Abstract
Phthalates are well-known endocrine-disrupting chemicals. Many detrimental health effects of phthalates were investigated, but studies on the association of phthalates with obesity in children showed inconsistent results. Thus, this systematic review and meta-analysis were performed to clarify whether prenatal and postnatal exposures to phthalates are associated with physical growth disturbances in children. We performed the systematic review and meta-analysis following the PRISMA 2020 statement guidelines, and found 39 studies that met our inclusion criteria, including 22 longitudinal and 17 cross-sectional studies. We observed a significant negative association between the prenatal exposure to DEHP and the body mass index (BMI) z-score of the offspring (β = − 0.05; 95% CI: − 0.10, − 0.001) in the meta-analysis, while no significant association between the prenatal exposure to DEHP and the body fat percentage of the offspring was observed (β = 0.01; 95% CI: − 0.41, 0.44). In the systematic review, studies on the association between phthalates exposure in childhood and obesity were inconsistent. Prenatal exposure to phthalates was found to be associated with decreased BMI z-score in children, but not associated with body fat percentage. Our findings suggest that phthalates disturb the normal muscle growth of children, rather than induce obesity, as previous studies have hypothesized.
Subject terms: Environmental sciences, Endocrinology, Risk factors
Introduction
Phthalates are wildly used chemicals to improve the utility of plastics and personal care products. Due to phthalates’ low price and usefulness, the annual global production of phthalates is estimated up to 5.5 million tonnes1. Phthalates can be classified as high-molecular-weight phthalates (HMWPs) and low-molecular-weight phthalates (LMWPs). HMWPs can give plastics flexibility, and were used in toys, building materials, medical devices, and paints. Di-(2-ethylhexyl) phthalate (DEHP), the most widely used HMWP, accounts for 65.2% of the total consumption of phthalates, and is produced approximately 2 million tonnes per year2,3. Meanwhile, LMWPs are usually used in cosmetics such as shampoos, cosmetics, lotions, nail care products, and other personal hygiene products; dibutyl phthalate (DBP) is one of the most widely used LMWPs4.
Phthalate is a well-known endocrine-disrupting chemical with anti-androgenic effects. Due to its anti-androgenic properties, previous studies have focused on the health effects of phthalates, including abnormal sexual development such as hypospadias and anogenital distance, adverse birth outcomes, precocious puberty, and hormonal disturbances of testosterone and thyroid hormone5–9. However, the relationship between phthalates and obesity remains unclear and inconclusive, although phthalates can interfere with growth and metabolism10–14.
Recently, the negative association between the perinatal exposure to phthalates and the body weight was reported in a systematic review and meta-analysis from animal studies15. A rodent study reported that prenatal exposure to DEHP could induce decreased muscle mass16. Although a recent systematic review and meta-analysis of human studies was performed, a definitive conclusion in children was not reached17. Considering these evidence, the hypothesis that phthalates exposure is associated with obesity should be revised as phthalate exposure are associated with disturbing normal growth. Therefore, it is necessary to review and summarize the direction and size of associations found in the studies using the latest results.
The present study aimed to clarify whether prenatal and postnatal exposures to phthalates are associated with physical growth as measured by body composition indices in children. Thus, we performed a systematic literature review and meta-analysis for the association of phthalates with body composition indices among children.
Materials and methods
Search strategy and selection methods
This study was registered in PROSPERO, a prospective international register of systematic reviews (CRD42021235007). The review question was as follows: “Does the prenatal and postnatal exposures to phthalates affect the physical growth of children?” According to PECO formulation guidance18, more specifically, the review question was “among the children, what is the effect of one-unit of natural log of DEHP (or DBP) metabolites versus one-unit incremental increase on the physical growth measured by BMI, body fat percentage, and other indices”. We used PubMed, EMBASE, and Google Scholar to search articles that reported associations between the DEHP and DBP levels and the physical growth of children between January 1, 1980, and December 31, 2021, using the search string (Supplementary Table S1). The inclusion criteria were: (1) the epidemiologic study in a cohort, case–control, or cross-sectional design; and (2) the size of association reported in beta estimates (β) with 95% confidence intervals (CIs), or in the form that can be converted to β and 95% CIs. The exclusion criteria were: (1) presented outcomes in irrelevant forms; (2) not able to use the size of the association; (3) letter, commentary, or review articles; (4) investigated the identical study population to other included study; (5) articles not written in English; and (6) non-human.
Following the PRISMA statement guidelines for reporting systematic reviews and meta-analysis19, we systematically searched the literature databases. The search results of each search were downloaded into a reference management software program (EndNote, version X8) for identifying duplicate articles and for further review. Two authors (DW-L and HM-L) screened records and selected articles according to the inclusion and exclusion criteria. If two authors disagreed about eligibility of a study, the authors agreed after discussion and understanding with a third author (YC-H). Finally, the authors manually checked the reference lists of the included articles.
Data extraction
We extracted the following data from all articles using a data-extraction sheet: first author, year, country, type of study, sample size, the timing of exposure assessment, measured metabolites and the corresponding range, statistical analysis, adjustment variables, timing of outcome assessment, outcome variables, findings, estimates type (β estimate and/or odds ratio [OR]), and estimates and 95% CIs of the association between prenatal phthalate exposure and outcome variables.
The classes of DEHP metabolites were measured not identically across studies. Included studies reported the size of associations of outcome variables with each DEHP metabolite, with or without their sum (∑DEHP). We preferred the size of association of outcome variables with ∑DEHP as an exposure indicator for our meta-analysis. Among secondary metabolites, the available metabolites were selected in the following order according to the molar fraction of excretion to absorbed DEHP in the human body: mono-2-ethyl-5-carboxypentyl phthalate (MECCP), mono-(2-ethyl-5-hydroxy-hexyl) phthalate (MEHHP), and mono-(2-ethyl-5-oxo-hexyl) phthalate (MEOHP)20. Among the DBP metabolites, mono-n-butyl phthalate (MnBP) was preferentially selected, followed by mono-isobutyl phthalate (MiBP)21. If it was not available to use the size of association of secondary metabolites of DEHP, mono-(2-ethylhexyl) phthalate was used as an indicator of exposure. In each study, we used β estimates with 95% CIs with the model with the most adjustment variables was used for meta-analysis.
Quality assessment
The Newcastle–Ottawa quality assessment scale (NOS) was used22. The NOS for cohort and cross-sectional studies consists of selection, comparability, and outcome assessment items. By using the assessment tool, the quality of cohort studies was scored from 0 to 9 and classified as low (0–3), moderate (4–6), or high (7–9). The quality of cross-sectional studies was scored from 0 to 10 and classified into low (0–3), moderate (4–7), or high (8–10).
Statistical analysis
Four meta-analyses for prenatal exposure and body composition in children were performed according to the phthalates (DEHP or DBP) × outcome (body mass index [BMI] z-score and body fat percentage). For the studies on the association between postnatal exposure and body composition in children, we could not perform a meta-analysis for the heterogeneity in the age of study participants, measured outcome, and presented the size of the association. The standardized regression coefficient for effect size and its standard error were used for meta-analyses of the association between phthalates and body composition23. The heterogeneity of results across studies was examined by Q test, with P < 0.10 implying substantial heterogeneity. The overall estimate was calculated using a random-effects model, considering the between-study variation. We evaluated publication bias by a Begg funnel plot and the Egger test. If the asymmetry of the funnel plot, and/or P < 0.05 from the Egger test were found, we considered the existence of publication bias. Statistical analyses were conducted using the R software, “metafor” package, version 2.13.2 (Wolfgang Viechtbauer, Maastricht, the Netherlands).
Ethics approval and consent to participate
Not applicable (no human subject participants will be involved).
Consent for publication
Not applicable.
Results
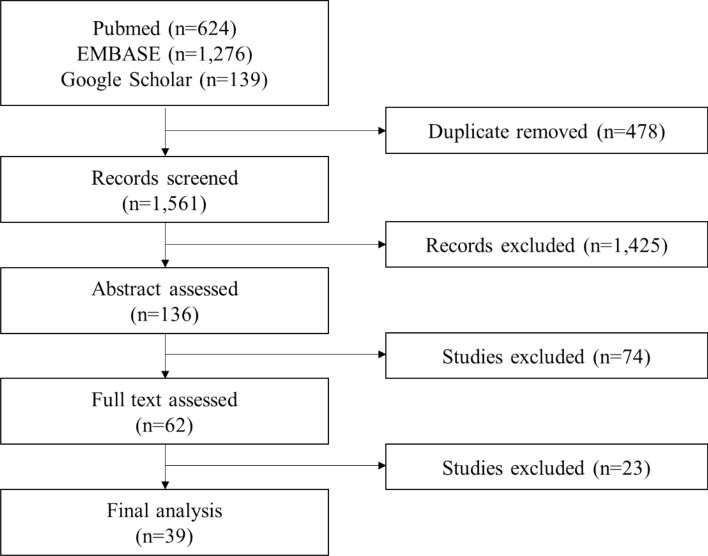
Figure 1 shows the process to include the relevant studies for the systematic review. We screened 1561 records, and excluded 1425 studies based on their titles. After the abstracts were reviewed, 74 irrelevant studies were excluded. The full texts of 62 studies were assessed, and we found 39 studies that met our inclusion criteria. The reference lists of the included studies were manually checked, and no additional studies were searched in this step.
Figure 1.
Flow diagram of the study selection process.
Table 1 summarizes the a total of 39 observational studies. The study size varied between 72 and 2,884 participants. Studies were conducted in the United States (n = 12), China (n = 6), South Korea (n = 4), Taiwan (n = 3), Spain (n = 2), Netherlands (n = 1), Australia (n = 1), France (n = 1), Greece (n = 1), Italy (n = 1), Germany (n = 1), Canada (n = 1), Sweden (n = 1), Thailand (n = 1), Iran (n = 1), Mexico (n = 1), and in multiple European countries (n = 1). Supplementary Table S2 shows reasons for exclusion in full-text review. The quality of the studies as assessed by the NOS is presented in Supplementary Table S3–4. The scores of the included longitudinal studies (n = 22) ranged from 8 to 9, and all were classified as good quality. Cross-sectional studies (n = 17) ranged from 5 to 8 and included 12 high-quality studies and 5 moderate-quality studies.
Table 1.
Summary of studies included in the systematic review.
| ID | First author | Year | Study design | Country | Sample Size | Study | Exposure assessment | Measured metabolites and range | Timing of outcome assessment | Outcome variables |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agay-Shay24 | 2015 | Cohort study | Spain | 470 | INMA Spanish Birth cohort | Maternal urine in the 1st and 3rd trimester of pregnancy |
GM of MECPP, MEHHP, MEOHP, and MEHP (40.8 µg/g Cr, 28.6 µg/g Cr, 27.8 µg/g Cr, and 14.6 µg/g Cr, respectively) ‘GM of MnBP and MiBP (32.4 µg/g Cr, 32.6 µg/g Cr, respectively) |
7 y | BMI z-scores |
| 2 | Berman25 | 2020 | Cohort study | Australia | 410 | Maternal urine in the 2nd and 3rd trimester of pregnancy | Median of ∑DEHP metabolites, and ∑DBP metabolites (9.34 µg/L, 4.10 µg/L, respectively) | 1, 2, 3, 5, 8, 10, 14, 17 and 20 y | Height, BMI, DXA (total fat %, total fat mass [g], total lean mass [g]) | |
| 3 | Botton26 | 2016 | Cohort study | France | 520 | EDEN mother–child cohort | Maternal urine in the 2nd trimester | Median of molar ∑DEHP metabolites, MnBP and MiBP (0.32 µM/L, 43 µg/L, and 39 µg/L, respectively) | 5 y | BMI |
| 4 | Buckley27 | 2016 | Cohort study | U.S | 707 | MSSM + CCCEH + HOME Study | Prenatal maternal urine | GM of molar ∑DEHP metabolites, MnBP and MiBP (0.277 µM/L, 30.6 µg/L, and 6.45 µg/L, respectively) | 4–9 y | BMI z-score and overweight/obese (BMI > = 85th percentile) |
| 5 | Buckley10 | 2016 | Cohort study | U.S | 180 | MSSM Study | Prenatal maternal urine | GM of molar ∑DEHP metabolites, MnBP and MiBP (0.284 µM/L, 32.9 µg/L, and 5.83 µg/L, respectively) | 4 and 9 y | Body composition (total fat %) |
| 6 | Buser28 | 2014 | Cross-sectional study | U.S | not described | NHANES 2007–2010 | Urine of the participants |
(Children and adolescent aged 6–19) GM of molar ∑DEHP metabolites, MnBP and MiBP (0.24 µM/L, 23.0 µg/L, and 10.43 µg/L, respectively) (adults > = 20 y) GM of ∑DEHP metabolites, MnBP and MiBP (0.18 µM/L, 15.21 µg/L, and 6.75 µg/L, respectively) |
Children and adolescent aged 6–19, adults > = 20 y |
(Children and adolescent) obese 95th percentile > = BMI z-score; overweight, 95th percentile > BMI z-score > = 85th percentile (adults) obese, BMI > = 30 kg/m2; overweight, 30 kg/m2 > BMI > = 25 kg/m2 |
| 7 | Chang29 | 2020 | Cross-sectional study | Taiwan | 152 | RAPIT program | Urine of the participants | GM of ∑DEHP metabolites, MnBP and MiBP (59.29 µg/g Cr, 49.44 µg/g Cr, and 28.85 µg/g Cr, respectively) | 5 y | BMI, total fat (%) |
| 8 | Deierlein30 | 2016 | Cohort study | U.S | 1,239 | The Breast Cancer and Environment Research Program | Urine of the participants at the baseline (6–8 y) | GM of ∑DEHP metabolites (182 µg/g Cr [6 y], 152 µg/g Cr [7 y], and 152 µg/g Cr [8 y]) and LMWH (184 µg/g Cr [6 y], 136 µg/g Cr [7 y], and 163 µg/g Cr [8 y]) | 3 times until the last visit when girls were on average 14 y old (11–16 y) | BMI |
| 9 | Heggeseth31 | 2019 | Cohort Study | U.S | 335 | CHAMACOS cohort study | Prenatal maternal urine | Median of MECPP, MEHHP, MEOHP, MnBP, and MiBP (24.05 µg/L, 14.8 µg/L, 10.75 µg/L, 20.7 µg/L, and 2.8 µg/L, respectively) | 11 follow-up visits between ages 2 and 14 y | BMI |
| 10 | Hou32 | 2015 | Cross-sectional study | Taiwan | 308 | 270 normal adolescents (6.5–15 y) and 38 complainants (6.5–8.5 y) | Urine of the participants | GM of ∑DEHP, MnBP and MiBP (193.73 µg/L, 75.42 µg/L, and 47.06 µg/L, respectively) | When assessing phthalate exposure (6.5–8.5 y) | Obese (BMI), waist-to-hip ratio, Subcutaneous fat thickness |
| 11 | Kim33 | 2016 | Cohort Study | South Korea | 128 | 128 healthy pregnant women and their infants in 2012 | Umbilical cord blood, newborns’ first urine | GM of MEHHP in maternal blood, maternal urine, cord blood, placenta, and newborns’ urine (0.31 µg/L, 18.23 µg/L, 0.,33 µg/L, 0.10 µg/L, and 5.83 µg/L, respectively), GM of MEOHP in maternal urine and newborns’ urine (15.88 µg/L, and 3.02 µg/L, respectively) | Perinatal | BMI z-score change during 3 months (Evaluation criterion for relative body mass increase was BMI z-score change over the 50th percentile) |
| 12 | Kim34 | 2018 | Cross-sectional study | South Korea | 137 | 65 overweight children (6–13 y) and 72 controls | Urine of the participants | GM OF MECPP, MEOHP, and MEHHP (87.3 µg/g Cr, 29.5 µg/g Cr, and 36.8 µg/g Cr, respectively) | When assessing phthalates exposure (6–13 y) | BMI percentile |
| 13 | Lee35 | 2020 | Cohort study | South Korea | 481 | EDC cohort | Prenatal maternal urine and urine of the participants |
GM of molar ∑DEHP in prenatal maternal urine and children’s urine at 6 years of age (0.11 µM/L, and 0.33 µM/L, respectively) GM of ∑MnBP in prenatal maternal urine and children’s urine at 6 years of age (39.68 µg/L, and 70.00 µg/L, respectively) |
6 y | BMI z-score, percentage of fat mass, fat mass index, percentage of skeletal muscle mass, skeletal muscle index |
| 14 | Maresca36 | 2016 | Cohort study | U.S | 424 | CCCEH cohort | Prenatal maternal urine | GM of molar ∑DEHP metabolites, MiBP, and MnBP (0.29 µM/L, 8.81 µg/L, 37.58 µg/L) | 5 y and 7 y | BMI z-score at 5 y and 7 y, percent of fat mass at 7 y, FMI at 7 y, WC at 7 y |
| 15 | Harley37 | 2017 | Cohort study | U.S | 219 | CHAMACOS cohort study | Prenatal maternal urine, two times | GM of ∑DEHP, MECPP, MEHHP, MEOHP, MnBP, and MiBP in each measurement (0.2 and 0.2 nmol/mL, 25.9 and 32.4 µg/L, 15.1 and 18.8 µg/L, 11.2 and 13.8 µg/L, 22.8 and 28.5 µg/L, and 2.7 and 3.4 µg/L, respectively) | 12 y | BMI z-score, WC |
| 16 | Saengkaew38 | 2017 | Cross-sectional study | Thailand | 155 | Children aged 7–18 y | Urine of the participants | Median of MBP (216.47 µg/g Cr), detection rate 82.58% | When assessing phthalate exposure | BMI z-score, WC |
| 17 | Shoaff39 | 2017 | Cohort study | U.S | 219 | HOME study | up to two times prenatally and six times from 1 to 8 y | GM of ∑DEHP, MiBP, and MnBP for children (86 µg/L, 4.8 µg/L, and 25 µg/L, respectively) | 8 y | BMI z-score, WC, body fat percent |
| 18 | Smerieri40 | 2015 | Cross-sectional study | Italy | 72 |
41 obese children and 31 controls (mean age 12 y) |
Urine of the participants | Detection rates of MECPP, MEOHP, and MEHHP were 80.5%, 87.8%, and 80.5% among obese group, and 38.7%, 74.2%, and 8.39% among control group, respectively | When assessing phthalate exposure | WC |
| 19 | Trasande13 | 2013 | Cross-sectional study | U.S | 2,884 | NHANES 2003–2008 (children 6–19 y) | Urine of the participants | GM of ∑DEHP metabolite (0.358 µM/L among male and 0.360 among female) and ∑LMW metabolite (0.593 µM/L among male and 0.680 µM/L among female) | When assessing phthalate exposure | BMI z-score, overweight (BMI z-score > = 85th percentile), and obesity (BMI z-score > = 95th percentile) |
| 20 | Tsai41 | 2016 | Cohort study | Taiwan | 88 | RAPIT program (6.0–10.5 y) | Estimated the total daily intake of DEHP, and urine of the participants | Mean of ∑DEHP metabolite 106.19 µg/g Cr | When participants were examined | Weight percentile and height percentile above 50th percentile (based on the standards provided by the Ministry of Health and Welfare) |
| 21 | Vafeiadi42 | 2018 | Cohort Study | Greece | 500 | Rhea Study | Prenatal maternal urine and urine of the participants |
GM of molar ∑DEHP, MiBP and MnBP in prenatal maternal urine (0.1 µM/g Cr, 33.5 µg/g Cr, and 37.1 µg/g Cr, respectively) GM of molar ∑DEHP, MiBP, and MnBP in children’s urine (0.3 µM/gCr, 41.1 µg/g Cr, and 21.7 µg/g Cr, respectively) |
4–6 y | BMI z-score |
| 22 | Valvi43 | 2015 | Cohort study | Spain | 391 | INMA Spanish birth cohort | Prenatal maternal urine at 1st and 3rd trimester | GM of ∑DEHP metabolites, MnBP and MiBP (99.6 µg/gCr, 32.7 µg/gCr, and 33.0 µg/gCr, respectively) | Birth to 6 mos., 1, 4, and 7 y of age | BMI z-score, weight gain z-score (0–6 months) |
| 23 | Vrijheid44 | 2020 | Cohort study | Europe | 1,031 | HELIX study (BiB in UK, EDEN in France, INMA in Spain, KANC in Lithuanina, MoBa and Rhea in Greece) | 77 prenatal exposure and 96 childhood exposure including air pollutants, built environments, and biomarkers of chemical pollutants | Not described | BMI z-score (age-and-sex standardized z-scores) | BMI z-score |
| 24 | Wu45 | 2020 | Cross-sectional study | U.S | 2372 | NHANES 2005–2010 (6–19 y) | Urine of the participants | GM of MiBP, 9.98 µg/L | When assessing phthalate exposure | BMI z-score |
| 25 | Xia46 | 2018 | Cross-sectional study | China | 159 | PTHEC study, 69 overweight/obese children and 80 normal weight children | Urine of the participants | Median of MEOHP, MEHHP, and MnBP among normal participants (2.97 µg/L, 7.57 µg/L, and 13.68 µg/L, respectively) and among overweight/obese participants (2.6 µg/L, 6.5 µg/L, and 18.68 µg/L, respectively) | When assessing phthalate exposure | Overweight/obese |
| 26 | Xie47 | 2015 | Case–control study | China | 167 | 57 boys with constitutional delay for growth and puberty and 110 controls (11 y) | Urine of the participants | Median of ∑DEHP metabolites and MnBP among cases (20.06 µg/L and 37.43 µg/L, respectively), and among controls (12.85 µg/L and 15.56 µg/L, respectively) | When assessing phthalate exposure | Constitutional delay of growth and puberty |
| 27 | Zettergren48 | 2021 | Cohort study | Sweden | 100 | BAMSE birth cohort | Urine of the participants at 4 years of age | GM of ∑DEHP metabolites and MnBP (331 µg/L, 296 µg/L) | 24 y | BMI, WC, Body fat %, trunk fat % (Bio-electrical impedance analysis) |
| 28 | Zhang11 | 2014 | Cross-sectional study | China | 497 | PTHEC study (8–13 y) | Urine of the participants | GM of ∑DEHP metabolites and MnBP; boys (8–10 y), 29.6 µg/L; boys (11–13 y), 21.9 µg/L; girls (8–10 y), 32.5 µg/L; girls (11–13 y), 16.5 µg/L | When assessing phthalate exposure | BMI z-score, body fat % (Yao’s formula) |
| 29 | Amin49 | 2017 | Cross-sectional study | Iran | 242 | Urine of the participants | Mean of MEOHP, MEHHP, MEHP, MBzP, MBP, and MMP were 257.98 µg/L, 149.44 µg/L, 104.46 µg/L, 233.01 µg/L, 218.17 µg/L, and 59.82 µg/L | 6–18 y | BMI z-score, WC | |
| 30 | Ashley-Martin50 | 2021 | Cross-sectional study | Canada | 200 | MIREC study | Urine of the participants | Twenty-two metabolites were measured. Median of ∑DEHP and ∑DiBP were 155 nmol/mL and 100 nmol/mL, respectively | 2–5 y | BMI z-score |
| 31 | Ding51 | 2021 | Cross-sectional study | China | 463 | Urine of the participants | Median of MECPP, MCMHP, MEOHP, MEHHP, MEHP, and ∑DEHP were 13.00 μg/L, 7.68 μg/L, 6.04 μg/L, 4.78 μg/L, 3.18 μg/L, and 34.56 μg/L | 16–19 y | BMI, WHR, WHtR | |
| 32 | Berger52 | 2021 | Cohort study | U.S | 309 | CHAMACOS cohort study | Prenatal maternal urine | GM of ∑DEHP (0.2 nmol/mL) | 5 y | BMI z-score |
| 33 | Li53 | 2021 | Cohort study | China | 814 | Maternal urine in the 1st, 2nd and 3rd trimester of pregnancy | Median of ∑DEHP at 1st, 2nd, and 3rd trimesters were 0.09 nmol/mL, 0.06 nmol/mL, and 0.07 nmol/mL | average BMI z-score of 6-, 12- and 24-month | BMI z-score | |
| 34 | Nidens54 | 2021 | Cohort study | Germany | 130 | Prenatal maternal urine | GM of ∑HMWP (31.31 μg/gCr) | 2 y | Weight gain (%) first 2 years of life | |
| 35 | On55 | 2021 | Cross-sectional study | South Korea | 240 | Urine of the participants | GM of MECPP, MEOHP, MEHHP, and MEHP (104.73 µg/g Cr, 33.96 µg/g Cr, and 14.54 µg/g Cr, respectively) | 5–16 y | BMI percentile, weight percentile, and height percentile | |
| 36 | Silva56 | 2021 | Panel study | Netherland | 471 | Urine of the participants | Median of MECCP, MEOHP, and MEHHP were 0.94 nmol/L, 0.14 nmol/L, and 0.27 nmol/L | 6y and 10 y | BMI z-score, Fat mass index | |
| 37 | Hatch57 | 2008 | Cross-sectional study | U.S | 1,009 (6–19 y) | NHANES 1999–2002 | Urine of the participants |
GM of MEHHP among boys in 6–11 y and 12–19y were 39.6 μg/gCr and 21.1 μg/gCr, respectively GM of MEHHP among girls in 6–11 y and 12–19y were 39.1 μg/gCr and 18.2 μg/gCr, respectively |
6–19y | BMI, WC |
| 38 | Wang58 | 2013 | Cross-sectional study | China | 259 | Urine of the participants | GM of ∑DEHP (117.3 nmol/mL) | 8–15y | BMI, WC | |
| 39 | Bowman59 | 2019 | Cohort study | Mexico | 229 | ELEMENT study | Urine of the participants |
(boys) GM of ∑DEHP at 1st, 2nd, and 3rd trimester in prenatal maternal urine were 65.07 μg/L and 63.42 μg/L, and 78.60 μg/L (girls) GM of ∑DEHP at 1st, 2nd, and 3rd trimester in prenatal maternal urine were 71.03 μg/L and 75.97 μg/L, and 76.69 μg/L |
8–14y (Visit 1) and 9–17y (Visit 2) | BMI z-score, WC, skinfold thickness |
MEHHP mono-(2-ethyl-5-hydroxy-hexyl) phthalate; MEOHP mono-(2-ethyl-5-oxo-hexyl) phthalate; MnBP mono-n-butyl phthalate (MnBP); MECCP Mono-2-ethyl-5-carboxypentyl phthalate; MBzP Monobenzyl phthalatete; BMI body mass index; WC waist circumference.
Prenatal exposure to phthalates and BMI z-scores
Supplementary Table S5 describes the studies investigating the association between prenatal exposure to phthalates and BMI. Among the 39 studies, 17 studies investigated the association between prenatal exposure to phthalates and BMI. The statistical significance of associations between phthalate metabolites and BMI of children is summarized in Supplementary Table S6.
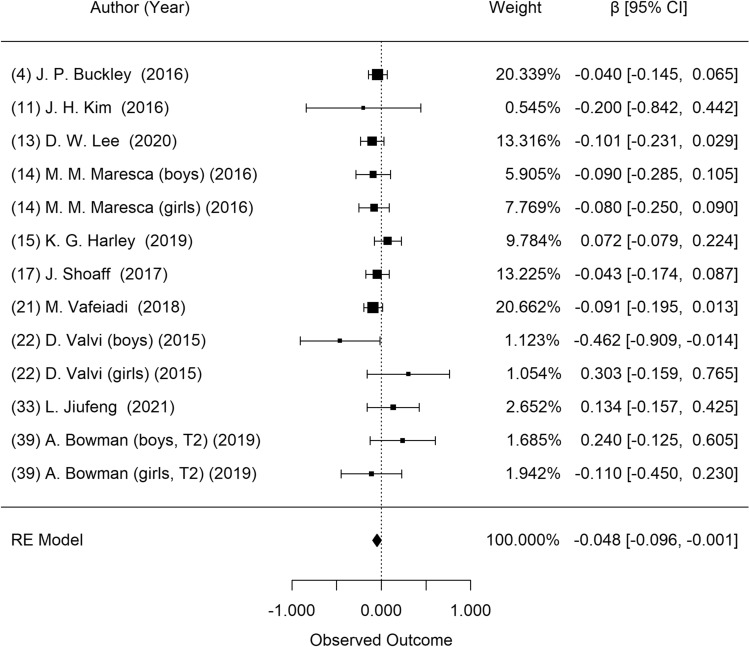
Figure 2 shows the results of a meta-analysis on the association between prenatal DEHP exposure and BMI z-score in children. Ten studies presented eligible results for the meta-analysis. Data from Agay-Shay et al. were not included because they were derived from the same study population (INMA cohort) as that from Shoaff et al. K. Data from Berger et al. were not included because they presented an unadjusted beta coefficient from the Bayesian hierarchical model, and they were derived from the same study population (CHAMACOS cohort) with Harley et al. Heterogeneity among these studies was also not significant (P = 0.338). In the random effect model, there was a significant negative association between prenatal DEHP exposure and BMI z-score index (β = − 0.05; 95% CI: − 0.10, − 0.001). Visual inspection of the funnel plot revealed no asymmetry (Supplementary Fig. S1), and the Egger test showed no publication bias (P = 0.542).
Figure 2.
Forest plot of studies on the association of DEHP exposure with BMI z-scores: longitudinal studies. Estimates were standardized as β and 95% confidence intervals as one unit increase of natural log of DEHP metabolites.
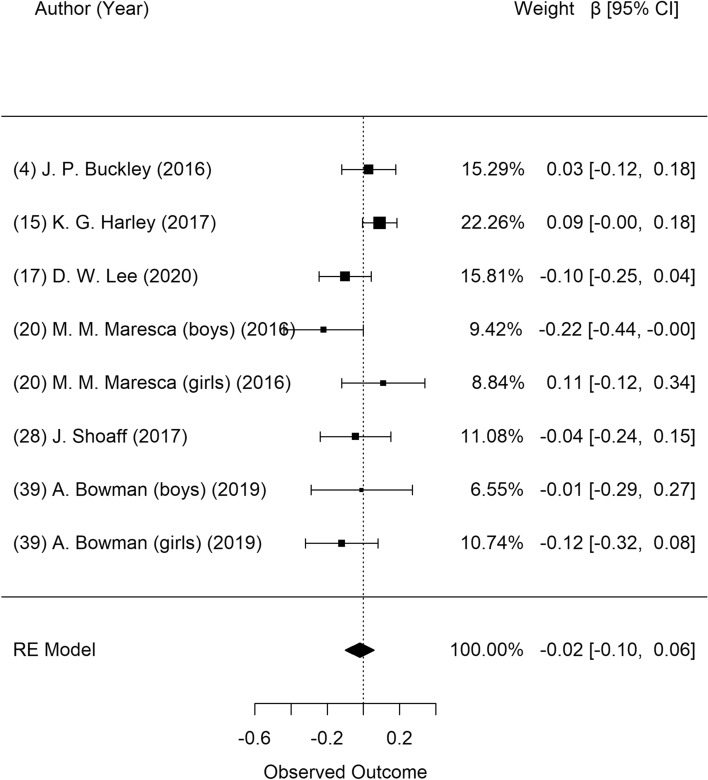
Figure 3 shows the meta-analysis results on the association between prenatal DBP exposure and BMI z-scores in children. Seven studies presented data on BMI z-scores, and these were selected for the meta-analysis. Heterogeneity among these studies was suspected, but it was not statistically significant (P = 0.161). In the random-effects model, there was no significant association between prenatal DBP exposure and BMI z-score (β = − 0.02; 95% CI: − 0.10, 0.06). Visual inspection of the funnel plot revealed no asymmetry (Supplementary Fig. S2), and the Egger test showed no publication bias (P = 0.271).
Figure 3.
Forest plot of studies on the association of DBP exposure with BMI z-scores: longitudinal studies. Estimates were standardized as β and 95% confidence intervals as one unit increase of natural log of DBP metabolites.
Prenatal exposure to phthalates and body fat percentage
Supplementary Table S7 describes studies that investigated the association between prenatal exposure to phthalates and body fat percentage. Among the 39 studies, seven were included. The results for this association were inconsistent, and only a limited number of studies reported statistical significance. The statistical significance of associations between phthalate metabolites and children's body fat percentage is summarized in Supplementary Table S8.
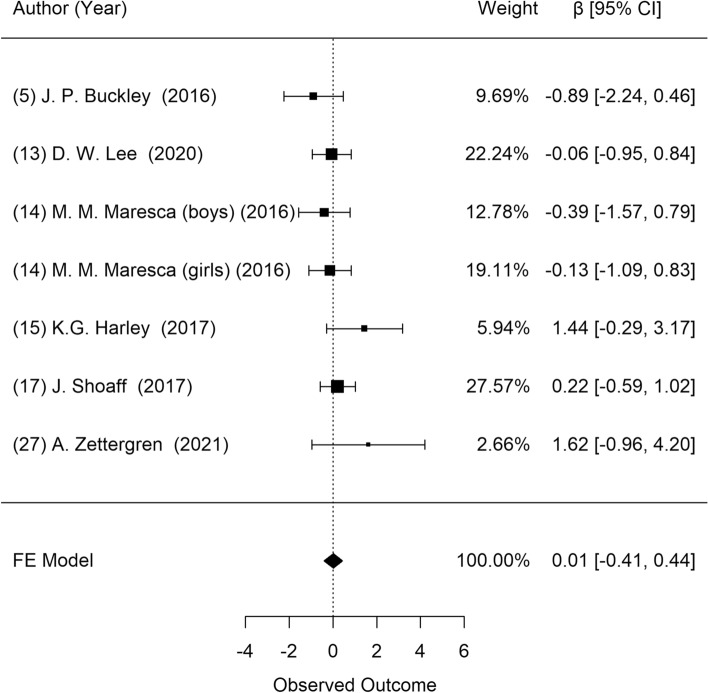
Figure 4 shows the results of the meta-analysis for the association between prenatal DEHP exposure and body fat percentage. Six studies presented body fat percentage data, which were chosen for the meta-analysis. Heterogeneity among these studies was not found (P = 0.358). In the random-effect model, no significant associations between prenatal DEHP exposure and body fat percentage were found (β = 0.01; 95% CI: − 0.41, 0.44). In addition, visual inspection of the funnel plot revealed no asymmetry (Supplementary Fig. S3), and the Egger test showed no publication bias (P = 0.287).
Figure 4.
Forest plot of studies on the association of DEHP exposure with body fat percentage: longitudinal studies. Estimates were standardized as β and 95% confidence intervals as one unit increase of natural log of DEHP metabolites.
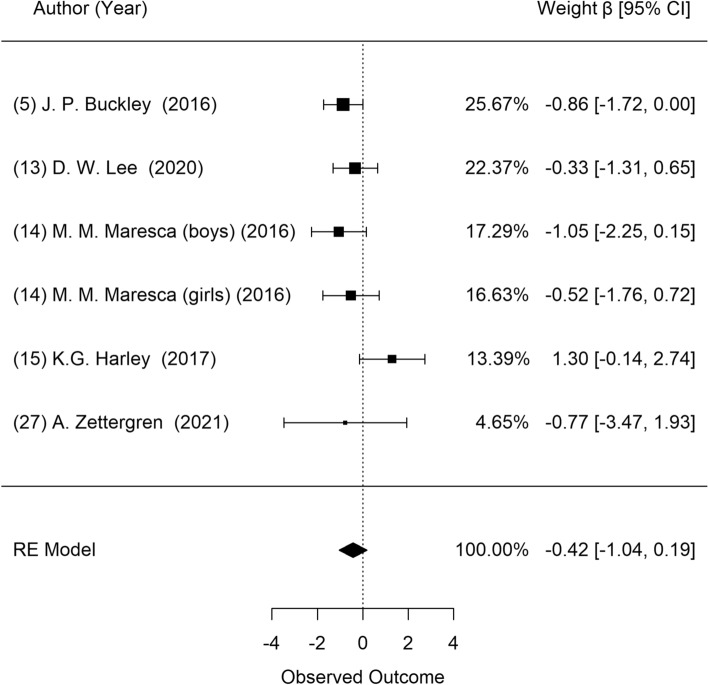
Figure 5 shows the results of a meta-analysis on the association between prenatal DBP exposure and body fat percentage. Five studies presented data regarding body fat percentage, and these were selected for the meta-analysis. Heterogeneity among these studies was not found (P = 0.184), and there were no significant associations between prenatal DBP exposure and body fat percentage (β = − 0.42; 95% CI: − 1.04, 0.19). Furthermore, visual inspection of the funnel plot revealed no asymmetry (Supplementary Fig. S4), and the Egger test showed no publication bias (P = 0.601).
Figure 5.
Forest plot of studies on the association of DBP exposure with body fat percentage: longitudinal studies. Estimates were standardized as β and 95% confidence intervals as one unit increase of natural log of DEHP metabolites.
Prenatal exposure to phthalates and other body composition indices
Supplementary Table S9 describes studies evaluating the association between the prenatal exposure to phthalates and body composition indices other than BMI or body fat percentage. Berman et al. assessed height, BMI, and body composition as measured by dual-energy X-ray absorptiometry (total fat percentage, total fat mass, and total lean mass), and the reported MiNP and MEHP were associated with decreased total lean mass25. Meanwhile, Buckley et al. used overweight/obesity defined by BMI z-score as the outcome variable27. Lee et al. also reported the association between phthalate metabolites and BMI z-score, fat mass percentage, fat mass index (FMI), skeletal muscle mass percentage, and skeletal muscle index (SMI) and reported that high levels of prenatal exposure to phthalates were significantly associated with decreased SMI among girls35. Maresca et al. reported that prenatal non-DEHP phthalate exposure was associated with lower BMI z-score, WC, and fat mass in boys during early childhood, contrary to their hypothesis36. Valvi et al. reported that weight gain Z-score was significantly associated with prenatal exposure to DEHP among boys43. Nidens et al. investigated the association between phthalate metabolites in prenatal maternal urine and weight gain (%) first 2 years of life, but it was not significant54.
Postnatal exposure to phthalates and body composition indices
Supplementary Table S10 summarizes the studies assessing the association between the postnatal exposure to phthalates and the BMI. The results of the included studies were inconsistent, and there were limited studies that reported the association of BMI with phthalate metabolites as continuous variables. Chang et al. studied 152 children in Taiwan and reported non-significant associations of BMI with DEHP metabolites, MnBP, and MiBP29. Shaoff et al. also analyzed the data of 219 children from the HOME study and reported associations between BMI z-score at 8 years and DEHP metabolites at prenatal, 1, 2, 3, 4, 5, and 8 years of age were not statistically significant39. The only significant association was between a ten-fold increase in DEHP metabolites at 5 years of age and a 0.04-unit increase in BMI z-score. Trasande et al. reported that a unit increase in the natural log-transformed sum of LMWP was associated with a 0.07-unit increase in BMI z-score using the data of children surveyed at National Health and Nutrition Examination Survey (NHANES) 2003–200813. Zettergren et al. investigated the participants’ phthalate metabolites at 4 years of age and their BMIs at 24 years of age and found that DiNP was associated with BMI, but DEHP and DBP were not48. A recent study in South Korea reported a significant association between urinary MEOHP and BMI percentile among children aged 5–16 years55, and Wang et al. also significant relationship between urinary sum of DEHP metabolites and BMI and WC. The statistical significance of associations between phthalate metabolites and BMI (and/or obesity) in children was summarized in Supplementary Table S11.
Supplementary Table S12 shows the studies on the association between the postnatal exposure to phthalates and the BMI. Chang et al. cross-sectionally studied 132 children and reported no association between phthalate metabolites and body fat percentage29, and Hou et al. studied 308 Taiwanese children and reported a significant association between the MnBP and MiBP and the waist-to-hip ratio32. Shaoff et al. analyzed the data of 219 children from the HOME study and showed significant associations between the waist circumference at 8 years of age and the sum of DEHP metabolites at 5 years of age39. There were significant associations between the body fat percentage at 8 years of age and the sum of DEHP metabolites at 1 and 5 years of age. In China, a case–control study on 57 boys with constitutional delay of growth and puberty and 110 controls reported that higher urinary phthalate metabolites were associated with constitutional delay of growth and puberty47. Another cohort study with 100 children reported non-significant associations between DEHP and DBP metabolites at 4 years of age and body indices until 24, including waist circumference, body fat percentage, and trunk fat percentage)48. Zhang et al. performed a cross-sectional study with 497 children in China and reported significant associations between phthalate exposure and fat distribution60. Ding et al. reported the significant association between waist-to-hip ratio and the sum of DEHP metabolites among children aged 16–19 years51.
Discussion
Main findings of the study
The systematic review and meta-analysis were performed to investigate the association between phthalates and physical growth in children. In the systematic literature review, a significant and negative association was found between the prenatal exposure to DEHP and the BMI z-score of the offspring, but there was no significant association between the prenatal exposure to DEHP and DBP and the body fat mass percentage of the offspring. Additionally, previous studies on the association between phthalates exposure in childhood and obesity were inconsistent in the systematic review.
Prenatal exposure to phthalates and growth disturbance
We found that prenatal phthalate exposure and decreased offspring’s BMI were significantly associated. It implies that phthalates could act as disrupting chemicals on normal development instead of obesogens. Previous researches have focused on obesity, and found inconsistent results. Among children aged 5–12 years in the U.S., prenatal exposures to DEHP and DBP were associated with increased obesity37. However, Vafeiadi et al. investigated five-hundred mother–child dyads, and found that prenatal phthalate exposure was not significantly associated with overweight at ages 4–6 years42. Buckley et al. studied 707 children in the U.S. and found that BMI z-scores in girls aged 4–7 years were negatively associated with prenatal exposure to DEHP27. These inconsistent results lead to the idea that phthalates could not be obesogen. Our recent study suggested the selective association of phthalate exposure with the development of muscle mass than fat mass could explain the inconsistent associations between prenatal exposure to phthalates and BMI in children35. A cross-sectional study in the U.S. also showed that an increased urinary concentration of phthalate metabolites is associated with decreased lean mass61. If phthalate exposure could disturb the growth of muscle mass rather than induce obesity, it could explain the inconsistencies reported in previous studies regarding the association between prenatal exposure to phthalates and BMI during childhood.
Possible mechanism
In the meta-analysis, prenatal exposure to phthalates was significantly associated with decreased BMI z-score but not with FMI. A possible explanation for this association is the antiandrogenic effects of phthalates on muscle development5,62. A murine study reported that androgen withdrawal mice showed decreased myofibrillar protein synthesis, and anabolic steroid administration reversed the effect63. Another study using mice also reported that testosterone had positive effects on muscle mass and the ultrastructure of muscles64. In an animal study, prenatal DEHP exposure led to decreased testosterone production in the offspring both in the fetal and postnatal period65. Several epidemiologic studies support that androgen is associated with muscle growth. A study with 50 boys and 50 girls aged 8–17 years reported that muscle strength was positively associated with testosterone levels66. Another study reported that testosterone is related with muscle mass and strength with a dose–response manner among hysterectomized women67. Furthermore, prenatal phthalate exposure is associated with decreased anogenital distance, which is positively related with antiandrogenic properties68,69. Increased phthalate metabolites were associated with decreased levels of serum testosterone in another human study70. Among children, the positive association between serum testosterone and SMI has been investigated71. Therefore, the antiandrogenic properties of phthalates could be an important link between prenatal exposure to phthalates and decreased SMI.
Inflammation is a possible mediator of disruption of muscle development following phthalate exposure. Phthalates exacerbate inflammatory response by increasing inflammatory cytokines72. A human study reported that DEHP exposure could induce interleukin-1β production in neonatal neutrophils73. In vitro study also reported that increased gene expression of inflammatory cytokines could be induced by DEHP74. Inflammatory cytokines are also associated with the inhibition of expression of myogenic miRNA in myoblasts and promoting muscle protein degradation75,76. Therefore, it could be inferred that inflammation due to phthalates could be associated with decreased SMIs.
Insulin-like growth factor-1 (IGF-1) could be another possible link of the association of phthalates with muscle mass. IGF-1 pathway is an important regulator of muscle growth processes in children77. Several epidemiologic studies have reported that urinary phthalate metabolites are negatively associated with IGF-141,78–80. These studies support that phthalates could lead to decreased muscle growth in children via IGF-1.
Phthalates exposure in children and body composition indices
The results of searched studies in the systematic review were inconsistent for the associations between the phthalates exposure in children and their body composition. Several researchers reported that phthalate exposure in children could be related with obesity, although obesity was inconsistently associated with phthalate metabolites, and the number of studies was limited to perform the meta-analysis. As one of the results with a significant association, a cross-sectional study involving 845 Danish children aged 4–9 years reported that children’s height and weight are negatively associated with urinary phthalate metabolites79. However, several studies showed a positive association between phthalates and obesity. A study that used NHANES data reported that LMWP could be associated with increased BMI z-score13, and a longitudinal study in the U.S. also noted that obesity at 8 years of age was associated with phthalate exposure at 5 years of age39. These studies suggested that the role of peroxisome proliferator activated receptors (PPARs), nuclear hormone receptors that have regulatory roles in adipogenesis and lipid storage, is important to induce adipogenesis and obesity81–83. Because phthalate exposure is associated with decreased thyroid hormone84, hormonal homeostasis can be disturbed due to phthalates, leading to fat accumulation and obesity. A Chinese metabolome study investigated 69 overweight/obese children and 80 normal-weight children. It was reported that urinary MnBP concentration differed between the two groups and was associated with arginine, proline, and butyraldehyde46. However, several studies had no significant associations between phthalates, obesity, and BMI13,28,29,38,39,44.
Some researchers argued that the association between urinary phthalates metabolites and obesity was not derived from the causal association between phthalates exposure in children and obesity. For instance, the recent study that explained the mechanism for cross-sectional studies for the association between phthalates and higher BMI demonstrated that the higher energy intake in the overweight and obese could result in the concomitant higher phthalates exposure85. Additionally, ultra-processed food consumption is associated with overweight and weight gain86, and is also associated with urinary phthalates metabolites87. Therefore, cross-sectionally observed association between phthalates metabolites and obesity might reflect the association of the dietary pattern and the amount of consumption with obesity. Additionally, urinary phthalate metabolite may be measured higher among children with more adipose and muscle mass. Given the absorption, distribution, metabolism, and excretion of phthalates, absorbed phthalates in the human body distribute mainly in the intestine and liver, and they are rapidly excreted.
On the other hand, a relatively small portion of absorbed phthalates is distributed in fat and muscle tissue. Still, they are excreted slower than those in the intestine and liver, resulting in a relatively higher proportion of phthalates in the human body88. Therefore, observed cross-sectional associations between phthalates and obesity in children might not be causal. Inconsistent results and related factors make it difficult to conclude the association between phthalates exposure in childhood and weight gain. Studies with longitudinal design and studies suggesting plausible mechanism, such as hormonal, epigenetic and/or metabolomic changes, are needed in the future.
Exposure assessment for phthalates
It has been assumed that a single measure of phthalate metabolites can adequately reflect exposure across the studies. All studies included in the meta-analysis also had the same assumption. Assessing DEHP exposures may be inconclusive because various metabolites of DEHP are rapidly metabolized in vivo and quickly excreted. As the excretion half-lives of DEHP metabolites are 0.5–3.0 days20, urine biomarkers can only reflect recent exposure. However, all studies included in the meta-analysis considered DEHP metabolites in the mothers’ or children’s urine. In all longitudinal studies, DEHP metabolites were assessed at a one-time point, rather than repeated measurements in a few-day interval. Included cross-sectional studies were also measured DEHP metabolites only once from children’s urine.
The temporal stability of DEHP metabolites over weeks to months has been studied. The daily variation of phthalates' urinary metabolites was investigated using urine samples of fifty participants on eight consecutive days, and reported intra-class coefficients of urinary DEHP metabolites as 0.20–0.3489. Another study reported one spot urine sample could predict the three-month average concentration of DEHP metabolites with sensitivity and specificity as 0.56 and 0.83, respectively90. It has been suggested that DEHP metabolites measured in the spot urine showed reasonable temporal stability for weeks to months, although it has limitations on stability91–95. In addition, a recent study investigated 805 urine samples of 16 volunteers for 6 months and suggested that adequately classifying the exposure level of participants requires several samples per subject96. In this systematic review, no studies measured phthalates repeatedly in a short time period to measure phthalates exposure more accurately. Therefore, all studies included in our meta-analyses assumed implicitly or explicitly that a single measurement could reflect exposure over a considerable period.
Strengths and limitations
To overcome the inconclusive results on the association between phthalate exposure and children’s growth17, we rationally and preferentially selected the estimate for the association between phthalates (exposure) and body composition indices (outcome). We used the sum of phthalate metabolites to assess the total exposure amount because the molar sum of several metabolites of DEHP is currently considered the best estimate of exposure rather than a simple mass sum of DEHP metabolites. Furthermore, the time points of measurement for phthalate exposure (prenatal or postnatal) and the methods for assessing body composition indices, including BMI, BMI z-score, and body fat percentage, differ across studies. In the present study, we attempted to collectively analyze the results in a meta-analysis with the abovementioned methods, which was also described in a previous meta-analysis study97. Therefore, we estimated the up-to-date summarized results for the association of prenatal phthalates exposure and body composition indices in children.
This study has several limitations. First, the calibration of the amount of exposure to phthalates considering the duration of exposure is not assessed in the systematic review and meta-analysis, because it is practically impossible. Second, the included studies had limited information and had methodological differences98, although standardized values from β estimates and 95% CIs were used to perform meta-analyses. If raw data can be obtained and pooled analysis is performed, more robust results may be expected. In the studies we reviewed, phthalate metabolites were measured from spot urine samples of participants. There is no study with repetitive measurement for accurate phthalates measurement for the association between phthalates and body composition indices. In the future, more repetitive methods such as using mean levels of various phthalate metabolites assessed at multiple time points could increase the precision and accuracy of predicting phthalate exposure99.
Conclusion
This systematic review and meta-analysis showed that prenatal exposure to phthalates is significantly associated with low BMI in children, but not with body fat mass. In addition, prenatal phthalate exposure may affect the disturbance of normal growth of children rather than act as an obesogen. Future studies on the health effects of phthalates should consider their detrimental effects on the expected growth of children. Furthermore, it is necessary to administer stricter and broader regulations on phthalates in living environments.
Supplementary Information
Author contributions
Conceived and designed the study: D.-W.L., Y.-C.H.; Performing a systematic review: D.-W.L., H.-M.L., Y.-C.H.; Analyzing the data and preparing the tables and figures: D.-W.L., Y.-C.H.; Wrote the paper: D.-W.L., Y.-C.H.; Critically revised for the paper: J.-Y.L., K.-B.M., C.-H.S., Y.-A.L.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13154-9.
References
- 1.Holland, M. Socio-economic assessment of phthalates. Organization for Economic Co-operation and Development Environment Working Papers No. 133.
- 2.Erythropel HC, Maric M, Nicell JA, Leask RL, Yargeau V. Leaching of the plasticizer di (2-ethylhexyl) phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014;98:9967–9981. doi: 10.1007/s00253-014-6183-8. [DOI] [PubMed] [Google Scholar]
- 3.Lorz PM, et al. Ullmann's Encyclopedia of Industrial Chemistry. Hoboken: Wiley-VCH Verlag GmbH & Co. KGaA; 2000. [Google Scholar]
- 4.Silva MJ, et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223:144–155. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Kim BN, et al. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol. Psychiat. 2009;66:958–963. doi: 10.1016/j.biopsych.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Lee BE, et al. Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children's Environmental Health) study. Int. J. Hyg. Environ. Health. 2014;217:328–334. doi: 10.1016/j.ijheh.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Moore RW, Rudy TA, Lin T-M, Ko K, Peterson RE. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer Di (2-ethylhexyl) phthalate. Environ. Health Perspect. 2001;109:229. doi: 10.1289/ehp.01109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley JP, et al. Prenatal phthalate exposures and childhood fat mass in a New York City cohort. Environ. Health Perspect. 2016;124:507–513. doi: 10.1289/ehp.1509788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty. PLoS ONE. 2014;9:e104852. doi: 10.1371/journal.pone.0104852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin MM, et al. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547–556. doi: 10.1016/j.chemosphere.2018.07.172. [DOI] [PubMed] [Google Scholar]
- 13.Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ. Health Perspect. 2013;121:501–506. doi: 10.1289/ehp.1205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009;304:19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassenaar PNH, Legler J. Systematic review and meta-analysis of early life exposure to di (2-ethylhexyl) phthalate and obesity related outcomes in rodents. Chemosphere. 2017;188:174–181. doi: 10.1016/j.chemosphere.2017.08.165. [DOI] [PubMed] [Google Scholar]
- 16.Neier K, et al. Longitudinal metabolic impacts of perinatal exposure to phthalates and phthalate mixtures in mice. Endocrinology. 2019 doi: 10.1210/en.2019-00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro C, et al. Association between the exposure to phthalates and adiposity: A meta-analysis in children and adults. Environ. Res. 2019;179:108780. doi: 10.1016/j.envres.2019.108780. [DOI] [PubMed] [Google Scholar]
- 18.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018;121:1027. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di (2-ethylhexyl) phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- 21.Council NR. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. US: National Academies Press; 2008. [PubMed] [Google Scholar]
- 22.Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (2000).
- 23.Nieminen, P. et al. Standardised regression coefficient as an effect size index in summarising findings in epidemiological studies. Epidemiol. Biostat. Public Health10.2427/8854 (2013).
- 24.Agay-Shay K, et al. Exposure to endocrine-disrupting chemicals during pregnancy and weight at 7 years of age: A multi-pollutant approach. Environ. Health Perspect. 2015;123:1030–1037. doi: 10.1289/ehp.1409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berman YE, et al. The influence of prenatal exposure to phthalates on subsequent male growth and body composition in adolescence. Environ. Res. 2020 doi: 10.1016/j.envres.2020.110313. [DOI] [PubMed] [Google Scholar]
- 26.Botton J, et al. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ. Res. 2016;151:601–609. doi: 10.1016/j.envres.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley JP, et al. Prenatal phthalate exposures and body mass index among 4- to 7-year-old children: A pooled analysis. Epidemiology. 2016;27:449–458. doi: 10.1097/ede.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int. J. Hyg. Environ. Health. 2014;217:687–694. doi: 10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CH, et al. The sex-specific association of phthalate exposure with DNA methylation and characteristics of body fat in children. Sci. Total Environ. 2020;737:139833. doi: 10.1016/j.scitotenv.2020.139833. [DOI] [PubMed] [Google Scholar]
- 30.Deierlein AL, et al. Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls. Epidemiology. 2016;27:492–499. doi: 10.1097/ede.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heggeseth BC, Holland N, Eskenazi B, Kogut K, Harley KG. Heterogeneity in childhood body mass trajectories in relation to prenatal phthalate exposure. Environ. Res. 2019;175:22–33. doi: 10.1016/j.envres.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou JW, et al. The effects of phthalate and nonylphenol exposure on body size and secondary sexual characteristics during puberty. Int. J. Hyg. Environ. Health. 2015;218:603–615. doi: 10.1016/j.ijheh.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, et al. Association of diethylhexyl phthalate with obesity-related markers and body mass change from birth to 3 months of age. J. Epidemiol. Community Health. 2016;70:466–472. doi: 10.1136/jech-2015-206315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SH, et al. Percentage fractions of urinary di(2-ethylhexyl) phthalate metabolites: Association with obesity and insulin resistance in Korean girls. PLoS ONE. 2018;13:e0208081. doi: 10.1371/journal.pone.0208081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DW, et al. Prenatal exposure to di-(2-ethylhexyl) phthalate and decreased skeletal muscle mass in 6-year-old children: A prospective birth cohort study. Environ. Res. 2020;182:109020. doi: 10.1016/j.envres.2019.109020. [DOI] [PubMed] [Google Scholar]
- 36.Maresca MM, et al. Prenatal exposure to phthalates and childhood body size in an urban cohort. Environ. Health Perspect. 2016;124:514–520. doi: 10.1289/ehp.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harley KG, et al. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr. Res. 2017;82:405–415. doi: 10.1038/pr.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saengkaew T, Jantarat C, Nosoognoen W, Supornsilchai V. Association between urinary phthalates and metabolic abnormalities in obese Thai children and adolescents. J. Pediatr. Endocrinol. Metab. JPEM. 2017;30:931–938. doi: 10.1515/jpem-2017-0172. [DOI] [PubMed] [Google Scholar]
- 39.Shoaff J, et al. Early-life phthalate exposure and adiposity at 8 years of age. Environ. Health Perspect. 2017;125:097008. doi: 10.1289/EHP1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smerieri A, et al. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS ONE. 2015;10:e0117831. doi: 10.1371/journal.pone.0117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai YA, et al. Effects of high di(2-ethylhexyl) phthalate (DEHP) exposure due to tainted food intake on pre-pubertal growth characteristics in a Taiwanese population. Environ. Res. 2016;149:197–205. doi: 10.1016/j.envres.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Vafeiadi M, et al. Association of early life exposure to phthalates with obesity and cardiometabolic traits in childhood: Sex specific associations. Front. Public Health. 2018;6:327. doi: 10.3389/fpubh.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valvi D, et al. Prenatal phthalate exposure and childhood growth and blood pressure: Evidence from the Spanish INMA-sabadell birth cohort study. Environ. Health Perspect. 2015;123:1022–1029. doi: 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vrijheid M, et al. Early-life environmental exposures and childhood obesity: An exposome-wide approach. Environ. Health Perspect. 2020;128:67009. doi: 10.1289/ehp5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu B, Jiang Y, Jin X, He L. Using three statistical methods to analyze the association between exposure to 9 compounds and obesity in children and adolescents: NHANES 2005–2010. Environ. Health Glob. Access Sci. Source. 2020;19:94. doi: 10.1186/s12940-020-00642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia B, et al. Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environ. Int. 2018;121:159–168. doi: 10.1016/j.envint.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Xie C, et al. Elevated phthalates' exposure in children with constitutional delay of growth and puberty. Mol. Cell Endocrinol. 2015;407:67–73. doi: 10.1016/j.mce.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Zettergren A, et al. Exposure to environmental phthalates during preschool age and obesity from childhood to young adulthood. Environ Res. 2021;192:110249. doi: 10.1016/j.envres.2020.110249. [DOI] [PubMed] [Google Scholar]
- 49.Amin MM, et al. Association of urinary phthalate metabolites concentrations with body mass index and waist circumference. Environ. Sci. Pollut. Res. Int. 2018;25:11143–11151. doi: 10.1007/s11356-018-1413-8. [DOI] [PubMed] [Google Scholar]
- 50.Ashley-Martin J, et al. Urinary phthalates and body mass index in preschool children: The MIREC Child Development Plus study. Int. J. Hyg. Environ. Health. 2021;232:113689. doi: 10.1016/j.ijheh.2021.113689. [DOI] [PubMed] [Google Scholar]
- 51.Ding S, et al. Relationships between di-(2-ethylhexyl) phthalate exposure and lipid metabolism in adolescents: Human data and experimental rat model analyses. Environ. Pollut. 2021 doi: 10.1016/j.envpol.2021.117570. [DOI] [PubMed] [Google Scholar]
- 52.Berger K, et al. Prenatal exposure to mixtures of phthalates, parabens, and other phenols and obesity in five-year-olds in the chamacos cohort. Int. J. Environ. Res. Public Health. 2021;18:1–18. doi: 10.3390/ijerph18041796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, et al. Trimester-specific and sex-specific effects of prenatal exposure to di(2-ethylhexyl) phthalate on fetal growth, birth size, and early-childhood growth: A longitudinal prospective cohort study. Sci. Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.146146. [DOI] [PubMed] [Google Scholar]
- 54.Nidens N, et al. Associations of prenatal exposure to phthalates and one phthalate substitute with anthropometric measures in early life: Results from the German LIFE Child cohort study. Best Pract. Res. Clin. Endocrinol. Metabol. 2021 doi: 10.1016/j.beem.2021.101532. [DOI] [PubMed] [Google Scholar]
- 55.On J, et al. Urinary di(2-ethylhexyl)phthalate metabolite ratios in obese children of South Korea. Environ. Sci. Pollut. Res. Int. 2021;28:29590–29600. doi: 10.1007/s11356-021-12823-y. [DOI] [PubMed] [Google Scholar]
- 56.Silva CCV, et al. Phthalate and bisphenol urinary concentrations, body fat measures, and cardiovascular risk factors in dutch school-age children. Obesity. 2021;29:409–417. doi: 10.1002/oby.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatch EE, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ. Health. 2008;7:1–15. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, et al. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS ONE. 2013;8:e56800. doi: 10.1371/journal.pone.0056800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowman, A. et al. Phthalate exposures, DNA methylation and adiposity in Mexican children through adolescence. Front.Public Health10.3389/fpubh.2019.00162 (2019). [DOI] [PMC free article] [PubMed]
- 60.Zhang Y, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019;123:325–336. doi: 10.1016/j.envint.2018.11.076. [DOI] [PubMed] [Google Scholar]
- 61.Corbasson I, Hankinson SE, Stanek EJ, Reeves KW. Urinary bisphenol-A, phthalate metabolites and body composition in US adults, NHANES 1999–2006. Int. J. Environ. Health Res. 2016;26:606–617. doi: 10.1080/09603123.2016.1233524. [DOI] [PubMed] [Google Scholar]
- 62.Stroheker T, et al. Evaluation of anti-androgenic activity of di-(2-ethylhexyl) phthalate. Toxicology. 2005;208:115–121. doi: 10.1016/j.tox.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 63.White JP, et al. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol. Cell. Endocrinol. 2013;365:174–186. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sinha I, Sinha-Hikim AP, Wagers AJ, Sinha-Hikim I. Testosterone is essential for skeletal muscle growth in aged mice in a heterochronic parabiosis model. Cell Tissue Res. 2014;357:815–821. doi: 10.1007/s00441-014-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parks LG, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 66.Round J, Jones DA, Honour J, Nevill AM. Hormonal factors in the development of differences in strength between boys and girls during adolescence: a longitudinal study. Ann. Hum. Biol. 1999;26:49–62. doi: 10.1080/030144699282976. [DOI] [PubMed] [Google Scholar]
- 67.Huang G, et al. Testosterone dose-response relationships in hysterectomized women with and without oophorectomy: Effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause (New York, NY) 2014;21:612. doi: 10.1097/GME.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bornehag C-G, et al. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ. Health Perspect. 2014;123:101–107. doi: 10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swan SH, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J. Clin. Endocrinol. Metab. 2014;99:4346–4352. doi: 10.1210/jc.2014-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou WW, Tse MA, Lam TH, Leung GM, Schooling CM. Adolescent testosterone, muscle mass and glucose metabolism: Evidence from the ‘Children of 1997’ birth cohort in Hong Kong. Diabet. Med. 2015;32:505–512. doi: 10.1111/dme.12602. [DOI] [PubMed] [Google Scholar]
- 72.Rael LT, et al. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxid. Med. Cell. Longev. 2009;2:166–171. doi: 10.4161/oxim.2.3.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vetrano AM, et al. Inflammatory effects of phthalates in neonatal neutrophils. Pediatr. Res. 2010;68:134–139. doi: 10.1203/PDR.0b013e3181e5c1f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishioka J, et al. Di-(2-ethylhexyl) phthalate induces production of inflammatory molecules in human macrophages. Inflamm. Res. 2012;61:69–78. doi: 10.1007/s00011-011-0390-x. [DOI] [PubMed] [Google Scholar]
- 75.Georgantas RW, et al. Inhibition of myogenic microRNAs 1, 133, and 206 by inflammatory cytokines links inflammation and muscle degeneration in adult inflammatory myopathies. Arthritis Rheumatol. 2014;66:1022–1033. doi: 10.1002/art.38292. [DOI] [PubMed] [Google Scholar]
- 76.Szewczyk NJ, Jacobson LA. Signal-transduction networks and the regulation of muscle protein degradation. Int. J. Biochem. Cell Biol. 2005;37:1997–2011. doi: 10.1016/j.biocel.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 77.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 78.Huang P-C, et al. Characterization of phthalate exposure in relation to serum thyroid and growth hormones, and estimated daily intake levels in children exposed to phthalate-tainted products: A longitudinal cohort study. Environ. Pollut. 2020;264:114648. doi: 10.1016/j.envpol.2020.114648. [DOI] [PubMed] [Google Scholar]
- 79.Boas M, et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ. Health Perspect. 2010;118:1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu W, et al. Exposure to phthalates in children aged 5–7years: Associations with thyroid function and insulin-like growth factors. Sci. Total Environ. 2017;579:950–956. doi: 10.1016/j.scitotenv.2016.06.146. [DOI] [PubMed] [Google Scholar]
- 81.Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol. Cell Endocrinol. 2009;304:43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 82.Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 2013;33:185. doi: 10.1042/BSR033e017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. Int. J. Androl. 2012;35:437–448. doi: 10.1111/j.1365-2605.2012.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morgenstern R, et al. Phthalates and thyroid function in preschool age children: sex specific associations. Environ. Int. 2017;106:11–18. doi: 10.1016/j.envint.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell JL, Jr, et al. Excretion of Di-2-ethylhexyl phthalate (DEHP) metabolites in urine is related to body mass index because of higher energy intake in the overweight and obese. Environ. Int. 2018;113:91–99. doi: 10.1016/j.envint.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 86.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 2018;120:90–100. doi: 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 87.Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019;131:105057. doi: 10.1016/j.envint.2019.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fay, M., Donohue, J. M. & De Rosa, C. ATSDR evaluation of health effects of chemicals. VI. Di (2-ethylhexyl) phthalate. Toxicol. Ind. Health15 (1999). [DOI] [PubMed]
- 89.Fromme H, et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int. J. Hyg. Environ. Health. 2007;210:21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect. 2004;112:1734. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adibi JJ, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008;116:467. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ. Health Perspect. 2009;117:86. doi: 10.1289/ehp.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ. Health Perspect. 2002;110:515. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki Y, et al. Exposure assessment of phthalate esters in Japanese pregnant women by using urinary metabolite analysis. Environ. Health Prev. Med. 2009;14:180. doi: 10.1007/s12199-009-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Teitelbaum S, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 96.Faÿs F, et al. Is there an optimal sampling time and number of samples for assessing exposure to fast elimination endocrine disruptors with urinary biomarkers? Sci. Total Environ. 2020;747:141185. doi: 10.1016/j.scitotenv.2020.141185. [DOI] [PubMed] [Google Scholar]
- 97.Lee D-W, Kim M-S, Lim Y-H, Lee N, Hong Y-C. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environ. Res. 2018;167:558–566. doi: 10.1016/j.envres.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 98.Becker BJ, Wu M-J. The synthesis of regression slopes in meta-analysis. Stat. Sci. 2007;22(3):414–429. doi: 10.1214/07-STS243. [DOI] [Google Scholar]
- 99.Factor-Litvak P, et al. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS ONE. 2014;9:e114003. doi: 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.