Abstract
Gastroesophageal reflux disease (GERD) has consistently been the most frequently diagnosed gastrointestinal malady in the USA. The mainstay of therapy has traditionally been medical management, including lifestyle and dietary modifications as well as antacid medications. In those patients found to be refractory to medical management or with a contraindication to medications, the next step up has been surgical anti-reflux procedures. Recently, though innovative advancements in therapeutic endoscopy have created numerous options for the endoscopic management of GERD, in this review, we discuss the various endoscopic therapy options, as well as suggested strategies we use to recommend the most appropriate therapy for patients.
Keywords: Gastroesophageal reflux disease, Stretta, Transoral incisionless fundoplication, Esophyx, Endoscopic suturing, Resection and plication, Overstitch

David P. Lee

Kenneth J. Chang
Introduction
Gastroesophageal reflux disease (GERD) is the most prevalent gastrointestinal disorder in the USA [1], and results from incompetent resistance to the retrograde movement of gastric contents into the esophagus. Its clinical manifestations classically include heartburn and regurgitation, although a wide variety of symptoms have also been associated with GERD, including dysphagia, odynophagia, water brash, globus sensation, atypical chest pain, chronic cough, hoarseness, and wheezing.
For years, the mainstay of initial therapy for GERD has been medical management. This includes lifestyle and dietary modifications, such as sleeping with the head of bed elevated, eliminating offending foods from the diet, and not eating within 3 to 4 h of lying down for bed. Medication options have included antacids for mild symptoms, stepping up to histamine-2 receptor antagonists or proton pump inhibitors (PPIs) for more severe symptoms.
In those patients who have a contraindication to medications, or simply find it impractical to remain on medical therapy for the long term, the next step has typically been a surgical anti-reflux procedure, classically a hiatal hernia repair with Nissen fundoplication. More recently, though, a variety of endoscopic options have emerged to bridge the gap between medical and surgical management of GERD.
This need for filling the therapeutic gap comes both from the patient and physician’s point of view. For surgeons performing laparoscopic anti-reflux surgery (LARS), there has been a trend away from the 360-degree Nissen fundoplication due to a higher incidence of postoperative gas/bloat and dysphagia [2]. While some of these issues may be partially addressed with a partial fundoplication (Dor or Toupet), these procedures are less standardized and long-term outcomes may not be as predictable.
Endoscopic treatments for GERD are now considered appropriate for those patients early in the GERD spectrum, as well as those with altered anatomy where standard laparoscopic surgical approaches are limited. There are currently three endoscopic devices approved by the Food and Drug Administration (FDA) in the USA being used to treat GERD: Stretta® for radiofrequency therapy (Restech, Houston, TX), Esophyx-Z® for Transoral Incisionless Fundoplication (TIF) (EndoGastric Solutions, Redmond, WA), and Overstitch® for endoscopic suturing (Apollo Endosurgery, Austin, TX). In this article, we will review these endoscopic anti-reflux procedures in detail.
Pathophysiology of GERD
To evaluate the role of endoscopic anti-reflux procedures, one must first understand the anatomy of the gastroesophageal junction (GEJ) and the pathologic abnormalities that then lead to GERD.
Pressure gradients between the abdominal stomach and thoracic esophagus would favor retrograde movement of gastric contents into the esophagus during much of human activity, and were not for a complex anti-reflux mechanism at the juncture of the esophagus, stomach, and diaphragm. The lower esophageal sphincter complex is one of the two primary components to this anti-reflux barrier and is intrinsic to the esophagus and comprises the lower esophageal sphincter and esophagogastric junction (hereby referred to as LES). The second component is the crural diaphragm, which in normal individuals acts in concert with the LES to open during swallowing and then contract, pinching the esophagus, to maximize the threshold preventing gastric reflux. The two components taken together constitute the high-pressure zone (HPZ) found during esophageal manometry, and high-resolution manometry demonstrates that both the crural diaphragm and the LES open synchronously during swallowing and belching.
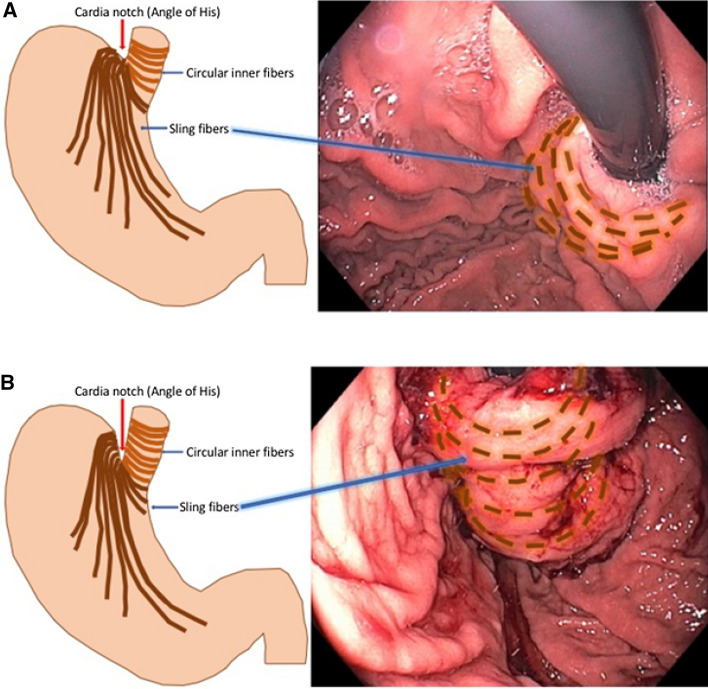
The LES has two components—the proximal portion is made up of the intrinsic muscles of the distal esophagus and the distal portion consists of the sling fibers of the proximal stomach [3] (Fig. 1). Mechanistically, one can consider the LES functioning as an “internal sphincter,” whereas the crural diaphragm constitutes the “external sphincter.” The phrenoesophageal ligament anchors the distal esophagus to the crural diaphragm, thus, coupling the internal and external sphincters.
Fig. 1.
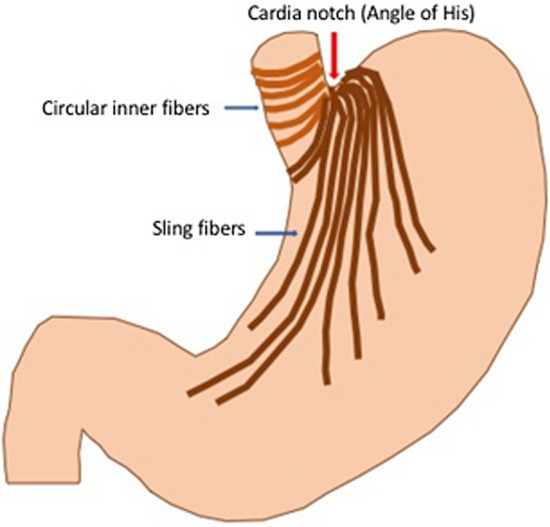
Muscle fibers of the proximal stomach that make up the distal portion of the LES. The gastric sling fibers are a continuation of the inner circular fibers of the distal esophagus and when contracted will “pull down” and accentuate the cardiac notch as an anti-reflux mechanism
Transient or permanent dysfunction of one or both components constitutes the pathophysiology of GERD. In the early stages of GERD, transient opening of the HPZ occurs too frequently and is too often accompanied by reflux of gastric contents rather than merely air. Whether this occurs due to a neurologic reflex or due to transient shortening of the lower esophageal sphincter leading to loss of sphincteric competence is still uncertain; nevertheless, anti-reflux procedures such as the Nissen fundoplication have been found to decrease both the frequency of these transient events as well as the amount of gastric liquid reflux during these transient events. Transient lower esophageal sphincter relaxation (TLESR), one of the principal mechanisms of reflux, especially for daytime reflux, is neurologically mediated [3]. The afferent signals for such relaxation may originate in the pharynx, the larynx, or the stomach. The efferent pathway is in the vagus nerve, and nitric oxide is the postganglionic neurotransmitter. In more advanced stages of GERD, a chronic loss of lower esophageal sphincter length and pressure, and separation of the crural diaphragm from the LES due to hiatal hernia, can lead to more severe reflux.
Even though the proton pump inhibitor (PPI) class of medications are the mainstay of treating GERD, these medications do not decrease the frequency of reflux events, and persistent symptoms related to ongoing reflux often require physical revision of the compromised anatomy. Laparoscopic anti-reflux surgery (LARS) is considered the “gold standard” procedure to restore the anti-reflux barrier, as they restore both the crural component by way of hiatal hernia repair as well as the lower esophageal sphincter by creating a flap valve via fundoplication. However, both the level of invasiveness and the gas-bloat side effects related to a supra-competent flap valve have motivated physicians and patients alike to search for alternative interventions.
In patients who have a largely intact crural sphincter (i.e., absence or a very limited hiatal hernia, Hill Grade 1 or 2), the potential for an endoluminal approach to restoring the lower esophageal sphincter exists. Conceptually, this could entail decreasing the distensibility of the whole or just the bottom of the LES to prevent shortening and loss of LES competence during gastric distention, increasing the resting pressure of the LES, reinforcing the sling fibers at the GE junction (Fig. 1), or locally altering vagal neuromodulation of transient LES openings.
Radiofrequency Energy Treatment of GERD (Stretta)
The Stretta® system was FDA approved for the endoscopic treatment of GERD in 2000 and over 25,000 procedures have been performed to date. This procedure utilizes radiofrequency (RF) energy which is applied to the muscles of the lower esophageal sphincter (LES) and the gastric cardia by way of four needle electrodes that extend out from a balloon catheter into the muscle at six levels across the GEJ, resulting in an improvement of reflux symptoms.
Stretta Mechanisms of Action
It is not entirely clear how the Stretta procedure produces its effects, though a number of theories have been proposed. Some have proposed that RF energy causes a limited coagulative necrosis of the tissue, which is healed by fibrosis [4, 5]. However, since the mucosal temperature is kept well below the accepted level of tissue ablation (100 °C), it is unlikely that tissue destruction followed by fibrosis occurs [6].
Other studies have postulated that Stretta affects the neuromuscular functions of the LES. One study examining the effect of Stretta on the LES pressure and gastric yield pressure in pigs after LES botulinum toxin injection [7] found significant restoration of LES pressure following Stretta compared to sham, with increased gastric yield pressure in the Stretta group. The authors concluded that Stretta reversed the majority of the LES pressure reduction accomplished with botulinum toxin injection and increased gastric yield pressure by 75% when compared to the controls. Another animal study exploring the effect of Stretta to the gastric cardia on the activation of TLESRs and GERD [8] concluded that Stretta delivery to only the gastric cardia in dogs prevents the triggering of TLESRs, thereby reducing gastroesophageal reflux. In a double-blind, randomized crossover study, Arts et al. tested the hypothesis that Stretta alters GEJ resistance [9]. Twenty-two patients participated in the study—11 in the Stretta and 11 in the sham treatment groups. The authors concluded that Stretta decreased GEJ compliance, which may, in turn, contribute to symptomatic benefit by decreasing refluxate volume.
We hypothesize that the treatment at the cardia may be the more important component for the mechanism of action of Stretta. The RF energy is being applied directly onto the sling and clasp fibers of the gastric cardia, which then decrease tissue compliance with less effacement [10], and therefore decrease the TLESRs.
Stretta Patient Selection
Stretta is indicated for patients with GERD who have a contraindication to medical therapy or have concerns regarding the long-term side effects of the PPI class of medications, and either do not qualify or refuse surgical options for the treatment of GERD. Contraindications for Stretta include: age under 18, pregnant women, patients without a diagnosis of GERD, hiatal hernia > 2 cm, achalasia or incomplete LES relaxation in response to swallow, poor surgical candidate, and ASA IV classification.
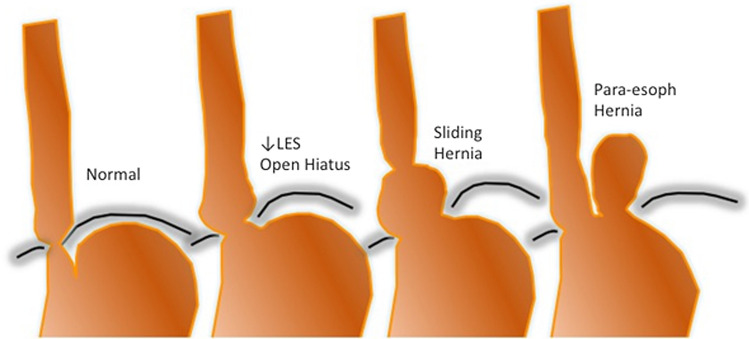
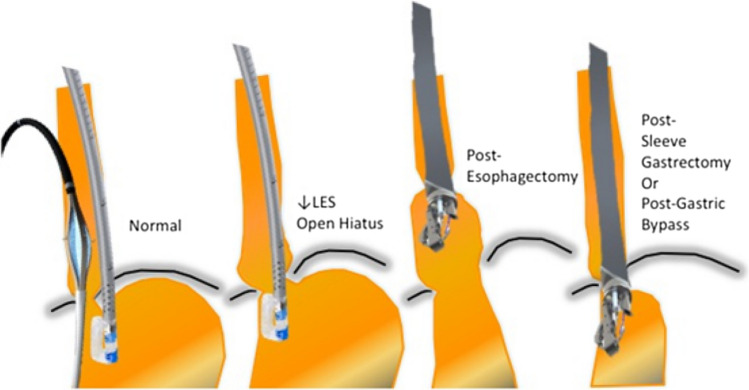
In our experience, the patient most ideally suited for this procedure is one who falls into the first group of the GERD spectrum (Fig. 2). These patients have normal LES tone, no hiatal hernia, and a closed diaphragmatic hiatus (Hill Grade I). This category has been called “Dynamic Failure,” as there are no obvious anatomical defects. These patients have the phenotype of daytime reflux, no esophagitis or Barrett’s, and on ambulatory pH monitoring will have predominantly upright reflux. The main mechanism for GERD in these patients is inappropriate TLESRs.
Fig. 2.
Spectrum of anatomical defects among patients with gastroesophageal reflux disease. (LES: Lower esophageal sphincter)
Failure to respond to medical therapy portends poorer responses to anti-reflux procedures. Alternative causes of their symptoms, including hypersensitive esophagus and functional heartburn should be considered. Achalasia or incomplete lower esophageal sphincter (LES) relaxation in response to swallow must be evaluated for prior to therapy considerations, due to the risk of worsening these conditions following therapy.
Stretta Safety and Efficacy
The Stretta procedure is arguably the safest, least invasive, most well-tolerated and easiest to perform among the clinically available anti-reflux procedures. To date, more than 25,000 Stretta procedures have been performed globally. The most common complications reported have been gastroparesis and ulcerative esophagitis, which are rare. Transient epigastric pain or chest pain, low-grade fever, dysphagia, and odynophagia have also been reported [11, 12]. Liu et al. in their study involving 90 patients report five cases of dyspepsia, nine transient chest pain, two superficial mucosal injury, three mucosal bleeding, and two low-grade fever after the procedure [13]. Very rare serious complications had been described in the early years of clinical experience, such as esophageal perforation in three patients and two deaths due to aspiration pneumonia [14]. The perforations were attributed to either poor patient selection or operator error.
There have been multiple studies evaluating the short- and long-term efficacy of the Stretta procedure. Stretta has been shown to be effective at improving heartburn scores and quality of life in multiple randomized, controlled trials [9, 15–17] as well as nonrandomized prospective trials [13, 18] that appears to be sustained for up to 12 months. In addition, some longer follow-up studies have been published reporting the improvement in patient heartburn scores, patient satisfaction, and decreased PPI use seem sustained at up to 48 months [19–21], and even at 8-year follow-up [22] and 10-year follow-up [23].
Transoral Incisionless Fundoplication (TIF)
Trans-oral incisionless fundoplication (TIF) using the EsophyX® device was FDA approved in 2007 for the endoscopic treatment of GERD, and over 25,000 procedures have been performed to date. The device is designed to create full-thickness serosa-to-serosa plications and reconstruct valves approximately 3 cm in length, and 270 to 300 degrees in circumference, guided by the surgical principles of the laparoscopic fundoplication.
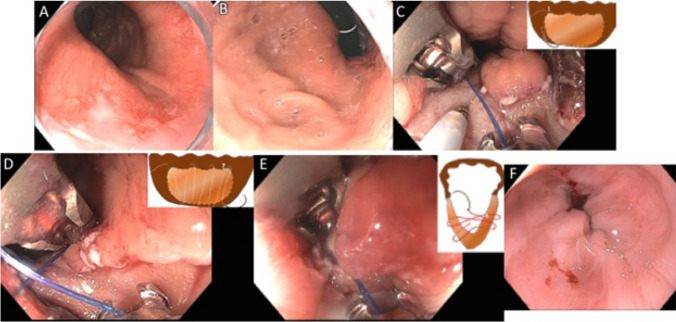
While early attempts at endoscopic fundoplication were unsuccessful and lacked durability, more robust devices and techniques designed to physically reconstruct a flap valve, namely the TIF procedure, have resulted in more successful and durable restoration of LES function, and have done so without the degree of side effects seen with a Nissen fundoplication. In its current technique iteration (TIF 2.0), this procedure is anatomically and physiologically similar to surgical fundoplication (Fig. 3). During the procedure, the gastric fundus is folded up and around the distal esophagus, which has been retracted below the diaphragm, and anchored with polypropylene fasteners. This results in tightening and reinforcing the sling fibers (Fig. 4a, b) of the proximal stomach (the lower portion of the LES), accentuating the cardiac notch, steepening the angle of His, and re-establishing the flap valve mechanism.
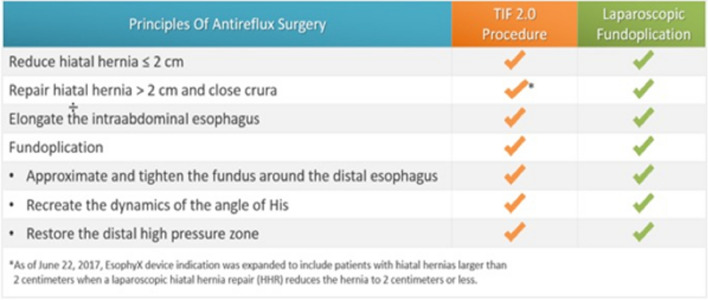
Fig. 3.
Applying the principles of anti-reflux surgery to laparoscopic fundoplication and TIF 2.0. All of the principal elements are preserved in both
Fig. 4.
Diagram and image of the muscle fibers in the distal esophagus (circular inner fibers) and proximal stomach (sling fibers) that make up the entirety of the lower esophageal sphincter. a Pre-TIF valve showing “flat” angle of His (Cardia Notch), short valve, and loose gastric sling fibers. b Post-TIF valve showing “steep” angle of His (Cardia Notch), tall valve, and tighter gastric sling fibers
TIF Mechanisms of Action
The mechanism of action of the TIF procedure in many ways mirrors that of Nissen fundoplication [24]. One paper published by Rinsma et al. [25] characterizes the mechanisms. In their study involving fifteen patients, they performed 90-min postprandial measurements using combined high-resolution manometry and impedance-pH monitoring followed by an ambulatory 24-h pH-impedance monitoring. Patients were studied before and 6 months after the TIF procedure. EGJ distensibility was evaluated using an endoscopic functional luminal imaging probe (EndoFLIP) before and directly after the procedures. With regard to the stationary esophageal manometry and impedance-pH monitoring performed directly after the procedure, TIF resulted in a marked reduction in both the number of TLESRs (16.8 ± 1.5 vs. 9.2 ± 1.3; p < 0.01) and the number of TLESRs associated with liquid-containing reflux (from 11.1 ± 1.6 vs. 5.6 ± 0.6; p < 0.01). TIF also led to a decrease in the number and proximal extent of reflux episodes and an improvement of acid exposure in the upright position; conversely, TIF had no effect on the number of gas reflux episodes, corroborating the low incidence of post-TIF gas-bloat symptoms. EGJ distensibility was reduced after the procedure (2.4 ± 0.3 mm2 /mmHg vs. 1.6 ± 0.2 mm2 /mmHg; p < 0.05). Also of note, the basal LES pressure in the fasted state was increased after TIF (from 13.9 ± 1.0 to 20.5 ± 1.8 mmHg; p < 0.01). Thus, TIF reduces EGJ distensibility, thereby decreasing TLESRs, which is the main mechanism for upright refluxers. It also creates a 3-cm high-pressure zone at the distal esophagus in the configuration of a flap valve, which should decrease both upright and supine reflux. However, since it is a 270° partial fundoplication, and the flap valve luminal diameter is controlled by the diameter of the device (to prevent over-tightening), gas can still escape from the stomach into the esophagus, minimizing the side effect of gas-bloat. Thus, we believe that the TIF valve, with its diameter, length, floppiness, and orientation—represents the optimal flap valve reconstruction [26].
TIF Patient Selection
Patient selection for TIF is most critical. First, the patient must have a clear indication for an anti-reflux procedure. From here, one must discern which patients are good candidates for TIF alone, and which patients are better served with a laparoscopic or combined approach (i.e., Concomitant Laparoscopic Hernia Repair and TIF, see below).
There are three components of the anti-reflux anatomy to assess: (1) whether there is a hiatal hernia that needs to be reduced, (2) whether the right crura, which acts like a sling or noose around the GEJ [27, 28], needs to be tightened, and (3) whether the LES needs to have a valve reconstruction. The vertical length of the hiatal hernia can be assessed by an esophagram or upper endoscopy. Neither modality is perfect, as sliding hernias can often be missed. Even more tricky, however, is the assessment of the crural tightness (diaphragmatic hiatus). The axial width of the crural opening is best assessed using the Hill classification performed during a retroflex view. However, this can often be misleading (i.e., underestimating the Hill grade) for the following reasons: (1) insufficient time and insufflation during retroflexion (Fig. 5), and (2) a fat pad can fill the open hiatus, creating a “stuffing” effect. We recommend a full 60 s be spent in retroflexion with active insufflation to determine the Hill classification. A Hill grade 1 or 2 is acceptable for TIF alone. However, if the hiatus is open more than 2 cm (or diameter of 2 scopes, i.e., Hill 3), or there is an axial hernia length of more than 2 cm (Hill 4), the patient will most likely need a crural repair, which cannot be accomplished with TIF alone. We call this the 2 × 2 rule. In our experience, underestimation of the Hill grade is the most common reason for TIF failure. This cannot be over-emphasized. In a recent paper describing salvage laparoscopic surgery among five patients who failed TIF, three of the five patients were found to have significant hiatal hernia that required repair at the time of revision [29].
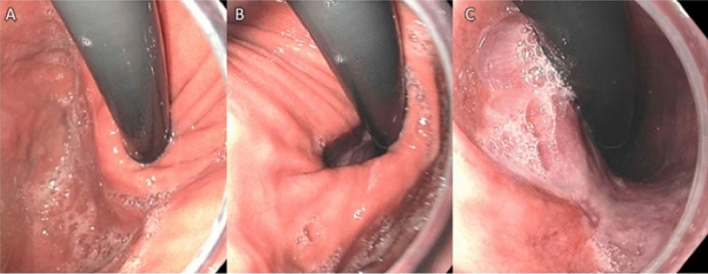
Fig. 5.
The “60 s rule” for assessing the diaphragmatic hiatus in retroflexed position underscores the common mistake of underestimating the Hill Grade. a Initially, after only a few seconds of inspection, assessment appears to be a Hill Grade 1. b About 30 s later, with continuous CO2 insufflation and scope rotation, the hiatus opens up to Hill Grade 2, approximately 2 cm in diameter. c At close to 60 s, the hiatus opens even wider to approximately 3 cm in diameter
Efficacy of TIF
The efficacy of the TIF 2.0 procedure has been evaluated on multiple non-comparative studies [30–41], as well as in randomized, controlled trials.
The TEMPO trial consisted of 63 patients randomized to TIF (40 patients) vs high dose PPI (23 patients) [42]. The primary outcome was elimination of daily troublesome regurgitation or extraesophageal symptoms. Secondary outcomes were normalization of esophageal acid exposure, PPI usage, and healing of esophagitis. At the 6-month follow-up, troublesome regurgitation was eliminated in 97% of TIF patients versus 50% of PPI patients (p = 0.006). Globally, 62% of TIF patients experienced elimination of regurgitation and extraesophageal symptoms versus 5% of PPI patients (p = 0.009). Esophageal acid exposure was normalized in 54% of TIF patients versus 52% of PPI patients (p = 0.914). 90% of TIF patients were off PPIs. The authors concluded that at the 6-month follow-up, TIF was more effective than maximum standard dose PPI therapy in eliminating troublesome regurgitation and extraesophageal symptoms of GERD.
Of the 63 patients receiving TIF, 5-year follow-up data was available as follows: 60 were available at 1 year, 52 at 3 years, and 44 at 5 years [40]. Troublesome regurgitation was eliminated in 88% of patients at 1 year, 90% at 3 years, and 86% at 5 years. Resolution of troublesome atypical symptoms was achieved in 82% of patients at 1 year, 88% at 3 years, and 80% at 5 years. No serious adverse events occurred. There were three reoperations by the end of the 5-year follow-up (5%). At the 5-year follow-up, 34% of patients were on daily PPI therapy as compared with 100% of patients at screening. The total GERD health-related quality-of-life score improved by decreasing from 22.2 to 6.8 at 5 years (p < 0.001). This paper concluded that the majority of patients undergoing TIF experienced a durable elimination of troublesome GERD symptoms with no SAEs or safety concerns, and that TIF could be a cost-effective alternative to laparoscopic Nissen fundoplication.
The RESPECT trial [43] was a prospective, sham-controlled trial to determine if TIF reduced troublesome regurgitation to a greater extent than PPIs in patients with GERD. Six hundred and ninety-six patients with troublesome regurgitation despite daily PPI with three validated GERD-specific symptom scales, on and off PPIs, were initially screened. Eighty-seven patients with GERD and hiatal hernias ≤ 2 cm were randomly assigned to groups that underwent TIF and then received 6 months of placebo, or sham surgery and 6 months of once or twice daily omeprazole (controls, n = 42). Patients were blinded to therapy during the follow-up period and reassessed at 2, 12, and 26 weeks. At 6 months, patients underwent 48-h esophageal pH monitoring and esophagoduodenoscopy. By intention-to-treat analysis, TIF eliminated troublesome regurgitation in a larger proportion of patients (67%) than PPIs (45%) (p = 0.023). A larger proportion of controls had no response at 3 months (36%) than patients who received TIF (11%) (p = 0.004). Control of esophageal pH improved after TIF (mean 9.3% before and 6.3% after; p < 0.001), but not after sham surgery (mean 8.6% before and 8.9% after). Patients from both groups who completed the protocol had similar reduction in GERD symptom scores. The authors concluded that TIF was an effective treatment for patients with GERD symptoms, particularly in those with persistent regurgitation despite PPI therapy, based on evaluation 6 months after the procedure.
A third clinical trial performed in Europe was a double-blind sham-controlled study in GERD patients who were chronic PPI users [44]. Forty-four patients were randomized equally to 22 patients in each group. The primary effectiveness endpoint was the proportion of patients in clinical remission after 6-month follow-up. Secondary outcomes were: PPI consumption, esophageal acid exposure, reduction in Quality of Life in Reflux and Dyspepsia and Gastrointestinal Symptom Rating Scale scores and healing of reflux esophagitis. Results showed that the time in remission after TIF procedure (197 days) was significantly longer compared to those submitted to the sham intervention (107 days), p < 0.001. After 6 months, 13/22 (59%) of the chronic GERD patients remained in clinical remission after TIF.
A recent meta-analysis [45] was conducted using data only from these three randomized studies that assessed the TIF procedure compared to control. The purpose of the meta-analysis was to determine the efficacy and long-term outcomes associated with performance of the TIF procedure in patients with chronic long-term refractory GERD on optimized PPI therapy, including esophageal pH, PPI utilization and quality of life. Results from this meta-analysis, including data from 233 patients, demonstrated that TIF subjects at 3 years had improved esophageal pH, a decrease in PPI utilization, and improved quality of life. Other recent publications are also showing favorable durability with long-term outcomes at 5 years [37, 40] and even preliminary data at 10 years [46].
Concomitant Laparoscopic Hernia Repair and TIF
There continues to be an increased interest in performing TIF along with concomitant laparoscopic hiatal hernia repair (cTIF) [47]. For surgeons performing both LARS and TIF, the rationale for cTIF includes: (1) a trend away from Nissen Fundoplication due to higher incidence of postoperative gas/bloat and dysphagia coupled with established data that TIF produces much less gas/bloat, dysphagia [2], and emerging data suggesting that cTIF also produces less gas/bloat than traditional LARS [34, 35, 48], (2) the non-standardization of the partial fundoplication (Dor or Toupet), and (3) the concern over potential effects of stronger MRI machines in the future on the magnetic sphincter augmentation device [49].
The LOTUS trial brought to light in a 5-year randomized, open, parallel-group trial in Europe the fact that while heartburn and regurgitation were better controlled in the LARS group compared to the esomeprazole group, the surgery patients had significantly more long-term dysphagia, bloating, and flatulence [50]. The prevalence and severity of symptoms at 5 years in the esomeprazole (266 patients) and LARS groups (248 patients), respectively, were 16% and 8% for heartburn (p = 0.14), 13% and 2% for acid regurgitation (p < 0.001), 5% and 11% for dysphagia (p < 0.001), 28% and 40% for bloating (p < 0.001), and 40% and 57% for flatulence (p < 0.001). Experience and data such as these continue to prompt further investigation into procedural strategies that minimize post-procedure side effects while maximizing therapeutic benefits of LARS.
From a surgical technical point of view, performing an anatomical repair of the hiatal defect alone avoids the more extensive LARS dissection that may require a larger retroesophageal window creation, takedown of the short gastric vessels for complete fundic mobilization, which may increase the risk of bleeding and injury to the spleen, and needing to reposition the bulk of the fundus in the retroesophageal space. While the EsophyX device was FDA approved in 2007, the FDA further approved in 2017 its use in patients with hiatal hernias larger than 2 cm in conjunction with laparoscopic hernia repair. In 2011, Ihde published a retrospective community-based study evaluating the safety and symptomatic outcomes of a series of 42 patients who had either undergone TIF (24 patients) or cTIF (18 patients) based on the presence of a hiatal hernia 3 cm or larger [34]. There were no long-term postoperative complications. GERD health-related quality-of-life scores indicated heartburn resolution in 63% of patients. The need for daily PPI therapy was eliminated in 76% of patients. Atypical symptom relief measured by the median reflux symptom index score reduction was significant (5 [0–47] vs. 22 [2–42] on PPIs, p < 0.001).
A more recent study by Idhe et al. evaluated pre- and post-procedure pH analyses on a subset of patients who underwent cTIF. In this study, 55 patients had cTIF, with 29 patients (53%) having matched preoperative and postoperative validated surveys and pH evaluations [35]. The results showed no serious complications over a mean follow-up of 296 days. The mean GERD-HRQL score improved from 33.7 (SD, 22.0) to 9.07 (SD, 13.95), p < 0.001. The mean Reflux Symptom Index score improved from 20.32 (SD, 13) to 8.07 (SD, 9.77), p < 0.001. The mean pH score improved from 35.3 (SD, 2.27) to 10.9 (SD, 11.5), p < 0.001. Twenty-two of the 29 patients were judged to have an intact hiatal repair with transoral incisionless fundoplication (76%). Of the 22 patients with an intact hiatal repair and intact fundoplication, 21 (95%) had normalized their pH exposure.
Emerging Applications for TIF
With TIF firmly established as an effective endoscopic anti-reflux procedure for select patients with GERD, there are several clinical scenarios where TIF is being explored. TIF after per-oral endoscopic myotomy (POEM) is a very exciting area of exploration—as the benefits of POEM over laparoscopic Heller myotomy (LHM) with partial fundoplication for patients with achalasia may be outweighed by the incidence of post-POEM GERD. Recent meta-analyses show that POEM may have better results than LHM [51–54] for improvement of dysphagia, but the issue of post-POEM GERD being higher than post-LHM still needs to be addressed [55]. We need to keep in mind that LHM alone has an incidence of postoperative GERD of approximately 50%, while LHM in combination with a partial fundoplication reduces postoperative GERD to approximately 10% [56]. Therefore, most surgeons will automatically perform both operations together. If a substantial number of patients require anti-reflux surgery after POEM, then it could tip the balance back toward LHM plus partial fundoplication as the preferred first line option. Fortunately, TIF may represent the endoscopic solution to post-POEM GERD [57]. In our experience of over 60 consecutive POEM procedures, only three patients were refractory to PPI medications and the TIF procedure was able to control GERD symptoms and esophagitis in all three patients [58]. Further studies examining both efficacy and durability of TIF post-POEM are underway.
TIF can be considered in obese patients prior to laparoscopic sleeve gastrectomy (LSG), given the higher rate of GERD with LSG compared to Roux-En-Y gastric bypass (RYGB) [59–62]. Since TIF does not incorporate much of the gastric fundus into the fundoplication, LSG is still quite feasible after a TIF procedure. This strategy may decrease the number of patients going to RYGB due to preoperative GERD. TIF post-LSG is possible, although it requires a sufficient gastric luminal diameter for the device to close.
Finally, there is now a recognized association between lung transplant outcomes and GERD with data supporting an association between GERD and allograft injury, encouraging a strategy of early diagnosis and aggressive reflux management in lung transplant recipients to improve transplant outcomes [63, 64]. There are centers that are currently exploring the role of TIF in the management of these patients.
Endoscopic Suturing for GERD
Early experiences with cinching or suturing devices were fraught with disappointment. Developed in the 1980’s, the Endocinch suturing device was the first such suturing device on the market for the endoscopic treatment of GERD [65], with the main goal of creating a full-thickness intussusception of the gastroesophageal junction, resulting in an inverted gastroplication, or in other words, a gastro-gastric plication. The suture bites, however, were not reproducibly full-thickness and the plications were mucosa to mucosa—which readily loosened over time.
After Endocinch, the NDO Plicator was introduced. This device created a gastroplication below the gastroesophageal junction with serosa-to-serosa apposition of the anterior gastric cardia in order to enhance the flap valve competency. Clinical data showed improved esophageal acid exposure in the short-term, and decreased medication usage and improved GERD-related symptoms for up to 5 years [66–69]. Despite this interesting outcomes data, the Plicator was taken off the market for unclear reasons.
Evolution of Full-Thickness Endoscopic Suturing for GERD
The OverStitch® device (Apollo Endosurgery, Austin, Texas) was FDA approved in 2008 for endoscopic placement of suture(s) and approximation of soft tissue. We first reported a pilot series of ten patients in which endoscopic augmentation of the GEJ was performed using the OverStitch affixed to a double channel gastroscope with suturing performed in an antegrade fashion (unlike TIF which is performed in retrograde position) [70]. When first applied as an anti-reflux procedure, interrupted sutures were placed on the gastric side of the GEJ in two layers in order to create a narrowed and elongated GEJ. Technical success was achieved in all patients, including those with a history of previous anti-reflux procedures (n = 7) and those with a hiatal hernia (n = 6). Adverse events were limited to one patient who developed self-limited nausea and vomiting. However, while the median pre-procedure GERD-HRQL improved from 20 (range 11–45) to a post-procedure score of 6 (range 3–25) (p = 0.001), the median duration of GERD symptom improvement after the procedure was only 1 month (range 0.5–4).
We then adopted a concept from our endoscopic gastric bypass revision techniques—that of mucosal ablation with argon plasma coagulation (APC) prior to suturing. The rationale is the mucosal ablation induces an inflammatory response may lead to more adherence when tissue is plicated together. We named this technique Mucosal Ablation and Suturing of the EG Junction (MASE). [71].
The MASE technique consists of first applying APC ablation to gastric mucosa below the GE junction to improve tissue apposition prior to suturing (Fig. 6). Next, in the forward viewing position, three sutures are placed along the lesser curvature of the cardia immediately below the esophagogastric junction to create a tightening and tissue bulge to prevent stomach contents from refluxing into the esophagus. Patients were discharged home after the procedure without overnight stay. The primary outcome evaluated was reduction or cessation of medications used for treating GERD. The secondary outcomes evaluated were procedure tolerance and safety. Among the 27 patients who underwent the MASE procedure with a mean follow-up time of 124 days (range 34–338), seven patients (26%) had altered anatomy from prior surgery: fundoplication (N = 4), Billroth II (N = 1), Roux-en-Y (N = 1), and sleeve gastrectomy (N = 1). Pre-procedure, 22 patients (82%) were on once or twice daily PPI therapy and five patients were on H2-receptor antagonists/topical antacids. Of the 22 patients on daily PPI, 13 patients (59%) were able to discontinue their medication, and three patients (14%) were able to reduce their dose. Of the seven patients with altered anatomy, four patients (57%) were able to discontinue or reduce their PPI after the procedure. With regard to tolerance, the most common side effect was self-limited epigastric pain post-procedure (22%). One patient required an overnight stay in the hospital for intravenous pain control. There were no other early or late complications. Thus, the MASE procedure represents a novel endoscopic treatment for GERD which may be particularly useful in managing complex patients with GERD, including those with altered anatomy who may not be candidates for surgical or endoscopic fundoplication.
Fig. 6.
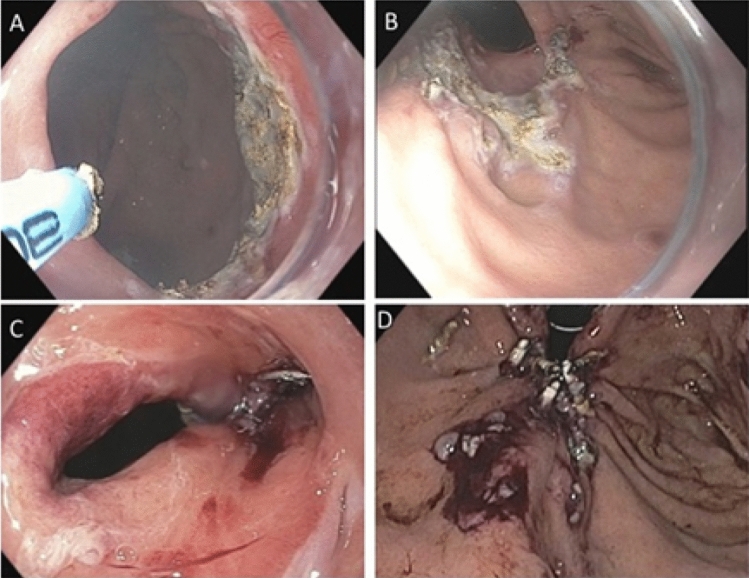
Mucosal ablation and suturing of the esophageal gastric junction (MASE) in a patient with severe GERD post-esophagectomy. a Wide open anastomosis with application of argon plasma coagulation (APC) to ablate mucosa; b: Retroflex view showing open anastomosis and extent of APC mucosal ablation; c: Post-MASE antegrade appearance showing significant narrowing at the anastomosis; d: Post-MASE retroflex view showing gastro-gastric plication successfully applied even with stomach in the chest
As various endoscopic approaches to GERD were being reported, a case series from Japan published in 2014 by Inoue et al. described a novel anti-reflux mucosectomy (ARMS) technique as a treatment for GERD in patients without a hiatal hernia [72]. This was initially a serendipitous observation that some patients after mucosectomy for neoplastic lesions in the cardia noticed improvement in their reflux symptoms. They next applied this technique to a series of patients with GERD symptoms specifically for this indication. Indeed, in their case series of ten patients, this technique appeared to be effective. In the DeMeester score, mean heartburn score decreased from 2.7 to 0.3 (p = 0.0011), regurgitation score from 2.5 to 0.3 (p = 0.0022), and total score from 5.2 to 0.67 (p = 0.0011). At endoscopic examination, the flap valve grade decreased from 3.2 to 1.2 (p = 0.0152). In 24-h esophageal pH monitoring the fraction of time at pH < 4 improved from 29.1% to 3.1% (p = 0.1). Fraction time absorbance more than > 0.14 of bile reflux was controlled from 52 to 4% (p = 0.05). In two cases of total circumferential resection, repeat balloon dilation was necessary to control stenosis. In all cases, proton pump inhibitor prescription could be discontinued with no ill effects. This initial case series demonstrated the potential anti-reflux effect of ARMS, with a crescentic mucosal resection appearing adequate. Several centers subsequently trialed this technique for safety, feasibility, and efficacy, with similar encouraging results, good safety profile and over two-thirds of patients in all studies improving their symptom scores, as well as some decreasing PPI use and improving esophageal acid exposure [73–77].
During this same time period, Benias et al. started developing an endoscopic suturing strategy that involved mucosal resection as opposed to mucosal ablation prior to suturing [78], which is called “Resection and Plication” or RAP. In addition to resection, the suture pattern was different, utilizing a single running suture instead of 2–3 interrupted sutures. RAP consists of semi-circumferential mucosectomy along with full-thickness plication of the LES and cardia. In this study, RAP was performed on ten patients with GERD refractory to PPI therapy. All patients underwent RAP without adverse events and were discharged on the same day. Only half of the patients required general anesthesia. Follow-up ranged from 5 to 24 months (median 9 months), and all patients had significant improvement in their GERD-HRQL scores (p < 0.0001, 95% CI 19.3—25.3); eight of ten eliminated their daily PPI dependence. The main advantages of RAP include a short procedure time, simple approach using readily available equipment, and possible avoidance of general anesthesia. Our group recently performed RAP in 26 patients with 100% technical success79. Eighteen patients (70%) had altered anatomy: esophagectomy (4), Roux-en-Y gastric bypass (4), sleeve gastrectomy (1), prior failed Nissen fundoplication (5), prior failed TIF (2), endoscopic sleeve gastroplasty (2). Mean follow-up was 6 months. Sixty percent of patients were able to stop or decrease PPI use. Reflux disease questionnaire for symptom severity was 14.6 pre-RAP and 3.0 post-RAP (p < 0.01) and for symptom frequency was 16.1 pre-RAP and 3.1 post-RAP (p < 0.01). GERD-HQRL was 25.7 pre-RAP and 3.7 post-RAP (p < 0.01). There were no adverse events with 90% of patients discharged the same day. The technique was slightly different from that of Benias et al. with the resection and plications performed more posterior and toward the lesser curve (Fig. 7). In addition, some patients receive a second reinforcing suture (Fig. 7e).
Fig. 7.
Resection and plication (RAP) technique for GERD in a patient after gastric bypass. a Endoscopic appearance of a wide open, patulous GEJ above the gastric pouch. b Retroflex view from the gastric pouch showing open hiatus, Hill Grade II-III. c After completion of piecemeal endoscopic mucosal resection (EMR) using a band ligation technique, the exposed muscularis propria is seen and suturing begins with the first bite taken distally at 8 o’clock position. d Bites are taken from 8 o’clock to 3 o’clock, alternating in a zigzag pattern (see inset) between distal and proximal sides of the mucosectomy area. The seventh bite seen here is at 4 o’clock at distal edge. e After the first running suture is cinched down, a second reinforcing suture is placed in a “V” formation. f Post-RAP appearance of the GEJ which is now much narrower with the creation of a short flap valve
Currently, we mainly consider performing the RAP procedure in GERD patients with altered anatomy where options for other anti-reflux surgeries or procedures are limited. In patients where mucosal resection is not feasible (prior resection, ablation, scarring), we will consider the MASE technique. The main advantage of endoscopic suturing in these altered anatomy cases is that retroflexion is not necessary and the gastro-gastric plication can be performed even if the GEJ is displaced in the thorax, as in the case of post-esophagectomy patients.
Conclusion
GERD is a spectrum disorder and endoscopic treatment options are now considered appropriate treatment in patients early in the GERD spectrum as well as some salvage situations, including altered anatomy.
Understanding the GEJ anatomy and physiology as it pertains to GERD is paramount to successfully appropriating these endoscopic modalities.
We have reviewed the proposed mechanism, patient selection, technical aspects, and clinical outcomes data for Stretta, TIF, and endoscopic suturing for GERD. Each of these procedures play a role in the treatment of GERD patients on different parts of the GERD spectrum as well as altered anatomy (Fig. 8).
Further studies are underway evaluating the role of endoscopic anti-reflux therapies in specific clinical scenarios, including post-POEM GERD, post-bariatric surgery anatomy, and even lung transplant.
Additional studies would be helpful to evaluate the long-term durability of these endoscopic techniques.
Fig. 8.
Summary diagram suggesting the various endoscopic anti-reflux procedures for the early part of the GERD spectrum as well as altered anatomy. (LES = Lower Esophageal Sphincter)
Declarations
Conflict of interest
Dr. Chang has served as consultant for Apollo Endosurgery, Boston Scientific, Cook, Covidien, Erbe, Endogastric Solutions, Mauna Kea, Mederi, Medtronics, Olympus, Ovesco, Pentax, and Torax. Dr. Lee has served as a speaker for GI Supply.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. 2018;154:267–276. doi: 10.1053/j.gastro.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazerbachi F, Krishnan K, Abu Dayyeh BK. Endoscopic GERD therapy: A primer for the transoral incisionless fundoplication procedure. Gastrointest Endosc. 2019;90(3):370–383. doi: 10.1016/j.gie.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–932. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- 4.Fry LC, Monkemuller K, Malfertheiner P. Systematic review: Endoluminal therapy for gastro-oesophageal reflux disease: Evidence from clinical trials. Eur J Gastroenterol Hepatol. 2007;19:1125–1139. doi: 10.1097/MEG.0b013e3282f16a21. [DOI] [PubMed] [Google Scholar]
- 5.Triadafilopoulos G. Stretta: An effective, minimally invasive treatment for gastroesophageal reflux disease. Am J Med. 2003;115:192S–200S. doi: 10.1016/S0002-9343(03)00224-9. [DOI] [PubMed] [Google Scholar]
- 6.Triadafilopoulos G. Stretta: A valuable endoscopic treatment modality for gastroesophageal reflux disease. World J Gastroenterol. 2014;20:7730–7738. doi: 10.3748/wjg.v20.i24.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utley DS, Kim M, Vierra MA, et al. Augmentation of lower esophageal sphincter pressure and gastric yield pressure after radiofrequency energy delivery to the gastroesophageal junction: A porcine model. Gastrointest Endosc. 2000;52:81–86. doi: 10.1067/mge.2000.105981. [DOI] [PubMed] [Google Scholar]
- 8.Kim MS, Holloway RH, Dent J, et al. Radiofrequency energy delivery to the gastric cardia inhibits triggering of transient lower esophageal sphincter relaxation and gastroesophageal reflux in dogs. Gastrointest Endosc. 2003;57:17–22. doi: 10.1067/mge.2003.23. [DOI] [PubMed] [Google Scholar]
- 9.Arts J, Bisschops R, Blondeau K, et al. A double-blind sham-controlled study of the effect of radiofrequency energy on symptoms and distensibility of the gastro-esophageal junction in GERD. Am J Gastroenterol. 2012;107:222–230. doi: 10.1038/ajg.2011.395. [DOI] [PubMed] [Google Scholar]
- 10.Ayazi S, Tamhankar A, DeMeester SR, et al. The impact of gastric distension on the lower esophageal sphincter and its exposure to acid gastric juice. Ann Surg. 2010;252:57–62. doi: 10.1097/SLA.0b013e3181e3e411. [DOI] [PubMed] [Google Scholar]
- 11.Perry KA, Banerjee A, Melvin WS. Radiofrequency energy delivery to the lower esophageal sphincter reduces esophageal acid exposure and improves GERD symptoms: A systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:283–288. doi: 10.1097/SLE.0b013e3182582e92. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Barber C, McLoughlin P, et al. Systematic review of endoscopic treatments for gastro-oesophageal reflux disease. Br J Surg. 2009;96:128–136. doi: 10.1002/bjs.6440. [DOI] [PubMed] [Google Scholar]
- 13.Liu HF, Zhang JG, Li J, et al. Improvement of clinical parameters in patients with gastroesophageal reflux disease after radiofrequency energy delivery. World J Gastroenterol. 2011;17:4429–4433. doi: 10.3748/wjg.v17.i39.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gersin KRF. The Stretta procedure: Review of catheter and technique evolution, efficacy and complications 2 years after introduction. Surg Endosc. 2002;16:199. doi: 10.1007/s00464-001-8134-6. [DOI] [Google Scholar]
- 15.Corley DA, Katz P, Wo JM, et al. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: A randomized, sham-controlled trial. Gastroenterology. 2003;125:668–676. doi: 10.1016/S0016-5085(03)01052-7. [DOI] [PubMed] [Google Scholar]
- 16.Coron E, Sebille V, Cadiot G, et al. Clinical trial: Radiofrequency energy delivery in proton pump inhibitor-dependent gastro-oesophageal reflux disease patients. Aliment Pharmacol Ther. 2008;28:1147–1158. doi: 10.1111/j.1365-2036.2008.03790.x. [DOI] [PubMed] [Google Scholar]
- 17.Aziz AM, El-Khayat HR, Sadek A, et al. A prospective randomized trial of sham, single-dose Stretta, and double-dose Stretta for the treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:818–825. doi: 10.1007/s00464-009-0671-4. [DOI] [PubMed] [Google Scholar]
- 18.Triadafilopoulos G, DiBaise JK, Nostrant TT, et al. The Stretta procedure for the treatment of GERD: 6 and 12 month follow-up of the US open label trial. Gastrointest Endosc. 2002;55:149–56. doi: 10.1067/mge.2002.121227. [DOI] [PubMed] [Google Scholar]
- 19.Dughera L, Navino M, Cassolino P, et al. Long-term results of radiofrequency energy delivery for the treatment of GERD: Results of a prospective 48-month study. Diagn Ther Endosc. 2011;2011:507157. doi: 10.1155/2011/507157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noar MD, Lotfi-Emran S. Sustained improvement in symptoms of GERD and antisecretory drug use: 4-year follow-up of the Stretta procedure. Gastrointest Endosc. 2007;65:367–372. doi: 10.1016/j.gie.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Reymunde A, Santiago N. Long-term results of radiofrequency energy delivery for the treatment of GERD: Sustained improvements in symptoms, quality of life, and drug use at 4-year follow-up. Gastrointest Endosc. 2007;65:361–366. doi: 10.1016/j.gie.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Dughera L, Rotondano G, De Cento M, et al. Durability of Stretta radiofrequency treatment for GERD: Results of an 8-year follow-up. Gastroenterol Res Pract. 2014;2014:531907. doi: 10.1155/2014/531907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noar M, Squires P, Noar E, et al. Long-term maintenance effect of radiofrequency energy delivery for refractory GERD: A decade later. Surg Endosc. 2014;28:2323–2333. doi: 10.1007/s00464-014-3461-6. [DOI] [PubMed] [Google Scholar]
- 24.Jobe BA, O'Rourke RW, McMahon BP, et al. Transoral endoscopic fundoplication in the treatment of gastroesophageal reflux disease: The anatomic and physiologic basis for reconstruction of the esophagogastric junction using a novel device. Ann Surg. 2008;248:69–76. doi: 10.1097/SLA.0b013e31817c9630. [DOI] [PubMed] [Google Scholar]
- 25.Rinsma NF, Smeets FG, Bruls DW, et al. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc. 2014;28:941–949. doi: 10.1007/s00464-013-3250-7. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen NT, Chinn J, Chang K. Collaboration between GI surgery & gastroenterology improves understanding of the optimal antireflux valve-the omega flap valve. Surg Endosc 2021. [DOI] [PubMed]
- 27.Mittal RK, Zifan A, Kumar D, et al. Functional morphology of the lower esophageal sphincter and crural diaphragm determined by three-dimensional high-resolution esophago-gastric junction pressure profile and CT imaging. Am J Physiol Gastrointest Liver Physiol. 2017;313:G212–G219. doi: 10.1152/ajpgi.00130.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yassi R, Cheng LK, Rajagopal V, et al. Modeling of the mechanical function of the human gastroesophageal junction using an anatomically realistic three-dimensional model. J Biomech. 2009;42:1604–1609. doi: 10.1016/j.jbiomech.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashfaq A, Rhee HK, Harold KL. Revision of failed transoral incisionless fundoplication by subsequent laparoscopic Nissen fundoplication. World J Gastroenterol. 2014;20:17115–17119. doi: 10.3748/wjg.v20.i45.17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes WE, Hoddinott KM, Mundy S, et al. Transoral incisionless fundoplication offers high patient satisfaction and relief of therapy-resistant typical and atypical symptoms of GERD in community practice. Surg Innov. 2011;18:119–129. doi: 10.1177/1553350610392067. [DOI] [PubMed] [Google Scholar]
- 31.Bell RC, Fox MA, Barnes WE, et al. Univariate and multivariate analyses of preoperative factors influencing symptomatic outcomes of transoral fundoplication. Surg Endosc. 2014;28:2949–2958. doi: 10.1007/s00464-014-3557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell RC, Freeman KD. Clinical and pH-metric outcomes of transoral esophagogastric fundoplication for the treatment of gastroesophageal reflux disease. Surg Endosc. 2011;25:1975–1984. doi: 10.1007/s00464-010-1497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chimukangara M, Jalilvand AD, Melvin WS, et al. Long-term reported outcomes of transoral incisionless fundoplication: An 8-year cohort study. Surg Endosc. 2019;33:1304–1309. doi: 10.1007/s00464-018-6403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ihde GM, Besancon K, Deljkich E. Short-term safety and symptomatic outcomes of transoral incisionless fundoplication with or without hiatal hernia repair in patients with chronic gastroesophageal reflux disease. Am J Surg 2011;202:740–6; discussion 746–7. [DOI] [PubMed]
- 35.Ihde GM, 2nd, Pena C, Scitern C, et al. pH scores in hiatal repair with transoral incisionless fundoplication. JSLS. 2019;23(1):e2018.00087. doi: 10.4293/JSLS.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narsule CK, Burch MA, Ebright MI, et al. Endoscopic fundoplication for the treatment of gastroesophageal reflux disease: Initial experience. J Thorac Cardiovasc Surg. 2012;143:228–234. doi: 10.1016/j.jtcvs.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Stefanidis G, Viazis N, Kotsikoros N, et al. Long-term benefit of transoral incisionless fundoplication using the esophyx device for the management of gastroesophageal reflux disease responsive to medical therapy. Dis Esophagus. 2017;30:1–8. doi: 10.1111/dote.12525. [DOI] [PubMed] [Google Scholar]
- 38.Testoni PA, Corsetti M, Di Pietro S, et al. Effect of transoral incisionless fundoplication on symptoms, PPI use, and ph-impedance refluxes of GERD patients. World J Surg. 2010;34:750–757. doi: 10.1007/s00268-010-0394-7. [DOI] [PubMed] [Google Scholar]
- 39.Testoni PA, Testoni S, Mazzoleni G, et al. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: A prospective single-center study. Surg Endosc. 2015;29:2770–2780. doi: 10.1007/s00464-014-4008-6. [DOI] [PubMed] [Google Scholar]
- 40.Trad KS, Barnes WE, Prevou ER, et al. The TEMPO Trial at 5 years: Transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov. 2018;25:149–157. doi: 10.1177/1553350618755214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson EB, Barnes WE, Mavrelis PG, et al. The effects of transoral incisionless fundoplication on chronic GERD patients: 12-month prospective multicenter experience. Surg Laparosc Endosc Percutan Tech. 2014;24:36–46. doi: 10.1097/SLE.0b013e3182a2b05c. [DOI] [PubMed] [Google Scholar]
- 42.Trad KS, Barnes WE, Simoni G, et al. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: The TEMPO Randomized Clinical Trial. Surg Innov. 2015;22:26–40. doi: 10.1177/1553350614526788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter JG, Kahrilas PJ, Bell RC, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology. 2015;148:324–333 e5. doi: 10.1053/j.gastro.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Hakansson B, Montgomery M, Cadiere GB, et al. Randomised clinical trial: Transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther. 2015;42:1261–70. doi: 10.1111/apt.13427. [DOI] [PubMed] [Google Scholar]
- 45.Gerson L, Stouch B, Lobontiu A. Transoral incisionless fundoplication (TIF 2.0): A meta-analysis of three randomized, controlled clinical trials. Chirurgia (Bucur) 2018;113:173–184. doi: 10.21614/chirurgia.113.2.173. [DOI] [PubMed] [Google Scholar]
- 46.Testoni PA, Distefano G, Mazzoleni G, et al. Transoral incisionless fundoplication with Esophyx (TIF 2.0) for gastro-esophageal reflux disease: Three to ten year outcomes in a prospective observational single-center study. Gastrointest Endosc. 2018;87:AB 262–AB 263. doi: 10.1016/j.gie.2018.04.1564. [DOI] [Google Scholar]
- 47.Choi AY, Roccato MK, Samarasena JB, et al. Novel interdisciplinary approach to GERD: Concomitant laparoscopic hiatal hernia repair with transoral incisionless fundoplication. J Am Coll Surg. 2021;232:309–318. doi: 10.1016/j.jamcollsurg.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Janu P, Shughoury AB, Venkat K, et al. Laparoscopic hiatal hernia repair followed by transoral incisionless fundoplication with EsophyX Device (HH + TIF): Efficacy and safety in two community hospitals. Surg Innov. 2019 doi: 10.1177/1553350619869449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith CD, Ganz RA, Lipham JC, et al. Lower esophageal sphincter augmentation for gastroesophageal reflux disease: The safety of a modern implant. J Laparoendosc Adv Surg Tech A. 2017;27:586–591. doi: 10.1089/lap.2017.0025. [DOI] [PubMed] [Google Scholar]
- 50.Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: The LOTUS randomized clinical trial. JAMA. 2011;305:1969–1977. doi: 10.1001/jama.2011.626. [DOI] [PubMed] [Google Scholar]
- 51.Schlottmann F, Luckett DJ, Fine J, et al. Laparoscopic Heller Myotomy versus peroral endoscopic myotomy (POEM) for achalasia: A systematic review and meta-analysis. Ann Surg. 2018;267:451–460. doi: 10.1097/SLA.0000000000002311. [DOI] [PubMed] [Google Scholar]
- 52.Awaiz A, Yunus RM, Khan S, et al. Systematic review and meta-analysis of perioperative outcomes of peroral endoscopic myotomy (POEM) and laparoscopic Heller Myotomy (LHM) for achalasia. Surg Laparosc Endosc Percutan Tech. 2017;27:123–131. doi: 10.1097/SLE.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Wang H, Chen X, et al. Per-oral endoscopic myotomy versus laparoscopic Heller Myotomy for achalasia: A meta-analysis of nonrandomized comparative studies. Medicine (Baltimore) 2016;95:e2736. doi: 10.1097/MD.0000000000002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marano L, Pallabazzer G, Solito B, et al. Surgery or peroral esophageal myotomy for achalasia: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3001. doi: 10.1097/MD.0000000000003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Repici A, Fuccio L, Maselli R, et al. GERD after per-oral endoscopic myotomy as compared with Heller's myotomy with fundoplication: A systematic review with meta-analysis. Gastrointest Endosc. 2018;87:934–943 e18. doi: 10.1016/j.gie.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 56.Richards WO, Torquati A, Holzman MD, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: A prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405–12; discussion 412–5. [DOI] [PMC free article] [PubMed]
- 57.Tyberg A, Choi A, Gaidhane M, et al. Transoral incisional fundoplication for reflux after peroral endoscopic myotomy: A crucial addition to our arsenal. Endosc Int Open. 2018;6:E549–E552. doi: 10.1055/a-0584-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang KJ. Endoscopic foregut surgery and interventions: The future is now. The state-of-the-art and my personal journey. World J Gastroenterol. 2019;25:1–41. doi: 10.3748/wjg.v25.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bou Daher H, Sharara AI. Gastroesophageal reflux disease, obesity and laparoscopic sleeve gastrectomy: The burning questions. World J Gastroenterol. 2019;25:4805–4813. doi: 10.3748/wjg.v25.i33.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu L, Chen B, Du N, et al. Relationship between bariatric surgery and gastroesophageal reflux disease: A systematic review and meta-analysis. Obes Surg. 2019;29:4105–4113. doi: 10.1007/s11695-019-04218-3. [DOI] [PubMed] [Google Scholar]
- 61.Popescu AL, Ionita-Radu F, Jinga M, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. Rom J Intern Med. 2018;56:227–232. doi: 10.2478/rjim-2018-0019. [DOI] [PubMed] [Google Scholar]
- 62.Sharples AJ, Mahawar K. Systematic review and meta-analysis of randomised controlled trials comparing long-term outcomes of Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2017;30(2):664–672. doi: 10.1007/s11695-019-04235-2. [DOI] [PubMed] [Google Scholar]
- 63.Hathorn KE, Chan WW, Lo WK. Role of gastroesophageal reflux disease in lung transplantation. World J Transpl. 2017;7:103–116. doi: 10.5500/wjt.v7.i2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood RK. Esophageal dysmotility, gastro-esophageal reflux disease, and lung transplantation: What is the evidence? Curr Gastroenterol Rep. 2015;17:48. doi: 10.1007/s11894-015-0474-9. [DOI] [PubMed] [Google Scholar]
- 65.Swain CP, Mills TN. An endoscopic sewing machine. Gastrointest Endosc. 1986;32:36–38. doi: 10.1016/S0016-5107(86)71727-6. [DOI] [PubMed] [Google Scholar]
- 66.Pleskow D, Rothstein R, Lo S, et al. Endoscopic full-thickness plication for the treatment of GERD: A multicenter trial. Gastrointest Endosc. 2004;59:163–171. doi: 10.1016/S0016-5107(03)02542-2. [DOI] [PubMed] [Google Scholar]
- 67.Pleskow D, Rothstein R, Lo S, et al. Endoscopic full-thickness plication for the treatment of GERD: 12-month follow-up for the North American open-label trial. Gastrointest Endosc. 2005;61:643–649. doi: 10.1016/S0016-5107(04)02648-3. [DOI] [PubMed] [Google Scholar]
- 68.Pleskow D, Rothstein R, Kozarek R, et al. Endoscopic full-thickness plication for the treatment of GERD: Long-term multicenter results. Surg Endosc. 2007;21:439–444. doi: 10.1007/s00464-006-9121-8. [DOI] [PubMed] [Google Scholar]
- 69.Pleskow D, Rothstein R, Kozarek R, et al. Endoscopic full-thickness plication for the treatment of GERD: Five-year long-term multicenter results. Surg Endosc. 2008;22:326–332. doi: 10.1007/s00464-007-9667-0. [DOI] [PubMed] [Google Scholar]
- 70.Han J, Chin M, Fortinsky KJ, et al. Endoscopic augmentation of gastroesophageal junction using a full-thickness endoscopic suturing device. Endosc Int Open. 2018;6:E1120–E1125. doi: 10.1055/a-0603-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fortinsky KJ, Shimizu T, Chin M, et al. Mucosal ablation and suturing at the esophagogastric junction (MASE): A novel procedure for the management of patients with gastroesophageal reflux disease. Gastroinest Endosc. 2018;87:AB552. doi: 10.1016/j.gie.2018.04.2198. [DOI] [Google Scholar]
- 72.Inoue H, Ito H, Ikeda H, et al. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: A pilot study. Ann Gastroenterol. 2014;27:346–351. [PMC free article] [PubMed] [Google Scholar]
- 73.Ota K, Takeuchi T, Harada S, et al. A novel endoscopic submucosal dissection technique for proton pump inhibitor-refractory gastroesophageal reflux disease. Scand J Gastroenterol. 2014;49:1409–1413. doi: 10.3109/00365521.2014.978815. [DOI] [PubMed] [Google Scholar]
- 74.Hedberg HM, Kuchta K, Ujiki MB. First experience with banded anti-reflux mucosectomy (ARMS) for GERD: Feasibility, safety, and technique (with video) J Gastrointest Surg. 2019;23:1274–1278. doi: 10.1007/s11605-019-04115-1. [DOI] [PubMed] [Google Scholar]
- 75.Monino L, Gonzalez JM, Vitton V, et al. Anti-reflux mucosectomy with band ligation in the treatment of refractory gastroesophageal reflux disease. Endoscopy. 2019;51:E215–E216. doi: 10.1055/a-0875-3479. [DOI] [PubMed] [Google Scholar]
- 76.Patil G, Dalal A, Maydeo A. Feasibility and outcomes of anti-reflux mucosectomy (ARMS) for proton pump inhibitor dependent gastroesophageal reflux disease: First Indian study (with video) Dig Endosc. 2020;32:745–752. doi: 10.1111/den.13606. [DOI] [PubMed] [Google Scholar]
- 77.Yoo IK, Ko WJ, Kim HS, et al. Anti-reflux mucosectomy using a cap-assisted endoscopic mucosal resection method for refractory gastroesophageal disease: A prospective feasibility study. Surg Endosc. 2020;34:1124–1131. doi: 10.1007/s00464-019-06859-y. [DOI] [PubMed] [Google Scholar]
- 78.Benias PC, D'Souza L, Lan G, et al. Initial experience with a novel resection and plication (RAP) method for acid reflux: A pilot study. Endosc Int Open. 2018;6:E443–E449. doi: 10.1055/s-0044-101453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sowa P, Ortizo R, Samarasena J, et al. Resection and plication (RAP): A novel procedure for the management of gastroesophageal reflux disease. Gastroinest Endosc. 2020;XX:Abstract submitted.