Abstract
Introduction
Oxidative stress has been considered as one of many contributor in developing risk of cancer. Oxidative stress may also promote the increasing number of free radical. Malondialdehyde (MDA) is one of radical oxidative marker, while Superoxide Dismutase (SOD) play role as endogenous antioxidant. It has been postulated that in cancer cells there is an increase of oxidative stress compared to normal cell.
Method
This study is a case controlled analytical study to find the relationship between levels of MDA and SOD in patients with brain tumours. The sample obtained was 35 people who met the inclusion and exclusion criteria. Based on this analysis, it will be determined whether there is a significant relationship between levels of MDA and SOD in each type of brain tumours.
Result
There is no significant relationship from all groups brain tumour and all tumours have a low correlation (r = 0.187) in the value of superoxide dismutase level. There is also no significant relationship from all groups (p = 0.302) and a low correlation (r = 0.187) to the value of Malondialdehyde level.
Discussion
There was no relationship between superoxide dismutase in any type of intracranial tumour in this study. These concluded that superoxide values could not be a risk factor for primary intracranial tumours. Levels of MDA which is an indicator of lipid peroxidation, were significantly higher in patients consisting of meningiomas and gliomas. In high grade gliomas, the MDA increases due to the progressive progression of glioma tumours due to an increase in Reactive oxygen species levels.
Conclusion
This study shows no correlation between SOD as an endogenous antioxidant and MDA as radical oxidative marker in primary brain tumour.
Keywords: Brain tumour, Malondialdehyde, Superoxide dismutase, Endogenous antioxidant, Neurooncology
Highlights
-
•
Brain Tumour.
-
•
Malondialdehyde.
-
•
Superoxide Dismutase.
-
•
Endogenous Antioxidant.
-
•
Neurooncology.
1. Introduction
There are two state of oxidative stress, such are the formation of reactive oxygen species and antioxidant effect on cell. The formation of oxidative stress require endogenous and exogenous source. The source may be formed from mitochondria and peroxisome [1].
Oxidative stress has been considered as one of many contributor in developing risk of cancer. There is an increase of reactive oxygen species (ROS) in patients with continuous inflammation, and thus may be a recruitment of large amount of activated cells and thus lead to preneoplastic event, and may overcome the level of antioxidant in cell level, and thus may alter the genetic which will lead to increasing oncogene [2].
Oxidative stress may also promote the increasing number of free radical. Malondialdehyde (MDA) is one of free radical, which is formed from the reaction of free radical and lipid and may alter the structure of cell membrane, and later may cause DNA alteration in cell level [3].
Many studies have reported superoxide dismutase (SOD) in cellular level. There are three types of SOD, the cuprum SOD or so called SOD1, located within cytosol, inner membrane mitochondrial and nucleus. SOD2 or called the Manganese SOD as the most significant type of SOD in cellular level. EcSOD (SOD3) predominantly an antioxidant enzyme [4]. Many studies have reported the role of SOD2 in brain tumour, such as gliomas. It has been reported that the SOD2 increased in glioma tissues in comparison to the control tissues [5].
It has been postulated that in cancer cells, there is an increase of oxidative stress compared to normal cell. Brain tumour may be caused by alteration in gene and promoting oncogene. Thus, inflammation still play an important role in oncogenesis in primary brain tumour. This study will observe the relationship of SOD and MDA in brain tumour case.
2. Method
This study is a case controlled analytical study to find the relationship between levels of MDA and SOD in patients with brain tumours. Samples were collected from patients at Haji Adam Malik General Hospital in Medan, Indonesia. The patient's blood serum was taken to be checked for these variables.
The sample obtained was 35 people who met the inclusion and exclusion criteria. The sample included are patient willing to be included as sample, have complete medical record and have undergo diagnostic procedure to confirm the diagnosis of brain tumour. Patients over 70 years old, with high comorbidities and with a previous brain surgery are excluded in this study.
Samples were categorized based on demographic data (age and gender), then the samples were categorized based on the type of brain tumour suffered. Once categorized, the levels of MDA and SOD were examined. Specimens were taken from the patient's peripheral blood examination and analyzed in laboratorium. The results of these examinations are grouped on a nominal scale and analyzed statistically.
Analysis using SPSS Program to determine the relationship between this variable. Based on this analysis, it will be determined whether there is a significant relationship between levels of MDA and SOD in brain tumours.
3. Result
3.1. Sample characteristic
In the demographic results, it was found that the most sample was male as many as 20 people (57%) and the most age was 41–50 years as many as 15 people (43%). This data can be seen in Table 1.
Table 1.
Sample gender and age.
| Description | Parameter | n |
|---|---|---|
| Gender | Men | 20 |
| Women | 15 | |
| Total | 35 | |
| Age | <20 | 3 |
| 21–30 | 3 | |
| 31–40 | 5 | |
| 41–50 | 15 | |
| 51–60 | 5 | |
| 61–70 | 4 | |
| Total | 35 |
Types of tumours are divided into 3 categories, Meningioma, Glioma and Brain metastases based on Head CT scan images, head MRI with contrast and histopathology. The most common type of tumour was meningioma as many as 15 people (44%). The data is attached in Table 2.
Table 2.
Type of brain tumour.
| Description | Type | n |
|---|---|---|
| Type of Brain Tumour | Meningioma | 15 |
| Glioma | 10 | |
| Brain Metastasis | 10 | |
| Total | 35 |
The sample obtained was then carried out an assessment of the degree of consciousness based on GCS and the value of karnofsky performance status (KPS).
3.2. Superoxide Dismutase (SOD) value in sample
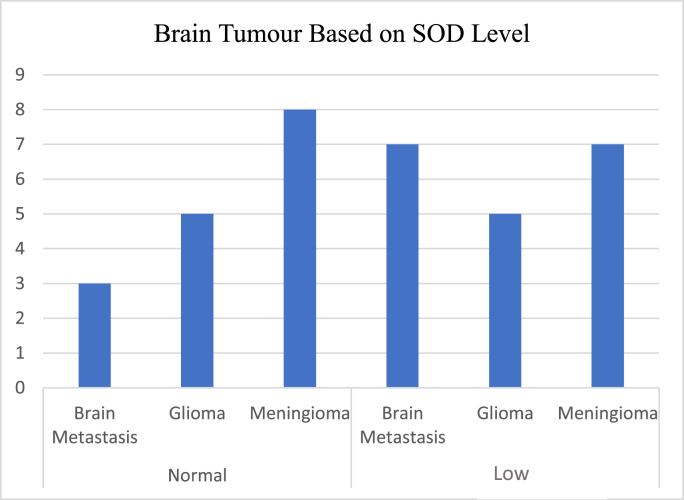
In meningioma tumours, 8 samples (53%) had normal SOD values, while 7 samples (47%) had decreased SOD values. In glioma type tumours, 5 samples (50%) had normal SOD values and 5 samples (50%) had decreased SOD values. In brain metastatic tumours, 7 samples (70%) had decreased SOD values (Fig. 1).
Fig. 1.
SOD value in brain tumour.
Based on Fig. 1 and Table 4, it can be concluded that in all groups of brain tumours there is no significant relationship with superoxide dismutase (p = 0.66) (see Table 3). Based on the correlation value, all tumours have a low correlation (r = 0.187) to the value of superoxide dismutase level. Between all tumour group using post hoc test there are also no significant correlation between every group regarding SOD level based on Table 5.
Table 4.
Brain tumour and SOD.
| Brain Tumour | Superoxide Dismutase | p | r |
|---|---|---|---|
| Glioma | Normal | 0.66 0.66 |
0.187 0.187 |
| Meningioma | Low | ||
| Brain Metastases |
Table 3.
GCS and KPS.
| No | Description | Parameter | n |
|---|---|---|---|
| 1 | Glassgow coma Scale (GCS) | 3–8 | 0 |
| 9–12 | 2 | ||
| 13–15 | 33 | ||
| Total | 35 | ||
| 2 | Karnofsky Perfomance Score (KPS) | 100 | 33 |
| 90 | 2 | ||
| 80 | 0 | ||
| 70 | 0 | ||
| 60 | 0 | ||
| 50 | 0 | ||
| 40 | 0 | ||
| 30 | 0 | ||
| 20 | 0 | ||
| 10 | 0 | ||
| 0 | 0 | ||
| Total | 35 |
Table 5.
Tukey HSD test in SOD level between tumour group.
| Brain Tumour | SOD level |
|
|---|---|---|
| Δ | P value | |
| Meningioma vs glioma | −0.853 | 0.998 0.669 0.751 |
| Meningioma vs brain metastasis | −1.102 | |
| Glioma vs brain metastasis | −1.1017 | |
3.3. Malondyaldehyde (MDA) value in sample
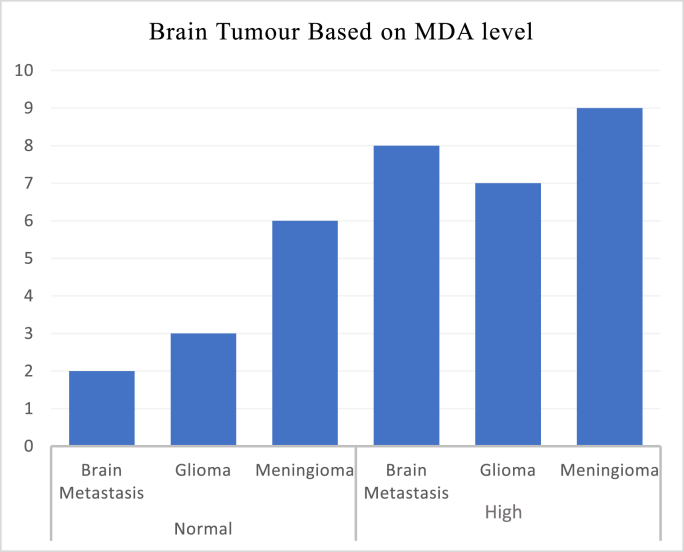
In meningioma tumours, 6 samples (40%) had normal MDA values, while 9 samples (60%) had decreased higher values. In glioma type tumours, 3 samples (30%) had normal MDA values and 7 samples (70%) had higher MDA values. In brain metastatic tumours, 9 samples (90%) had higher MDA values (Fig. 2).
Fig. 3.
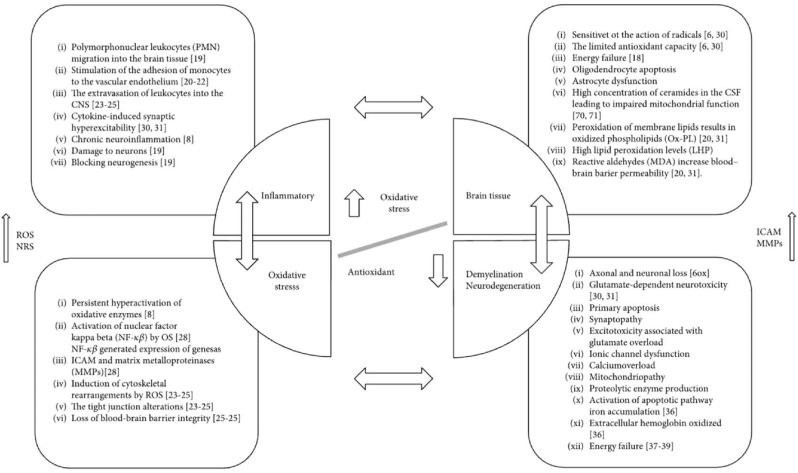
Schematic relationship of antioxidant and oxidative stress in brain tissue [8].
Fig. 2.
MDA value in brain tumour.
Based on Fig. 2 and Table 6, it can be concluded that in all groups of brain tumours there is no significant relationship from all groups (p = 0.173). Based on the correlation value, all tumours have a low correlation (r = 0.179) in the value of Malondialdehyde level. Between all tumour group using post hoc test there are also no significant correlation between every group regarding MDA level based on Table 5 (see Table 7).
Table 6.
Brain tumour and MDA.
| Brain Tumour | MDA | p | r |
|---|---|---|---|
| Glioma | Normal | 0.173 0.173 |
0.179 0.179 |
| Meningioma | High | ||
| Brain Metastases |
Table 7.
Tukey HSD test in MDA level between tumour group.
| Brain tumour |
MDA Level |
|
|---|---|---|
| Δ | P value | |
| Meningioma vs Glioma | −3,876 | 0.155 |
| Meningioma vs brain metastasis | −2,992 | 0.319 |
| Glioma vs brain metastasis | 0.878 | 0.918 |
4. Discussion
Reactive oxygen species (ROS) are important modulators of metabolism, signal transduction and stress responses by acting as secondary messengers for redox sensitive substrates, altering protein structure and function. ROS are spatially restricted due to the limited diffusion distance afforded by the short half-life. Oxidative stress may also promote the increasing number of free radical [4].
Malondialdehyde (MDA) is one of free radical, which is formed from the reaction of free radical and lipid and may alter the structure of cell membrane, and later may cause DNA alteration in cell level [3].
One of the important enzymatic components of the antioxidant defense system is superoxide dismutase (SODs). There are three members of the SOD that exist physiologically, with a tightly regulated localization pattern [4]. The enzyme SOD, which catalyzes the spontaneous dismutation of superoxide radicals to hydrogen peroxide, is present in all parts of the nervous system, including: mitochondrial intermembrane space (SOD1; copper/zinc SOD), mitochondrial matrix (SOD2; manganese SOD), and plasma, lymph, and synovial fluid (SOD3; extracellular SOD). According to Rajaraman, an increase in SOD3 in the blood can be a risk factor for the presence of meningiomas or gliomas, but according to a 2019 study, that there was no relationship between superoxide dismutase and any type of intracranial tumour, such as gliomas and meningiomas, and concluded that superoxide values could not be a factor. Risk for primary intracranial tumours [6,7].
Normalization of SOD levels contributed to the reduction of the cancer cell phenotype. Current research has suggested that SOD may regulate cancer progression and, therefore, may be used as a new target for cancer treatment. SOD liposomes/mimetics have experimentally shown promising results to produce animal models of cancer prevention [9].
It has been conceptualized that the overexpression of SOD may inhibit cell proliferation, and thus the decrease of SOD may promote the tumoural cell growth. This study found no correlation between SOD and brain tumour in patients. A study stated that perturbation of SOD level may favour tumour growth [10].
Brain tissue has many fatty acid, predominantly arachidonic acid and decosahexaenoic acid, and perform as main structure of lipid membrane. The enzymatic metabolism may produce proinflammatory mediator, and may lead to production of ROS thus may increase the level of lipid peroxidation [11].
In the study by Yilmaz N, levels of MDA, which is an indicator of lipid peroxidation, were significantly higher in patients consisting of meningiomas and gliomas compared to healthy controls. In another study, the MDA level in gliomas increased with tumour grading. In high grade gliomas, the MDA increases due to the progressive progression of glioma tumours due to an increase in ROS levels [12]. In this study, there is no correlation between MDA level in primary brain tumour, and, may be caused by fewer grade of brain tumour in our institution, opposite to the study which stated that higher grade glioma may increase MDA level. Also, a study from Wozniak B et al. stated that higher level of MDA was found in brain tumour patient, as a result of higher level of lipid peroxidation in central nervous system tumour group [13]. Another study also stated that the level of lipid peroxidation is lower in adjacent peritumoral tissue, which from the study stated that glioma has higher level than meningioma level and brain metastasis level [14]. A study from Cirak et al. stated that the level of MDA is also correlate with the grade of brain tumour [15].
5. Conclusion
This study shows no correlation between SOD and MDA in primary brain tumour. This correlates with another study that stated there is no increase in oxidative stress and primary brain tumour, caused by lower glioma grade in our institution.
Ethical Approval
Approval has been given by ethical committee of Universitas Sumatera Utara
Consent
Not Applicable
Sources of funding
None
Author contribution
Ridha Dharmajaya : Author
Dina Keumala Sari: Co- Author
Registration of Research Studies
Name of the registry: None
Unique Identifying number or registration ID: None
Hyperlink to your specific registration (must be publicly accessible and will be checked): None
Guarantor
Ridha Dharmajaya: Author
Email : dharmajayaridha@gmail.com
Declaration of competing interest
There is no conflict of interest in this study
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.103231.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sullivan L.B., Chandel N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metabol. 2014;2(1):1–12. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whongsiri P., Pimratana C., Wijitsettakul U., Sanpavat A., Jindatip D., Hoffmann M.J., Boonla C. Oxidative stress and LINE-1 reactivation in bladder cancer are epigenetically linked through active chromatin formation. Free Radic. Biol. Med. 2019;134:419–428. doi: 10.1016/j.freeradbiomed.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Woźniak B., Woźniak A., Kasprzak H.A., Drewa G., Mila-Kierzenkowska C., Drewa T., Planutis G. Lipid peroxidation and activity of some antioxidant enzymes in patients with glioblastoma and astrocytoma. Journal of neuro-oncology. 2007;81(1):21–26. doi: 10.1007/s11060-006-9202-5. [DOI] [PubMed] [Google Scholar]
- 4.Griess B., Tom E., Domann F., Teoh-Fitzgerald M. Extracellular superoxide dismutase and its role in cancer. Free Radic. Biol. Med. 2017;112:464–479. doi: 10.1016/j.freeradbiomed.2017.08.013. Halliwell, B. (2006). Oxidative stress and neurodegeneration: where are we now?. Journal of neurochemistry, 97(6), 1634-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghantasala S., Pai M.G.J., Biswas D., Gahoi N., Mukherjee S., Kp M., Srivastava S. Multiple reaction monitoring-based targeted assays for the validation of protein biomarkers in brain tumours. Front. Oncol. 2021;11:1623. doi: 10.3389/fonc.2021.548243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajaraman P., Hutchinson A., Rothman N., et al. Oxidative response gene polymorphisms and risk of adult brain tumours. Neuro Oncol. 2008;10(5):709–715. doi: 10.1215/15228517-2008-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taş A., Silig Y., Pinarbaşi H., Gürelik M. Role of SOD2 Ala16Val polymorphism in primary brain tumours. Biomedical reports. 2019;10(3):189–194. doi: 10.3892/br.2019.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamcyk B., Wawrzyniak S., Kasperczyk S., Adamczyk-Sowa M. The evaluation of oxidative stress parameters in serum patient with relapsing-remitting multiple sclerosis treated with II-line immunomodulatory therapy. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/9625806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younus H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018;12(3):88. [PMC free article] [PubMed] [Google Scholar]
- 10.Dhar S.K., Clair D.K.S. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012;52(11–12):2209–2222. doi: 10.1016/j.freeradbiomed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Huang T.-T., Zou Y., Corniola R. Oxidative stress and adult neurogenesis—effects of radiation and superoxide dismutase deficiency. Semin. Cell Dev. Biol. 2012;23(7):738–744. doi: 10.1016/j.semcdb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardiany N.S., Mulyawan W., Wanandi S.I. Correlation between oxidative stress and tumour grade in glioma cells from patients in Jakarta. Med. J. Indones. 2012;21(3):122–127. [Google Scholar]
- 13.Woźniak B., Woźniak A., Kasprzak H.A., Drewa G., Mila-Kierzenkowska C., Drewa T., Planutis G. Lipid peroxidation and activity of some antioxidant enzymes in patients with glioblastoma and astrocytoma. Journal of neuro-oncology. 2007;81(1):21–26. doi: 10.1007/s11060-006-9202-5. [DOI] [PubMed] [Google Scholar]
- 14.Zengin E., Atukeren P., Kokoglu E., Gumustas M.K., Zengin U. Alterations in lipid peroxidation and antioxidant status in different types of intracranial tumors within their relative peritumoral tissues. Clin. Neurol. Neurosurg. 2009;111(4):345–351. doi: 10.1016/j.clineuro.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Cirak B., Inci S., Palaoglu S., Bertan V. Lipid peroxidation in cerebral tumors. Clinica chimica acta. 2003;327(1–2):103–107. doi: 10.1016/s0009-8981(02)00334-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.