Abstract
Eight concentration and purification methods were evaluated to determine percentages of recovery of Cryptosporidium parvum oocysts from calf feces. The NaCl flotation method generally resulted in the highest percentages of recovery. Based on the percentages of recovery, the amounts of fecal debris in the final oocyst preparations, the relatively short processing time (<3 h), and the low expense, the NaCl flotation method was chosen for further evaluation. Extraction efficiency was evaluated by using oocyst concentrations of 25, 50, 102, 103, 104, and 105 oocysts g of bovine feces−1. The percentages of recovery ranged from 10.8% (25 oocysts g−1) to 17.0% (104 oocysts g−1) (r2 = 0.996). A conservative estimate of the detection limit for bovine feces is ca. 30 oocysts g of feces−1. Percentages of recovery were determined for six different types of animal feces (cow, horse, pig, sheep, deer, and chicken feces) at a single oocyst concentration (104 oocysts g−1). The percentages of recovery were highest for bovine feces (17.0%) and lowest for chicken feces (3.2%). Percentages of recovery were determined for bovine manure after 3 to 7 days of storage. The percentages of recovery ranged from 1.9 to 3.5% depending on the oocyst concentration, the time of storage, and the dispersing solution. The percentages of oocyst recovery from soils were evaluated by using different flotation solutions (NaCl, cold sucrose, ZnSO4), different dispersing solutions (Triton X-100, Tween 80, Tris plus Tween 80), different dispersion techniques (magnetic stirring, sonication, blending), and different dispersion times (5, 15, and 30 min). Twenty-five-gram soil samples were used to reduce the spatial variability. The highest percentages of recovery were obtained when we used 50 mM Tris–0.5% Tween 80 as the dispersing solution, dispersion for 15 min by stirring, and saturated NaCl as the flotation solution. The percentages of oocyst recovery from freshly spiked sandy loam, silty clay loam, and clay loam soils were ca. 12 to 18, 8, and 6%, respectively. The theoretical detection limits were ca. 1 to 2 oocysts g of soil−1 depending on the soil type. The percentages of recovery without dispersant (distilled H2O or phosphate-buffered saline) were less than 0.1%, which indicated that oocysts adhere to soil particles. The percentages of recovery decreased with storage time, although the addition of dispersant (Tris-Tween 80) before storage appeared to partially prevent adhesion. These data indicate that the NaCl flotation method is suitable for routine detection and enumeration of oocysts from feces, manures, soils, or soil-manure mixtures.
Cryptosporidium parvum, the causal agent of cryptosporidiosis, is a widespread protozoan parasite that infects numerous mammalian species. C. parvum is an important human pathogen, as evidenced by several outbreaks of cryptosporidiosis in the past decade; the most severe of these outbreaks occurred in Milwaukee, Wis., where more than 400,000 people were infected (13). C. parvum is a particularly serious health threat to immunodeficient individuals (e.g., AIDS and cancer patients) because there are no effective treatments for the disease.
An important mode of C. parvum transmission to humans is believed to be via contaminated drinking water or recreational water. Studies have shown that waterborne C. parvum oocysts (the infectious stage found outside the body) may remain viable for several months (8). Although wildlife and sewage outflows have been implicated in watershed contamination (3, 11, 16, 19), farm animals are also believed to be major contributors. Sheep, horses, and pigs are susceptible to infection by C. parvum and shed oocysts (21, 29, 30); however, dairy and beef calves are generally considered to present the greatest risk because of their numbers, distribution, incidence of infection, and high levels of oocyst excretion.
Neonatal calves are particularly susceptible to infection (scours) and can excrete up to 30 billion oocysts or more over a 1- to 2-week period. Based on a survey of 7,369 calves from 1,103 dairy farms located in 28 states, Garber et al. (10) found that more than 50% of 2-week-old calves and 22.4% of all calves (ages, 1 to 17 weeks) tested positive for C. parvum. These authors concluded that virtually all herds with more than 100 cows are infected with C. parvum. Limited data suggests that adult cows may also shed oocysts. Scott et al. (18) found up to 18,000 oocysts per g of feces from apparently healthy adult cows. Based on an average content of 900 oocysts per g of feces and a total excretion of ca. 40 kg of feces per cow per day, a single adult bovine could potentially excrete more than 36 million oocysts per day.
These data suggest that contaminated manures from dairy or beef cattle operations can be major sources of C. parvum oocysts unless manure management or treatment strategies are used to minimize oocyst viability or transport to water. In addition to direct fecal deposition, possible modes of transport to potable or recreational water include surface transport from land-applied manures or leaching through the soil to groundwater (e.g., karst groundwater). Land application of manures is recommended in order to recycle nutrients (e.g., nitrogen and phosphorus) for crop growth. The Environmental Protection Agency has proposed manure management recommendations to minimize nutrient transport to surface water (7). It is important to determine if the proposed recommendations also minimize transport of C. parvum oocysts to surface water.
Evaluations of the efficacy manure management strategies depend on accurate determinations of oocyst numbers in feces, manures, and soils. Methods for detecting Cryptosporidium oocysts in fecal samples have been described previously. Fecal smears are commonly used for clinical purposes to detect oocysts in stool samples. Although quick and relatively quantitative, smears have limited sensitivity and are applicable only to watery or diluted samples (samples with low percentages of solids). Several concentration and purification methods in which a variety of flotation solutions are used for extraction and recovery of oocysts from fecal samples have been described (2, 5, 12, 17, 20, 22, 32). In general, these methods have not been rigorously evaluated with respect to extraction efficiencies and/or detection limits.
Few studies have addressed the transport of oocysts over or through soils, in large measure because of difficulties associated with detection and enumeration of oocysts in soil samples or soil-manure mixtures. Mawdsley et al. (14) have described a method for extraction and enumeration of oocysts in soil in which sucrose flotation is used. These authors reported extraction efficiencies of up to 61.6% for 1-g soil samples processed shortly after spiking; however, the extraction efficiencies declined to 4% after 24 h. Walker et al. (23) obtained comparable results by using a procedure adapted from the method of Mawdsley et al.; in this study the percentage of recovery was 43% ± 5.7% (average ± 95% confidence interval) for freshly spiked samples. This method is suitable for laboratory experiments in which oocysts are likely to be relatively homogeneously distributed throughout the soil. However, for field scale experiments, in which the oocyst distribution is likely to be more heterogeneous, larger sample sizes are preferable in order to reduce spatial variability.
We describe here an evaluation of concentration and purification methods that were used in conjunction with immunofluorescence antibody staining for detection and enumeration of C. parvum oocysts in feces, manures, and soils. Our goal was to identify a relatively fast, inexpensive method that could be used for routine detection and quantitation of low levels of oocysts in feces, manures, soils, or soil-manure mixtures.
MATERIALS AND METHODS
Sample preparation.
Purified C. parvum oocysts were obtained from infected calves as previously described (9). Oocysts (ca. 107 oocysts ml−1) were stored in sterile phosphate-buffered saline (PBS) (pH 7.2) at 4°C until they were used. Oocyst suspensions used to spike fecal, manure, or soil samples were prepared immediately prior to use; precise numbers were determined with a Neubauer hemocytometer. Purified oocysts were used in all experiments unless indicated otherwise.
One-gram aliquots of fresh calf feces and feces from lactating cows at the Beltsville Agricultural Research Center dairy farm were spiked with 0.5-ml portions of oocyst suspensions (serial dilutions of a known stock suspension) to give final concentrations of 2 × 103, 104, and 105 oocysts g of calf feces−1 and 25, 50, 200, 103, 104, and 105 oocysts g of cow feces−1, respectively. One-gram aliquots of fresh feces from adult swine, sheep, chicken, horse, and deer samples were spiked with 0.5-ml portions of an oocyst suspension to give a concentration of 104 oocysts g of feces−1. Samples of swine, sheep, and chicken feces were obtained from research animals at the Beltsville Agricultural Research Center, Beltsville, Md.; horse feces were obtained from a stable (Glen Dale Farms, Greenbelt, Md.); and deer feces from feral animals were collected in the field at the Patuxent Wildlife Refuge, U.S. Fisheries and Wildlife Service, Bowie, Md. Prior to spiking, the feces were examined (by the NaCl flotation method) to ensure that no detectable oocysts were present. Samples were processed immediately after spiking. We prepared six samples of each type of feces at each concentration.
To determine percentages of recovery from bovine manure, 100-g aliquots of a manure slurry (containing feces, urine, and water) that was collected fresh from the Beltsville Agricultural Research Center dairy barn were each spiked with 1 ml of an oocyst suspension to give a final concentration of 103 or 104 oocysts g of manure−1. After thorough mixing with a magnetic stirrer the manure was stored at 4°C.
To determine percentages of recovery from soils, 25-g aliquots of air-dried soil were each spiked with 0.5 ml of a purified oocyst suspension. Experiments were conducted with the following three soil types: sandy loam, silty clay loam, and clay loam. A textural analysis was conducted by using the hydrometer method (27). The sandy loam soil contained 66.9% sand, 16.6% silt, and 16.5% clay, and its organic matter content was 1.1%; the silty clay loam soil contained 18.4% sand, 53.2% silt, and 28.4% clay, and its organic matter content was 3.1%; and the clay loam soil contained 25.7% sand, 42.5% silt, and 31.8% clay, and its organic matter content was 2.8%. Except as noted below, experiments were conducted with the sandy loam soil. All experimental treatments were replicated six times unless indicated otherwise.
Extraction procedures used for feces.
Twenty-milliliter portions of PBS were added to samples after they were thoroughly mixed with oocysts. Fecal suspensions were filtered through a stainless steel mesh sieve (pore size, 45 μm) and rinsed with ca. 30 ml of PBS. The suspensions were centrifuged at 500 × g for 10 min in 50-ml polypropylene centrifuge tubes. Each resulting supernatant was decanted, the sediment was resuspended in PBS, and the process was repeated once. Most of the sediments were resuspended in 5 ml of PBS to form a slurry; the exceptions were (i) after discontinuous sucrose gradient centrifugation, when the sediment was resuspended in 5 ml of 2.5% aqueous potassium dichromate (K2Cr2O7), and (ii) after flotation with ZnSO4 · 7H2O, when the sediment was resuspended in 5 ml of distilled water.
The following four flotation solutions were examined: MgSO4 (575 g liter−1; specific gravity, 1.27), ZnSO4 · 7H2O (703 g liter−1; specific gravity, 1.3), cold sucrose (700 g liter−1; specific gravity, 1.18), and NaCl (360 g liter−1; specific gravity, 1.21). Sediment slurries were emulsified with 45-ml portions of the flotation solutions and centrifuged at 500 × g for 10 min. The upper 5 ml of each supernatant was transferred to a 50-ml tube. The other methods examined included cesium chloride gradient centrifugation, discontinuous Sheather’s gradient centrifugation, discontinuous Percoll gradient centrifugation, and formalin-ethyl acetate sedimentation.
For cesium chloride gradient centrifugation (12), solutions were prepared from stock solutions of CsCl (specific gravity, 1.8) and Tris buffer (50 mM Tris, 10 mM EDTA; pH 7.2) by using the following proportions of CsCl and Tris buffer: 1:1 (density, 1.4 g ml−1), 1:7 (density, 1.1 g ml−1), and 1:15 (density, 1.05 g ml−1). Three milliliters of each CsCl solution was layered into a 15-ml tube. The sediment was centrifuged at 1,500 × g for 10 min in Tris buffer, the supernatant was removed, and the sediment was resuspended in 1 ml of Tris buffer. The contents of each tube were overlaid with 1 ml of suspension, and the tubes were centrifuged at 16,000 × g for 60 min at 4°C. Following centrifugation, the band between the 1.1- and 1.05-g ml−1 densities in each tube was aspirated with a glass pipette and transferred to a 50-ml tube.
For discontinuous Sheather’s gradient centrifugation (2), a sucrose solution (500 g of sucrose and 6.5 g of phenol in 320 ml of water) was diluted 1:2 and 1:4 with sterile PBS. Ten milliliters of the 1:2 dilution was transferred to a 50-ml centrifuge tube and overlaid with 10 ml of the 1:4 dilution. The contents of each tube were overlaid with 5 ml of a PBS suspension in K2Cr2O7, and the tubes were centrifuged at 1,500 × g for 30 min at 4°C. Following centrifugation, the upper yellow potassium dichromate layer was discarded, while the pellet and next two layers (yellow turbid and white clear layers) were transferred to 50-ml tubes.
For discontinuous Percoll gradient centrifugation (17), Percoll gradients were prepared in 15-ml centrifuge tubes, and each gradient consisted of the following four 2-ml layers: 100% Percoll (density, 1.13 g ml−1), 75% Percoll in distilled water (density, 1.09 g ml−1), 33% Percoll (density, 1.05 g ml−1), and 10% Percoll (density, 1.01 g ml−1). The contents of each tube were overlaid with 1 ml of suspension, and the tubes were centrifuged at 650 × g for 15 min at 4°C. The layer between the 1.09- and 1.05-g ml−1 densities in each tube was aspirated with a glass pipette and transferred to a 50-ml centrifuge tube.
For formalin-ethyl acetate sedimentation (31), sediment slurries were mixed with 9 ml of neutral buffered 10% formalin and then with 4 ml of ethyl acetate. Samples were shaken in an inverted position for 30 s and then centrifuged at 500 × g for 2 min. The upper three layers were transferred to 50-ml tubes, enough PBS was added to bring the volume to 50 ml, and the tubes were centrifuged twice at 500 × g for 10 min. The sediment in each tube was resuspended in MgSO4 and centrifuged at 500 × g for 10 min, and the upper 5 ml was transferred to a 50-ml tube.
Following each procedure, the volume of the oocyst suspension was brought to 50 ml with distilled water, and the preparation was centrifuged at 500 × g for 10 min. The pellet was washed once more with 50 ml of water and then with 15 ml of water, and then it was centrifuged in a 1-ml Eppendorf tube at ca. 1,500 × g for 3 min. The final pellet was resuspended in 100 μl of distilled water.
Extraction procedures used for manure.
One-gram aliquots of manure were processed on days 3 and 6 (104 oocysts g−1) or days 4 and 7 (103 oocysts g−1). Samples were diluted with 50 ml of 50 mM Tris and 0.5% (vol/vol) Tween 80 or 50 ml of PBS and dispersed for 15 min with a magnetic stirrer. Manure solutions were filtered through a stainless steel mesh sieve (pore size, 45 μm) and washed with ca. 50 ml of Tris-Tween 80 or PBS. After centrifugation (500 × g for 10 min) in 100-ml tubes, the supernatants were decanted, and the sediments were transferred to 50-ml tubes and processed by using the NaCl flotation method as described above. Percentages of recovery and standard deviations were calculated based on six replicates.
Extraction procedures used for soils.
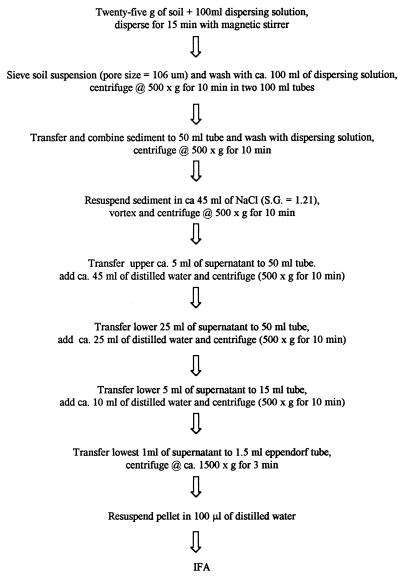
The flow diagram in Fig. 1 shows the generic extraction procedure used for soils. The flotation solutions evaluated included ZnSO4 · 7H2O, cold sucrose, and NaCl. All other experiments were conducted by using the NaCl flotation method. The dispersing solutions evaluated included distilled water, PBS (pH 7.2), 1% (wt/vol) Tween 80, 1% (wt/vol) Triton X-100, and 50 mM Tris and 0.5% (vol/vol) Tween 80. Dispersion times of 5, 15, and 30 min were evaluated in conjunction with magnetic stirring and Tris-Tween 80. Samples were processed as previously described.
FIG. 1.
Flow diagram showing the NaCl flotation method for extracting oocysts from soils. S.G., specific gravity; IFA, immunofluorescence antibody.
The dispersion methods evaluated included magnetic stirring for 15 min, sonication for 15 min with a model 2210 Ultrasonic Cleaner (Branson, Danbury, Conn.) at 47 kHz, and blending for 2 min with a Waring blender at low speed (19,000 rpm). Twenty-five-gram soil samples were obtained from the upper 2-cm portions of sandy loam or loam soil cores previously used for leaching experiments (unpublished data). Three samples per core were obtained approximately 48 h after leaching, and the percentages of recovery were determined for each dispersion method (n = 2). Since the percentages of oocyst recovery from the sandy loam and loam soils were almost identical, the data are presented below as the average levels of recovery for both cores. The samples were processed as described above by using Tris-Tween 80 dispersing solution and magnetic stirring for 15 min.
The percentages of recovery as a function of storage time were evaluated by using oocysts obtained from diluted calf diarrhea samples; the final concentration used was 104 oocysts per 25 g of soil. Five milliliters of distilled water or dispersing solution (50 mM Tris and 0.5% [vol/vol] Tween 80) was added to 25 g of air-dried soil (moisture content, ca. 20%), and then 0.5 ml of the oocyst suspension was added immediately. The soil samples were stored at 4°C until they were processed. The samples were processed after 1 h and 1, 3, 7, 10, 14, and 21 days as described above by using the Tris-Tween 80 dispersing solution and magnetic stirring for 15 min.
Oocyst detection and enumeration.
After each Eppendorf tube was thoroughly vortexed, three 10-μl aliquots (of the 100-μl sample) were pipetted into slide wells (diameter, 5 mm), dried with a slide warmer, and stained by the direct immunofluorescence antibody method by using a commercial kit (Merifluor; Meridian Diagnostic, Inc., Cincinnati, Ohio). Samples were examined with an epifluorescence microscope (Olympus) by using a magnification of ×200. The number of oocysts per gram of feces or manure or per 25 g of soil was determined by multiplying the average number of oocysts counted in three wells by 10. Percentages of recovery and standard deviations were calculated based on six replicates unless indicated otherwise.
RESULTS
Recovery from feces and manure.
The percentages of oocyst recovery from calf feces varied from <1 to 18.7% for the eight concentration and purification methods evaluated (Table 1). The NaCl and sucrose flotation methods gave significantly higher percentages of recovery (P < 0.05) at the lowest oocyst concentration used (2 × 103 oocysts g−1). The NaCl flotation method gave significantly higher percentages of recovery (P < 0.05) at oocyst concentrations of 104 and 105 oocysts g−1 than most of the other methods gave; the only exception was CsCl gradient centrifugation. In general, the percentages of recovery were highest for the lowest oocyst concentration (2 × 103 oocysts g−1). Subjectively, CsCl gradient centrifugation and discontinuous Percoll gradient centrifugation resulted in the smallest amounts of fecal debris in final oocyst preparations, formalin-ethyl acetate sedimentation, MgSO4 flotation, ZnSO4 flotation, and sucrose flotation resulted in the most fecal debris, and Sheather’s discontinuous gradient centrifugation and NaCl flotation resulted in intermediate amounts of fecal debris. The flotation methods were more cost and time efficient than the gradient centrifugation methods because of the inexpensive materials and fewer, less complex procedures. However, except for NaCl flotation, they generally resulted in larger amounts of fecal debris. The NaCl flotation method was chosen for further evaluation because of the higher percentages of recovery, intermediate amounts of fecal debris, relatively short processing times (<3 h), and low expense associated with it.
TABLE 1.
Percentages recovery from calf feces at three oocyst concentrations obtained with the following eight concentration and purification methods: cesium chloride gradient centrifugation, Sheather’s discontinuous sucrose gradient centrifugation, discontinuous Percoll gradient centrifugation, formalin-ethyl acetate sedimentation, MgSO4 flotation, ZnSO4 flotation, sucrose flotation, and NaCl flotation
| Method | % Recovery at the following oocyst concna:
|
||
|---|---|---|---|
| 2 × 103 oocysts g−1 | 104 oocysts g−1 | 105 oocysts g−1 | |
| CsCl centrifugation | 13.2 ± 3.2b | 10.3 ± 3.5 | 9.9 ± 3.8 |
| Sheather’s centrifugation | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.2 |
| Percoll centrifugation | 7.0 ± 1.3 | 2.4 ± 0.2 | 1.9 ± 0.4 |
| Formalin-ethyl acetate sedimentation | 1.5 ± 0.4 | 2.1 ± 0.7 | 0.8 ± 0.3 |
| MgSO4 flotation | 10.8 ± 1.4 | 2.8 ± 0.5 | 2.6 ± 0.5 |
| ZnSO4 flotation | 3.6 ± 1.0 | 2.6 ± 0.7 | 2.3 ± 0.6 |
| Sucrose flotation | 17.9 ± 2.7 | 7.4 ± 0.6 | 4.0 ± 1.3 |
| NaCl flotation | 18.7 ± 5.9 | 8.7 ± 1.4 | 8.1 ± 1.9 |
The least significant differences (P < 0.05) for oocyst concentrations of 2 × 103, 104, and 105 oocysts g−1 were 2.7, 0.8, and 0.9%, respectively.
Mean ± standard deviation (n = 6).
Percentages of recovery from adult bovine feces were determined by using oocyst concentrations ranging from 25 to 105 oocysts g of feces−1 (Table 2). The percentages of recovery from adult feces were generally comparable to the percentages of recovery from calf feces and were comparable at different oocyst concentrations. The coefficients of variation were consistent at oocyst concentrations of >103 oocysts g−1 but increased as the oocyst concentrations decreased. Linear regression plots (log number of oocysts added versus log number of oocysts recovered) gave an r2 value of 0.996. A conservative estimate of the detection limit in bovine feces is ca. 30 oocysts g−1 (reciprocal of fraction recovered [ca. 0.12] × fraction of suspension counted [0.3]). The percentages of recovery from different animal feces varied from 3.2% for chicken feces to 17% for bovine feces (Table 3).
TABLE 2.
Recovery of oocysts from adult bovine feces at different initial concentrations when the NaCl flotation method was used
| Oocyst concn (oocysts g−1) | % Recovery | No. of oocysts/10 μl | Coefficient of variation (%) | Range (oocysts/ 10 μl) |
|---|---|---|---|---|
| 105 | 11.1 | 1,111.6 ± 314.2a | 28 | 649–1,792 |
| 104 | 17.0 | 169.8 ± 54.1 | 32 | 105–298 |
| 103 | 17.7 | 17.7 ± 6.9 | 39 | 9–30 |
| 200 | 12.8 | 2.5 ± 2.5 | 100 | 0–8 |
| 50 | 13.4 | 0.67 ± 0.84 | 125 | 0–2 |
| 25 | 10.8 | 0.27 ± 0.46 | 170 | 0–1 |
Mean ± standard deviation (n = 6). The values must be multiplied by 10 to obtain percentages of recovery.
TABLE 3.
Recovery of oocysts from adult animal feces when the NaCl flotation method was used
| Animal | % Recoverya | Coefficient of variation (%) |
|---|---|---|
| Cow | 17.0 ± 5.4b | 32 |
| Horse | 9.6 ± 1.6 | 16 |
| Pig | 7.0 ± 2.9 | 42 |
| Sheep | 6.9 ± 2.0 | 29 |
| Deer | 4.6 ± 1.9 | 41 |
| Chicken | 3.2 ± 2.2 | 68 |
The initial concentration was 104 oocysts g of feces−1.
Mean ± standard deviation (n = 6).
The percentages of recovery from a bovine manure slurry were determined as a function of the storage and dispersing solution (Table 4). The percentages of recovery were substantially lower than the percentages of recovery for fresh bovine feces. The percentages of recovery when PBS was used as the dispersing solution were consistent (2.3 to 2.5%) regardless of the oocyst concentration or storage time. The percentages of recovery when Tris-Tween 80 was used as the dispersing solution were initially higher (3.2 to 3.5%) but decreased after an additional 3 days of storage (to 1.9 to 2.1%). The coefficients of variation were consistently higher at lower oocyst concentrations but were generally consistent at a given concentration regardless of the storage time.
TABLE 4.
Recovery of oocysts from a bovine manure slurry as a function of concentration, storage, and dispersion solution when the NaCl flotation method was used
| Oocyst concn (oocysts g−1) | Dispersion solution | No. of days after spiking | % Recovery | Coefficient of variation (%) |
|---|---|---|---|---|
| 104 | Tris-Tween 80 | 3 | 3.2 ± 0.36a | 11 |
| 6 | 2.1 ± 0.36 | 17 | ||
| PBS | 3 | 2.3 ± 0.30 | 13 | |
| 6 | 2.4 ± 0.46 | 19 | ||
| 103 | Tris-Tween 80 | 4 | 3.5 ± 0.14 | 40 |
| 7 | 1.9 ± 0.08 | 42 | ||
| PBS | 4 | 2.5 ± 1.4 | 56 | |
| 7 | 2.4 ± 0.7 | 29 |
Mean ± standard deviation (n = 6).
Recovery from soils.
Initial experiments were conducted with NaCl, ZnSO4, and cold sucrose flotation solutions (n = 6) by using a Tris-Tween 80 dispersing solution and magnetic stirring for 15 min. The percentages of recovery were as follows: ZnSO4, 0.8% ± 0.4%; cold sucrose, 2.8% ± 0.9%; and NaCl, 16.6% ± 2.3%. The percentages of recovery with NaCl were significantly higher (P < 0.05) than the percentages of recovery with ZnSO4 or cold sucrose.
PBS, H2O, and three detergent dispersing solutions were evaluated in conjunction with magnetic stirring for 15 min. The order for percentage of oocyst recovery was as follows: PBS and H2O < 1% Tween 80 <1% Triton X-100 < 50 mM Tris–0.5% Tween 80 (Table 5). The percentages of recovery with Tris-Tween 80 were significantly higher (P < 0.05) than the percentages of recovery with the other dispersants. The time of dispersion was evaluated by using 50 mM Tris–0.5% Tween 80. The percentages of recovery increased ca. threefold from 5 min (5.7% ± 0.5%) to 15 min (18.1% ± 1.9%) but did not increase after 30 min (18.0% ± 3.2%). The percentages of recovery after 15 or 30 min were significantly higher (P < 0.05) than the percentages of recovery after 5 min.
TABLE 5.
Percentages of recovery of purified oocysts from sandy loam soil as a function of the dispersing solution
| Dispersing solution | % Recoverya | No. of oocysts/10 ml | Coefficient of variation (%) |
|---|---|---|---|
| Distilled H2O | 0.06 | 2.5 ± 2.6b | 104 |
| PBS | 0.02 | 0.7 ± 0.3b | 43 |
| Tween 80 | 3.2 | 172 ± 12.3c | 7.2 |
| Triton X-100 | 5.9 | 236 ± 52.9d | 22 |
| Tris-Tween 80 | 14.7 | 588 ± 121c | 21 |
The initial concentration was 4 × 104 oocysts per 25 g of soil. The least significant difference (P < 0.05) was 2.2%.
Mean ± standard deviation (n = 4). The value must be multiplied by 10 to obtain the percentage of recovery.
Mean ± standard deviation (n = 6). The value must be multiplied by 10 to obtain the percentage of recovery.
Mean ± standard deviation (n = 12). The value must be multiplied by 10 to obtain the percentage of recovery.
Different methods of dispersion were evaluated by using soil leaching cores ca. 48 h after experimental treatments were used (unpublished data). The numbers of oocysts detected were as follows: blender, 14.0 ± 0.5 oocysts/10 μl; sonication, 21.5 ± 0.7 oocysts/10 μl; and magnetic stirrer, 25.0 ± 1.4 oocysts/10 μl. Because of limited replication (n = 2), we could not conclude that the percentages of oocyst recovery with magnetic stirring were significantly different than the percentages of oocyst recovery with sonication.
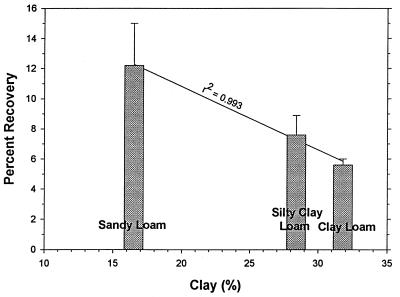
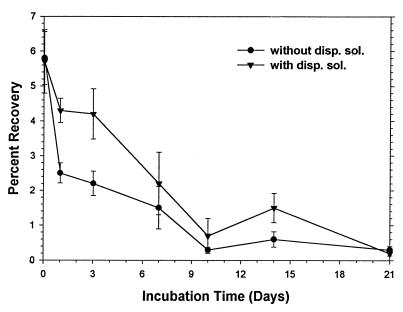
Percentages of oocyst recovery were determined for freshly spiked sandy loam, silty clay loam, and clay loam soils (Fig. 2). The percentages of recovery decreased as the clay content increased. A linear regression analysis of percentage of recovery versus clay content gave an r2 value of 0.993. Percentages of recovery were evaluated as a function of storage with and without the addition of a dispersing solution (Tris-Tween 80). With or without the dispersing solution, the percentages of recovery decreased ca. threefold after 1 h (Fig. 3). The percentages of recovery continued to slowly decrease for 10 days, although the percentages of recovery were generally higher when the dispersing solution was added.
FIG. 2.
Mean percentages of oocyst (purified) recovery from freshly spiked sandy loam, silty clay loam, and clay loam soils. The error bars indicate standard deviations (n = 6). The initial oocyst concentration was 104 oocysts per 25 g of soil.
FIG. 3.
Plot of percentages of oocyst (from diluted calf diarrhea) recovery from sandy loam soil versus storage time with and without dispersing solution (disp. sol.) (Tris-Tween 80). The error bars indicate standard deviations (n = 6). The initial oocyst concentration was 104 oocysts per 25 g of soil. The first sample was obtained 1 h after spiking.
DISCUSSION
The goal of these experiments was to identify a method which can be used for routine detection and enumeration of low levels of C. parvum oocysts in feces, manures, soils, and soil-manure mixtures. Primary consideration was given to percentages of recovery, the amounts of debris in the final oocyst preparations, processing time, and expense.
In our hands, the NaCl flotation method generally gave the highest percentages of recovery from calf feces; in addition, the processing times with this method were relatively short (<3 h), and this method was the least expensive method tested. Although other methods gave cleaner preparations (CsCl and Percoll gradient centrifugation) or resulted in less variability (MgSO4 flotation), they were deficient in other areas considered. Commonly used methods, such as formalin-ethyl acetate sedimentation (6, 24–26, 30, 31) and sucrose flotation (5, 15, 20, 26), were generally inferior because of lower percentages of recovery, greater background fecal debris levels, or greater variability. Xiao and Herd (28) have described a method (“quantitative FA”) which requires no flotation or gradient centrifugation procedure. Using a concentration of 103 oocysts g of calf feces−1, these authors obtained a percentage of recovery of 14.8% and a coefficient of variation of 47.1%. Given the simplicity of their method (one clarification step and one centrifugation step) and the excellent percentages of recovery at higher oocyst concentrations, it appears that this method is preferable for relatively highly contaminated fecal samples. However, as described by Xiao and Herd, the quantitative FA method has a detection limit of ca. 700 oocysts g−1 (reciprocal of 0.15 × 0.01). By comparison, the detection limit of our method is ca. 30 oocysts g−1 because of the 10-fold concentration step and because 30% of the final oocyst suspension is counted.
The percentages of oocyst recovery from fresh bovine feces were relatively consistent over a wide range of oocyst concentrations (25 to 105 oocysts g−1) when the NaCl flotation method was used; the r2 value was 0.996. Therefore, it appears to be reasonable to quantify oocyst concentrations based on a single correction factor (percentage of recovery) for a given feces type; separate correction factors must be determined for different fecal types. Note that previously described methods have been evaluated by extracting fecal samples immediately after spiking. Consequently, the reported percentages of recovery may overestimate the levels of recovery from stored fecal samples depending on the extent of oocyst adhesion to fecal solids.
Since manures typically are stored for different periods of time, we attempted to simulate a contaminated manure slurry by spiking a preparation with an infected calf diarrhea sample, thoroughly mixing it, and then storing it. The percentages of oocyst recovery were substantially lower for the stored bovine manure than for fresh fecal samples, indicating that correction factors for feces are not applicable to manures. We suspect that the lower percentages of oocyst recovery were primarily due to enhanced adhesion of oocysts to fecal particles during storage, although the possibility that decomposition occurred cannot be ruled out. Experiments were conducted with Tris-Tween 80 as the dispersing solution in an attempt to improve the percentages of recovery. The percentages of recovery were initially higher (on days 3 and 4), but then they decreased. It is unclear why this occurred. Low percentages of recovery complicate attempts to quantify oocyst loading rates from manures (the numbers of oocysts applied per square meter or hectare). For example, if it is assumed that manure accumulates from a 100-animal herd for 1 week, that the fecal excretion rate is 40 kg of feces cow−1 day−1, that the preparation is diluted ca. 50% with urine and water, and that the percentage of recovery is 2.5%, a minimum of ca. 7.5 billion oocysts would be required to obtain a positive sample, and each 0.1% change in the percentage of recovery would correspond to ca. 300 million oocysts.
Experiments were conducted to assess the levels of oocyst recovery from soils with several previously described flotation solutions, including NaCl, ZnSO4, and cold sucrose. The ideal flotation solution should have (i) a relatively high specific gravity, (ii) low viscosity, and (iii) the ability to disperse clay and silt soil particles. In theory, because of the great difference in the densities of oocysts and soil particles (ca. 1.05 and 2.65 g ml−1, respectively), separation of oocysts from soil particles should be simple. In contrast to a previous report (1), our data indicates that oocysts adhere to soil particles, as shown by percentages of recovery of <0.1% when distilled water or PBS was used as the dispersant. Consequently, the levels of oocyst recovery depend on both the physical separation of oocysts from soil particles and the dispersion of soil particles during sedimentation, as well as the specific gravity of the flotation solution. For example, if it is assumed that an oocyst with a spherical radius of 2.5 μm adheres to a soil particle that is half its size (spherical radius, 1.25 μm), the resulting oocyst-soil particle aggregate should have a composite density of ca. 1.24 g ml−1.
We obtained the highest percentages of recovery with NaCl flotation. We suspect that this was due primarily to the ability of monovalent cations to disperse soil particles, which minimized entrapment of oocysts or oocyst-soil particle aggregates during sedimentation. Substantially lower percentages of recovery were obtained with ZnSO4 flotation, despite the higher specific gravity of ZnSO4. We suspect that this was due primarily to the tendency of divalent cations to precipitate soil particles, which entrapped oocysts or oocyst-soil particle aggregates during sedimentation. We also obtained lower percentages of recovery with cold sucrose flotation. We suspect that this was due to a combination of lower specific gravity and higher viscosity. Oocyst detection was difficult due to high soil particle background levels in wells resulting from poor sedimentation of soil particles.
Different dispersing solutions, dispersion times, and dispersion procedures were evaluated to optimize levels of oocyst recovery. Our results obtained with dispersing solutions are consistent with the results of Mawdsley et al. (14), who obtained their highest levels of recovery with Tris-Tween 80. The highest levels of oocyst recovery were obtained with magnetic stirring, which is a relatively mild procedure. By comparison, the highest levels of bacterial recovery from soils are typically observed with blending (4). We suspect that the shear forces created by blending were too severe for oocyst walls. After blending, large numbers of what appeared to be fluorescent wall fragments were observed in oocyst preparations.
The percentage of oocyst recovery was linearly correlated (r2 = 0.993) with clay content (soil particle diameters, ≤2 μm). These data suggest that for mineral soils, it may be possible to predict levels of oocyst recovery based on soil texture data. More soil types are required, however, to verify this relationship. The detection limit of our method is ca. 1 to 2 oocysts g of soil−1 depending on the soil type (reciprocal of percentage of recovery × fraction counted). The detection limit reported by Mawdsley et al. (14) was 529 oocysts g of clay loam soil−1. Walker et al. (23) obtained detection limits of <40 oocysts g of silt loam soil−1 by including a final concentration step. Although our extraction procedure is somewhat more complicated than that of Walker et al. (23), the larger sample size means that fewer samples can be used.
Levels of oocyst recovery from soils also depend on incubation or storage time. The percentages of recovery from sandy loam soil decreased to <1% within 10 days. Our results are similar to those of Mawdsley et al. (14), who observed a >99% decrease in the level of recovery after 1 week of incubation. It is unclear to what extent this is due to adhesion to soil particles or to decomposition. The addition of a dispersing solution (Tris-Tween 80) to soil samples enhanced the levels of oocyst recovery, suggesting that the initial decreases in percentages of recovery were due primarily to adhesion to soil particles. In addition, storage of soil samples at 4°C should have minimized the decomposition rates. Adhesion of oocysts to soil particles does not preclude decomposition. It does, however, complicate attempts to estimate decomposition rates and to quantify oocyst loading rates.
It is unclear whether the NaCl flotation method is compatible with oocyst viability testing. Gradient centrifugation methods, such as the CsCl and discontinuous Percoll methods, are most commonly used to purify oocysts from feces for viability testing (9). ZnSO4 and cold sucrose flotation methods have also been shown to be compatible with viability testing, although they selectively concentrate viable oocysts (5). It is questionable whether there is any method which is suitable for quantitative recovery of oocysts from a wide range of environmental matrices and is compatible with viability testing.
In conclusion, the NaCl flotation method appears to be suitable for routine detection and enumeration of C. parvum oocysts in a variety of environmental matrices, including feces, manures, soils, and soil-manure mixtures. Further research is needed to elucidate the mechanisms of oocyst-manure and oocyst-soil interactions in order to improve the levels of recovery and to estimate decomposition and mortality rates in manures and soils.
ACKNOWLEDGMENTS
We thank Valerie McPhatter and Nicolle Farmer for technical assistance and Ron Fayer, Jim Trout, and Colleen Carpenter (Immunology and Disease Resistance Laboratory, Beltsville Agricultural Research Center, Beltsville, Md.) for oocyst preparations.
REFERENCES
- 1.Anguish L J, Ghiorse W C. Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl Environ Microbiol. 1997;63:724–733. doi: 10.1128/aem.63.2.724-733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 3.Atwill E R, Sweitzer R A, Perira M, Gardiner I, van Vuren D, Boyce W. Prevalence of and associated risk factors for shedding Cryptosporidium oocysts and Giardia cysts within feral pig populations in California. Appl Environ Microbiol. 1997;63:3946–3949. doi: 10.1128/aem.63.10.3946-3949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloem J, Bolhuis P R, Veninga M R, Wieringa J. Microscopic methods for counting bacteria and fungi in soil. In: Kassem A, Nannipieri P, editors. Methods in applied soil microbiology and biochemistry. New York, N.Y: Academic Press; 1995. pp. 162–173. [Google Scholar]
- 5.Bukhari Z, Smith H U. Effect of three concentration techniques on viability of Cryptosporidium parvum oocysts recovered from bovine feces. J Clin Microbiol. 1995;33:2592–2595. doi: 10.1128/jcm.33.10.2592-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chichino G, Bruno A, Cevini C, Atzori C, Gatti S, Scaglia M. New rapid staining methods of Cryptosporidium oocysts in stools. J Protozool. 1991;38:212S–214S. [PubMed] [Google Scholar]
- 7.Environmental Protection Agency. Proposed general NPDES permit for concentrated animal feeding operations (CAFO) Fed Reg. 1995;60:44489–44498. [Google Scholar]
- 8.Fayer R, Graczyk T K, Lewis E J, Trout J M, Farley C A. Survival of infectious Cryptosporidium parvum oocysts in seawater and eastern oysters (Crassostrea virginica) in the Chesapeake Bay. Appl Environ Microbiol. 1998;64:1070–1074. doi: 10.1128/aem.64.3.1070-1074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fayer R, Ellis W. Paromomycin is effective as prophylaxis for cryptosporidiosis in dairy calves. J Parasitol. 1993;79:771–774. [PubMed] [Google Scholar]
- 10.Garber L P, Salman M D, Hurd H S, Keefe T, Schlater J L. Potential risk factors for Cryptosporidium infection in dairy calves. J Am Vet Med Assoc. 1994;205:86–91. [PubMed] [Google Scholar]
- 11.Hansen J S, Ongerth J E. Effects of time and watershed characteristics on the concentration of Cryptosporidium oocysts in river water. Appl Environ Microbiol. 1991;57:2790–2795. doi: 10.1128/aem.57.10.2790-2795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilani R, Sekla L. Purification of Cryptosporidium oocysts and sporozoites by cesium chloride and Percoll gradients. Am J Trop Med Hyg. 1987;36:505–508. doi: 10.4269/ajtmh.1987.36.505. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie W R, Hoxie N J, Proctor M E, Gradus M S, Blair K A, Peterson D E, Kazmierczak J J, Addiss D A, Fox K R, Rose J R, Davis J P. A massive outbreak of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 14.Mawdsley J L, Brooks A E, Merry R J. Movement of the protozoan pathogen Cryptosporidium parvum through three contrasting soil types. Biol Fertil Soils. 1996;21:30–36. [Google Scholar]
- 15.Mtambo M M A, Nash A S, Blewett D A, Wright S. Comparison of staining and concentration techniques for detection of Cryptosporidium oocysts in cat faecal specimens. Vet Parasitol. 1992;45:49–57. doi: 10.1016/0304-4017(92)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Ong C, Moorehead W, Ross A, Isaac-Renton J. Studies of Giardia spp. and Cryptosporidium spp. in two adjacent watersheds. Appl Environ Microbiol. 1996;62:2798–2805. doi: 10.1128/aem.62.8.2798-2805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeters J, Villacarta I. Cryptosporidium. In: Eckert J, Braun R, Shirley M W, Coudert P, editors. Guidelines on techniques in coccidiosis research. European Commission Directorate-General XII, Science, Research and Development Environment Research Program. 1995. p. 220. [Google Scholar]
- 18.Scott C A, Smith H V, Gibbs H A. Excretion of Cryptosporidium parvum oocysts by a herd of beef suckler cows. Vet Rec. 1994;134:172. doi: 10.1136/vr.134.7.172. [DOI] [PubMed] [Google Scholar]
- 19.States S, Stadterman K, Ammon L, Vogel P, Baldizar J, Wright D, Conley L, Sykora J. Protozoa in river water: sources, occurrence, and treatment. J Am Water Works Assoc. 1997;74:75–83. [Google Scholar]
- 20.Suresh P, Jerold R. Comparative evaluation of several techniques for purification of Cryptosporidium parvum oocysts from rat feces. J Clin Microbiol. 1996;34:38–40. doi: 10.1128/jcm.34.1.38-40.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacal V, Jr, Sobieh M, El-ahraf A. Cryptosporidium in market pigs in southern California, USA. Vet Rec. 1987;120:615–617. doi: 10.1136/vr.120.26.615. [DOI] [PubMed] [Google Scholar]
- 22.Vetterling J. Continuous-flow differential density flotation of coccidial oocysts and a comparison with other methods. J Parasitol. 1969;55:412–417. [PubMed] [Google Scholar]
- 23.Walker M J, Montemagno C, Bryant J C, Ghiorse W. Method detection limits of PCR and immunofluorescence assay for Cryptosporidium parvum in soil. Appl Environ Microbiol. 1998;64:2281–2283. doi: 10.1128/aem.64.6.2281-2283.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber R, Ralph B, Bishop H, Wahlquist S, Sullivan J, Juranek D. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J Clin Microbiol. 1991;29:1323–1327. doi: 10.1128/jcm.29.7.1323-1327.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber R, Bryan R T, Juranek D D. Improved stool concentration procedure for detection of Cryptosporidium oocysts in fecal specimens. J Clin Microbiol. 1992;30:2869–2873. doi: 10.1128/jcm.30.11.2869-2873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webster K A, Smith H V, Giles M, Dawson L, Robertson L J. Detection of Cryptosporidium parvum oocysts in feces: comparison of conventional coproscopical methods and the polymerase chain reaction. Vet Parasitol. 1996;61:5–13. doi: 10.1016/0304-4017(95)00811-x. [DOI] [PubMed] [Google Scholar]
- 27.Weil R R. A laboratory manual for general soils. 4th ed. Needham Heights, Mass: Ginn Press; 1989. pp. 23–32. [Google Scholar]
- 28.Xiao L, Herd R P. Quantitation of Giardia and Cryptosporidium oocysts in fecal samples by direct immunofluorescence assay. J Clin Microbiol. 1993;31:2944–2956. doi: 10.1128/jcm.31.11.2944-2946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao L, Herd R P. Review of equine Cryptosporidium infection. Equine Vet J. 1994;26:9–13. doi: 10.1111/j.2042-3306.1994.tb04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao L, Herd R P, McClure K E. Periparturient rise in the excretion of Giardia sp. cysts and Cryptosporidium parvum oocysts as a source of infection for lambs. J Parasitol. 1994;80:55–59. [PubMed] [Google Scholar]
- 31.Young K H, Bullock S L, Melvin D M, Sprivil C L. Ethylacetate as a substitute for diethylether in the formalin-ether sedimentation technique. J Clin Microbiol. 1979;10:852–853. doi: 10.1128/jcm.10.6.852-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zierdt W. Concentration and identification of Cryptosporidium sp. by use of a parasite concentrator. J Clin Microbiol. 1984;20:860–861. doi: 10.1128/jcm.20.5.860-861.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]