Abstract
Background
The gold standard for detecting bladder cancer is white light cystoscopy (WLC) and resection of suspicious lesions. In this study, we evaluate two miniaturized Optical Coherence Tomography (OCT) probes for endoscopic use, regarding their applicability in diagnosing urothelial cancer.
Materials and methods
In total, 33 patients who underwent a radical cystectomy were included. Preoperative oncological staging and determining the indication for the surgical intervention were done following the latest European Association of Urology (EAU) guidelines. Samples were taken from bladder tissue after bladder removal and prepared for OCT measurement. Additionally, porcine bladder samples were used as reference tissue. We took measurements using two miniaturized probes: a bimodal probe and a single modality OCT probe. A non-miniaturized standard OCT scanner was used as a reference.
Results
Histopathological examination revealed urothelial cancer in all but three patients. Measurements on porcine tissue revealed a clear distinction between the urothelial layers for all probes. Furthermore, we detected improved image quality thanks to the stretching of the tissue. We took 271 measurements in human samples. While the urothelial layers were well delineated in healthy tissue, all the probes revealed a loss of these structures in cancerous regions. While the single-modality probe delivered an image quality equaling the reference images, it was possible to detect cancerous areas with the bimodal probe.
Conclusion
We demonstrate that endoscopic probes for OCT imaging are technologically feasible and deliver acceptable image quality. A distinction between healthy and abnormal tissue is possible. We propose combining different endoscopic imaging modalities as a promising approach for urothelial cancer diagnostics.
Keywords: Endoscopy, Tomography, Optical coherence, Urinary bladder neoplasms, Diagnostic techniques and procedures
Highlights
-
•
Application of Optical Coherence Tomography (OCT) probes for the detection of bladder cancer.
-
•
Evaluation of image quality on porcine bladder samples as well as human tissue samples of bladder cancer.
-
•
Detection of cancerous tissue was possible in all samples.
-
•
Image quality was acceptable in all miniaturized probes.
The gold standard for detecting bladder cancer is white light cystoscopy (WLC) and resection of suspicious lesions. In this study, we evaluate two miniaturized Optical Coherence Tomography (OCT) probes for endoscopic use, regarding their applicability in diagnosing urothelial cancer. In total, 33 patients who underwent a radical cystectomy were included. Preoperative oncological staging and determining the indication for the surgical intervention were done following the latest European Association of Urology (EAU) guidelines. Samples were taken from bladder tissue after bladder removal and prepared for OCT measurement. Additionally, porcine bladder samples were used as reference tissue. We took measurements using two miniaturized probes: a bimodal probe and a single modality OCT probe. A non-miniaturized standard OCT scanner was used as a reference. Histopathological examination revealed urothelial cancer in all but three patients. Measurements on porcine tissue revealed a clear distinction between the urothelial layers for all probes. Furthermore, we detected improved image quality thanks to the stretching of the tissue. We took 271 measurements in human samples. While the urothelial layers were well delineated in healthy tissue, all the probes revealed a loss of these structures in cancerous regions. While the single-modality probe delivered an image quality equaling the reference images, it was possible to detect cancerous areas with the bimodal probe. We demonstrate that endoscopic probes for OCT imaging are technologically feasible and deliver acceptable image quality. A distinction between healthy and abnormal tissue is possible. We propose combining different endoscopic imaging modalities as a promising approach for urothelial cancer diagnostics.
1. Introduction
Innovative imaging modalities for detecting anatomic anomalies and pathological transformation have changed every day clinical practice over recent decades. Optical modalities such as Narrow Band imaging (NBI), Raman spectroscopy (RS) and optical coherence tomography (OCT) have proven to be valuable tools in various fields. OCT has become a standard diagnostic tool especially in ophthalmology for assessing pathologies in the human retina [1]. OCT, first described in 1991 [2], is a technique relying on low coherence light interferometry, that allows cross-sectional views of tissue structure manifested in refractive index variations [3]. Since it is non-invasive and has no destructive effect on the tissue being examined, it is being applied to diagnose various types of carcinomas such as lung, breast, and colon cancer [4]. The focus on applying OCT in urology has been in diagnosing bladder cancer. The current gold standard for detecting bladder cancer is white light cystoscopy (WLC) and transurethral resection for macroscopically suspicious lesions. This diagnosis depends on the resected tissue's being examined by a pathologist. As bladder cancer has the highest recurrence rate among all cancer types [5], a non-invasive alternative for assessing macroscopically suspicious lesions is urgently needed. There is evidence that OCT can reveal a structural difference in healthy bladder tissue when compared to various stages of bladder cancer [6]; a recent meta-analysis including 11 studies and a total of 1933 OCT-evaluated bladder lesions demonstrated 94.9% sensitivity and 84.6% specificity in detecting bladder cancer [7]. This technology can be therefore potentially applied to differentiate between malignant and benign bladder lesions and avoid an unnecessary transurethral bladder resection. It remains difficult, however, to apply this technology during standard cystoscopy as there are no commercially available endoscopic tools small enough for intravesical application during cystoscopy, and most studies have been done in animal models or on explanted tissue so far. We evaluated two miniaturized OCT probes developed for endoscopic use regarding their applicability and utility in diagnosing bladder cancer; we also assessed their image quality using ex vivo human tissue samples originating from post-cystectomy patients.
2. Methods
2.1. Patient cohort
A total of 33 patients who underwent a radical cystectomy for bladder cancer in our department between March 2017 and March 2019 were included in this study. Preoperative oncological staging and determining the indication for the aforementioned surgical intervention were done following the latest European Association of Urology (EAU) guidelines on bladder cancer prior to study inclusion. The study was approved by our local ethics committee (Institutional Ethics Committee of the University of Freiburg, Germany (Project-ID: 579/16) and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments). All participants gave their informed consent before being enrolled in this study. Exclusion criteria were involvement in any other clinical trial, dementia, emergency patients and patients without the ability to consent, but none of these applied since no patient screened fulfilled any exclusion criterion. The study was designed and conducted in accordance with the consort criteria.
2.2. Human tissue experiments
Samples were taken from bladder tissue immediately after bladder removal during radical cystectomy. For this purpose, the removed organ was inspected by a board-certified pathologist and a representative sample measuring about 1.5 cm × 0.5 cm × 0.5 cm was removed from the organ for OCT measurements. The sample was taken from the edge of the macroscopic tumor in order to contain tumor tissue as well as healthy urothelial mucosa. The sample's lateral edges l were color-oded to document the orientation. The tissue sample was fixated using a 4% formaldehyde solution. After completing the OCT measurements, the tissue was returned to the pathologist for a full histopathological examination for diagnostic purposes and as a reference for assessing the OCT images. After preservation at room temperature for 72–96 h, the tissue was embedded in paraffin. Slices for staining were cut using a microtome and HE staining was performed afterwards according to standard protocol of the Institute of Surgical Pathology at the University of Freiburg Medical Center.
2.3. Animal tissue experiments
Porcine bladder tissue was examined as reference tissue to evaluate the OCT measurements on unchanged, unfixated, healthy urothelial tissue. Samples were taken from fresh bladder specimens immediately after the animal was slaughtered for food-production purposes at Emil Färber GmbH & Co. KG, Freiburg, Germany. The porcine bladder specimens were cut to obtain bladder samples measuring 2 cm × 1 cm x 0.8 cm. OCT measurements were then taken on the unfixated tissue. For reference purposes, we also took measurements on samples fixated in 4% formaldehyde solution for 48 h.
2.4. OCT engines
We applied a custom-developed OCT system featuring an akinetic swept laser source from Insight Photonic Solutions (Lafayette, CO USA)with a maximum A-Scan rate of 174 KHz and a sweep range of 90 nm around the center wavelength of 1310 nm. The expected axial resolution for the given center wavelength and sweep range is 8.3 μm in air. The light source was connected to a fiber-based Michelson interferometer with a 90:10 splitting ratio between the sample arm and reference arm, respectively. The back-coupled light from the two arms was decoupled from the rest of the setup using two optical circulators, which were connected to a 50:50 beam splitter to interfere with the signals from the reference and sample arms. A dual-balanced photodetector recorded the OCT interferogram [8].
2.5. Miniaturized OCT probes and reference system
In this study, we evaluated two different miniaturized probe prototypes developed for endoscopic OCT imaging, and compared their imaging performance with that of a bench-top system. The first probe (OCT probe 1; OCT-only) was 11.3 mm long and had an outer diameter of 4 mm. With 12.4 μm lateral resolution, and a maximum 1.6 mm field-of-view (FOV), this probe had been optimized for OCT imaging. To scan the imaging beam, and thus provide 3-dimensional tomograms, the probe featured a scanner comprising a fiber cantilever driven by a tubular piezoelectric actuator. The particularly compact design, which renders the probe suitable for flexible potential endoscopy applications, was enabled by the direct integration of a scan lens at the tip of the fiber cantilever with a 3D nano-printed mechanical interface. Further information on the design and opto-mechanical performance of this probe is found in Shah et al., 2019 [9].
The second probe (OCT probe 2; bimodal) evaluated in this study combines two optical modalities, OCT and full-field white light videomicroscopy in one miniaturized probe. This probe is 15 mm long and its maximum diameter 3.6 mm. The OCT mode's lateral resolution is 43.71 μm, the FOV is 1.2 mm, and depth-of-field is 2.7 mm. It has two isolated beam paths that share the same field of view, therefore giving the user the means of employing both modalities simultaneously. In the present study, we only applied/engaged the OCT modality. As the first probe, the bimodal one also features a similar beam scanner as well. Further information on the bimodal probe's design and opto-mechanical performance is found in Kretschmer et al., 2018 [10].
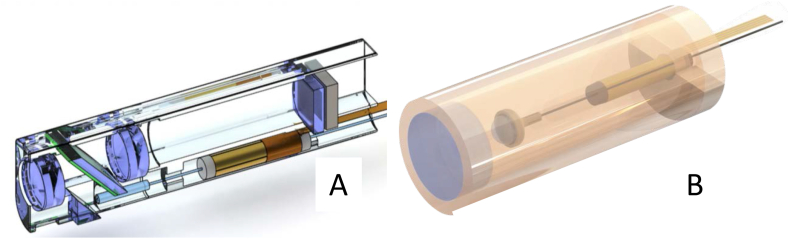
All probes used in this study are depicted in Fig. 1. For reference measurements, we used a conventional bench-top OCT setup with a two-dimensional galvanometric scanner from Thorlabs (Ely, Cambridgeshire, England). Combined with a scan-lens, this setup provided a FOV of 15 mm and lateral resolution of 13 μm.
Fig. 1.
Diagram of all probes used in this study. (A = OCT probe 2, bimodal; B=OCT probe 3, nanoscribe).
2.6. Data evaluation
All OCT images were optimized for contrast and brightness using specifically programmed post-processing software. All OCT images recorded by the reference probe were then correlated with their respective counterpart on the HE stain of its respective tissue sample. For all measurements taken using the reference probe, each sample was completely covered longitudinally and transversally. For the miniaturized probes, due to their limited FOV, sample measurements were taken covering not the entire sample, but taking representative measurements in the tumor area, the healthy tissue area, as well as in the transition zone. All morphological abnormalities any histological structures such as blood vessels, fatty tissue and others correctly detected in the OCT image were marked. In addition, we evaluated the location of the HE stain corresponding to the respective OCT scan regarding the presence of tumor tissue, as well as the penetration depth and tumor-tissue grading. An experienced histopathologist carried out these steps (G. K). Each of the marked reference images were compared consecutively with the respective OCT measurements taken by the miniaturized probes in terms of detecting marked structures and for general image quality and any artefacts.
3. Results
3.1. Demographics and histopathology
This study included 12 female and 21 male patients. Average age was 69.9 years ranging from 47 to 86 years. Histopathological examination of the bladder specimens revealed urothelial cancer in all except for 3 patients; one was a suspected recurring bladder cancer not proven in the pathological examination, in another, no tumor was detected following neoadjuvant chemotherapy, and the third patient had an invasive adenocarcinoma. Three patients had a pT1 tumor, eight a pT2a tumor, one a pT2b, another three a pT3a, nine had a pT3b, and four a pT4a. One carcinoma was detected in situ and another, a patient after radiation therapy, presented a pTa tumor.
3.2. Measurements on porcine tissue samples
We took 193 measurements on 15 different porcine tissue samples via the SS-OCT setup. 60 measurements had to be excluded because of artefacts. We assessed a total of 33 measurements using the reference probe, 44 using the bimodal OCT probe, and 56 using the OCT-only probe. Comparison of the HE stain and images from the reference probe revealed a clear distinction between the urothelial layers in the OCT scan including the detection of the M. detrusor vesicae. Comparing the images generated by the miniaturized probes yielded a similar result; however, the bimodal probe to distinguish the individual urothelial layers in OCT was inferior to the other probes. The OCT-only probe delivered an image quality equivalent to the reference probe. Fig. 2 illustrates an example of the image quality resulting from these measurements. Scans with each modality were done on stretched bladder tissue and on relaxed tissue. We observed significantly improved image quality through the stretched tissue. In all samples the tissue layers were more distinguished and since the thickness of the urothelial layer decreased the penetration of tissue was improved as well.
Fig. 2.
OCT measurements of porcine bladder tissue. The images above depict the scan developed with the reference probe (left side on stretched bladder tissue, right side on relaxed bladder tissue), the scans of the miniaturized probes (stretched tissue) are depicted below.(U = urothelial layer, LP = lamina propria, M = M. detrusor vesicae).
3.3. Correlation with histological tissue features in human tissue samples
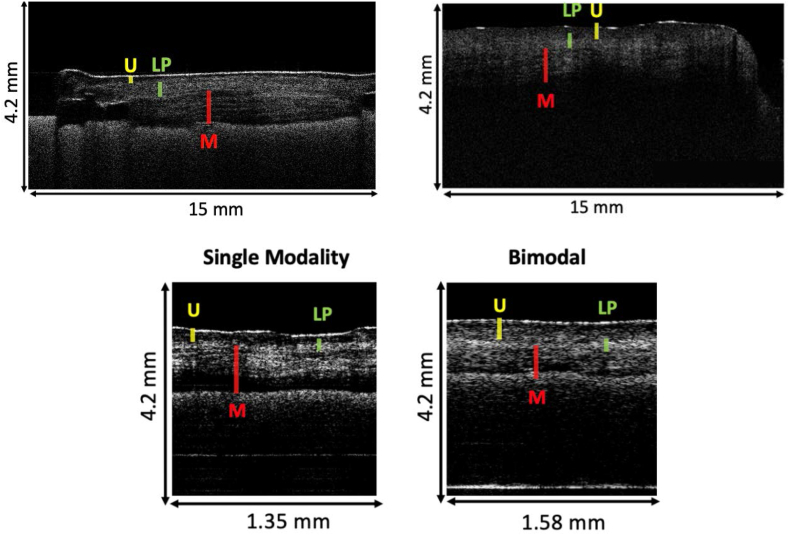
We took 128 reference measurements in human tissue samples, 104 with the bimodal probe and 39 with the OCT-only probe. While the urothelial layers could be distinguished in healthy tissue with all probes, when the tissue was cancerous, the clarity between these structures was lost - they lost their homogeneity when the probes were measuring cancerous tissue, and it became impossible to differentiate between histological tumor stages. Fig. 3 provides an example of images resulting from these measurements. While a single-modality probe with a 2.7 mm depth of focus and 12.4 μm lateral resolution yielded image quality equaling the reference image's, cancerous areas were detectable with the bimodal probe, however, with a slight loss of image quality (Fig. 4).
Fig. 3.
Depiction of OCT measurement with the reference OCT probe and respective HE stain in the same region. While the urothelial layers can be clearly distinguished on the right side of the OCT scan, this distinction is lost on the left side, where a pT3 urothelial cancer is present (arrow) in the histology. Because of the OCT signal's limited penetration, it is not possible to determine the invasion depth on the OCT scan. (U = urothelial layer, LP = lamina propria, M = M. detrusor vesicae).
Fig. 4.
Depiction of an OCT scan using the miniaturized bimodal OCT probe of healthy human bladder tissue (right side) and urothelial cancer tissue (T3 tumor in the respective histological examination; left side, arrow). While in the healthy region a clear distinction can be made between the bladder wall layers, this distinction is clearly lost in the tumor region. (U = urothelial layer, LP = lamina propria, M = M. detrusor vesicae).
4. Discussion
OCT is a promising technology in the field of urology for diagnosing urothelial cancer. In this study we demonstrate the feasibility of miniaturized, endoscopic OCT probes to provide comparable image quality similar to conventional OCT systems. Various studies have shown the ability of OCT to distinguish the distinct layers of the bladder wall and structural differences in malignant areas. A recent meta-analysis reported OCT's 94.9% sensitivity and 84.6% specificity for detecting bladder cancer [7]. We were able in this study to reproduce these results using miniaturized probes suitable for application in standard cystoscopy. Our data shows for the first time, that it is technologically feasible to miniaturize the OCT technology while still providing sufficient image quality to detect the structural tissue characteristics specific for bladder cancer. This provides a potential benefit for the clinical practice, since this technology allows it to non-invasivally evaluate potentially malignant bladder lesions, reducing the number of unnecessary transurethral bladder resections. The current challenge associated with applying this technology is the diagnostic accuracy when taking real time measurements. Only one study to date has provided a real-time analysis [11]. In our study, real-time images were processed by a specifically developed image processing software, and the evaluation was done on the processed images, thus a real-time evaluation was not possible and will have to be investigated in future studies. However, the implementation of ad hoc processing and the display of real-time images are technically feasible provided enough computation power is available. Furthermore, while the image quality suffices for detecting bladder cancer, assessing the invasion depth is difficult, since the OCT's penetration depth is limited. Many new optical technologies with intriguing capabilities are being developed, like label-free specific spectroscopy-based approaches such as Raman spectroscopy (RS). By combining different modalities, we may eventually prove able to harvest the full potential of the diagnostic capabilities of modern optical technologies. We show in our study the feasibility of manufacturing a multi-modal OCT probe combining white-light cystoscopy and OCT while providing sufficient image quality. A recent study [12] on the ex vivo detection and grading of non-muscle invasive bladder cancer assessed a multimodal probe combining RS and OCT and demonstrated the feasibility of using these complementary technologies in one probe while providing 78% sensitivity and 69% specificity when detecting non-muscle-invasive bladder (OCT) and 81% sensitivity and 61% specificity (RS). It will be up to future in vivo studies on the real-time application of these technologies to provide strong evidence of the clinical applicability of these technologies.
There are certain limitations of our study. Firstly, we only included human tissue samples originating from patients after cystectomy, therefore the tumor stages originated from invasive urothelial carcinoma, and we could not assess the ability of our OCT probes to distinguish between invasive cancer and superficial tumor stages. However, the ability of our miniaturized probes for the differentiation between malignant lesions and healthy bladder tissue does already provide a significant clinical benefit for the patient, since further invasive diagnostics can be avoided. It is to be assumed, that in cases of a malignant tumor, the degree of invasiveness of the tumor will still have to be assessed by transurethral resection, since this information will also determine, if a cystectomy has to be performed. Since a transurethral resection is also the standard therapeutic approach, this does depict a significant disadvantage of our technology. Also, the number of test subjects and the study design did not allow for comparative statistical calculations. Furthermore, although done on fresh tissue samples, all measurements were taken ex vivo. Future in vivo studies will have to confirm the functionality of our miniaturized probes during cystoscopy.
5. Conclusions
We show that the application of endoscopic probes for OCT imaging is technologically feasible and miniaturized probes deliver acceptable image quality. While a distinction between healthy and abnormal tissue is possible the infiltration depth is a limiting factor in OCT technology. We therefore propose combining different endoscopic imaging modalities (OCT, white light, etc.) as the most promising approach in the search for improvements in minimally invasive diagnostics in bladder cancer.
Sources of funding
No funding was received.
Ethical approval
This study was approved by our local ethics committee (Project-ID: 579/16, Ethics Committee of the University Medical Center Freiburg) and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent
All participants gave their informed consent prior to their inclusion in the study.
Author contribution
Dominik Stefan Schöb (D. S. S) Protocol/project development, conducting experiments, data collection and management, data analysis, manuscript writing.
Carolin Wollensak (C.W.) Data analysis, conducting experiments, data collection and management, data analysis.
Simon Kretschmer (S. K.) conducting experiments, data analysis.
Gerardo González-Cerdas (G. G-C.) conducting experiments, data analysis.
Caglar Ataman (C. A.) conducting experiments, data analysis.
Gian Kayser (G. K.) conducting experiments, data analysis.
Franz Friedrich Dressler (F.F.D.) conduction of experiments, data analysis.
Christian Gratzke (C. G.), M. D., Ph. D. manuscript and figure writing/editing, supervision.
Hans Zappe (H. Z.) Protocol/project development, manuscript and figure writing/editing, supervision.
Arkadiusz Miernik (A. M.), M.D., Ph.D. Protocol/project development, manuscript and figure writing/editing, supervision.
Registration of research studies
German Trial registry, ID: DRKS00011591, prospectively registered 13.01.2017: https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00011591.
Guarantor
Dominik Schöb.
Arkadiusz Miernik.
Hans Zappe.
Christian Gratzke.
Declaration of competing interest
Other authors have no conflict of interest or financial ties to disclose.
Contributor Information
Dominik Stefan Schoeb, Email: Dominik.stefan.schoeb@uniklinik-freiburg.de.
Carolin Wollensak, Email: carolin.wollensak@uniklinik-freiburg.de.
Simon Kretschmer, Email: simon.kretschmer@imtek.uni-freiburg.de.
Gerardo González-Cerdas, Email: gerardo.gonzalez@imtek.uni-freiburg.de.
Caglar Ataman, Email: caglar.ataman@imtek.uni-freiburg.de.
Gian Kayser, Email: gian.kayser@uniklinik-freiburg.de.
Franz Friedrich Dressler, Email: franzFriedrich.Dressler@uksh.de.
Christian Gratzke, Email: christian.gratzke@uniklinik-freiburg.de.
Hans Zappe, Email: hans.zappe@imtek.uni-freiburg.de.
Arkadiusz Miernik, Email: arkadiusz.miernik@uniklinik-freiburg.de.
References
- 1.Götze A. Universität; 2017. Die optische Kohärenztomographie zur Diagnostik früher neurodegenerativer Veränderungen der Retina bei pädiatrischen Patienten mit einem Diabetes mellitus Typ 1 oder einer chronischen Nierenerkrankung. [Google Scholar]
- 2.Huang D., Swanson E.A., Lin C.P., Schuman J.S., Stinson W.G., Chang W., Hee M.R., Flotte T., Gregory K., Puliafito C.A. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fercher A.F., Drexler W., Hitzenberger C.K., Lasser T. Optical coherence tomography-principles and applications. Rep. Prog. Phys. 2003;66(2):239. [Google Scholar]
- 4.van Manen L., Dijkstra J., Boccara C., Benoit E., Vahrmeijer A.L., Gora M.J., Mieog J.S.D. The clinical usefulness of optical coherence tomography during cancer interventions. J. Cancer Res. Clin. Oncol. 2018;144(10):1967–1990. doi: 10.1007/s00432-018-2690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauberg E.C., de Bruin D.M., Faber D.J., van Leeuwen T.G., de la Rosette J.J., de Reijke T.M. A new generation of optical diagnostics for bladder cancer: technology, diagnostic accuracy, and future applications. Eur. Urol. 2009;56(2):287–297. doi: 10.1016/j.eururo.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Schmidbauer J., Remzi M., Klatte T., Waldert M., Mauermann J., Susani M., Marberger M. Fluorescence cystoscopy with high-resolution optical coherence tomography imaging as an adjunct reduces false-positive findings in the diagnosis of urothelial carcinoma of the bladder. Eur. Urol. 2009;56(6):914–919. doi: 10.1016/j.eururo.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 7.Xiong Y.-Q., Tan J., Liu Y.-M., Li Y.-Z., You F.-F., Zhang M.-Y., Chen Q., Zou K., Sun X. Diagnostic accuracy of optical coherence tomography for bladder cancer: a systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2019;27:298–304. doi: 10.1016/j.pdpdt.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Kretschmer S. Albert-Ludwigs-Universität Freiburg im Breisgau; 2019. Multi-modal Endoscopic Probes for in Situ Tissue Inspection. [Google Scholar]
- 9.Shah R.N., Kretschmer S., Nehlich J., Ataman Ç., Zappe H. MOEMS and Miniaturized Systems XVIII. International Society for Optics and Photonics; 2019. Compact OCT probe for flexible endoscopy enabled by piezoelectric scanning of a fiber/lens assembly; p. 109310A. [Google Scholar]
- 10.Kretschmer S., Jäger J., Vilches S., Ataman Ç., Zappe H. A bimodal endoscopic imager in a glass package. J. Micromech. Microeng. 2018;28(10):105009. [Google Scholar]
- 11.Lerner S.P., Goh A.C., Tresser N.J., Shen S.S. Optical coherence tomography as an adjunct to white light cystoscopy for intravesical real-time imaging and staging of bladder cancer. Urology. 2008;72(1):133–137. doi: 10.1016/j.urology.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Placzek F., Bautista E.C., Kretschmer S., Wurster L.M., Knorr F., González-Cerdas G., Erkkilä M.T., Stein P., Ataman Ç., Hermann G.G. Morpho-molecular ex vivo detection and grading of non-muscle-invasive bladder cancer using forward imaging probe based multimodal optical coherence tomography and Raman spectroscopy. Analyst. 2020;145(4):1445–1456. doi: 10.1039/c9an01911a. [DOI] [PubMed] [Google Scholar]