Abstract
Objective To evaluate postoperative pain, using the visual analog scale (VAS), in patients undergoing anterior cruciate ligament reconstruction (ACLR) and receiving intra-articular anesthetic solutions.
Methods The present is a randomized clinical trial with a sample of 48 patients divided into 4 groups: Group I (n = 12) – 20 mL of saline solution (control); Group II (n = 12) – 20 mL of 0.5% bupivacaine; Group III (n = 12) – 20 mL of 0.5% bupivacaine + 0.1 mg of epinephrine; and Group IV (n = 12) – 20 mL of saline solution + 0.1 mg of epinephrine. These solutions were injected into the knee at the end of the surgery. Pain was assessed using the VAS immediately and 6, 12, 24 and 48 hours after the procedure.
Results The VAS scores were highly variable among the groups. A Kruskal-Wallis analysis of variance (ANOVA), considering a level of significance of 5%, revealed that all intra-articular anesthetic solutions influenced the assessment of pain ( p = 0.003), and that Group-III subjects presented less postoperative pain. There was no evidence of a higher or lower use of supplemental analgesic agents, or of adverse effects resulting from these anesthetic solutions.
Conclusion Bupivacaine combined with epinephrine was the most effective solution for pain control in patients undergoing ACLR, but with no statistically significant differences when compared to Group II ( p = 0.547). There was no decrease or increase in the use of supplemental analgesics or in the occurrence of adverse systemic effects ( p > 0.05).
Keywords: anterior cruciate ligament, pain assessment, analgesia, drug therapy, arthroscopy
Introduction
Postoperative pain relief remains a major medical challenge. Despite the great advances in the understanding of the pathophysiology of acute pain and the development of new analgesic agents and administration techniques, the control of postoperative pain is still an issue in a significant number of patients. 1
Local intra-articular (IA) anesthetic agents are often used to prevent acute pain after arthroscopic knee surgery. However, the severity of this pain varies in each individual patient. In an effort to find the ideal method for an effective, long-lasting control of the postoperative pain, many different drugs, including opioids, non-steroidal anti-inflammatory drugs (NSAIDs), ketamine, clonidine, and neostigmine, have been added to IA anesthetic solutions, 2 3 with no consensus on which drugs should be used or on dose standardization.
Bupivacaine is often used in IA pain control because of its active period and effectiveness. Especially in a single administration, its effectiveness has been studied because its action on postoperative pain is conceptually simple. 4
The visual analog scale (VAS) is the most common score for the assessment of pain; this instrument evaluates a characteristic or attitude that is believed to vary through a continuous range, and cannot be measured in an easy and direct way. It is also useful to analyze the success of the treatment, to determine which procedures have better outcomes, and to define any therapeutic failure resulting in improvement or worsening of the pain. The amount of pain experienced by the patient ranges from none (0) to extreme pain (10), as described by Wewers and Lowe. 5
The primary objective of the present study was to assess, using the VAS, the postoperative acute pain in patients undergoing ACLR and receiving IA anesthetic solutions; a secondary objective was to determine which anesthetic solution was most effective for the control of the pain and when the pain was better controlled, as well as to observe the adverse effects and the need for supplemental pain-control measures.
Methods
The present is a randomized, triple-blinded clinical trial conducted in four groups of patients diagnosed with chronic knee instability and with an indication for surgical treatment. The sample size was estimated based on the total number of patients with this condition cared for at the institution, which was determined as 3.33% per month. Considering a level of confidence of 95% and a level of accuracy (margin of error) of 5%, the final sample consisted of 48 patients.
The sample included patients: with a diagnosis of chronic knee instability; older than 18 years of age; with physical status classified as I and II according to the score of the American Society of Anesthesiologists (ASA); with absence of local and systemic inflammatory diseases; with isolated injuries of the ACL (but no other ligamentous injury), with no history of fractures or previous surgery at the refgion of the knee; in whose procedures the medial flexor tendons were used as graft material; operated on by the main researcher and his team. The exclusion criteria were the following: skeletal immaturity; chondral lesions greater than 2 cm 2 ; additional surgeries, such as osteotomies and other ligament-related procedures; patients with clinical conditions, including hypertension or coagulopathies; pregnant subjects; chronic use of anticoagulant agents; use of analgesic agents up to 24 hours before the surgical procedure; refusal to sign the free and informed consent form (FICF); procedures not performed at our institution; non-compliance of the anesthesiologist in following the project protocol; patients who self-declared as members of indiginous populations.
A data collection instrument specific to this research was prepared, and patient- and surgery-related data were recorded, including name, weight, age and identification number, surgery date, operated side, experimental group (I , II, III, IV), ischemia time during surgery, and heart rate (HR) and blood pressure (BP) immediately after surgery (T0), 6 hours after surgery (T1), 12 hours after surgery (T2), 24 hours after surgery (T3), and 48 hours after surgery (T4). The possible adverse effects and the need for supplemental pain control were documented. A color-coded VAS was used at each experimental time interval. Associated injuries (both meniscal and chondral lesions) were noted, as well as the diameter of the bone tunnels in the femur and tibia for ligament reconstruction.
The present study was conducted according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines. 6
The patients were allocated per randomization result; authorization for hospitalization was requested so that they could be referred to surgery according to the availability of vacancies and the patient waiting list.
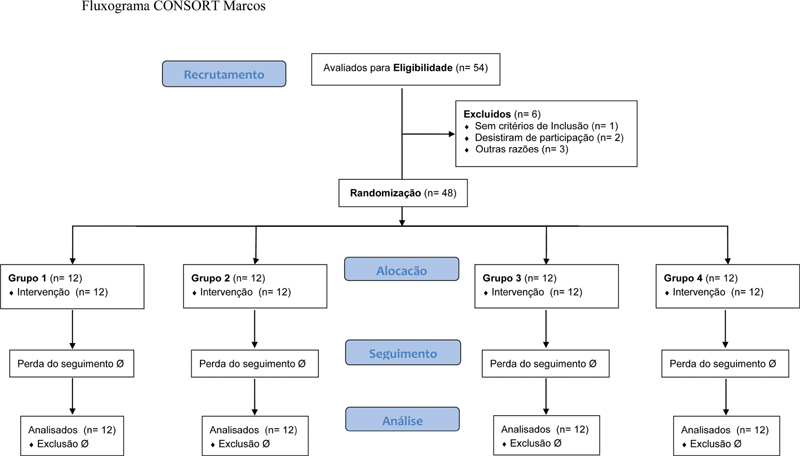
Using the free software available on www.randomization.com , 48 patients were randomly distributed in 12 blocks of 4 equal groups. Each group received an IA solution in the knee according to the following allocation ( Figure 1 ):
Fig. 1.
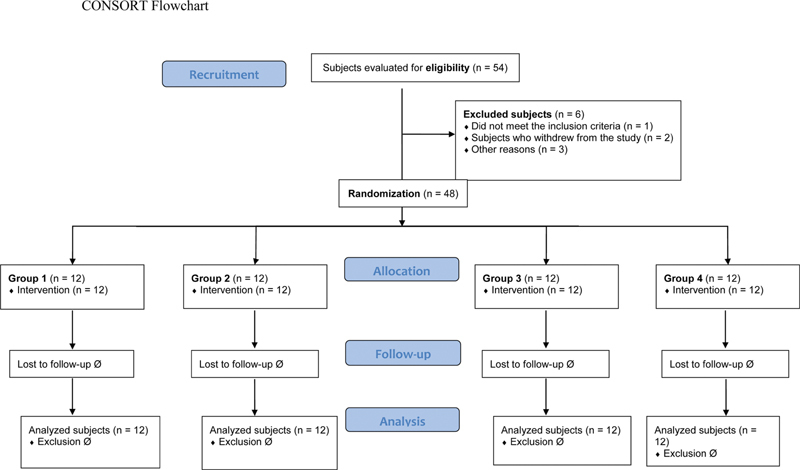
CONSORT flow chart.
Group I (n = 12): 20 mL of 0.9% saline solution (SS) (control);
Group II (n = 12): 20 mL of 0.5% bupivacaine;
Group III (n = 12): 20 mL of 0.5% bupivacaine + 0.1 mg of epinephrine; and
Group IV (n = 12): 20 mL of 0.9% SS + 0.1 mg of epinephrine.
Since capsular distention due to hemorrhage contributes to immediate postoperative pain, we theorized that a reduction in bleeding could decrease pain; this is why the patients in groups III and IV received epinephrine alone or associated with bupivacaine.
The solutions were prepared by an auxiliary member of the research team (an anesthesiology resident) who had no contact with the patient before or after surgery, about 20 minutes before the end of the surgery, following a direct communication from the researcher. The surgeon was not aware of the content of the injected solution.
The anesthesia consisted of a subarachnoid block with 0.5% hyperbaric bupivacaine (dose of 15 mg to 20 mg) and 20 mcg of fentanyl. Other multimodal analgesia modalities were not administered to maximize the control of pain-related variables during the postoperative period.
All surgeries were performed arthroscopically with an articular approach, using high anterolateral (AL) and anteromedial (AM) portals. Femoral and tibial tunnels were drilled in the anatomical position, at the center of the previous ACL footprint, via an AM transportal for the passage and subsequent fixation of the graft. The ACL was reconstructed using the ipsilateral knee medial flexors, that is, the semitendinosus (ST) and gracilis (G) muscles tendons. The grafts were collected through an incision over the pes anserinus at the proximal third of the leg, with approximately 3 cm in length, before the arthroscopy per se; meniscal injuries were treated with a partial or subtotal meniscectomy.
After the suture of the skin, the solution was injected into the knee through the AL portal according to group allocation. The pneumatic tourniquet was deflated after the sutures had been performed and a compressive dressing had been placed.
The severity of the pain was assessed using the VAS ( Figure 2 ); the painful stimulus was caused by knee flexion at 45°, which was repeated 3 times, and measured with a goniometer at T0, T1, T2, T3 and T4 by a doctor who was blinded to the allocation of the groups. The need for analgesic supplementation was noted in the data-collection instrument: intravenous (IV) NSAID (40 mg of tenoxicam, once a day) or opioids (50 mg pf tramadol every 4 hours, and 10 mg of morphine in a slow IV drip as required, up to every 4 hours).
Fig. 2.

Visual Analog Scale for pain.
During the hospitalization, pain control was performed with 1 g of IV dipyrone, which was systematically administered every 4 hours; thromboprophylaxis was achieved with 40 mg of subcutaneous sodium enoxaparin, once a day, for 10 days. In addition, cryotherapy was applied in the anterior region of the knee for 20 minutes every 4 hours. Early deambulation and movement were encouraged and performed according to individual tolerance, with the help of a physical therapist. The patients were discharged as allowed by the clinical conditions, usually 48 hours after the surgical procedure, when the last research data were collected, for this is part of the routine of the service.
The HR and BP were recorded after the painful stimulus. In addition, the systemic adverse effects of the solutions and medications, such as sweating, tremors, nausea and vomiting, hypotension, pruritus, urinary retention requiring tube placement, tachycardia or bradycardia, skin rash and headache, if any, were noted.
The absolute (n) and relative (%) frequency distribution of the numerical data (attributes or nominal data) and the descriptive statistics of the quantitative data (specific or variable data) were performed. The Chi-squared test or, if required, the Fisher exact test were used to compare nominal parameters. The data was analyzed descriptively, and the results were presented in contingency tables (attributes) or statistics (values) with frequency distribution, graphs, and descriptive measures. An analysis of variance (ANOVA) was performed to determine the influence of intra-articular analgesia among the groups. A significance level of 5% ( p = 0.05) was adopted for the decision-making regarding all relationships between the variables and the hypothesis tests.
The ANOVA technique was used in the present study. The Tukey test was used to compare the mean values. The Kruskal-Wallis test, a non-parametric method to determine whether a set of samples comes from the same distribution, was used as an extension of the Mann-Whitney test in more than two samples.
The data were tabulated using Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, US) spreadsheets, and analyzed using the Minitab (Minitab, LLC, State College, PA, US) statistical software , version 14.1.
The project was submitted to the institutional Ethics in Research Committee (under CAAE number 50651315.8.0000.0007), and received a consolidated opinion under number 1.774.789 on October 13, 2016. In addition, the study was approved on Clinical Trials under registration RBR – 7PCKQT. All patients who agreed to participate in the research signed an FICF.
Results
A total of 36 (75.0%) patients were male, and 12 (25.0%) were female. Their ages ranged from 18 to 54 years, with a mean standard deviation of 31 ± 10. The median age was 33 years old. Regarding laterality, 18 (37.5%) patients were operated on the left knee, and 30 (62.5%), on the right knee. Ischemia time ranged from 75 to 120 minutes, with an average value of 102.02 ± 15.02 minutes ( Table 1 ).
Table 1. Clinical characteristics of the study sample.
| CHARACTERISTICS | FREQUENCY (n = 48) | % |
|---|---|---|
| Side | ||
| Left | 18 | 37.5 |
| Right | 30 | 62.5 |
| Ischemia time (minutes) | ||
| 75 to 85 | 9 | 18.8 |
| 86 to 96 | 6 | 12.5 |
| 97 to 107 | 16 | 33.3 |
| 108 to 118 | 11 | 22.9 |
| > 118 | 6 | 12.5 |
| Tunnels | ||
| Seven | 6 | 12.5 |
| Eight | 34 | 70.8 |
| Nine | 8 | 16.7 |
| Lesions | ||
| Present | 31 | 64.6 |
| Absent | 17 | 35.4 |
| Lesion Type | (n = 38) | |
| Associated lesion | 1 | 2.6 |
| Trochlear chondral lesion | 1 | 2.6 |
| Sequela of tibial eminence fracture | 1 | 2.6 |
| Lateral meniscus | 14 | 36.8 |
| Medial meniscus | 21 | 55.3 |
In total, 31 (64.6%) patients presented associated injuries, and 17 (35.4%) subjects had no other injury. These 31 patients had 38 lesions, including a trochlear lesion and a chondral lesion (associated injury) at the medial femoral condyle; medial-meniscus injury was the most frequently observed lesion ( Table 1 ).
Postoperative pain was assessed using the VAS, as shown in Table 2 .
Table 2. Descriptive analysis of postoperative pain in the study sample.
| TIME | GROUP | n | MEAN | STANDARD DEVIATION | MINIMUM | MEDIAN | MAXIMUM |
|---|---|---|---|---|---|---|---|
| Immediately after surgery (T0) | I | 12 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| II | 12 | 0.3 | 0.9 | 0.0 | 0.0 | 3.0 | |
| III | 12 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| IV | 12 | 0.1 | 0.3 | 0.0 | 0.0 | 1.0 | |
| 6 hours after surgery (T1) | I | 12 | 5.1 | 1.2 | 3.0 | 5.5 | 6.0 |
| II | 12 | 3.1 | 2.1 | 1.0 | 2.0 | 8.0 | |
| III | 12 | 2.4 | 2.2 | 0.0 | 1.0 | 6.0 | |
| IV | 12 | 4.8 | 2.4 | 2.0 | 4.5 | 9.0 | |
| 12 hours after surgery (T2) | I | 12 | 4.4 | 2.5 | 0.0 | 6.0 | 7.0 |
| II | 12 | 3.6 | 2.5 | 0.0 | 3.0 | 7.0 | |
| III | 12 | 2.8 | 1.7 | 0.0 | 2.5 | 5.0 | |
| IV | 12 | 5.3 | 2.3 | 1.0 | 5.5 | 9.0 | |
| 24 hours after surgery (T3) | I | 12 | 4.3 | 2.6 | 0.0 | 5.0 | 7.0 |
| II | 12 | 3.3 | 2.1 | 0.0 | 3.0 | 7.0 | |
| III | 12 | 2.6 | 2.3 | 0.0 | 2.0 | 7.0 | |
| IV | 12 | 4.8 | 2.5 | 1.0 | 4.5 | 9.0 | |
| 48 hours after surgery (T4) | I | 12 | 3.6 | 2.4 | 0.0 | 4.5 | 7.0 |
| II | 12 | 2.8 | 1.9 | 0.0 | 2.5 | 6.0 | |
| III | 12 | 2.7 | 2.8 | 0.0 | 2.5 | 8.0 | |
| IV | 12 | 3.8 | 2.5 | 0.0 | 3.5 | 7.0 |
Considering the VAS classification at each time point, 47 (97.9%) patients had mild pain, and 1 (2.1%) presented moderate pain at T0. At T1, 18 (37.5%) subjects had mild pain, 27 (56.3%), moderate pain, and 3 (6.3%), severe pain. At T2, 15 (31.3%) subjects presented mild pain, 31 (64.6%), moderate pain, and 2 (4.2%), severe pain. At T3, 16 (33.3%) patients had mild pain, 30 (62.5%), moderate pain, and 2 (4.2%), severe pain. At T4, 20 (41.7%) patients had mild pain, 27 (56.3%), moderate pain, and 1 (2.1%), severe pain.
Considering a level of significance of 5%, the Kruskal-Wallis ANOVA revealed that the IA anesthetic solutions influenced the assessment of pain in each group ( p = 0.003) ( Table 3 ).
Table 3. Analysis of variance of pain in the operated patients.
| GROUP | n | MEDIAN | RANK | p -value |
|---|---|---|---|---|
| I | 12 | 4.50 | 133.6 | 0.003 |
| II | 12 | 2.00 | 112.5 | |
| III | 12 | 1.50 | 97.1 | |
| IV | 12 | 4.00 | 138.8 |
Note: Kruskal Wallis analysis of variance.
The ANOVA for pain revealed a strong influence of the IA analgesia in each group ( p < 0.0001) and of the time interval ( p < 0.0001). There were no significant differences in the interaction of the time interval with the IA analgesia ( p < 0.286) ( Table 4 ).
Table 4. Analysis of variance of pain according to the visual analog scale (VAS) in admitted patients.
| CAUSE OF THE VARIATION | DEGREES OF FREEDOM | SUM OF SQUARE VALUES | MEAN SQUARE VALUE | p -value |
|---|---|---|---|---|
| Time 1 | 4 | 17.23 | 17.2333 | 0.000 |
| Group 2 | 3 | 4.37 | 4.3667 | 0.000 |
| Time versus Group | 12 | 3.30 | 3.3 | 0.286 |
| Residue | 220 | 50.50 | 50.5 | |
| TOTAL | 239 | 75.40 |
Notes: 1 6, 12, 24, and 48 hours after surgery. 2 I, II, III, and IV.
A multiple comparison analysis using the Tukey test found that the moment most related to pain variability was T0 compared to all other time intervals ( p < 0.0001).
Multiple comparisons of average pain scores revealed significant differences when comparing Group I with Group III ( p = 0.014) and Group II with Group IV ( p = 0.042). In addition, there was a significant difference in the mean pain scores when Group III was compared with Group IV ( p = 0.001) ( Table 5 ).
Table 5. Multiple comparisons of average pain scores among the study groups.
| Tukey | ||||||
|---|---|---|---|---|---|---|
| GROUP | MEAN DIFFERENCE (I-J) | STANDARD MODEL | p -value | 95% CONFIDENCE INTERVAL | ||
| Inferior cut-off value | Superior cut-off value | |||||
| Group I | Group II | 0.088 | 0.323 | −0.08 | 0.38 | |
| Group III | 0.088 | 0.014 | 0.04 | 0.49 | ||
| Group IV | 0.088 | 0.779 | −0.31 | 0.14 | ||
| Group II | Group I | 0.088 | 0.323 | −0.38 | 0.08 | |
| Group III | 0.088 | 0.547 | −0.11 | 0.34 | ||
| Group IV | 0.088 | 0.042 | −0.46 | −0.01 | ||
| Group III | Group I | 0.088 | 0.014 | −0.49 | −0.04 | |
| Group II | 0.088 | 0.547 | −0.34 | 0.11 | ||
| Group IV | 0.088 | 0.001 | −0.58 | −0.12 | ||
| Group IV | Group I | 0.088 | 0.779 | −0.14 | 0.31 | |
| Group II | 0.088 | 0.042 | 0.01 | 0.46 | ||
| Group III | 0.088 | 0.001 | 0.12 | 0.58 | ||
Adverse effects occurred in 2 (4.2%) patients, and 46 (95.8%) subjects presented no adverse effects. These 2 patients included 1 subject from Group I, who had nausea, and 1 from Group 3, who complained of a headache.
In total, 7 (14.6%) patients required adjuvant analgesia, including 6 subjects who received opioids and one treated with opioids and anti-inflammatory agents. The remaining 41 (85.4%) patients required no supplemental pain control.
To prove that adjuvant analgesia was not an experimental confounding factor, the relationship between the use of IA solutions and additional pain control was evaluated using the Pearson Chi-Squared test. At a 5% level of significance, there was no significant relationship between the IA solutions and the supplemental analgesia ( p = 0.606) ( Table 6 ).
Table 6. Relationship between intra-articular solutions and additional pain control.
| GROUP | ADDITIONAL PAIN CONTROL | Total | |||
|---|---|---|---|---|---|
| Yes | % | No | % | ||
| I | 3 | 25.0 | 9 | 75.0 | 12 |
| II | 1 | 8.3 | 11 | 91.7 | 12 |
| III | 1 | 8.3 | 11 | 91.7 | 12 |
| IV | 2 | 16.7 | 10 | 83.3 | 12 |
| TOTAL | 7 | 14.6 | 41 | 85.4 | 48 |
Note: Chi-square test: p = 0.606.
Discussion
The success of any medical intervention is also determined by the perception of the patients regarding the benefits obtained with the treatment, be it conservative or surgical. The search for an ideal pain control after arthroscopic ACLR has become increasingly important due to the greater number of surgeries performed.
As demonstrated in the present study, ACLR is associated with significant postoperative pain, which may limit the possibility of its performance in outpatient facilities (with hospital discharge on the same day of the surgery; day hospital). In our service, surgery is still performed with traditional admission and hospital discharge after 24 to 48 hours due to inadequate pain control and even cultural reasons. In many institutions, including some in Brazil, ACLR is performed on an outpatient basis. In the United States, more complex surgeries, such as total-knee and hip replacement surgeries, have been performed on selected subjects on an outpatient basis, demonstrating their success in postoperative pain control. Some of our patients scored 9 points on the postoperative VAS for pain, and all were discharged 48 hours after surgery. Difficulties in adapting common methods for the relief of postoperative pain used in inpatient procedures to outpatient procedures resulted in inadequate pain management after surgery. In the present study, even subjects treated with IA bupivacaine infiltration scored 8 points on the VAS 48 hours after the procedure, showing the need for satisfactory pain control.
In sports medicine, ACLR is considered a highly successful procedure, but the management of postoperative pain has not yet been adequately achieved. Although the general pain level after ACLR has not been deemed intolerable, surgery can lead to considerable discomfort during the immediate postoperative period. In addition, it has been reported that significant postoperative pain has a negative effect on healing and results in patient dissatisfaction; 7 these data are consistent with those of our study, in which the need for supplemental pain control was minimal. However, some patients in Group II (0.5% bupivacaine alone) scored up to 8 points on the VAS.
Osborne and Keene 8 failed to prove the effectiveness of 0.5% bupivacaine alone or associated with 0.2 mg of epinephrine when compared to placebo. It is likely that preoperative factors, such as discomfort, tolerance to acute or chronic pain, and other medical conditions are the most important determinants of postoperative pain, that is, the preoperative status of the knee is the best predictor of the outcome of the procedure. However, these variables were not analyzed in the present study, even though its design and results were similar to those of the aforementioned study.
Some reports 9 show that the combination of morphine and ketorolac to IA ropivacaine increases the analgesic efficacy of the local anesthetic agent, reduces the requirements for pain control after hospital discharge, and improves some aspects of the activities of daily life (pain-related sleeping problems, appetite, concentration, need for assistance, the ability to walk on flat ground without pain, the ability to resume work) with no increased incidence of side effects. We did not use ketorolac and ropivacaine because they were not available in our institution, but IV morphine was administered in case of severe, unbearable pain, or VAS scores higher than 6. The quantification of the use of analgesic agents after hospital discharge was not an objective of the present study, and the outcomes were assessed up to 48 hours after surgery.
An impacting variable in IA injections is the use of epinephrine. 10 Epinephrine has been recommended to prevent local anesthetic toxicity. Bupivacaine is injected IA at the end of the procedure, and it is considered a good analgesic agent; in addition, its systemic action is not related to major adverse effects. This was well demonstrated in the present study, since pain control within the first 12 hours was higher in Group III (bupivacaine and epinephrine), whose patients had lower VAS scores compared to those of the other groups.
In the present study, tramadol was administered IV only in the case of moderate pain (VAS > 5), unlike a report by Zeidan et al., 11 in which an IA mixture of 100 mg of tramadol and 0.25% bupivacaine decreased the VAS score and achieved better postoperative pain control than the IA administration of either drug alone. This solution was also associated with a faster return to unsupported walk and early hospital discharge. Tramadol was prescribed in five patients, but it was not associated with a decrease in VAS scores.
Kristensen et al. 12 showed that local infiltration with ropivacaine and epinephrine is similar to femoral nerve block (FNB) for pain control after ACLR with medial flexor tendon graft. Until randomized studies investigate the FNB combined with infiltration at the donor site, the authors recommend analgesia with local infiltration in ACLR with medial hamstring graft.
Additional pain control was only required in 7 (14.1%) of our patients, demonstrating that IA analgesia is useful in reducing postoperative disability, preventing the onset of pain, and helping to avoid the need for additional drugs. Good pain control can be achieved in the immediate postoperative period with IV or oral analgesic drugs. However, these substances are not consistently successful because they are nonspecific, and can cause many side effects, such as acute gastric lesions. An IA injection of 150 mg of ropivacaine with 30 mg of ketorolac at the end of arthroscopic knee surgery increases the analgesic efficacy of local anesthetics without increasing the side effects; in addition, it had an increased sedative effect in this group of 7 patients. 13 In the present investigation, adverse effects only occurred in two patients and were considered mild (headache and nausea), with no correlation with the studied groups or drugs.
In the study by Jazayeri et al., 14 the postoperative analgesic effect of an IA injection of morphine and tramadol after minor arthroscopic knee surgery was maximal within 6 hours. 14 In the present research, analgesic effects were best observed during the first 6 hours, without the inconvenience of opioid-related adverse effects; as such, pain control was more efficient during the first 12 hours, especially in the first 6 hours.
Local anesthetics containing epinephrine can cause significant chondrotoxicity in human chondrocytes cultivated in vitro. Neither epinephrine alone, at concentrations of 1:100,000 and 1:200,000, or its preservative (methylparaben) reduced the viability of the chondrocytes. 15 This is why we used epinephrine at a concentration of 1:200,000.
The IA admonistration of bupivacaine and/or morphine did not have an analgesic effect strong enough to explain its frequent use in patients undergoing ACLR with flexor tendon grafting and spinal anesthesia, 16 despite the lower pain detected at all time intervals in Group III (bupivacaine and morphine). The VAS score for pain was low in all groups and at all times, and the pain was controlled with simpler, low-cost medications. Our results showed greater analgesic efficacy with bupivacaine alone or combined with epinephrine when compared with placebo.
Group III presented lower VAS scores at all time intervals, especially in the first 24 hours after surgery, unlike a previous Brazilian report by Souza et al., 17 who found no differences in postoperative analgesia when using IA morphine, bupivacaine, fentanyl and normal saline in arthroscopic knee surgery under subarachnoid anesthesia in most time intervals (immediately after surgery and every 6 hours for 24 hours). Six hours after surgery, patients receiving fentanyl presented significantly less pain, but those treated with morphine required more additional analgesia.
Subarachnoid anesthesia for ACLR was associated with postoperative nausea and vomiting. Prophylaxis with dexamethasone and perphenazine resulted in fewer adverse effects. 18 In the present study, nausea was not a frequent finding (since it was only observed in one patient), and there were no cases of vomiting. Per the anesthetic technique, all subjects received dexamethasone during induction to avoid such complications.
We observed no correlation between the increased use of opioid agents, side effects and the studied group. Dal et al. 19 noticed a reduction in postoperative pain and adequate use analgesic drugs when IA ketamine, bupivacaine, or neostigmine were administered. These authors did not observe any psychomimetic side effects, particularly those associated with higher doses or systemic use, and the IA administration of ketamine provided long-lasting, effective analgesia, similar to neostigmine, but less effective than bupivacaine, after knee arthroscopy, with no adverse effects.
All groups receiving IA solutions, whether combined or not, showed decreased VAS scores for pain, which is in line with the results of a prospective, randomized, double-blinded clinical study with different design and drugs; 20 this suggests that, in knee arthroscopy, combined analgesic injections consisting of morphine, bupivacaine, epinephrine and epinephrine plus bupivacaine resulted in lower pain levels and decreased use of narcotics at the anesthetic recovery room, with statistically significant differences when compared to epinephrine alone. These results were independent of the timing of the injection, whether pre- or postoperatively.
In Group III, the solutions enabled a satisfactory pain control in patients undergoing ACLR with spinal anesthesia. In Brazil, spinal block is routine, and hospital discharge usually occurs in 24 or 48 hours, as in the present study. The international literature is abundant with descriptions of pain control in patients undergoing ACLR with general anesthesia and FNB who are often discharged on the same day. In patients undergoing knee arthroscopy performed under general anesthesia due to the greater painful stimulus, Eroglu et al. 21 noted that the IA administration of 5 mg of morphine and 20 mL of 0.25% bupivacaine resulted in decreased pain levels when compared to placebo (in this case, normal saline solution).
In the present study, pain control was superior with bupivacaine alone (Group II) compared to placebo or epinephrine alone (Group IV), which is consistent with the study by Wei et al., 22 which demonstrated that a single IA dose of bupivacaine proved to be significantly better than placebo in pain relief after arthroscopic knee surgery. More high-quality, randomized, controlled clinical trials with longer follow-up periods are required to determine the safety of a single dose of bupivacaine. Even so, the routine use of a single IA dose of bupivacaine is an effective way to control pain after arthroscopic knee surgery. In short, it cannot be concluded that a single IA dose of bupivacaine is toxic or clinically safe.
Recently, a meta-analysis by Zhou et al. 23 concluded that a single IA dose of ropivacaine at the end of arthroscopic knee surgery provides effective pain relief in the immediate and early postoperative periods, with no increase in short-term side effects. Since ropivacaine was not available in our institution, we used bupivacaine, which has a similar mechanism of action, and we obtained similar analgesia outcomes.
Iwasaki et al . 24 demonstrated that there is no strong evidence that an IA injection of bupivacaine induces degenerative changes in the articular cartilage, without differences in cell viability, cell density or in the scores of cartilage assessment; therefore, the results may apply to both normal and osteoarthritic joints.
The present study has some limitations. Even though the preoperative status of the knee can alter the perception of pain by patients, and longer periods of time since the injury correlate with worse knee conditions, the time elapsed from injury to surgery was not considered. The activity level of the patients, that is, if it remains unchanged, was not determined. Data collection could have been performed at shorter intervals to determine pain patterns with greater accuracy. Pain could have been stratified by gender and age to enable the collection of more accurate information on this issue, but these measurements were not part of the goals of the present study. There are other scales to measure pain, but the VAS was adopted because it is easily understood by the patients. The patients' mental status can also modify pain perception, but this assessment was not an objective of the present study. There is controversy surrounding the IA use of local anesthetics regarding chondrotoxicity, but this could only be verified with a longer follow-up period and through a histological study, which would require a new surgical procedure. The coexistence with other IA lesions (meniscal and chondral injuries, involving the trochlea and medial femoral condyle) may contribute to pain, in addition to the ACLR per se, but there was also no relationship between the groups and associated injuries. The graft donor site is a potential source of pain, and may be studied in a further work. The adverse systemic effects of the drugs were recorded by the nursing staff, and may have been underreported. Adjuvant pain control can cause confusion, since pain can improve both with the solution and analgesia, resulting in a confusion bias. There are other potential ways to control pain, such as the FNB and adductor canal block, but these procedures are not routinely performed in our service.
Conclusion
The present study concludes that epinephrine does not present benefits in reducing postoperative pain; regardless of the IA anesthesia, pain control is better in the early postoperative period (up to 12 hours); the presence or absence of epinephrine and/or bupivacaine in the IA space does not induce systemic adverse effects; there was no quantitative difference in the postoperative use of adjuvant analgesic/anti-inflammatory agents among the groups; and there was no correlation between the systemic effects of the adjuvant analgesic/anti-inflammatory medications used and the groups studied.
Conflito de Interesses Os autores declaram não haver conflito de interesses.
Suporte Financeiro
Não houve suporte financeiro de fontes públicas, comerciais, ou sem fins lucrativos.
Financial Support
There was no financial support from public, commercial, or non-profit sources.
Trabalho desenvolvido no Departamento de Ortopedia e Traumatologia , Fundação Hospital Adriano Jorge, Cachoeirinha, Manaus, AM, Brasil.
Work developed at the Orthopedics and Traumatology Department, Fundação Hospital Adriano Jorge, Cachoeirinha, Manaus, AM, Brazil.
Referências
- 1.Rawal N. Postoperative pain relief using regional anaesthesia. Curr Anaesth Crit Care. 2007;18(03):140–148. [Google Scholar]
- 2.Alagol A, Calpur O U, Usar P S, Turan N, Pamukcu Z. Intraarticular analgesia after arthroscopic knee surgery: comparison of neostigmine, clonidine, tenoxicam, morphine and bupivacaine. Knee Surg Sports Traumatol Arthrosc. 2005;13(08):658–663. doi: 10.1007/s00167-004-0612-7. [DOI] [PubMed] [Google Scholar]
- 3.Raja S N, Dickstein R E, Johnson C A. Comparison of postoperative analgesic effects of intraarticular bupivacaine and morphine following arthroscopic knee surgery. Anesthesiology. 1992;77(06):1143–1147. doi: 10.1097/00000542-199212000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Sun Q B, Liu S D, Meng Q J, Qu H Z, Zhang Z. Single administration of intra-articular bupivacaine in arthroscopic knee surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2015;16:21. doi: 10.1186/s12891-015-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wewers M E, Lowe N K. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(04):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 6.CONSORT Group . Schulz K F, Altman D G, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 7.Koh I J, Chang C B, Seo E S, Kim S J, Seong S C, Kim T K. Pain management by periarticular multimodal drug injection after anterior cruciate ligament reconstruction: a randomized, controlled study. Arthroscopy. 2012;28(05):649–657. doi: 10.1016/j.arthro.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Osborne D, Keene G. Pain relief after arthroscopic surgery of the knee: a prospective, randomized, and blinded assessment of bupivacaine and bupivacaine with adrenaline. Arthroscopy. 1993;9(02):177–180. doi: 10.1016/s0749-8063(05)80370-6. [DOI] [PubMed] [Google Scholar]
- 9.Ng H P, Nordström U, Axelsson K. Efficacy of intra-articular bupivacaine, ropivacaine, or a combination of ropivacaine, morphine, and ketorolac on postoperative pain relief after ambulatory arthroscopic knee surgery: a randomized double-blind study. Reg Anesth Pain Med. 2006;31(01):26–33. doi: 10.1016/j.rapm.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 10.White A P, Laurent S, Wilkinson D J. Intra-articular and subcutaneous prilocaine with adrenaline for pain relief in day case arthroscopy of the knee joint. Ann R Coll Surg Engl. 1990;72(06):350–352. [PMC free article] [PubMed] [Google Scholar]
- 11.Zeidan A, Kassem R, Nahleh N. Intraarticular tramadol-bupivacaine combination prolongs the duration of postoperative analgesia after outpatient arthroscopic knee surgery. Anesth Analg. 2008;107(01):292–299. doi: 10.1213/ane.0b013e31816ba364. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen P K, Pfeiffer-Jensen M, Storm J O, Thillemann T M. Local infiltration analgesia is comparable to femoral nerve block after anterior cruciate ligament reconstruction with hamstring tendon graft: a randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(02):317–323. doi: 10.1007/s00167-013-2399-x. [DOI] [PubMed] [Google Scholar]
- 13.Rokhtabnak F, Ale Bouyeh M R, Seyed Siamdust A, Masoomshahi M, Aghajani M. Comparison of the effects of intra-articular sole ropivacaine and combined ketorolac and ropivacaine for pain control after knee arthroscopy surgery. Br J Pain. 2015;9(03):149–156. doi: 10.1177/2049463714553312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jazayeri S M, Mosaffa F, Abbasian M, Hosseinzadeh H R. Comparing the efficacy of intra-articular application of morphine and tramadol on postoperative pain after arthroscopic knee surgery. Anesth Pain Med. 2012;2(01):28–31. doi: 10.5812/aapm.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dragoo J L, Korotkova T, Kim H J, Jagadish A. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med. 2010;38(06):1154–1159. doi: 10.1177/0363546509359680. [DOI] [PubMed] [Google Scholar]
- 16.Danieli M V, Cavazzani Neto A, Herrera P A. Intra-articular bupivacaine or bupivacaine and morphine after ACL reconstruction. Acta Ortop Bras. 2012;20(05):258–261. doi: 10.1590/S1413-78522012000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza R H, Issy A M, Sakata R K. Analgesia intra-articular com morfina, bupivacaína ou fentanil após operação de joelho por videoartroscopia. Rev Bras Anestesiol. 2002;52(05):570–580. [PubMed] [Google Scholar]
- 18.Williams B A, Vogt M T, Kentor M L, Figallo C M, Kelly M D, Williams J P. Nausea and vomiting after outpatient ACL reconstruction with regional anesthesia: are lumbar plexus blocks a risk factor? J Clin Anesth. 2004;16(04):276–281. doi: 10.1016/j.jclinane.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Dal D, Tetik O, Altunkaya H, Tetik O, Doral M N. The efficacy of intra-articular ketamine for postoperative analgesia in outpatient arthroscopic surgery. Arthroscopy. 2004;20(03):300–305. doi: 10.1016/j.arthro.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin R C, Amjadi F, Parker R D. Short-term analgesic effects of intra-articular injections after knee arthroscopy. Arthroscopy. 2005;21(03):307–312. doi: 10.1016/j.arthro.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Eroglu A, Saracoglu S, Erturk E, Kosucu M, Kerimoglu S. A comparison of intraarticular morphine and bupivacaine for pain control and outpatient status after an arthroscopic knee surgery under a low dose of spinal anaesthesia. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1487–1495. doi: 10.1007/s00167-010-1061-0. [DOI] [PubMed] [Google Scholar]
- 22.Wei J, Yang H B, Qin J B, Kong F J, Yang T B. Single-dose intra-articular bupivacaine after knee arthroscopic surgery: a meta-analysis of randomized placebo-controlled studies. Knee Surg Sports Traumatol Arthrosc. 2014;22(07):1517–1528. doi: 10.1007/s00167-013-2543-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Yang T B, Wei J. Single-dose intra-articular ropivacaine after arthroscopic knee surgery decreases post-operative pain without increasing side effects: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016;24(05):1651–1659. doi: 10.1007/s00167-015-3656-y. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki K, Sudo H, Kasahara Y. Effects of Multiple Intra-articular Injections of 0.5% Bupivacaine on Normal and Osteoarthritic Joints in Rats. Arthroscopy. 2016;32(10):2026–2036. doi: 10.1016/j.arthro.2016.02.011. [DOI] [PubMed] [Google Scholar]