Abstract
We produced monoclonal antibodies (MAbs) to the extracellular proteins of Listeria monocytogenes EGD grown in Chelex-treated improved minimal medium. Ten of the positive hybridomas generated were chosen for further characterization. Seven of the MAbs reacted with a protein having a molecular mass of 60 kDa. These MAbs inhibited listeriolysin (LLO)-mediated hemolysis, and two of them were specific for LLO and none of the other thiol-activated toxins tested. In an enzyme-linked immunosorbent assay and Western blot analysis, five of the anti-LLO MAbs reacted with ivanolysin from Listeria ivanovii. Three of the 10 MAbs reacted with a 29-kDa protein on Western blots and neutralized the phosphatidylcholine-specific phospholipase C (PC-PLC) activity of L. monocytogenes. These three anti-PC-PLC MAbs did not react with phospholipases from five different gram-positive bacteria. However, the anti-PC-PLC MAbs recognized a 27-kDa extracellular protein from L. ivanovii and neutralized sphingomyelinase activity in a hemolysis test that demonstrates the antigenic relatedness of listerial phospholipases. These data indicate that listerial thiol-activated toxins possess species-specific epitopes and share group-specific epitopes. This is the first description of MAbs that neutralize listerial PC-PLC, and the data suggest that there is antigenic similarity between L. monocytogenes PC-PLC and L. ivanovii sphingomyelinase. The reactions of the MAbs with catfish isolates of L. monocytogenes suggested that some of the isolates examined lack the LLO and/or PC-PLC required for pathogenicity. The MAbs described here differentiated some catfish isolates from previously described type strain-pathogenic isolates and could be useful for detecting and determining the virulence of L. monocytogenes in food and clinical samples and for detecting L. ivanovii in veterinary clinical samples.
Listeria monocytogenes has been known to be a human pathogen for more than 50 years. Fetuses, newborns, the elderly, and immunocompromised individuals are especially at risk of L. monocytogenes infection (23). Increased reports of human listeriosis in the last few decades and the direct association of many cases with contaminated foods have generated much interest in the etiologic agent, L. monocytogenes (5). In a recent survey workers found that the annual incidence of listeriosis was 7.4 cases per million people in the United States (23). Of the 13 known serotypes of L. monocytogenes, many of which are found in foods or the environment, only 3 (serotypes 1/2a, 1/2b, and 4b) are associated with the majority of human listeriosis cases. Serotype 4b accounts for 40% of sporadic listeriosis cases (44). L. monocytogenes is capable of growing over wide ranges of temperature (1 to 45°C), pH (pH 5 to 9), and osmolarity (1 to 10% NaCl), which makes this bacterium an ideal postprocessing food-contaminating agent (35, 39). Several reports have described the presence of L. monocytogenes in vegetable, dairy, and some meat products (19, 21, 29). One of the first documented cases of Listeria-contaminated seafood involved the detection of Listeria sp. in crabmeat in 1987. A review of the incidence of L. monocytogenes in fish and seafood has recently been published (30). Listeriosis is also of major veterinary importance, and the primary clinical manifestations in cattle are abortion, encephalitis, and mastitis (39).
Several molecules associated with L. monocytogenes have been implicated as potential virulence factors; these include listeriolysin (LLO) and phosphatidylcholine-specific phospholipase C (PC-PLC), also known as lecithinase.
LLO is a 58.6- to 60-kDa extracellular protein which is encoded by the hly gene and is a member of the sulfydryl (SH)-activated group of bacterial toxins expressed by diverse species of gram-positive bacteria. Listeria ivanovii produces a similar toxin, ivanolysin (ILO). LLO and ILO are the only thiol-activated toxins produced by intracellular bacteria (27, 36). A gene located in the lecithinase operon, plcB, encodes PC-PLC, which hydrolyzes phosphatidylcholine (lecithin); hence, this compound is considered a lecithinase. Phospholipase C enzymes have been isolated from a variety of gram-positive and gram-negative bacteria.
Listerial lecithinase catalyzes the hydrolysis of a broad spectrum of phospholipids, including phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and, to a lesser extent, sphingomyelin (25). In L. monocytogenes, lecithinase is necessary for the breakdown of the two plasma membranes that surround the bacterium after it enters host cells and facilitates cell-to-cell spread during infection (13, 51). PC-PLC is produced by all virulent strains of L. monocytogenes, while distinct lecithin degradation is not expressed by other Listeria spp. (13).
Several detection systems have been developed to monitor the incidence of L. monocytogenes in foods. Some of the techniques, including isolation and identification of L. monocytogenes by conventional selective culture and biochemical methods, are very effective (9, 37) but time-consuming. New methods for rapid detection and identification of L. monocytogenes in foods in which monoclonal antibodies (MAbs) (3, 8, 38, 41), DNA probes (15, 17, 33), or DNA amplification is used in conjunction with PCR (2, 42) have been developed. Molecular biology has revolutionized our ability to detect nucleic acid sequences foreign to a host. Furthermore, the sensitivity and specificity of nucleic acid probes are unmatched in other methods. However, several concerns arise when nucleic acid probes are used for the detection of L. monocytogenes and subsequent determinations of virulence. Nucleic acid probes do not discriminate between living and dead organisms. In addition, nucleic acid probes only detect a gene; this detection does not necessarily indicate that the gene is being expressed (32). For these reasons, we sought to produce MAbs against crucial virulence factors of strain EGD of L. monocytogenes for the purpose of determining the presence of the virulence factors in channel catfish isolates.
MATERIALS AND METHODS
Bacterial strains and growth media.
L. monocytogenes reference strains ATCC 15313 (serovar 1), ATCC 19115 (serovar 4b), and EGD (= NCTC 7973) (serovar 1/2a), two L. monocytogenes strains isolated from channel catfish fillets (CCF1 [serovar 1] and CCF4 [serovar 4]), and two L. monocytogenes strains isolated from various organs of healthy channel catfish (HCC7 [serovar 1] and HCC23 [serovar 4]) were used in this study. Bacterial cultures that were to be analyzed for virulence factor production were cultivated on 5% sheep blood agar plates at 37°C for 24 h. Bacteria were harvested, washed, and inoculated into 250 ml of the improved minimal medium (IMM) described by Phann-Thanh and Gormon (43) at densities ranging from 105 to 106 CFU/ml. To enhance LLO and PC-PLC production, Chelex 100 beads (Bio-Rad Laboratories, Hercules, Calif.) were added to the medium at a final concentration of 0.2%, and the preparation was incubated overnight at 37°C in order to reduce the iron availability (10, 14). The resin was removed by filtration through a 0.22-μm-pore-size membrane filter prior to inoculation with bacteria. The cultures were incubated overnight with shaking at 37°C.
Preparation of LEP.
To produce Listeria extracellular proteins (LEP) from each strain, bacteria were grown in IMM aerobically overnight in a stirred bioreactor (Cytostir; Kontes, Vineland, N.J.) at 37°C. Each culture was terminated in the late log phase of growth, the cells were harvested by centrifugation at 3,200 × g for 30 min at 4°C, and the supernatant fluid was filtered through a 0.22-μm-pore-size membrane filter. Phenylmethylsulfonyl fluoride and EDTA were each added to the supernatant at a final concentration of 0.1 mM in order to inactivate proteases. To prevent oxidation, dithiothreitol was added at a final concentration of 1 mM. The filtered supernatant was then concentrated 20-fold by using an N2 pressure-driven ultrafiltration cell equipped with a 20-kDa cutoff membrane (Spectrum Medical Industries, Inc., Houston, Tex.). The concentration of extracellular proteins was determined by the bicinchoninic acid method (Pierce Chemical Co., Rockford, Ill.). The concentrated extracellular proteins were stored at −20°C until they were needed.
MAb production.
MAbs were generated by a method that is routinely used in our laboratory (1) and was adapted from previously described methods (22, 48). The extracellular proteins of L. monocytogenes EGD that were used as antigens were concentrated by ultrafiltration from 18-h Chelex-treated IMM culture supernatants. Production of the proteins of interest was enhanced in this medium (43), as determined by polyacrylamide gel electrophoresis analysis. Two RBF/Dn mice (Jackson Laboratories, Bar Harbor, Maine) were inoculated on five occasions at 2-week intervals. For the first three inoculations, the mice were injected subcutaneously with 7, 12, and 35 μg of LEP, respectively, in TiterMax Gold adjuvant (CytRx Corporation, Norcross, Ga.). The last two inoculations (35 and 33 μg) were intraperitoneal injections without adjuvant. When an intense antibody response was present, as determined by an enzyme-linked immunosorbent assay (ELISA) performed with serum, the fusion was done with one of the mice. Hybridomas were screened for reactions to the L. monocytogenes EGD LEP by performing an ELISA (1, 12, 20). Positive hybridomas were expanded and were tested further by performing a Western blot analysis (4, 34) in which the LEP were used as test antigens. Based on the Western blot results, hybridomas were chosen for cloning and expansion. Once cloned, the MAbs were tested with the Western blot procedure again to confirm their reactivities with either LLO or PC-PLC. To confirm that our MAbs reacted with LLO, a comparison with an anti-LLO MAb, kindly supplied by P. Cossart of the Pasteur Institute (41), was made in a Western blot analysis (4). All MAbs were isotyped by using reagents obtained from Southern Biotech (Birmingham, Ala.), and 10 selected MAbs were used in the assays described below. All of the MAbs in hybridoma culture supernatants were stored at 4°C and contained 0.02% sodium azide.
Characterization of MAbs.
One representative anti-LLO MAb and one anti-PC-PLC MAb were chosen for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (34) and Western blot (4) analyses in order to determine the presence of LLO and PC-PLC proteins in the seven L. monocytogenes strains described above. Extracellular proteins from each of the seven strains were electrophoresed on SDS–12.5% polyacrylamide gels and electroeluted onto nitrocellulose membranes, and the membranes were then probed with the representative MAbs.
Detection of thiol-activated toxins.
An ELISA was performed to determine the specificities of the seven anti-LLO MAbs for ILO, seeligerolysin, streptolysin, perfringolysin, and cereolysin. For antigen production, we used L. monocytogenes ATCC 15313 and EGD, L. ivanovii ATCC 19119, Listeria seeligeri ATCC 35967, Clostridium perfringens ATCC 13124, a Streptococcus pyogenes diagnostic isolate, and a Bacillus cereus diagnostic isolate. The diagnostic isolates were obtained from the Mississippi State University College of Veterinary Medicine bacterial repository. Most of the bacterial strains were grown in charcoal-treated IMM (45) for 18 h at 37°C; the C. perfringens culture was incubated anaerobically for 36 h at 37°C. After incubation, all of the cultures were centrifuged at 10,000 × g for 10 min at 4°C. The supernatants were collected, and the protein concentrations of the supernatants were determined by using the bicinchoninic acid method mentioned above. The culture supernatant of L. monocytogenes ATCC 15313 was used as a negative control. The ELISA plate wells were coated with a solution containing 10 μg of protein from each bacterial culture per ml (100 ng/well). All of the MAbs were used in an ELISA performed by using standard procedures (1, 20).
Detection of bacterial phospholipase C.
The specificities of the three anti-PC-PLC MAbs for L. ivanovii ATCC 19119 and four other bacterial phospholipase C enzymes from C. perfringens ATCC 13124, the B. cereus diagnostic isolate, Staphylococcus aureus ATCC 25923, and a Rhodococcus equi diagnostic isolate were evaluated by ELISA and Western blot techniques. L. seeligeri ATCC 35967 culture supernatant was used as a negative control. The diagnostic isolates were obtained from the Mississippi State University College of Veterinary Medicine bacterial repository. The antigens used to coat the ELISA plate wells were prepared and the ELISA was performed as described previously. For immunoblotting, equal amounts of extracellular proteins from the bacterial strains were resolved on SDS–12.5% polyacrylamide gels, electroeluted onto nitrocellulose membranes, and probed with three anti-PC-PLC MAbs.
Effects of MAbs on the hemolytic activity of L. monocytogenes.
Anti-LLO MAbs were examined to determine their ability to inhibit hemolytic activity by using a microtiter plate hemolysis test (41). One hundred microliters of phosphate-buffered saline (pH 6) supplemented with 0.1% bovine serum albumin and 20 mM cysteine was added to each well of U-bottom microtiter plates. Serial twofold dilutions of each anti-LLO MAb were made across the columns of the plates. The last row of each plate was used as a positive control, and therefore no MAb was added to it. Fifty microliters of supernatant from an 18-h charcoal-treated IMM culture of L. monocytogenes EGD was added to each well. This amount of supernatant contained enough toxin to lyse all of the erythrocytes. After 10 min of incubation at 37°C, 50 μl of a preparation containing 3% human erythrocytes (HRBC) was added to each well. The plates were incubated for an additional 30 min at 37°C and then centrifuged at 1,000 × g for 3 min. Neutralization of hemolytic activity by MAbs was evaluated visually by looking for the presence of red cell pellets consisting of unlysed erythrocytes in the wells.
Effects of MAbs on the lecithinase activity of L. monocytogenes.
Three anti-PC-PLC MAbs, designated PLC1, PLC2, and PLC3, were screened to determine their toxin-neutralizing potentials. This was accomplished by evaluating the abilities of the MAbs to inhibit the PC-PLC activity that is responsible for the formation of an opaque zone on egg yolk agar (24, 46). In this assay, 100-μl portions of supernatants from charcoal-treated peptone-glucose-yeast extract broth cultures of strains ATCC 15313 and HCC7 supplemented with 0.1 mM ZnSO4 and 100-μl portions of hybridoma culture supernatants containing the three MAbs were added to wells punched in egg yolk agar. Irrelevant MAb culture supernatants were added to the appropriate wells as negative controls. After the plates were incubated for 24 h at 37°C, neutralization of lecithinase activity was determined visually. In order to clarify if anti-PC-PLC MAbs neutralized the sphingomyelinase activity of L. ivanovii, a CAMP test was performed (11). For this test, a blood agar plate was streaked vertically with R. equi. Near the vertically streaked R. equi, two wells were punched, and 100 μl of sterile L. ivanovii culture supernatant was added to each well. One well also received an equal amount of anti-PC-PLC MAb. After 24 h of incubation, the plate was evaluated for neutralization of sphingomyelinase activity, which was characterized by the absence of the classic arrowhead-shaped zones of hemolysis.
RESULTS
Production and characterization of MAbs.
Of the 200 wells from the fusion, 48 had positive ELISA reactions to LEP. In the primary screening analysis in which Western blotting was used, all of the culture supernatants recognized three proteins having molecular masses of approximately 29, 33, and 58 kDa. Based on the screening reactions, 24 hybridomas were chosen for cloning and expansion. After cloning, the MAbs were retested by performing Western blotting to confirm their reactivities with either LLO or PC-PLC. Screening of tissue culture supernatants obtained from cloned hybridomas yielded eight MAbs that reacted with the same 58-kDa antigen, as did an anti-LLO MAb supplied by P. Cossart (Pasteur Institute). One of the eight MAbs reacted weakly with LLO and was not tested further. An isotype analysis of the remaining seven anti-LLO MAbs revealed that SE2, SE3, SE4, SE5, and SE6 were immunoglobulin G1 (IgG1), while SE1 and SE8 were IgG3 (Table 1). All of the MAbs were κ-light-chain molecules. Three hybridomas produced MAbs that reacted only with the 29-kDa antigen. An isotype analysis of the three anti-PC-PLC MAbs revealed that all of them were IgG1 with κ light chains (Table 2).
TABLE 1.
Properties of anti-LLO MAbs
| MAb | Isotype | Light chain | LLO-neutralizing activity | Cross-reactivity of anti-LLO MAbs with other thiol-activated toxinsa
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| C. perfringens | S. pyogenes | B. cereus | L. ivanoviib | ATCC 15313c | L. seeligeri | ||||
| SE1 | IgG3 | κ | + | − | − | − | − | − | − |
| SE2 | IgG1 | κ | + | − | − | − | + | − | − |
| SE3 | IgG1 | κ | + | − | − | − | + | − | − |
| SE4 | IgG1 | κ | + | − | − | − | + | − | − |
| SE5 | IgG1 | κ | + | − | − | − | + | − | − |
| SE6 | IgG1 | κ | + | − | − | − | + | − | − |
| SE8 | IgG3 | κ | + | − | − | − | − | − | − |
An ELISA was used to determine cross-reactivity.
Identical cross-reactivity results were obtained for L. ivanovii by Western blotting.
L. monocytogenes ATCC 15313.
TABLE 2.
Properties of anti-PC-PLC MAbs
| MAb | Isotype | Light chain | PC-PLC-neutralizing activity | Cross-reactivity of anti-PC-PLC MAbs with other bacterial phospholipases
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
C. perfringens
|
B. cereus
|
S. aureus
|
R. equi
|
L. ivanovii
|
L. seeligeri
|
||||||||||
| ELISA | Western blot | ELISA | Western blot | ELISA | Western blot | ELISA | Western blot | ELISA | Western blot | ELISA | Western blot | ||||
| PLC1 | IgG1 | κ | + | − | − | − | − | − | − | − | − | + | + | − | − |
| PLC2 | IgG1 | κ | + | − | − | − | − | − | − | − | − | + | + | − | − |
| PLC3 | IgG1 | κ | + | − | − | − | − | − | − | − | − | + | + | − | − |
Detection of thiol-activated toxins.
Two MAbs, SE1 and SE8, did not cross-react with the thiol-activated toxins produced by L. ivanovii, L. seeligeri, C. perfringens, S. pyogenes, and B. cereus. Five of the MAbs cross-reacted with L. ivanovii culture supernatant (Table 1). None of the MAbs reacted with strain ATCC 15313 culture supernatant. After positive ELISA reactions between MAbs SE2, SE3, SE4, SE5, and SE6 and culture supernatant of L. ivanovii were obtained, a Western blot analysis was done to confirm the cross-reactivity with ILO. In the Western blot analysis, MAbs SE1 and SE8 did not react with L. ivanovii culture supernatant, while the other MAbs (SE2, SE3, SE4, SE5, and SE6) cross-reacted with ILO (Table 1). All of the L. monocytogenes strains except ATCC 15313 and CCF4 were positive for LLO production, as judged by MAb probing of Western blots (Fig. 1). HCC7 was only weakly positive for LLO (Table 3).
FIG. 1.
Western blot analysis of extracellular proteins from different L. monocytogenes strains performed with a representative anti-LLO MAb obtained in the present study. Lane 1, molecular weight markers; lane 2, ATCC 15313; lane 3, HCC23; lane 4, ATCC 19115; lane 5, HCC7; lane 6, CCF4; lane 7, EGD; lane 8, CCF1.
TABLE 3.
Use of MAbs to detect the presence of virulence factors in L. monocytogenes channel catfish isolates and correlation with pathogenicity
| L. monocytogenes catfish isolatea | Serovar | LLO | PC-PLC | Pathogenicityb |
|---|---|---|---|---|
| ATCC 15313 | 1 | − | + | − |
| ATCC 19115 | 4b | + | + | + |
| EGD | 1/2a | + | + | + |
| CCF1 | 1 | + | + | + |
| CCF4 | 4 | − | − | − |
| HCC7 | 1 | +c | + | + |
| HCC23 | 4 | + | − | − |
CCF, channel catfish fillet; HCC, healthy channel catfish organs.
Pathogenicity data for CCF1, CCF4, HCC7, and HCC23 have not been published.
HCC7 is weakly positive for LLO.
Detection of bacterial phospholipase C.
The specificities of the three anti-PC-PLC MAbs for L. ivanovii phospholipase and four other bacterial phospholipases were determined by ELISA and Western blot techniques. The MAbs did not cross-react with the phospholipase of C. perfringens, B. cereus, S. aureus, or R. equi. Culture supernatant from L. seeligeri was used as a negative control. None of the MAbs cross-reacted with L. seeligeri. However, the anti-PC-PLC cross-reacted with L. ivanovii culture supernatant in the ELISA and Western blot analyses. The MAbs recognized a 27-kDa protein from L. ivanovii (Fig. 2 and Table 2). All of the L. monocytogenes strains except CCF4 and HCC23 were positive for PC-PLC production based on Western blot results (Fig. 3 and Table 3).
FIG. 2.
Western blot analysis of extracellular proteins from different L. monocytogenes strains performed with a representative anti-PC-PLC MAb obtained in the present study. Lane 1, molecular weight markers; lane 2, ATCC 15313; lane 3, HCC23; lane 4, ATCC 19115; lane 5, HCC7; lane 6, CCF4; lane 7, EGD; lane 8, CCF1.
FIG. 3.
Western blot analysis of extracellular proteins from different bacterial strains performed with anti-PC-PLC MAb PLC1. Lane 1, molecular weight markers; lane 2, L. monocytogenes ATCC 15313; lane 3, L. seeligeri; lane 4, S. aureus; lane 5, B. cereus; lane 6, L. ivanovii; lane 7, L. monocytogenes HCC7; lane 8, C. perfringens; lane 9, R. equi. Anti-PC-PLC MAbs PLC2 and PLC3 gave identical results.
Effects of MAbs on the hemolytic activity of L. monocytogenes.
The anti-LLO MAbs were capable of inhibiting LLO-mediated lysis of HRBC to different degrees (Table 1). Because the MAb concentrations of the tissue culture supernatants were not standardized, we did not attempt to determine neutralization titers. Isotype-matched negative control MAbs did not inhibit LLO-induced lysis of HRBC.
Effects of MAbs on the lecithinase activity of L. monocytogenes.
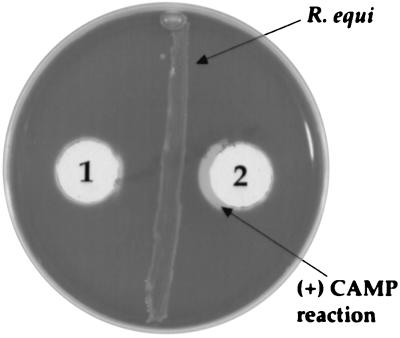
All of the anti-PC-PLC MAbs neutralized PC-PLC-induced opacity on egg yolk agar caused by strains ATCC 15313 and HCC7 (Table 2). Three irrelevant negative control MAbs did not neutralize PC-PLC-induced opacity on egg yolk agar caused by culture supernatants of strains ATCC 15313 and HCC7. Wells containing only strain ATCC 15313 culture supernatant (as a positive control) resulted in a larger opacity zone than the strain HCC7 zone. A modified CAMP test was used to assess the capability of anti-PC-PLC MAbs to neutralize the sphingomyelinase activity of L. ivanovii. After the incubation period, the characteristic arrowhead-shaped zone of hemolysis observed with the positive control was not present around the anti-PC-PLC MAb well (Fig. 4).
FIG. 4.
Inhibition of the classic arrowhead-shaped reaction in the CAMP test by MAb PLC1 (well 1). A negative control sample (well 2) produced the typical arrowhead-shaped reaction.
DISCUSSION
The fact that food products have been implicated in epidemics of listeriosis suggests that there is a need to develop detection systems to monitor the incidence of L. monocytogenes in foods. Currently, Listeria spp. are detected by using microbiological culture methods that can take as long as 3 weeks to determine if food products are Listeria free (44). New detection systems in which MAbs (3, 8, 31, 47), nucleic acid probes (15–17), and PCR (2, 6) are used are more efficient, rapid methods for detecting Listeria spp. in food. However, all of these techniques have some drawbacks. There is some evidence which suggests that detection of Listeria spp. with DNA probes requires large numbers of target organisms (17); however, this drawback has been overcome in the 1990s. The presence of polymerase inhibitors in foods and the mechanical problems associated with extracting low numbers of bacteria from a food mass are major limitations when the PCR technique is used (22). The MAbs described to date are not species specific for L. monocytogenes, and none of them have been used to determine the virulence of isolates. Except for pathogenicity testing in a living system, which can take up to 10 days, there is no other detection method that can differentiate virulent L. monocytogenes strains from strains that are avirulent. Production of highly specific MAbs against known virulence factors, such as the production described in this paper, could play an important role in detecting virulent L. monocytogenes.
In the present study all of the L. monocytogenes strains examined except ATCC 15313 and CCF4 were positive for LLO production in immunoblot analyses performed with our MAbs and gave the same pattern that the anti-LLO MAbs produced by Nato et al. (41) gave. Based on inhibition of LLO-mediated hemolysis, it is evident that the anti-LLO MAbs produced in this study targeted the epitopes responsible for the lytic activity of the toxin or induced detrimental structural changes in the toxin. Two of the seven MAbs examined, SE1 and SE8 (both IgG3), reacted only with the strain EGD culture supernatant, and no other cross-reactions occurred. LLO is antigenically closely related to ILO (80% homology) and seeligerolysin (26, 28); thus, the cross-reactions of some of the anti-LLO MAbs with ILO were predictable. However, LLO does have the L. monocytogenes-specific epitopes which were demonstrated by SE1 and SE8, and MAbs SE1 and SE8 should be useful for distinguishing expression of LLO from expression of other thiol-activated toxins. Nato et al. (41) suggested that there are six antigenic determinants on LLO; however, the various reaction patterns which we observed with our MAbs and the results of Nato et al. (41) suggest that more than seven antigenic determinants are present on LLO.
The three MAbs that react with the 29-kDa antigen are most likely anti-PC-PLC MAbs. This conclusion is supported by the observation that PC-PLC-mediated lecithin degradation on egg yolk agar was inhibited by our 29-kDa antigen-specific MAbs. To our knowledge, this is the first description of MAbs against PC-PLC. All three anti-PC-PLC MAbs which we produced are highly specific, although the L. monocytogenes PC-PLC is broadly similar to phospholipase C enzymes of C. perfringens and B. cereus (49). Limited cross-reactions did occur; we observed cross-reactions with L. ivanovii culture supernatant in the ELISA analysis and with a 27-kDa antigen from L. ivanovii in the Western blot analysis. The 27-kDa antigen is most likely the sphingomyelinase C antigen of L. ivanovii which is responsible for a positive CAMP reaction with R. equi (40, 50). Sphingomyelinase C is considered a member of the phospholipase C family because phosphatidylcholine and sphingomyelin have the same head group, phosphoryl choline (49). Neutralization of the sphingomyelinase activity of L. ivanovii by our MAbs is not out of the ordinary, and the fact that this occurs supports the hypothesis that L. monocytogenes PC-PLC exhibits broad homology or identity with other PC-PLC or PC-PLC-like molecules.
Nucleic acid sequences homologous to the prfA, plcA, plcB, mpl, hly, and actA genes of L. monocytogenes are present in two other hemolytic species of the genus Listeria, L. ivanovii and L. seeligeri (27). Therefore, it is reasonable to expect that there are homologous regions in the phospholipases of the two pathogenic Listeria species, L. monocytogenes and L. ivanovii. However, Geoffroy et al. (24), using rabbit antiserum raised against PC-PLC, showed that the 29-kDa PC-PLC of L. monocytogenes was not present in L. ivanovii culture supernatants. Because of the lack of PC-PLC activity in L. ivanovii and the absence of antigenic similarity, Geoffroy et al. (24) concluded that PC-PLC of L. monocytogenes is different from sphingomyelinase C of L. ivanovii. Although L. ivanovii does not exhibit PC-PLC activity, it produces a positive reaction on egg yolk agar (24, 50). This suggests that sphingomyelinase C can degrade lecithin, and the cross-reactions of our anti-PC-PLC MAbs were probably related to the composition of the head group (phosphoryl choline).
The incidence of Listeria spp. in seafood and fish varies widely depending on the method used for isolation, the sample type, and the age of the sample, among other things. The incidence of different Listeria spp. in seafood and fish samples analyzed by the Food and Drug Administration between 1991 and 1996 was 8.7% (30). There has been one documented report of Listeria spp. detected in catfish products (7), and the majority of Listeria contamination cases in fish have involved cold or hot smoked salmon (18, 30). However, in all of these cases decisions concerning product removal were based on detection of Listeria spp., not determinations of virulence. L. monocytogenes EGD and ATCC 19115, which were used in this study, are well-known pathogens and express both LLO and PC-PLC, as verified by our results. Two of our catfish isolates (CCF1 and HCC7) expressed both of these virulence factors, suggesting that these isolates are potential pathogens. The other two catfish isolates, CCF4 and HCC23, did not express either LLO and PC-PLC or PC-PLC, which placed these organisms in the nonpathogen category. Buchanan et al. (7) detected Listeria spp. other than L. monocytogenes; however, catfish samples which we analyzed in addition to the samples described in this paper were positive for L. monocytogenes and no other Listeria spp. (data not shown). The critical question to be answered is, are the L. monocytogenes strains detected in catfish virulent? At this time in vivo pathogenicity testing is the only way to determine the pathogenicity of L. monocytogenes.
Other tests for determining the virulence properties of L. monocytogenes have been proposed and developed; these tests include tests for cell culture invasiveness, virulence gene detection (15), antibodies to LLO (41), and lecithinase production on egg yolk agar (24). However, although these methods are valuable, none is totally reliable for determining L. monocytogenes virulence. As mentioned above, nucleic acid probes are highly specific and sensitive; however, these probes do not differentiate between gene presence and gene expression. Considering the specific reactions of the anti-LLO MAbs and anti-PC-PLC MAbs produced in the present study, we could speculate that MAbs should be useful for detecting pathogenic L. monocytogenes strains in food and clinical specimens. In addition, the reaction of our MAbs with L. ivanovii makes them useful for detecting this organism in veterinary clinical samples. Our results suggest that some channel catfish isolates lack crucial virulence factors (LLO and/or PC-PLC) that are required for pathogenicity. This finding is important to catfish producers and processors because recalls based on detection of L. monocytogenes rather than virulence could have devastating consequences. Therefore, additional studies should be performed with these MAbs in order to verify that they are useful in methods for rapidly detecting L. monocytogenes in foods and clinical specimens.
ACKNOWLEDGMENTS
This research was supported by Agricultural Research Service/USDA agreement 58-6202-5-083, by the Mississippi Agricultural and Forestry Experiment Station (MAFES) under project MISV-0889, and by the College of Veterinary Medicine, Mississippi State University. S. Erdenlig thanks the AFC agency for providing financial support through the Turkish Ministry of Agriculture.
We thank D. Adams and B. Boyd for their expert technical assistance.
Footnotes
This paper is MAFES publication number J-9433.
REFERENCES
- 1.Ainsworth A J, Dexiang C, Greenway T. Characterization of monoclonal antibodies to channel catfish, Ictalurus punctatus, leucocytes. Vet Immunol Immunopathol. 1990;26:81–92. doi: 10.1016/0165-2427(90)90134-e. [DOI] [PubMed] [Google Scholar]
- 2.Bessesen M T, Luo Q, Rotbart H A, Blaser M J, Ellison R T., III Detection of Listeria monocytogenes by using the polymerase chain reaction. Appl Environ Microbiol. 1990;56:2930–2932. doi: 10.1128/aem.56.9.2930-2932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhunia A K, Ball P H, Fuad A T, Kurz B W, Emerson J W, Johnson M G. Development and characterization of a monoclonal antibody specific for Listeria monocytogenes and Listeria innocua. Infect Immun. 1991;59:3176–3184. doi: 10.1128/iai.59.9.3176-3184.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake M S, Johnston H, Russel-Jones G V, Gotschlich E C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 5.Brackett R E, Beuchat L R. Pathogenicity of Listeria monocytogenes grown on crabmeat. Appl Environ Microbiol. 1990;56:1216–1220. doi: 10.1128/aem.56.5.1216-1220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubert A, Riebe J, Schnitzler N, Schonberg A, Goebel W, Schubert P. Isolation of catalase-negative Listeria monocytogenes strains from listeriosis patients and their rapid identification by anti-p60 antibodies and/or PCR. J Clin Microbiol. 1997;35:179–183. doi: 10.1128/jcm.35.1.179-183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan R L, Stahl H G, Bencivengo M M, del Corral R. Comparison of lithium chloride-phenylethanol-moxalactam and modified Vogel Johnson agars for detection of Listeria spp. in retail-level meats, poultry and seafood. Appl Environ Microbiol. 1989;55:599–603. doi: 10.1128/aem.55.3.599-603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butman B T, Plank M C, Durgam R F, Mattingly J A. Monoclonal antibodies which identify a genus-specific Listeria antigen. Appl Environ Microbiol. 1988;54:1564–1569. doi: 10.1128/aem.54.6.1564-1569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassiday P K, Brackett R E. Methods and media to isolate and enumerate Listeria monocytogenes: a review. J Food Prot. 1989;52:207–217. doi: 10.4315/0362-028X-52.3.207. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty T, Goebel W. Recent developments in the study of virulence in Listeria monocytogenes. Curr Top Microbiol Immunol. 1988;138:41–58. [PubMed] [Google Scholar]
- 11.Christie R, Atkins F E, Muench-Petersen E. A note on the lytic phenomenon shown by group B streptococci. Aust J Exp Biol Med. 1944;22:197–200. doi: 10.1038/icb.1945.30. [DOI] [PubMed] [Google Scholar]
- 12.Cobbold S P, Waldmann H. A rapid solid-phase enzyme-linked binding assay for screening monoclonal antibodies to cell surface antigens. J Immunol Methods. 1981;44:125–133. doi: 10.1016/0022-1759(81)90340-9. [DOI] [PubMed] [Google Scholar]
- 13.Coffey A, Romboutus F M, Abee T. Influence of environmental parameters on phosphatidylcholine phospholipase C production in Listeria monocytogenes: a convenient method to differentiate L. monocytogenes from other Listeria species. Appl Environ Microbiol. 1996;62:1252–1256. doi: 10.1128/aem.62.4.1252-1256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowart R E, Foster B G. Differential effects of iron on the growth of Listeria monocytogenes: minimum requirements and mechanism of acquisition. J Infect Dis. 1985;151:721–730. doi: 10.1093/infdis/151.4.721. [DOI] [PubMed] [Google Scholar]
- 15.Datta A R, Wentz B A, Hill W E. Detection of hemolytic Listeria monocytogenes by using DNA colony hybridization. Appl Environ Microbiol. 1987;53:2256–2259. doi: 10.1128/aem.53.9.2256-2259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta A R, Wentz B A, Russell J. Cloning of the listeriolysin O gene and development of specific gene probes for Listeria monocytogenes. Appl Environ Microbiol. 1990;56:3874–3877. doi: 10.1128/aem.56.12.3874-3877.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta A R, Wentz B A, Shook D, Truckess M W. Synthetic oligodeoxyribonucleotide probes for detection of Listeria monocytogenes. Appl Environ Microbiol. 1988;54:2933–2937. doi: 10.1128/aem.54.12.2933-2937.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillion R, Patel T, Ratnam S. Occurrence of Listeria in hot and cold smoked seafood products. Int J Food Microbiol. 1994;22:73–77. doi: 10.1016/0168-1605(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 19.Doyle M P, Glass K A, Beery J T, Garcia G A, Pollard D J, Schultz R D. Survival of Listeria monocytogenes in milk during high-temperature, short-time pasteurization. Appl Environ Microbiol. 1987;53:1433–1438. doi: 10.1128/aem.53.7.1433-1438.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA): quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 21.Farber J M, Hughes A, Holley R, Brown B. Thermal resistance of Listeria monocytogenes in sausage meat. Acta Microbiol Hung. 1989;36:273–275. [PubMed] [Google Scholar]
- 22.Fitter S, Heuzenroeder M, Thomas C J. A combined PCR and selective enrichment method for rapid detection of Listeria monocytogenes. J Appl Bacteriol. 1992;73:53–59. doi: 10.1111/j.1365-2672.1992.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 23.Gellin B G, Broome C V. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 24.Geoffroy C, Raveneau J, Beretti J L, Lecroisey A, Vazquez-Boland J A, Alouf J E, Berche P. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect Immun. 1991;59:2382–2388. doi: 10.1128/iai.59.7.2382-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldfine H, Johnston N C, Knob C. Nonspecific phospholipase C of Listeria monocytogenes: activity on phospholipids in Triton X-100-mixed micelles and in biological membranes. J Bacteriol. 1993;175:4298–4306. doi: 10.1128/jb.175.14.4298-4306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gormley E, Mengaud J, Cossart P. Sequences homologous to the listeriolysin O gene region of Listeria monocytogenes are present in virulent and avirulent haemolytic species of the genus Listeria. Res Microbiol. 1989;140:631–643. doi: 10.1016/0923-2508(89)90195-2. [DOI] [PubMed] [Google Scholar]
- 27.Gouin E, Mengaud J, Cossart P. The virulence gene cluster of Listeria monocytogenes is also present in Listeria ivanovii, an animal pathogen, and Listeria seeligeri, a nonpathogenic species. Infect Immun. 1994;62:3550–3553. doi: 10.1128/iai.62.8.3550-3553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas A, Dumbsky M, Kreft J. Listeriolysin genes: complete sequence of ILO from Listeria ivanovii and of LSO from Listeria seeligeri. Biochim Biophys Acta. 1992;1130:81–84. doi: 10.1016/0167-4781(92)90466-d. [DOI] [PubMed] [Google Scholar]
- 29.Heisick J E, Wagner D E, Nierman M L, Peeler J T. Listeria spp. found on fresh market produce. Appl Environ Microbiol. 1989;55:1925–1927. doi: 10.1128/aem.55.8.1925-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jinneman K C, Wekell M M, Eklund M W. Incidence and behavior of Listeria monocytogenes in fish and seafood. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. New York, N.Y: Marcel Dekker, Inc.; 1999. pp. 601–629. [Google Scholar]
- 31.Kathariou S, Mizumoto C, Allen R D, Fok A K, Benedict A A. Monoclonal antibodies with a high degree of specificity for Listeria monocytogenes serotype 4b. Appl Environ Microbiol. 1994;60:3548–3552. doi: 10.1128/aem.60.10.3548-3552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kathariou S, Pine L. The type strain(s) of Listeria monocytogenes: a source of continuing difficulties. Int J Syst Bacteriol. 1991;41:328–330. doi: 10.1099/00207713-41-2-328. [DOI] [PubMed] [Google Scholar]
- 33.Kohler S, Leimeister-Wachter M, Chakraborty T, Lottspeich F, Goebel W. The gene coding for protein of Listeria monocytogenes and its use as a specific probe for Listeria monocytogenes. Infect Immun. 1990;58:1943–1950. doi: 10.1128/iai.58.6.1943-1950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lamont R J, Postlethwaite R, MacGowan A P. Listeria monocytogenes and its role in human infection. J Infect. 1988;17:7–28. doi: 10.1016/s0163-4453(88)92236-0. [DOI] [PubMed] [Google Scholar]
- 36.Leimeister-Wachter M, Chakraborty T. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect Immun. 1989;57:2350–2357. doi: 10.1128/iai.57.8.2350-2357.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loessner M J, Bell R H, Jay J M, Shelef L A. Comparison of seven plating media for enumeration of Listeria spp. Appl Environ Microbiol. 1988;54:3003–3007. doi: 10.1128/aem.54.12.3003-3007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loiseau O, Cottin J, Robert R, Tronchin G, Mahaza C, Senet J M. Development and characterization of monoclonal antibodies specific for the genus Listeria. FEMS Immunol Med Microbiol. 1995;11:219–230. doi: 10.1111/j.1574-695X.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 39.Low J C, Donachie W. A review of Listeria monocytogenes and listeriosis. Vet J. 1997;153:9–29. doi: 10.1016/s1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 40.Mencikova E. Phospholipase C in Listeria. Acta Microbiol Hung. 1989;36:321–325. [PubMed] [Google Scholar]
- 41.Nato F, Riech K, Lhopital S, Rouyre S, Geoffroy C, Mazie J C, Cossart P. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect Immun. 1991;59:4641–4646. doi: 10.1128/iai.59.12.4641-4646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederhauser C, Candrian U, Hofelein C, Jermini M, Buhler H P, Luthy J. Use of polymerase chain reaction for detection of Listeria monocytogenes in food. Appl Environ Microbiol. 1992;58:1564–1568. doi: 10.1128/aem.58.5.1564-1568.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phann-Thanh L, Gormon T. A chemically defined minimal medium for the optimal culture of Listeria. Int J Food Microbiol. 1997;35:91–95. doi: 10.1016/s0168-1605(96)01205-6. [DOI] [PubMed] [Google Scholar]
- 44.Pusch D J. A review of current methods used in the United States for isolating Listeria from food. Int J Food Microbiol. 1989;8:197–204. doi: 10.1016/0168-1605(89)90014-7. [DOI] [PubMed] [Google Scholar]
- 45.Ripio M T, Dominguez-Bernal G, Suarez M, Brehm K, Berche P, Vazquez-Boland J A. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res Microbiol. 1996;147:371–384. doi: 10.1016/0923-2508(96)84712-7. [DOI] [PubMed] [Google Scholar]
- 46.Schuchat A, Swaminathan B, Broome C V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siragusa G R, Johnson M G. Monoclonal antibody specific for Listeria monocytogenes, Listeria innocua, and Listeria welshimeri. Appl Environ Microbiol. 1990;56:1897–1904. doi: 10.1128/aem.56.6.1897-1904.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taggart R T, Samloff I M. Stable antibody-producing murine hybridomas. Science. 1983;219:1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- 49.Titball R W, Hunter S E C, Martin K L, Morris B C, Shuttleworth A D, Rubidge T, Anderson D W, Kelly D C. Molecular cloning and nucleotide sequence of the alpha-toxin (phospholipase C) of Clostridium perfringens. Infect Immun. 1989;57:367–376. doi: 10.1128/iai.57.2.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vazquez-Boland J A, Dominguez L, Rodriguez-Ferri E, Suarez G. Purification and characterization of two Listeria ivanovii cytolysins, a sphingomyelinase C and a thiol-activated toxin (ivanolysin) Infect Immun. 1989;57:3928–3935. doi: 10.1128/iai.57.12.3928-3935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazquez-Boland J A, Kocks C, Dramsi S, Ohayan H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]