Abstract
The Roadmap for Mental Health and Wellbeing Research in Europe (ROAMER) identified child and adolescent mental illness as a priority area for research. CAPICE (Childhood and Adolescence Psychopathology: unravelling the complex etiology by a large Interdisciplinary Collaboration in Europe) is a European Union (EU) funded training network aimed at investigating the causes of individual differences in common childhood and adolescent psychopathology, especially depression, anxiety, and attention deficit hyperactivity disorder. CAPICE brings together eight birth and childhood cohorts as well as other cohorts from the EArly Genetics and Life course Epidemiology (EAGLE) consortium, including twin cohorts, with unique longitudinal data on environmental exposures and mental health problems, and genetic data on participants. Here we describe the objectives, summarize the methodological approaches and initial results, and present the dissemination strategy of the CAPICE network. Besides identifying genetic and epigenetic variants associated with these phenotypes, analyses have been performed to shed light on the role of genetic factors and the interplay with the environment in influencing the persistence of symptoms across the lifespan. Data harmonization and building an advanced data catalogue are also part of the work plan. Findings will be disseminated to non-academic parties, in close collaboration with the Global Alliance of Mental Illness Advocacy Networks-Europe (GAMIAN-Europe).
Keywords: Childhood and adolescence psychopathology, Depression, Anxiety, Attention deficit hyperactivity disorder (ADHD), Psychiatric genetics
Introduction
The 2011 Roadmap for Mental Health and Wellbeing Research in Europe (ROAMER) [1–3] outlined six priorities for research in mental health, of which three are the focus of the EU funded Marie Skłodowska-Curie International Training Network CAPICE project: Childhood and Adolescence Psychopathology: unravelling the complex etiology by a large Interdisciplinary Collaboration in Europe:
Research into prevention, mental health promotion, and interventions in children, adolescents, and young adults;
Focus on the development and causal mechanisms of mental health symptoms, syndromes, and wellbeing across the lifespan (including older populations);
Develop and maintain international and interdisciplinary research networks and shared databases in the area of childhood and adolescence psychopathology.
To address these priorities, CAPICE brings together data from eight population-based birth and childhood cohorts, including twin cohorts, to focus on the causes of individual differences in childhood and adolescent psychopathology and its course (see Tables 1, 2). The CAPICE cohorts have collected longitudinal data on behavioral and emotional symptoms, lifestyle characteristics and environmental measures as well as genetic and epigenetic data (see Table 2), some also from parents. Data from other cohorts from the EArly Genetics and Life course Epidemiology (EAGLE) consortium [4], behaviour and cognition group, can also be involved in the studies, especially for genome-wide association meta-analysis (GWAMA). The EAGLE consortium is a collaboration of population-based birth, childhood and adolescent cohorts including the cohorts participating in CAPICE. These cohorts are mainly based in Western Europe and Australia as, to our knowledge, no comparable cohorts exist in other countries. If there are, they are very welcome to participate.
Table 1.
General descriptions of the CAPICE cohorts
| Cohort name | Descriptions |
|---|---|
| ALSPAC | The Avon Longitudinal Study of Parents and Children (ALSPAC) is a longitudinal pregnancy cohort which aimed to recruit all pregnant women in the former county of Avon with an expected due date between April 1991 and December 1992. Detailed information has continued to be collected on mothers, partners and children from 15,454 pregnancies in the cohort, this process has been described in detail elsewhere. Ethics approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time. Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). Please note that the study website contains details of all the data that is available through a fully searchable data dictionary and variable search tool: http://www.bristol.ac.uk/alspac/researchers/our-data/ |
| TEDS | The Twins Early Development Study (TEDS) is a longitudinal twin study that recruited over 16 000 twin pairs born between 1994 and 1996 in England and Wales through national birth records. More than 10 000 of these families are still involved in the study. TEDS is a representative sample of the population in England and Wales. Rich cognitive and behavioural data have been collected from the twins from infancy to emerging adulthood with data collection at ages 2, 3, 4, 7, 8, 9, 10, 12, 14, 16, 18, 19 and 21, enabling longitudinal genetically sensitive study designs. Data have been collected from twins themselves (including extensive web-based cognitive testing), from their parents and teachers, and from the UK National Pupil Database. More information may be found at: https://www.teds.ac.uk/. Ethical approval for TEDS has been provided by the King’s College London Ethics Committee (Reference number: PNM/09/10-104). Parental consent was obtained before data collection |
| GenR | The Generation R (Gen-R) study is a prospective cohort study from fetal life onwards that included pregnant women living in Rotterdam, the Netherlands, with an expected delivery date between April 2002 and January 2006 (n = 9778). The main aim of this study is to identify early environmental and genetic factors that affect growth, health and development. The Generation R Study is multidisciplinary and both prenatal and postnatal measures have included multiple domains of growth, health and development. Rotterdam is an ethnically diverse city and this is reflected in the Generation R participants. Of the enrolled mothers, 42% was of non-Dutch ethnic background, largely made by mothers from Surinamese (9%), Turkish (7%) and Moroccan (3%) background. Data has been collected in children up until the mean age of 10 years, with current on-going data collection at mean age 13 years. Study protocols were approved by the local ethics committee, and written informed consent and assent was obtained from all parents and children. More information may be found at: https://generationr.nl/researchers/ |
| NTR | The Netherlands Twin Register (NTR) is a population-based prospective cohort study which includes new-born twins and multiples from the Netherlands. Recruitment started with birth year 1986. NTR data collection has a focus on growth, development, emotional and behavioural problems and health. Phenotype data on emotional and behavioural problems were collected by surveys, in which parents and teachers were asked to rate their offspring/pupils’ behaviour using standardized instruments. At age 14 years and after, twins and their siblings were asked for self-assessments. Buccal cells and blood for DNA isolation were collected in multiple sub-projects. More information may be found at: https://tweelingenregister.vu.nl/. The study was approved by the Central Ethics Committee on Research Involving Human Subjects of the VU University Medical Centre, Amsterdam, an Institutional Review Board certified by the U.S. Office of Human Research Protections (IRB number IRB00002991 under Federal-wide Assurance- FWA00017598; IRB/institute codes, NTR 03-180) |
| TCHAD | The Swedish Twin study of CHild and Adolescent Development (TCHAD) is a longitudinal study of how genes and environments contribute to the development of health and behavioural problems from childhood to adulthood. The study includes 1480 twin pairs followed since 1994, when the twins were 8–9 years old. The last data collection was in 2005 when the twins were 19–20 years old. Both parents and twins have provided data. More information may be found at: https://ki.se/en/meb/twin-study-of-child-and-adolescent-development-tchad. All responding parents, legal guardians or twins provided informed consent; digitally, written or by participation, to the study, and all data were deidentified. This study received ethical approval from the Karolinska Institutet Ethical Review Board |
| NFBC’86 | Northern Finland Birth Cohort 1986 (NFBC1986) is a prospective longitudinal birth cohort which included pregnant women with expected date of delivery between July 1985 and June 1986 in the two Northern most provinces of Finland. In total, 9432 children were live-born in the cohort. At approximately age 16 years, the cohort members were asked to complete a postal questionnaire, including the Youth Self-Report (YSR). At age 16 years blood samples taken for DNA extraction for 6 266 adolescents attending the clinical examination. All participants and their parents provided consent to use their data and received Institutional Review Board approval by the University of Oulu, and the Ethics committee of the Ostrobotnia Hospital district. More information may be found at: https://www.oulu.fi/nfbc/ |
| CATSS | The Child and Adolescent Twin Study in Sweden (CATSS) is an ongoing longitudinal twin study targeting all twins born in Sweden since July 1, 1992. Parents of twins are interviewed regarding the children’s somatic and mental health and social environment in connection with their 9th or 12th birthdays and followed up over time. Follow-ups were conducted when the twins were 15 years of age and 18 years of age. All responding parents, legal guardians or twins provided informed consent; digitally, written or by participation, to the study, and all data were deidentified. This study received ethical approval from the Karolinska Institutet Ethical Review Board. DNA samples (from saliva) were obtained from the participants at study enrolment. More information may be found at: https://ki.se/en/meb/the-child-and-adolescent-twin-study-in-sweden-catss |
| MOBA | The Norwegian Mother, Father and Child Cohort Study (MoBa) is a population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health. Participants were recruited from all over Norway from 1999 to 2008. The women consented to participation in 41% of the pregnancies. The cohort now includes 114,500 children, 95,200 mothers and 75,200 fathers. Parent and infant DNA samples were collected at birth and stored in a biobank. The establishment of MoBa and initial data collection was based on a license from the Norwegian Data protection agency and approval from The Regional Committees for Medical and Health Research Ethics. The MoBa cohort is based on regulations based on the Norwegian Health Registry Act. The current study was approved by The Regional Committees for Medical and Health Research Ethics (REK 2013/863). More information may be found at: https://www.fhi.no/en/studies/moba/ |
Table 2.
Summary of available data in the CAPICE cohorts: number of children with survey data and genome-wide association data (N GWA) and epigenetics data (N epi) at ages 3–5 years, 6–8 years, 9–11 years, 12–13 years, 14–15 years, and 16–18 years
| Cohort | N GWA | N epi | 3–5 | 6–8 | 9–11 | 12–13 | 14–15 | 16–18 |
|---|---|---|---|---|---|---|---|---|
| ALSPAC | 8237 | 1018 | S | S | S | S | S | |
| TEDS* | 10,346 | S | S | S | S | S | ||
| GenR | 2211 | 1500 | A | A | A | A | ||
| NTR* | 6400 | 1400 | A | A | A | A | A | A |
| TCHAD* | 1120 | A | A | A | ||||
| NFBC’86 | 4000 | 530 | S | A | ||||
| CATSS* | 11,400 | O** | O** | S | ||||
| MOBA | 90,000 | A | O*** | |||||
| Overall total | 73,714 | 4448 |
The first rows summarize the cohorts that assessed mental health symptoms either with the strengths and difficulties questionnaire (S) or the Achenbach system of empirically based assessment (A), the following row the cohorts with S and A measures, and the final rows the cohorts with other (O) measures
*Number of twins. O** A-TAC: Autism–Tics, AD/HD and other Comorbidities inventory, O***SCARED: screen for child anxiety related emotional disorders, MFQ: mood and feelings questionnaire
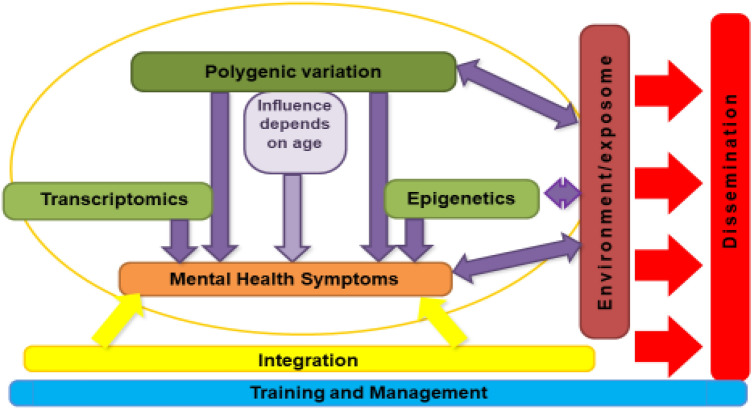
Efforts to prevent and treat childhood psychopathology need to be informed by a clear understanding of the aetiology of mental disorders, and factors that impact the development of a chronic course. Due to the genetically informative, longitudinal designs of the eight included cohorts, CAPICE is well positioned to address questions regarding the interplay of genetic and environmental factors in the development, course, and comorbidity patterns of child psychiatric conditions. Since CAPICE is an international training network, the analyses have been performed by 12 early stage researchers under the supervision of senior researchers at academic and non-academic sites across 5 European countries (Italy, The Netherlands, Norway, Sweden, and the United Kingdom). In this paper, we describe the six CAPICE objectives, as well as the methodological approaches that have been used and the initial results. The overview of the CAPICE research programme is illustrated in Fig. 1.
Fig. 1.
Overview of the CAPICE research programme: the aims are to investigate the influence of genetic, epigenetic, and transcriptomic variants on mental health symptoms, the interplay with the environment, and how these influences depend on age. Ultimately, these results will be integrated into a model predicting the persistence of symptoms
Objectives
-
Elucidate the role of genetic and environmental factors in mental health symptoms across childhood and adolescence, and to establish the overlap in genetic risk factors with other traits related to childhood mental health symptoms.
The projects that are part of this objective focus on the investigation of the overlap in genetic risk across phenotypes and ages, to explain comorbidity and persistence, respectively. Moreover, analyses will focus on disentangling the role of genetic and environmental factors in associations between childhood mental health symptoms and parental phenotypes or risk factors early in life.
Previous research suggests that both genetic and non-genetic factors play a role in the development and persistence of mental health symptoms and disorders. Depending on age, gender, and type of mental health symptoms, genetic factors explain between 40 and 80% of their symptom variance between individuals [5], indicating that the contributions of genetic and environmental factors vary across disorders. Identifying mechanisms that underlie the persistence of symptoms and patterns of comorbidity are of specific importance in childhood psychopathology due to the potential impact of these disorders throughout the life course. Epidemiological studies have shown that about 50% of children with mental disorders still suffer from mental disorders in adulthood [6], including severe mental illnesses such as schizophrenia (SCZ) [7]. As comorbidity has been associated with worse prognosis [6, 8], it is essential to comprehend the mechanisms behind this.
It is also still unknown to what extent the associations between environmental factors and mental disorders are explained by causal processes, in which the environmental risk factor directly increases the risk for psychopathology, or by other processes such as, for example, gene–environment correlation, where the same genetic variants that influence a child’s risk for a mental disorder are associated with the child’s risk to be exposed to an unfavourable environment.
-
Identify genetic, epigenetic, and transcriptomic variants associated with mental health symptoms during childhood and adolescence.
There has been considerable progress in the field of psychiatric genetics with over two hundred genomic regions significantly associated with SCZ [9], bipolar disorder (BD) [10], depression [11, 12], attention-deficit/hyperactivity disorder (ADHD) [13], and autism spectrum disorder (ASD) [14]. This has been achieved by large international collaborations such as the Psychiatric Genomics Consortium (PGC), performing meta-analyses with total sample sizes even up to 100,000 subjects [12]. The number of variants which have been associated with childhood psychopathology at genome-wide significance is still lower than for adult mental disorders [13, 14], but polygenic analyses have suggested that genetic variation in childhood mental disorders, as in adult disorders, is primarily due to many genetic variants of small effect [15]. This would mean that genome-wide association studies (GWAS) of child and adolescent psychopathology, which so far have been notably smaller than the large meta-analyses above, have likely been underpowered. By increasing the sample size and performing meta-analyses across the EAGLE cohorts, including the CAPICE cohorts, it may be possible to detect genome-wide significant associations.
In addition, because extended longitudinal data on mental health problems are available, it is possible to test whether genetic variants exert their influence across the lifespan or if their effect is restricted to a certain age period.
Finally, as previous work has also suggested that the impact of environmental factors on childhood psychopathology may be mediated by epigenetic variation (functional alterations in the genome that do not involve a change to DNA sequence [16]), identification of epigenetic differences associated to childhood mental disorders is also part of this objective.
-
Identify biological pathways associated with mental health symptoms and to validate potential drug targets based on these pathways.
The current identified genetic variants (SNPs) for psychiatric disorders only explain a small part of their variance [17–19]. However, the joint effect of all tested variants explains on average around 30% of the variance of psychiatric disorders [20], which makes it worth to investigate these variants further [21]. A key step in the pathway from variant identification in GWAS to the development of potential treatment or prevention approaches is the characterization of biologic pathways by which genetic variants affect disorders. For instance, follow-up analyses can specify whether associated variants are involved in the same biological pathways, which then can offer leads to novel drug targets or the repurposing of existing drugs that were initially developed for the treatment of other diseases [21–23].
In a recent study [22], a framework for drug repositioning is offered by associating transcriptomes imputed from GWAS data with drug-induced gene expression profiles from the Connectivity Map database. When applied to mental disorders, the method identified many candidates for repositioning, several upheld by preclinical or clinical evidence as they included known psychiatric medications or therapies considered in clinical trials [22]. Validation studies of these approaches have recognized antipsychotics as potential drug targets for mental disorders, confirming the approach may be a productive technique for developing drug therapies for childhood psychiatric conditions [24].
-
Build a prediction model that identifies children at the highest risk of developing chronic mental health symptoms.
To be able to provide treatment that takes into account the risk for persistence of symptoms, it is necessary to build a prediction model that supports stratifying children into groups at high and low risk for a severe, chronic symptom course. Similar risk calculators based on clinical symptoms and cognitive profiles have already been established for high-risk individuals for SCZ or BD [25–27]. Including environmental factors and multi-omic biomarkers may further improve the performance of the model [28].
-
Develop a sustainable international network of researchers in which collaboration is facilitated by data harmonization and information technology (IT) solutions enabling a joint analysis of data over cohorts.
The eight cohorts involved in CAPICE contain a wealth of data on childhood and adolescent mental health, but measures used to assess these traits differ across cohorts (see Table 2). One way to improve the power of studies combining results from multiple cohorts is to use common units of measurement. Using item-response theory (IRT) based test linking in these cohorts; it is possible to evaluate the extent to which dimensions from the individual instruments can be mapped onto dimensions that are shared across instruments. Such an analysis was successfully applied earlier to measures of personality [29].
-
Build a structure to disseminate the results to a broad audience of scientists, clinicians, patients and their parents, and the general public.
A limitation of research projects consists in the difficulty of properly disseminating their results, even if clinically relevant. Previous work has suggested that, even in targeted clinical research, it takes 17 years for 14% of discovery research to be integrated into physician practice [30]. Etiological research on mental disorders may include even broader dissemination gaps. To address this issue, CAPICE specifically aims to create structures allowing for the dissemination of project results to a broad audience, for instance, through a website and social network channels.
Methodology and results
Cohorts description
Before describing the approaches and results of each objective, we provide a general summary of the data collection of the eight CAPICE cohorts (see Tables 1, 2). All longitudinal cohorts have started either at birth or in childhood and use quantitative measures of psychopathology. These measures have been associated with clinical diagnoses [31–34] and are therefore widely used in clinical practice. The advantage of these dimensional measures is that they capture more of the variation present in the common population than dichotomous measures that only specify the presence or absence of a diagnosis. For genome-wide SNP data, methods to impute the genotypes are widely available allowing for the same genetic variants to be analysed over cohorts. All cohorts have been described in more detail elsewhere: ALSPAC [35–37], TEDS [38], GenR [39, 40], NTR [41–43], TCHAD [44], NFBC’86 [45], CATSS [46, 47], MOBA [48]. For a link to cohort-specific websites and for a detailed description of the cohorts (see Table 1). All data used for the analyses were collected under protocols that have been approved by the appropriate ethics committees, and studies were performed in accordance with the ethical standards.
Objective 1: Elucidate the role of genetic and environmental factors in mental health symptoms across childhood and adolescence, and to establish the overlap in genetic risk factors with other traits related to childhood mental health symptoms.
We refer to several excellent overviews for a description of the methods that can be used in genetic epidemiological studies analysing twin/family data and/or molecular genetic data [15, 49, 50]. In short, twin and family studies estimate the proportion of variance in a trait attributable to genetic and environmental factors by comparing the resemblance between pairs of relatives that differ in their relatedness. For example, if monozygotic twins, who are essentially 100% genetically identical, are more alike than dizygotic twins, who share on average 50% of their co-segregating alleles, this is an indication that genetic factors play a role in explaining differences between individuals for a certain trait. This model can be extended to include other family members, to longitudinal or multivariate designs, and to study gene–environment interaction (G × E), even without a direct measure of the environment [51–53].
It is also possible to address these questions using genotypic data obtained for GWAS. In GWAS, common single nucleotide polymorphisms (SNPs) (positions in the DNA sequence that vary between individuals) measured across the whole genome are tested for their association with a trait. Many complex traits, like mental disorders, are influenced by multiple genetic loci. In this case, polygenic analyses, taking into account many SNPs, can be applied to investigate the cumulative impact of these SNPs on a trait, as well as on the co-occurrence of phenotypes or the persistence of symptoms over time [15, 49]. To this point, CAPICE researchers have performed twin and polygenic risk score analyses of the correlation between common mental health symptoms, such as internalizing problems and ADHD problems, and the stability of symptoms over time, including into adulthood.
Regarding the co-occurrence of symptoms, the covariance could be explained by one common factor, the so-called p-factor, which was found to be for 50–60% heritable. Moreover, genetic factors explained stability in this factor across ages. A polygenic p-factor risk score based on adult psychiatric disorders was also associated with the childhood p-factor [54].
The genetic association between adult psychiatric disorders and childhood and adolescents traits was further investigated with polygenic analyses. The PGS for adult depression, neuroticism, BMI, and insomnia were significantly positively associated with childhood ADHD, internalizing and social problems, while the PGS for subjective well-being and educational attainment showed negative associations [55]. Only bipolar disorder PGS did not yield any significant associations. Effect sizes were in general similar across age and phenotype, although the PGS for educational attainment was more strongly associated with ADHD and the BMI PGS with ADHD and social problems [55]. A follow-up study is currently on the way, performing multivariate analyses to shed more light on the pattern of associations (https://osf.io/7nkw8).
Genetic data can also be leveraged to estimate the average causal effect of specific environmental factors, such as perinatal factors, on child or adolescent psychopathology, using a method known as Mendelian randomization (MR). Several ongoing CAPICE projects have applied this approach to estimate the effect of prenatal risk factors, such as maternal smoking, on childhood mental health problems. It is important to recognize that the assumptions that are required for MR may not hold for all prenatal exposures and offspring outcomes [56]. Therefore, methods to identify violations of the MR assumptions are also evaluated and approaches that require fewer assumptions are tested.
Another method to analyse the mechanisms underlying parent-offspring associations is maternal genome-wide complex trait analysis (M-GCTA), which can be applied when parental genotypic data are also available. M-GCTA can be used to calculate whether the association between parent and offspring psychopathology is explained by an environmental effect on top of the effect of the genetic transmission [57]. Applying this method to the MoBa data indicated no such effects for anxiety and depression at age 8 [58]. Analyses on a larger so more powerful sample and including externalizing problems are currently performed.
Objective 2: Identify genetic, epigenetic, and transcriptomic variants associated with mental health symptoms during childhood and adolescence.
GWAS have provided insight into the genetic basis of quantitative variation in complex traits in the past decade [20]. By increasing the sample size and performing meta-analyses across the EAGLE cohorts, including the CAPICE cohorts, it may be possible to detect genome-wide significant associations and to detect age effects. A large-scale GWAMA using a multivariate method to analyse summary statistics that are not independent [59] focused on identifying genetic variants that influence the development and course of internalising symptoms from ages 3 to 18 (https://osf.io/w5adg/). Three gene-wide significant effects were detected as well as significant genetic associations with adult depression and related traits as well as with childhood traits. Another study explores the effect of (ultra) rare and common variation in genes specific to brain cell types on neuropsychiatric disorders (https://osf.io/uyv2s).
Previous work has also suggested that the impact of environmental factors on childhood psychopathology may be mediated by epigenetic variation, which consists of functional alterations in the genome that do not involve a change to DNA sequence [16]. While there are several forms of epigenetic variation, most epigenetic studies have focused on alterations in DNA methylation. Epigenome-wide association studies (EWAS) are performed to test the effect of maternal mid-pregnancy vitamin D on offspring cord blood methylation and of the association between variation in child peripheral and cord blood methylation and the subsequent development of ADHD.
Under very strong assumptions, mediation of the possible average causal effect of prenatal exposures on offspring psychiatric outcomes might be tested using an extension of the MR approach, incorporating genetic variants as proposed instruments for a particular prenatal exposure and for methylation at a specific locus. In certain contexts, this might be considered as a follow up to the present studies.
The association of prenatal maternal smoking with offspring blood DNA methylation has been investigated in individuals aged 16–48 years, and MR and mediation analyses have been performed to evaluate whether methylation markers have causal effects on disease outcomes in the offspring [60]. 69 differentially methylated CpGs in 36 genomic regions (P-value < 1 × 10−7) were found to be associated with exposure to maternal smoking in adolescents and adults and MR analyses delivered evidence for a causal role of four maternal smoking-related CpG sites on an increased risk of SCZ or inflammatory bowel disease [60]. Further studies analyse whether alcohol, tobacco, and caffeine use in pregnancy might be causally related to ADHD in the offspring using negative control and MR approaches (https://osf.io/wxu58) (https://osf.io/aqrxp).
Objective 3: Identify biological pathways associated with mental health symptoms and to validate potential drug targets based on these pathways.
Applying drug pathway analyses to the CAPICE GWAS results may permit us to derive hypotheses about potential drug targets and consequently possibilities for drug repurposing. The GWAMA on internalizing problems did not detect biological pathways (https://osf.io/w5adg/) so could not identify drug targets. This is not surprising as there were not many significantly associated genes.
Objective 4: Build a prediction model that identifies children at the highest risk of developing chronic mental health symptoms.
Using cohort data, as well as Swedish registry data, studies have been performed to predict outcomes of psychiatric symptoms in childhood and adolescence, focusing not only on mental disorders but also on somatic medical outcomes. As part of these analyses, a machine learning model including 474 predictors has been developed that can predict mental health problems in adolescence using data from the Child and Adolescent Twin Study in Sweden (CATSS) [61]. The suggested model would not be appropriate for medical purposes, but it helps to build better models to predict mental health outcomes [61].
Moreover, longitudinal analyses of data from the Swedish and Dutch twin registers indicated that adolescent anxiety is associated with psychiatric disorders later in life, even when adjusting for other mental health issues [62].
Objective 5: Develop a sustainable international network of researchers in which collaboration is facilitated by data harmonization and information technology (IT) solutions enabling a joint analysis of data over cohorts.
Using Item-Response Theory (IRT) based test linking it has been evaluated whether internalizing and ADHD symptoms assessed by different instruments can be mapped onto dimensions that are shared across instruments. These analyses were possible as some of the EAGLE cohorts (ABCD [63], Raine [64], and TEDS [38]), had measured mental health symptoms of the same individuals at the same age with two or more instruments. This could allow combining individual raw item data from different instruments to maximize statistical power.
In addition, to facilitate data analyses over cohorts, a searchable data catalogue is created. The variables important for the current project include demographic and family characteristics, individual’s school achievements, mental health measures (both psychopathology as well as wellbeing) by various raters (mother, father, self-report, teacher), pregnancy/perinatal measures, several general health and anthropometric measures, parenting, parental mental health, and several genomic measures and biomarkers in children and parents. To build a search engine that returns items including the searched term as well as related terms, text mining of available data documentation has been used to identify relationships between words. These results can then be used to develop an advanced search engine for the data catalogue. If “mental health” is, for example, the search term, the results will also include “emotional problems”, “behavioural problems”, and “psychiatric history of the mother”.
Objective 6: Build a structure to disseminate the results to a broad audience of scientists, clinicians, patients and their parents, and the general public.
To engage the general public with the results from these studies, CAPICE researchers have also created content designed for a lay audience on the website (http://www.capice-project.eu/index), Twitter (https://twitter.com/capice_project), YouTube (https://www.youtube.com/channel/UCgq8uIHiHE69IlcHoYCjwKg/featured?view_as=subscriber), LinkedIn and Facebook. CAPICE was also represented at the Greenman Festival in Wales, UK, and ESRs presented multiple times on several international conferences.
Discussion
Genetic research, including psychiatric genetics, has substantially moved forward due to large-scale collaborations in consortia, with meta-analyses being the rule rather than the exception. Due to developments in methodology, it is also possible to use the genome-wide genotypic data for purposes other than the identification of genetic risk variants. Given the small effect sizes, these analyses need large samples to achieve adequate statistical power. The cohorts brought together in CAPICE and the close collaboration with the EAGLE behavior and cognition group (https://www.eagle-consortium.org/) has provided an opportunity to perform these analyses and progress the field of child psychiatry by addressing essential questions like “Which genetic variants and biological pathways underlie the continuity of symptoms from childhood into adulthood?”; “Which factors explain the associations of childhood psychopathology with early life and familial risk factors?”; “What is the role of epigenetic factors in the development of the child and adolescent psychopathology?”; “Can we predict which children are at higher risk for poorer outcomes?”.
The ultimate aim is that these results will inform the development of future treatment and prevention efforts, including supporting the identification of novel targets for existing pharmacological agents. Moreover, having better prediction tools will bring precision medicine closer for child psychiatry as it provides the opportunity to test interventions specifically targeted at children at high or at low risk for the persistence of symptoms.
We acknowledge that the studies performed in the framework of CAPICE are largely restricted to children and families with a Western European genetic ancestry. Extending genetic data collection to children and families from other backgrounds is essential to gain knowledge on similarities and differences between groups from various backgrounds.
These analyses have all been performed as part of a training program for Early Stage Researchers. These researchers receive not only mentoring from senior academics on their specific projects but also a structured curriculum of workshops on child psychiatry, statistics, and dissemination strategy throughout the grant period. These workshops as well as the secondments at other participating institutions aim to build a new generation of creative and innovative researchers who might exert a relevant impact on academic and non-academic organizations.
In conclusion, CAPICE provides a broad package of training in the field of psychiatric genetics, going from harmonizing the phenotypes, the creation of facilities for analyses across cohorts and the actual state-of-the-art analyses, to the translation of the results for drug target validation or prediction models that can be used in the clinic for targeted interventions.
Acknowledgements
All cohorts are grateful to all families and participants who took part in these studies. We also acknowledge and appreciate the unique efforts of the research teams and practitioners contributing to the collection of this wealth of data. We thank Sonja Swanson for her contribution.
Funding
This work has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie CAPICE Project grant agreement number 721567 CAPICE.
Compliance with ethical standards
Conflict of interest
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Avon Longitudinal Study of Parents and Children (ALSPAC)
The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. A comprehensive list of grants funding is available on the ALSPAC website. (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf).
GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe.
The Child and Adolescent Twin Study in Sweden study (CATSS)
CATSS was supported by the Swedish Council for Working Life, funds under the ALF agreement, the Söderström Königska Foundation and the Swedish Research Council (Medicine, Humanities and Social Science, and SIMSAM).
Generation R study
The generation and management of GWAS genotype data for the Generation R Study was done at the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, the Netherlands. We thank Pascal Arp, Mila Jhamai, Marijn Verkerk, Lizbeth Herrera and Marjolein Peters for their help in creating, managing and QC of the GWAS database. The general design of Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Netherlands Organisation for Scientific Research (NWO), the Ministry of Health, Welfare and Sport and the Ministry of Youth and Families. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreements No 633595 (DynaHEALTH) and 733206 (LIFECYCLE).
The Norwegian Mother and Child Cohort Study (MOBA)
MOBA are supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract noN01-ES-75558), NIH/NINDS (Grant No.1 UO1 NS 047537-01 and Grant No.2 UO1 NS 047537-06A1). Genotyping and data access was funded by the the Research Council of Norway grant agreements 229624 “HARVEST”, 223273 “NORMENT”, and 262177 “Intergenerational Transmission of Internalizing and Externalizing Psychopathological Spectra”.
The Netherlands Twin Register (NTR)
Data collection in the NTR was supported by NWO: Twin-family database for behavior genetics and genomics studies (480-04-004); “Spinozapremie” (NWO/SPI 56-464-14192; “Genetic and Family influences on Adolescent psychopathology and Wellness” (NWO 463-06-001); “A twin-sib study of adolescent wellness” (NWO-VENI 451-04-034); ZonMW “Genetic influences on stability and change in psychopathology from childhood to young adulthood” (912-10-020); “Netherlands Twin Registry Repository” (480-15-001/674); “Biobanking and Biomolecular Resources Research Infrastructure” (BBMRI-NL (184.021.007). We acknowledge FP7-HEALTH-F4-2007, grant agreement no. 201413 (ENGAGE), and the FP7/2007–2013 funded ACTION (grant agreement no. 602768) and the European Research Council (ERC-230374). Part of the genotyping was funded by the Genetic Association Information Network (GAIN) of the Foundation for the National Institutes of Health, Rutgers University Cell and DNA Repository (NIMH U24 MH068457-06), the Avera Institute, Sioux Falls, South Dakota (USA) and the National Institutes of Health (NIH R01 HD042157-01A1, MH081802, Grand Opportunity grants 1RC2 MH089951 and 1RC2 MH089995).
The Northern Finland Birth Cohorts 1986 (NFBC1986)
NFBCC1986 study has received financial support from EU QLG1-CT-2000-01643 (EUROBLCS) Grant no. E51560, NorFA Grant no. 731, 20056, 30167, USA/NIHH 2000 G DF682 Grant no. 50945, NIHM/MH063706, H2020-633595 DynaHEALTH action and Academy of Finland EGEA-project (285547).
Twin study of Child and Adolescent Development (TCHAD)
Supported by The Swedish Council for Working Life and the Swedish Research Council.
Twins Early Development Study (TEDS)
TEDS is supported by a program grant to RP from the UK Medical Research Council (MR/M021475/1), with additional support from the US National Institutes of Health (AG046938). The research leading to these results has also received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/grant agreement no. 602768. RP is supported by a Medical Research Council Professorship award (G19/2).
References
- 1.Wykes T, Haro JM, Belli SR, et al. Mental health research priorities for Europe. Lancet Psychiatry. 2015;2:1036–1042. doi: 10.1016/S2215-0366(15)00332-6. [DOI] [PubMed] [Google Scholar]
- 2.Haro JM, Ayuso-Mateos JL, Bitter I, et al. ROAMER: roadmap for mental health research in Europe. Int J Methods Psychiatr Res. 2014;23:1–14. doi: 10.1002/mpr.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ROAMER. http://www.roamer-mh.org/index.php?page=1_1. Accessed 19 Jun 2019
- 4.Middeldorp CM, Felix JF, Mahajan A, et al. The early growth genetics (EGG) and EArly Genetics and Lifecourse Epidemiology (EAGLE) Consortia: design, results and future prospects. Eur J Epidemiol. 2019;34:9. doi: 10.1007/s10654-019-00502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polderman TJC, Benyamin B, De Leeuw CA, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Publ Gr. 2015;47:702. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- 6.Costello EJ, Maughan B. Annual research review: optimal outcomes of child and adolescent mental illness. J Child Psychol Psychiatry. 2015;56:324. doi: 10.1111/JCPP.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maibing CF, Pedersen CB, Benros ME, et al. Risk of schizophrenia increases after all child and adolescent psychiatric disorders: a nationwide study. Schizophr Bull. 2015;41:963–970. doi: 10.1093/schbul/sbu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. doi: 10.1111/1469-7610.00424. [DOI] [PubMed] [Google Scholar]
- 9.Schizophrenia Working Group of the Psychiatric Genomics Consortium SWG of the PG. Ripke S, Neale BM, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl EA, Breen G, Forstner AJ, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wray NR, Ripke S, Mattheisen M, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard DM, Adams MJ, Clarke T-K, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–352. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grove J, Ripke S, Als TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–444. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wray NR, Lee SH, Mehta D, et al. Research review: polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014;55:1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- 16.Barker ED, Walton E, Cecil CAM. Annual research review: DNA methylation as a mediator in the association between risk exposure and child and adolescent psychopathology. J Child Psychol Psychiatry. 2018;59:303–322. doi: 10.1111/jcpp.12782. [DOI] [PubMed] [Google Scholar]
- 17.Duncan LE, Ostacher M, Ballon J. How genome-wide association studies (GWAS) made traditional candidate gene studies obsolete. Neuropsychopharmacology. 2019;44:1518–1523. doi: 10.1038/s41386-019-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins AL, Sullivan PF. Genome-wide association studies in psychiatry: what have we learned? Br J Psychiatry. 2013;202:1–4. doi: 10.1192/bjp.bp.112.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visscher PM, Wray NR, Zhang Q, et al. 10 Years of GWAS discovery: biology, function, and translation. Am J Hum Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breen G, Li Q, Roth BL, et al. Translating genome-wide association findings into new therapeutics for psychiatry. Nat Neurosci. 2016;19:1392–1396. doi: 10.1038/nn.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So H-C, Chau CK-L, Chiu W-T, et al. Analysis of genome-wide association data highlights candidates for drug repositioning in psychiatry. Nat Neurosci. 2017;20:1342–1349. doi: 10.1038/nn.4618. [DOI] [PubMed] [Google Scholar]
- 23.Gaspar HA, Breen G. Drug enrichment and discovery from schizophrenia genome-wide association results: an analysis and visualisation approach. Sci Rep. 2017;7:12460. doi: 10.1038/s41598-017-12325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaspar HA, Gerring Z, Hübel C, et al. Using genetic drug-target networks to develop new drug hypotheses for major depressive disorder. Transl Psychiatry. 2019;9:117. doi: 10.1038/s41398-019-0451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birmaher B, Merranko JA, Goldstein TR, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adolesc Psychiatry. 2018;57:755–763.e4. doi: 10.1016/j.jaac.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafeman DM, Merranko J, Goldstein TR, et al. Assessment of a person-level risk calculator to predict new-onset bipolar spectrum disorder in youth at familial risk. JAMA Psychiatry. 2017;74:841–847. doi: 10.1001/jamapsychiatry.2017.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon TD, Yu C, Addington J, et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173:980–988. doi: 10.1176/appi.ajp.2016.15070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee N, Shi J, García-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17:392–406. doi: 10.1038/nrg.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Berg SM, de Moor MHM, McGue M, et al. Harmonization of neuroticism and extraversion phenotypes across inventories and cohorts in the genetics of personality consortium: an application of item response theory. Behav Genet. 2014;44:295–313. doi: 10.1007/s10519-014-9654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownson RC, Kreuter MW, Arrington BA, True WR. Translating scientific discoveries into public health action: how can schools of public health move us forward? Public Health Rep. 2006;121:97–103. doi: 10.1177/003335490612100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achenbach T (2000) Manual for the ASEBA preschool forms & profiles : an integrated system of multi-informant assessment child behavior checklist for ages 1 1 2-5 language development survey caregiver-teacher. Univ. of Vermont Research Center for Children Youth & Families, Burlington Vt
- 32.Achenbach T (2001) Manual for the ASEBA school-age forms & profiles : an integrated system of multi-informant assessment. ASEBA, Burlington VT
- 33.Achenbach TM, Becker A, Döpfner M, et al. Multicultural assessment of child and adolescent psychopathology with ASEBA and SDQ instruments: research findings, applications, and future directions. J Child Psychol Psychiatry Allied Discip. 2008;49:251–275. doi: 10.1111/j.1469-7610.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 34.Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry Allied Discip. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 35.Boyd A, Golding J, Macleod J, et al. Cohort Profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort profile: the avon longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Northstone K, Lewcock M, Groom A, et al. Open peer review the avon longitudinal study of parents and children (ALSPAC): an update on the enrolled sample of index children in 2009. Wellcome Open Res. 2019;4:51. doi: 10.12688/wellcomeopenres.15132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haworth CMA, Davis OSP, Plomin R. Twins early development study (TEDS): a genetically sensitive investigation of cognitive and behavioral development from childhood to young adulthood. Twin Res Hum Genet. 2013;16:117–125. doi: 10.1017/thg.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–1264. doi: 10.1007/s10654-016-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina-Gomez C, Felix JF, Estrada K, et al. Challenges in conducting genome-wide association studies in highly admixed multi-ethnic populations: the Generation R Study. Eur J Epidemiol. 2015;30:317–330. doi: 10.1007/s10654-015-9998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Beijsterveldt CEM, Groen-Blokhuis M, Hottenga JJ, et al. The young Netherlands twin register (YNTR): Longitudinal twin and family studies in over 70,000 children. Twin Res Hum Genet. 2013;16:252–267. doi: 10.1017/thg.2012.118. [DOI] [PubMed] [Google Scholar]
- 42.Middeldorp CM, Hammerschlag AR, Ouwens KG, et al. A genome-wide association meta-analysis of attention-deficit/hyperactivity disorder symptoms in population-based pediatric cohorts. J Am Acad Child Adolesc Psychiatry. 2016;55:896–905.e6. doi: 10.1016/j.jaac.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kan KJ, Dolan CV, Nivard MG, et al. Genetic and environmental stability in attention problems across the lifespan: evidence from the Netherlands twin register. J Am Acad Child Adolesc Psychiatry. 2013;52:12–25. doi: 10.1016/j.jaac.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenstein P, Tuvblad C, Larsson H, Carlström E. The Swedish twin study of child and adolescent development: the TCHAD-study. Twin Res Hum Genet. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- 45.Järvelin MR, Hartikainen-Sorri A-L, Rantakallio P. Labour induction policy in hospitals of different levels of specialisation. BJOG An Int J Obstet Gynaecol. 1993;100:310–315. doi: 10.1111/j.1471-0528.1993.tb12971.x. [DOI] [PubMed] [Google Scholar]
- 46.Anckarsäter H, Lundström S, Kollberg L, et al. The child and adolescent twin study in Sweden (CATSS) Twin Res Hum Genet. 2011;14:495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- 47.Brikell I, Larsson H, Lu Y et al (2020) The contribution of common genetic risk variants for ADHD to a general factor of childhood psychopathology. Mol Psychiatry 25:18091821. 10.1038/s41380-018-0109-2 [DOI] [PMC free article] [PubMed]
- 48.Magnus P, Birke C, Vejrup K, et al (2016) Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol 382–388. 10.1093/ije/dyw029 [DOI] [PubMed]
- 49.Maier RM, Visscher PM, Robinson MR, Wray NR. Embracing polygenicity: a review of methods and tools for psychiatric genetics research. Psychol Med. 2018;48:1055–1067. doi: 10.1017/S0033291717002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 51.Molenaar D, Dolan CV. Testing systematic genotype by environment interactions using item level data. Behav Genet. 2014;44:212–231. doi: 10.1007/s10519-014-9647-9. [DOI] [PubMed] [Google Scholar]
- 52.Molenaar D, van der Sluis S, Boomsma DI, Dolan CV. Detecting specific genotype by environment interactions using marginal maximum likelihood estimation in the classical twin design. Behav Genet. 2012;42:483–499. doi: 10.1007/s10519-011-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwabe I, van den Berg SM. Assessing genotype by environment interaction in case of heterogeneous measurement error. Behav Genet. 2014;44:394–406. doi: 10.1007/s10519-014-9649-7. [DOI] [PubMed] [Google Scholar]
- 54.Allegrini AG, Cheesman R, Rimfeld K, et al. The p factor: genetic analyses support a general dimension of psychopathology in childhood and adolescence. J Child Psychol Psychiatry. 2020;61:30–39. doi: 10.1111/jcpp.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akingbuwa WA, Hammerschlag AR, Jami ES, et al. Genetic associations between childhood psychopathology and adult depression and associated traits in 42998 individuals: a meta-analysis. JAMA Psychiatry. 2020;77:1. doi: 10.1001/jamapsychiatry.2020.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans DM, Davey Smith G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. 2015;16:327–350. doi: 10.1146/annurev-genom-090314-050016. [DOI] [PubMed] [Google Scholar]
- 57.Eaves LJ, Pourcain BS, Smith GD, et al. Resolving the effects of maternal and offspring genotype on dyadic outcomes in genome wide complex trait analysis ("M-GCTA") Behav Genet. 2014;44:445–455. doi: 10.1007/s10519-014-9666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jami ES, Eilertsen EM, Hammerschlag AR, et al. Maternal and paternal effects on offspring internalizing problems: Results from genetic and family-based analyses. Am J Med Genet Part B Neuropsychiatr Genet. 2020;183:258–267. doi: 10.1002/ajmg.b.32784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baselmans BML, Jansen R, Ip HF, et al. Multivariate genome-wide analyses of the well-being spectrum. Nat Genet. 2019;51:445–451. doi: 10.1038/s41588-018-0320-8. [DOI] [PubMed] [Google Scholar]
- 60.Wiklund P, Karhunen V, Richmond RC, et al. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin Epigenetics. 2019;11:97. doi: 10.1186/s13148-019-0683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tate AE, McCabe RC, Larsson H, et al. Predicting mental health problems in adolescence using machine learning techniques. PLoS ONE. 2020;15:e0230389. doi: 10.1371/journal.pone.0230389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doering S, Lichtenstein P, Gillberg C, et al. Anxiety at age 15 predicts psychiatric diagnoses and suicidal ideation in late adolescence and young adulthood: results from two longitudinal studies. BMC Psychiatry. 2019;19:363. doi: 10.1186/s12888-019-2349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Eijsden M, Vrijkotte TG, Gemke RJ, van der Wal MF. Cohort profile: the Amsterdam born children and their development (ABCD) study. Int J Epidemiol. 2011;40:1176–1186. doi: 10.1093/ije/dyq128. [DOI] [PubMed] [Google Scholar]
- 64.Straker L, Mountain J, Jacques A, et al. Cohort profile: the Western Australian pregnancy cohort (Raine) study-generation 2. Int J Epidemiol. 2017;46:308. doi: 10.1093/ije/dyw308. [DOI] [PMC free article] [PubMed] [Google Scholar]