI] [PubMed] [Google Scholar]
- 12.Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci 11: 3630–3655, 1991. doi: 10.1523/JNEUROSCI.11-11-03630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez JA, Kirk MD. Cerebral-buccal pathways in Aplysia californica: synaptic connections, cooperative interneuronal effects and feedback during buccal motor programs. J Comp Physiol A 187: 801–815, 2001. doi: 10.1007/s00359-001-0251-0. [DOI] [PubMed] [Google Scholar]
- 14.Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol 15: 1712–1721, 2005. doi: 10.1016/j.cub.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 15.Chiel HJ, Weiss KR, Kupfermann I. An identified histaminergic neuron modulates feeding motor circuitry in Aplysia. J Neurosci 6: 2427–2450, 1986. doi: 10.1523/JNEUROSCI.06-08-02427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci USA 101: 9447–9452, 2004. doi: 10.1073/pnas.0402002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J Neurophysiol 90: 2074–2079, 2003. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- 18.Wu JS, Vilim FS, Hatcher NG, Due MR, Sweedler JV, Weiss KR, Jing J. Composite modulatory feedforward loop contributes to the establishment of a network state. J Neurophysiol 103: 2174–2184, 2010. doi: 10.1152/jn.01054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci 27: 3490–3502, 2007. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilim FS, Sasaki K, Rybak J, Alexeeva V, Cropper EC, Jing J, Orekhova IV, Brezina V, Price D, Romanova EV, Rubakhin SS, Hatcher N, Sweedler JV, Weiss KR. Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors. J Neurosci 30: 131–147, 2010. doi: 10.1523/JNEUROSCI.3282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman AK, Weiss KR, Cropper EC. Specificity of repetition priming: the role of chemical coding. J Neurosci 35: 6326–6334, 2015. doi: 10.1523/JNEUROSCI.4562-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkins MH, Cropper EC, Weiss KR. Cellular effects of repetition priming in the Aplysia feeding network are suppressed during a task-switch but persist and facilitate a return to the primed state. J Neurosci 38: 6475–6490, 2018. doi: 10.1523/JNEUROSCI.0547-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siniscalchi MJ, Cropper EC, Jing J, Weiss KR. Repetition priming of motor activity mediated by a central pattern generator: the importance of extrinsic vs. intrinsic program initiators. J Neurophysiol 116: 1821–1830, 2016. doi: 10.1152/jn.00365.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurwitz I, Goldstein RS, Susswein AJ. Compartmentalization of pattern-initiation and motor functions in the B31 and B32 neurons of the buccal ganglia of Aplysia californica. J Neurophysiol 71: 1514–1527, 1994. doi: 10.1152/jn.1994.71.4.1514. [DOI] [PubMed] [Google Scholar]
- 25.Hurwitz I, Susswein AJ. B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol 75: 1327–1344, 1996. doi: 10.1152/jn.1996.75.4.1327. [DOI] [PubMed] [Google Scholar]
- 26.Jing J, Weiss KR. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J Neurosci 22: 6228–6238, 2002. doi: 10.1523/JNEUROSCI.22-14-06228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing J, Cropper EC, Hurwitz I, Weiss KR. The construction of movement with behavior-specific and behavior-independent modules. J Neurosci 24: 6315–6325, 2004. doi: 10.1523/JNEUROSCI.0965-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabotyanski EA, Baxter DA, Byrne JH. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J Neurophysiol 79: 605–621, 1998. doi: 10.1152/jn.1998.79.2.605. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz I, Neustadter D, Morton DW, Chiel HJ, Susswein AJ. Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J Neurophysiol 75: 1309–1326, 1996. doi: 10.1152/jn.1996.75.4.1309. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki K, Jing J, Due MR, Weiss KR. An input-representing interneuron regulates spike timing and thereby phase switching in a motor network. J Neurosci 28: 1916–1928, 2008. doi: 10.1523/JNEUROSCI.4755-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurwitz I, Kupfermann I, Susswein AJ. Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica. J Neurophysiol 78: 1305–1319, 1997. doi: 10.1152/jn.1997.78.3.1305. [DOI] [PubMed] [Google Scholar]
- 32.Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the Aplysia feeding network. J Neurophysiol 97: 3046–3056, 2007. doi: 10.1152/jn.01301.2006. [DOI] [PubMed] [Google Scholar]
- 33.Due MR, Jing J, Weiss KR. Dopaminergic contributions to modulatory functions of a dual-transmitter interneuron in Aplysia. Neurosci Lett 358: 53–57, 2004. doi: 10.1016/j.neulet.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 34.Zhang G, Yu K, Wang T, Chen TT, Yuan WD, Yang F, Le ZW, Guo SQ, Xue YY, Chen SA, Yang Z, Liu F, Cropper EC, Weiss KR, Jing J. Synaptic mechanisms for motor variability in a feedforward network. Sci Adv 6: eaba4856, 2020. doi: 10.1126/sciadv.aba4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurwitz I, Kupfermann I, Weiss KR. Fast synaptic connections from CBIs to pattern-generating neurons in Aplysia: initiation and modification of motor programs. J Neurophysiol 89: 2120–2136, 2003. doi: 10.1152/jn.00497.2002. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez JA, Kirk MD. Short-term synaptic enhancement modulates ingestion motor programs of Aplysia. J Neurosci 20: RC85, 2000. doi: 10.1523/JNEUROSCI.20-14-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jing J, Vilim FS, Wu JS, Park JH, Weiss KR. Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J Neurosci 23: 5283–5294, 2003. doi: 10.1523/JNEUROSCI.23-12-05283.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dembrow NC, Jing J, Brezina V, Weiss KR. A specific synaptic pathway activates a conditional plateau potential underlying protraction phase in the Aplysia feeding central pattern generator. J Neurosci 24: 5230–5238, 2004. doi: 10.1523/JNEUROSCI.5649-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cropper EC, Jing J, Perkins MH, Weiss KR. Use of the Aplysia feeding network to study repetition priming of an episodic behavior. J Neurophysiol 118: 1861–1870, 2017. doi: 10.1152/jn.00373.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briggman KL, Kristan WB. Multifunctional pattern-generating circuits. Annu Rev Neurosci 31: 271–294, 2008. doi: 10.1146/annurev.neuro.31.060407.125552. [DOI] [PubMed] [Google Scholar]
- 41.Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci 12: 185–204, 1989. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- 42.Nadim F, Bucher D. Neuromodulation of neurons and synapses. Curr Opin Neurobiol 29: 48–56, 2014. doi: 10.1016/j.conb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daur N, Nadim F, Bucher D. The complexity of small circuits: the stomatogastric nervous system. Curr Opin Neurobiol 41: 1–7, 2016. doi: 10.1016/j.conb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bucher D, Marder E. SnapShot: neuromodulation. Cell 155: 482–482 e481, 2013. doi: 10.1016/j.cell.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 45.Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci 21: 4050–4058, 2001. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kintos N, Nusbaum MP, Nadim F. Convergent neuromodulation onto a network neuron can have divergent effects at the network level. J Comput Neurosci 40: 113–135, 2016. doi: 10.1007/s10827-015-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saideman SR, Blitz DM, Nusbaum MP. Convergent motor patterns from divergent circuits. J Neurosci 27: 6664–6674, 2007. doi: 10.1523/JNEUROSCI.0315-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez JC, Blitz DM, Nusbaum MP. Convergent rhythm generation from divergent cellular mechanisms. J Neurosci 33: 18047–18064, 2013. doi: 10.1523/JNEUROSCI.3217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perkins MH, Weiss KR, Cropper EC. Persistent effects of cyclic adenosine monophosphate are directly responsible for maintaining a neural network state. Sci Rep 9: 9058, 2019. doi: 10.1038/s41598-019-45241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarrard HE, Goldsmith BA, Abrams TW. In Aplysia sensory neurons, the neuropeptide SCPB and serotonin differ in efficacy both in modulating cellular properties and in activating adenylyl cyclase: implications for mechanisms underlying presynaptic facilitation. Brain Res 616: 188–199, 1993. doi: 10.1016/0006-8993(93)90209-6. [DOI] [PubMed] [Google Scholar]
- 52.Fox LE, Lloyd PE. Mechanisms involved in persistent facilitation of neuromuscular synapses in Aplysia. J Neurophysiol 87: 2018–2030, 2002. doi: 10.1152/jn.00142.2001. [DOI] [PubMed] [Google Scholar]
- 53.Reich G, Doble KE, Price DA, Greenberg MJ. Effects of cardioactive peptides on myocardial cAMP levels in the snail Helix aspersa. Peptides 18: 355–360, 1997. doi: 10.1016/s0196-9781(96)00335-x. [DOI] [PubMed] [Google Scholar]
- 54.Yamane T, Gelperin A. Aminergic and peptidergic amplification of intracellular cyclic AMP levels in a molluscan neural network. Cell Mol Neurobiol 7: 291–301, 1987. doi: 10.1007/BF00711305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferretti ME, Sonetti D, Pareschi MC, Biondi C. Effects of the Small Cardioactive Peptide-B (Scpb) on adenylate-cyclase of the central-nervous-system and peripheral organs of the fresh-water snail Planorbarius-corneus. Neurochem Int 22: 479–486, 1993. doi: 10.1016/0197-0186(93)90043-5. [DOI] [PubMed] [Google Scholar]
- 56.Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol 10: 1–26, 1974. doi: 10.1016/S0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- 57.Nargeot R, Petrissans C, Simmers J. Behavioral and in vitro correlates of compulsive-like food seeking induced by operant conditioning in Aplysia. J Neurosci 27: 8059–8070, 2007. doi: 10.1523/JNEUROSCI.1950-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nargeot R, Le Bon-Jego M, Simmers J. Cellular and network mechanisms of operant learning-induced compulsive behavior in Aplysia. Curr Biol 19: 975–984, 2009. doi: 10.1016/j.cub.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 59.Nargeot R, Baxter DA, Byrne JH. Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J Neurosci 17: 8093–8105, 1997. doi: 10.1523/JNEUROSCI.17-21-08093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nargeot R, Simmers J. Functional organization and adaptability of a decision-making network in Aplysia. Front Neurosci 6: 113, 2012. doi: 10.3389/fnins.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bédécarrats A, Puygrenier L, Castro O’Byrne J, Lade Q, Simmers J, Nargeot R. Organelle calcium-derived voltage oscillations in pacemaker neurons drive the motor program for food-seeking behavior in Aplysia. eLife 10: e68651, 2021. doi: 10.7554/eLife.68651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Proekt A, Wong J, Zhurov Y, Kozlova N, Weiss KR, Brezina V. Predicting adaptive behavior in the environment from central nervous system dynamics. PLoS One 3: e3678, 2008. doi: 10.1371/journal.pone.0003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Croll RP, Davis WJ. Motor program switching in Pleurobranchaea. 2. Ingestion and egestion in the reduced preparation. J Comp Physiol 147: 143–154, 1982. doi: 10.1007/BF00609839. [DOI] [Google Scholar]