Abstract
Symptoms of leaf blight, stem canker, and pod rot were observed on T. cacao during a series of samplings conducted in several states of Malaysia from September 2018 to March 2019. The identity of the pathogen that was responsible for the diseases was determined using morphological characteristics, DNA sequences, and phylogenetic analyses of multiple genes, namely, internal transcribed spacer (ITS), elongation translation factor 1-alpha (tef1-α), β-tubulin (tub2), and RNA polymerase subunit II (rpb2). A total of 57 isolates recovered from diseased leaves of T. cacao (13 isolates), stems (20 isolates), and pods (24 isolates) showed morphological features that resembled Lasiodiplodia sp. The identity of the isolates was further determined up to the species level by comparing DNA sequences and phylogenetic analyses of multiple genes. The phylogenetic analysis of the combined dataset of ITS, tef1-α, tub2, and rpb2 elucidated that all of the isolates obtained were Lasiodiplodia theobromae as supported by 97% bootstrap value. The results of pathogenicity tests revealed L. theobromae as the causal pathogen of leaf blight, stem canker, and pod rot of T. cacao.
Subject terms: Microbiology, Molecular biology, Plant sciences
Introduction
The cocoa tree (Theobroma cacao) is an evergreen shrub that is recognized by several names, including kakaw, pokok coklat, chocolate, cacao, koko, criollo, cacaoyer, and kakao1. Previously, T. cacao was classified under Sterculiaceae family, before being reclassified as a member of Malvaceae. It is originated in the Neotropical rainforest, particularly in the Amazon basin and on the Guyana plateau2–4. The word Theobroma means “Food of the Gods,” whereas cacao comes from the Mayans and Aztec languages, Kakaw and Cacahuatl, respectively5,6. Furthermore, T. cacao is the recognized species among the 22 Theobroma species that is commonly planted beyond its natural range and have an economic value1,6. Besides T. cacoa, the other species of Theobroma also have economic value such as T. grandiflorum in South America and T. bicolor in Mexico and Central America6. Clone seedling is preferred for plantation over hybrid seedling in almost all cocoa-producing countries because it will produce the same tree morphology, pod, and bean characteristics as the parent tree, where the clone tree has greater pod bearing capacities, bigger and more uniform beans, richer butter content, withstand to pest and pathogen attacks, and adaptable to a wide range of agro-climatic conditions1,7. The continued advancement of Malaysia's cocoa industry in the late 1970s and early 1980s resulted in the founding of the Malaysian Cocoa Board (MCB) in 1989, which is overseen by the Ministry of Plantation Industries and Commodities. The Board's goal was to grow Malaysia's cocoa industry so that it could be incorporated in the global market, as well as to boost the quality and performance of cocoa bean and downstream production8. Malaysia is now the leading country in the cocoa grinding industry8.
In addition, cocoa and its products have various nutritional values owing to their rich amounts of alkaloids, cardiac glycosides, catechin, enantiomer, epicatechin, flavanol, methylxanthines, procyanidin B2, saponin, tannins, and terpenoids9. Moreover, cocoa has several biological benefits, including high antioxidant activity, blood pressure reduction, anticancer activity, stress and depression reduction, reduced risk of heart attack and stroke, cholesterol control, antiplatelet effect, and anti-inflammatory activity10–14.
Theobroma cacao tree, similar to any other Malvaceae plants, has been shown to be fungus-prone. Among the most important diseases affecting cacao in Malaysia are black pod rot, canker, and vascular streak dieback (VSD), which affect the pod; trunk and stem; leaves and stems of the cacao tree, respectively1. Furthermore, several previous studies on the diseases of T. cacao caused by fungal and fungal-like pathogens have been reported worldwide namely, Ceratobasidium theobromae15, Colletotrichum gloeosporioides6, Colletotrichum siamense16,17, Colletotrichum theobromicola18, Colletotrichum tropicale17, Lasiodiplodia brasiliensis19, Lasiodiplodia pseudotheobromae17, Lasiodiplodia theobromae6,19–25, Moniliophthora perniciosa26, Moniliophthora roreri27, Neofusicoccum parvum28, Phytophthora palmivora6,25,29, and Phytophthora megakarya4,29.
In a series of samplings conducted from September 2018 to March 2019, the occurrences of leaf blight, stem canker, and pod rots of T. cacao were observed in cocoa plantations in several states of Malaysia. From observations during the sampling revealed the disease incidences of leaf blight, stem canker, and pod rots in cocoa plantations were 15%, 20%, and 25%, respectively, which may reduce cocoa production. The diseased samples were gathered and returned for further observation. Therefore, the present study sought to find the causative agent of leaf blight, stem canker, and pod rot of T. cacao in Malaysia using morphological, molecular, and pathogenicity analyses.
Results
Fungal isolation and morphological identification
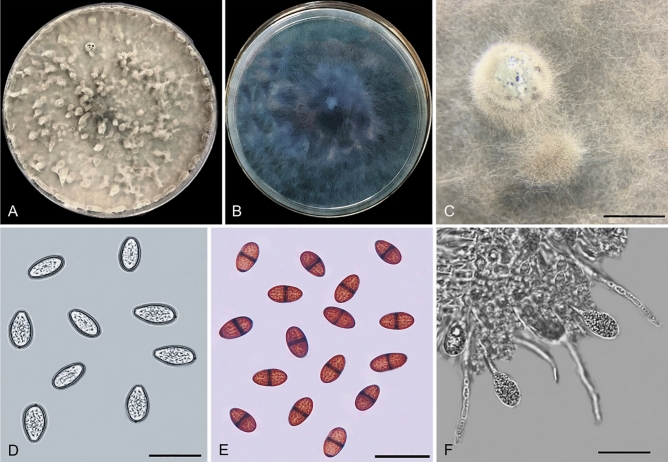
In total, 57 fungal isolates were retrieved from diseased leaves of T. cacao (13 isolates), stems (20 isolates), and pods (24 isolates). On PDA, the fungal isolates produced dense and fast-growing mycelia, white to pale greenish-gray colony and eventually becoming dark grayish (Fig. 1A). The pigmentation ranged from dark gray to black (Fig. 1B). The conidiomata were solitary, globose to subglobose, uniloculate, black, surrounded by dense grayish mycelia, and 3.32 ± 0.47 × 3.10 mm ± 0.27 mm (mean ± standard deviation (SD)) (length (L) × width (W)) in size (Fig. 1C). The conidia were observed as immature and mature conidia. Both immature and mature conidia were subovoid to ellipsoid-ovoid in shape, with a broadly rounded apex and a tapering to the truncated base. The immature conidia were initially double layered, hyaline, unicellular, and 25.0 ± 1.06 × 13.0 µm ± 0.48 µm (mean ± SD) (L × W) in size (Fig. 1D). The mature conidia appeared light to dark brown color with typical striate formation, one-septate, and 25.7 ± 1.73 × 13.1 µm ± 0.82 µm (mean ± SD) (L × W) in size (Fig. 1E). The conidiogenous cells were cylindrical, hyaline, thin walled, holoblastic, and smooth. The structure of the paraphyses was aseptate and septate, with rounded apex, hyaline, and cylindrical (Fig. 1F). Based on the characterization of the morphological features of the fungal isolates, it was tentatively identified as Lasiodiplodia sp., which is coherent with the morphology described by Alves et al.30 and Phillips et al.31.
Figure 1.
Morphological characteristics of Lasiodiplodia sp. recovered from diseased leaves, stem, and pods of Theobroma cacao. (A) Upper view of the colony appearance, (B) Reverse view colony appearance, (C) Conidiomata, (D) Immature conidia, (E) Mature conidia, (F) Conidiogenous cells and paraphyses. Scale bars: (C) = 1 mm; (D–F) = 50 µm.
Molecular identification and phylogenetic analysis
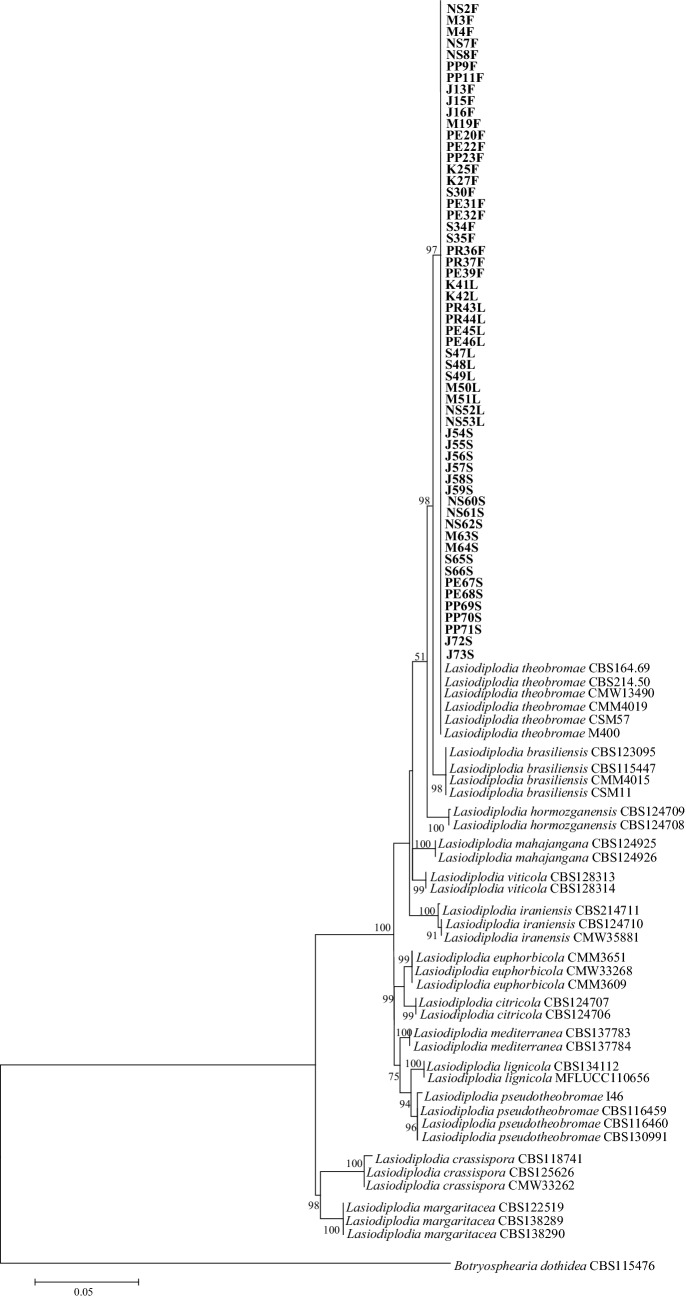
Molecular analysis of the sequences of ITS, tef1-α, tub2, and rpb2 clarified the species identification of all the 57 isolates of Lasiodiplodia sp. recovered from T. cacao. BLAST searches in the GenBank database revealed that the isolates showed 98–100% sequence homology to the KY473071 (ITS), JX464026 (tef1-α), EU673110 (tub2), and MT592333 (rpb2) of L. theobromae. A multi-locus analysis was performed to explicate the phylogenetic positions of these L. theobromae isolates. To construct the phylogenetic tree, the sequences of the isolates from the present study (57 isolates of L. theobromae) were aligned with 38 reference isolates of Lasiodiplodia species and one outgroup taxon (Botryosphaeria dothidea). Phylogenetic analysis revealed that the topologies of the ML trees generated from individual and concatenated genes (ITS, tef1-α, tub2, and rpb2) were similar (Figs. S1a–d and 2). The ML tree constructed from the concatenated sequences confirmed that the phylogenetic positions of the 57 isolates from T. cacao were clustered with the reference isolates of L. theobromae, supported by 97% bootstrap value (Fig. 2). As a result, all the present isolates were verified as L. theobromae by virtue of molecular identification and phylogenetic analysis.
Figure 2.
The maximum likelihood (ML) tree was generated with 1000 bootstrap replications using the Tamura-3-parameter model. The ML tree is inferred from concatenated sequence dataset of four genes (ITS, tef1-α tub2, and rpb2). Bootstrap support values greater than 50% are pointed out at the nodes. Isolates in bold represent isolates in the present study and Botryosphearia dothidea represents the outgroup. The bar indicates the substitutions number per position.
Pathogenicity test
The pathogenicity analysis of 13, 20, and 24 fungal isolates on healthy leaves, stems, and pods of T. cacao resulted in the production of typical symptoms of blight, canker, and rot, respectively as observed in the fields (Fig. 3A,G,R). There were no visible symptoms produced on control points of leaves, stems, and pods (Fig. 3B,H,S).
Figure 3.
Pathogenicity of Lasiodiplodia theobromae on leaves, stems, and pods of Theobroma cacao. (A) Blighted leaf observed in the field, (B) Asymptomatic control inoculated leaf, (C) Irregular black lesions with yellow halo observed after 4 days of inoculation (D,E) The lesions enlarged after 6 and 9 days of inoculation, respectively, (F) Presence of conidiomata on the diseased area (red arrow), (G) Cankered stem observed in the field, (H) Asymptomatic control inoculated stem, (I–K) Black necrotic lesions observed on the inoculation sites after 7, 14, and 21 days of inoculation, respectively, (L) Black necrotic lesions extending upwards and downwards after 28 days of inoculation, (M) Black sunken lesion on the inoculation site, (N) Incision of the stem inoculated site showed reddish-brown to black necrotic lesion, (O) Formation of gummosis on the necrotic lesion, (P) Vertical section of control (left) and fungal inoculated stems (right) showed symptomless and dark brown to black necrotic lesion, respectively, (Q) Transverse section of control (below) and fungal inoculated stems (above) showed symptomless and necrotic lesion, respectively, (R) Rotted pod observed in the field showed external and internal rotting symptoms, (S) Asymptomatic control inoculated pod, (T) Brown to black lesions observed on the inoculation sites after 5 days of inoculation, (U) The lesions enlarged after 7 days of inoculation (V), The lesion rapidly expanded after 9 days of inoculation, (W) The inoculated pod completely covered by the fungal mycelia after 12 days of inoculation, (X) Presence of black conidiomata (red circle) on the fungal inoculated pod, (Y) Cross-section of fungal inoculated pod showed rotting of the internal tissue.
After 4 days of inoculation, the fungal inoculated leaves exhibited small irregular black lesions bounded by yellow halos (Fig. 3C). The lesions and yellowing areas enlarged gradually during the incubation period (Fig. 3D,E). Conidiomata formed on the inoculation site (Fig. 3F). The lesion areas produced ranged from 3.0 to 4.6 cm2 (Table 1). There was no significant difference of lesion areas recorded among the tested isolates.
Table 1.
Lesion area produced on the leaves, stems and pods of Theobroma cacao inoculated with Lasiodiplodia theobromae.
| Isolate code | aLesion area (cm2) | ||
|---|---|---|---|
| Leaf | Stem | Pod | |
| K41L | 3.3 ± 0.7b | b– | – |
| K42L | 3.1 ± 0.1b | – | – |
| PR43L | 3.3 ± 0.7b | – | – |
| PR44L | 3.7 ± 1.0b | – | - |
| PE45L | 3.3 ± 0.3b | – | – |
| PE46L | 3.0 ± 0.3b | – | – |
| S47L | 4.6 ± 1.2b | – | – |
| S48L | 4.6 ± 1.2b | – | – |
| S49L | 3.5 ± 1.0b | – | – |
| M50L | 3.3 ± 0.4b | – | – |
| M51L | 3.1 ± 0.3b | – | – |
| NS52L | 4.0 ± 1.3b | – | – |
| NS53L | 3.2 ± 0.6b | – | – |
| J54S | – | 14 ± 0d | – |
| J55S | – | 14 ± 0d | – |
| J56S | – | 14 ± 0d | – |
| J57S | – | 14 ± 0d | – |
| J58S | – | 14 ± 0d | – |
| J59S | – | 14 ± 0d | – |
| NS60S | – | 12.3 ± 0c | – |
| NS61S | – | 12.3 ± 0c | – |
| NS62S | – | 14 ± 0d | – |
| M63S | – | 13.1 ± 0cd | – |
| M64S | – | 13.1 ± 0cd | – |
| S65S | – | 13.1 ± 0cd | – |
| S66S | – | 13.1 ± 0cd | – |
| PE67S | – | 13.1 ± 0cd | – |
| PE68S | – | 13.1 ± 0cd | – |
| PP69S | – | 12.0 ± 0c | – |
| PP70S | – | 12.0 ± 0c | – |
| PP71S | – | 13.1 ± 0cd | – |
| J72S | – | 13.1 ± 0cd | – |
| J73S | – | 13.1 ± 0cd | – |
| NS2F | – | – | 49.8 ± 5.3e |
| M3F | – | – | 50.3 ± 3.5e |
| M4F | – | – | 47.9 ± 4.0e |
| NS7F | – | – | 49.2 ± 3.8e |
| NS8F | – | – | 48.1 ± 4.4e |
| PP9F | – | – | 48.0 ± 2.6e |
| PP11F | – | – | 47.1 ± 6.8e |
| J13F | – | – | 49.8 ± 7.8e |
| J15F | – | – | 47.8 ± 10.1e |
| J16F | – | – | 49.9 ± 7.7e |
| M19F | – | – | 46.7 ± 8.0e |
| PE20F | – | – | 48.3 ± 10.6e |
| PE22F | – | – | 46.7 ± 8.0e |
| PP23F | – | – | 49.4 ± 4.8e |
| K25F | – | – | 48.3 ± 5.2e |
| K27F | – | – | 47.8 ± 8.9e |
| S30F | – | – | 47.2 ± 7.5e |
| PE31F | – | – | 50.8 ± 12.6e |
| PE32F | – | – | 49.2 ± 3.8e |
| S34F | – | – | 46.8 ± 2.3e |
| S35F | – | – | 46.2 ± 10.8e |
| PR36F | – | – | 49.2 ± 3.8e |
| PR37F | – | – | 49.6 ± 12.8e |
| PE39F | – | – | 50.5 ± 7.1e |
| Control | 0a | 0a | 0a |
aMeans ± standard deviation followed by different letters are significantly different (p < 0.05) according to Tukey’s test.
bNot applicable.
The fungal inoculated stems developed black necrotic lesions within the first to the third week of inoculation (Fig. 3I–K). After 4 weeks, the lesions extended longitudinally from the inoculation sites (Fig. 3L). The incision of the stem inoculated point displayed a reddish-brown to black necrotic lesion (Fig. 3M,N). Formation of gummosis on the necrotic lesion was also observed (Fig. 3O). Vertical and transverse sections of control and fungal inoculated stems showed symptomless and dark brown to black necrotic lesions, respectively (Fig. 3P,Q). There were significant differences of lesion areas produced on the L. theobromae inoculated stems that ranged from 12 to 14 cm2 (Table 1).
The fungal inoculated pods showed irregular brown to black lesions after 5 days of incubation (Fig. 3T). As the infection progressed, the lesions expanded and turned darker after 7 days of inoculation (Fig. 3U). After 12 days of inoculation, the lesions continued to expand, and the inoculated pods were completely colonized by the fungal grayish mycelia (Fig. 3V,W). Black conidiomata formed on the fungal inoculated pods (Fig. 3X). A cross-section of fungal inoculated pods showed rotting of the internal tissue (Fig. 3Y). The lesion areas ranged from 46.7 to 50.3 cm2 (Table 1). The lesion areas recorded on the fungal inoculated pods were significantly different compared to the control (Table 1).
The repetition of the pathogenicity assessment yielded the same outcomes as the first analysis. Koch's postulates were achieved by reisolating the same fungal isolates from the symptomatic inoculated leaves, stems, and pods of T. cacao and their identities were confirmed through morphological features.
Discussion
The present study identified L. theobromae isolates responsible to cause leaf blight, stem canker, and pod rot of T. cacao in Malaysia based on the morphological features, sequence comparison, and phylogenetic analysis of four genes (ITS, tef1-α, tub2, and rpb2). Fungi from genus Lasiodiplodia are cosmopolitan and belong to the Botryosphaeriaceae family, and most of the species can be primarily found in tropics and subtropics31–33. The genus consists of many phytopathogenic fungal species with widespread distribution33. Lasiodiplodia species responsible to cause over 500 plant diseases, including fruit rot, root rot, collar rot, stem-end rot, dieback, canker, and leaf necrosis32,34–43. In Malaysia, Lasiodiplodia species have been attributed to various destructive diseases, such as black rot of kenaf seeds44, leaf blight of Sansevieria trifasciata45, stem end-rot of Mangifera indica46, stem canker on Jatropha curcas and Acacia mangium47,48, and fruit rot of mango and guava49,50. Apart from that, they can act as secondary pathogens or endophytes, and they also can become pathogenic in response to a stressor34,36,40.
All the 57 fungal isolates recovered from diseased T. cacao in the present study was tentatively assigned as Lasiodiplodia sp. based on their macroscopic and microscopic characteristics. According to Hyde et al.51, the morphological approach has been widely used as the foundation for almost all studies of fungal taxonomy. Slippers and Wingfield34 also stated that Botryosphaeriaceae members are easily recognized from most other fungi through their colony appearance, aerial mycelium, and pigments, which can aid in the delimitation and rapid identification. However, due to the significant overlapping of key morphological characteristics among Lasiodiplodia species, clear-cut identification of the Lasiodiplodia isolates in the present study could not be achieved up to the species level by using traditional morphological descriptions such as conidial shape30,40.
Attributable to unresolve identity of Lasiodiplodia isolates based on morphological characteristics that could lead to uncertain and misleading results, phylogenetic analysis involving DNA sequences of multiple genes was applied to delineate species boundaries. Consistent with previous studies that also highlighted the importance of molecular work in defining Lasiodiplodia species34,39,40,52, the present study used several genes, namely, ITS, tef1-α, tub2, and rpb2, to explicitly characterize Lasiodiplodia isolates. The ITS region has been proposed and widely used in fungal taxonomic classification because of its straightforward amplification and it provides a high probability of successful fungal recognition, with the barcoding difference between inter- and intraspecific variations53,54. Nonetheless, the ITS region lacks interspecies variety and may even be vague in the identification of some fungi, thus the use of additional genes would provide better resolution in the phylogenetic analysis. Other studies also showed that a single gene is incapable of determining species in the genus Lasiodiplodia, implying that additional genes are required30,55. The tef1-α has become the marker of choice for fungal identification because of its distinct polymorphisms among similar species and consists of non-orthologous copies of the gene that are undetected in the genus56. The tub2 is another useful marker for delineating fungal species because it has fewer obscure aligned regions and less homoplasy across genera57. The rpb2 gene which codes for the second-largest protein subunit in fungi is a highly preserved single-copy gene54.
According to the results of phylogenetic analysis, it can be inferred that single gene analyses of ITS, tub2, and rpb2 are unable to resolve the identity of Lasiodiplodia isolates in the present study (Fig. S1a,c,d). Those phylogenetic trees displayed that L. theobromae was grouped with L. brasiliensis and L. hormozganensis. On the contrary, phylogenetic analysis of tef1-α sequences was able to differentiate isolates in the present study with other species of Lasiodiplodia by clustering them with several reference sequences of L. theobromae from the GenBank database with only 64% bootstrap value (Fig. S1b). Owing to the fact that single gene analysis could not accurately identify the Lasiodiplodia isolates in the present study, the combination of ITS, tef1-α, tub2, and rpb2 sequences was used for better characterization. The phylogenetic inferences based on multiple gene sequences revealed that the present isolates were grouped with L. theobromae with a higher bootstrap value (97%) (Fig. 2). The finding has been proven that phylogenetic analysis based on multigene provided robust resolution with clear-cut fungal identity. This is in line with the findings of Cruywagen et al.52.
Lasiodiplodia theobromae was confirmed to be the causal pathogen of leaf blight, stem canker, and pod rot of T. cacao in Malaysia. In 1895, L. theobromae was firstly described and reported to cause minor charcoal rot on cocoa in Ecuador31. Besides charcoal rot, L. theobromae was also reported to cause dieback on T. cacao since the late 1980s20. In Malaysia, documentations of relationship between L. theobromae and T. cacao are still limited. The present study represents the first report of leaf blight, stem canker, and pod rot of T. cacao caused by L. theobromae. Several studies have also found the incidence of L. theobromae causing foliar diseases in a wide range of hosts, including Camellia sinensis42, Catasetum fimbriatum58, Cocos nucifera59,60, Kadsura longipedunculata61, and S. trifasciata45. Moreover, the present study also revealed the ability of L. theobromae isolates to cause stem canker of T. cacao. Asman et al.24, previously reported L. theobromae as a causal agent of dieback and stem canker of cocoa by demonstrating internal discoloration with visible brown streaks in the vascular cambium. Furthermore, L. theobromae has been associated with cocoa dieback in Cameroon, India, and Venezuela19–21. It also responsible to cause dieback and stem canker on a number of plants, such as American ash (Fraxinus americana)62, blueberry bushes (Vaccinium spp.)63, strawberry (Fragaria × ananassa)41, mango (M. indica)64, cashew (Anacardium occidentale)65, sacha inchi (Plukenetia volubilis)66, Persian lime (Citrus latifolia)67, and grapevine (Vitis vinifera)68. In addition to infecting the leaf and stem, cocoa pod was also found to be susceptible to L. theobromae infection by showing rot symptoms. Several studies reported the occurrence of pod rot of T. cacao caused by L. theobromae6,22,25. Other pathogens were also identified to cause the same disease on the cocoa pod, namely C. gloeosporioides6, C. siamense17, C. tropicale17, L. pseudotheobromae17, N. parvum25, P. palmivora6,25,29, and P. megakarya4,29. From the pathogenicity tests, isolates of L. theobromae required wound to initiate infection and colonization on the host plant. Other studies have found that fungi from Botryosphaeriaceae can invade plants via endophytic conquest, injuries, seed-to-seedling conquest, contaminated soil, and insect infestation34,36.
In conclusion, the current study emphasized the first report of L. theobromae as a causal pathogen of leaf blight, stem canker, and pod rot of T. cacao in Malaysia. The pathogen was identified using morphological features supported by multigene DNA sequences and phylogenetic inference. The valid and precise identification of phytopathogen is critical for quarantine purpose and disease management strategies.
Materials and methods
Collecting samples and isolating fungi
From September 2018 to March 2019, sampling was conducted during rainy season in several states of Malaysia, including Johor, Kedah, Melaka, Negeri Sembilan, Perak, Perlis, Pulau Pinang, and Selangor (Fig. 4). The sampling sites and sampling activities were approved by the MCB comply with relevant institutional, national, and international guidelines and legislation. During the sampling, 50 blighted leaves, cankered stems, and rotted pods of T. cacao from the Koko Mardi (KM) clone were collected. The clone was used in the study because of its wide cultivation in Malaysia which showed susceptibility to a number of fungal diseases. Symptomatic leaves showed blighted symptoms, including circular to irregular blackish lesion surrounded by a yellow halo. The cankered stems were characterized as irregular blackish lesion, sometimes accompanied by gummosis on the disease area, expanded longitudinally, and internally became reddish-brown. The rotted pods were associated with dark brown to blackish lesions on the pods that eventually expanded and rotted.
Figure 4.

Sampling sites of diseased Theobroma cacao in several states of Malaysia.
The diseased and healthy margins of samples were cut into small pieces for fungal isolation. The small pieces of samples were surface-sterilized in 70% ethanol (C2H5OH) and 1% sodium hypochlorite (NaOCl) separately for 3 min. The samples were then rinsed in sterile distilled water three times in succession for 1 min each. The sterilized sample was blotted dry on sterile filter paper, transferred onto potato dextrose agar (PDA), and incubated at 25 °C ± 2 °C for 3–5 days. Pure cultures of fungal isolates obtained from single spore isolation were used for morphological and molecular assessments.
Morphological identification
In the present study, the fungal isolates obtained were provisionally examined based on morphological features, specifically macroscopic and microscopic characteristics. Colony appearance and pigmentation were observed at the macroscopic level. Under a dissecting microscope, the structure of the conidiomata was observed and photographed (EZ4, Leica Microsystem, Germany). The microscopic features such as conidia, conidiogenous cells, and paraphyses were observed using a light microscope (CX41, Olympus, Japan) and a camera (KY-F55BE, JVC, Japan). The average size of 30 randomized conidia was measured and recorded. Each fungal isolate was cultured onto carnation leaf agar (CLA) and incubated at 25 °C ± 2 °C for 7 days to observe the structures of conidiomata, conidia, conidiogenous cells, and paraphyses.
Molecular identification and phylogenetic analysis
To corroborate the identity of the fungal isolates of the present study, molecular identification and characterization was carried out. The fungal isolates were cultured in potato dextrose broth (PDB) and subjected to incubation at 25 °C ± 2 °C for 5 to 7 days. The mycelia that grew on the surface of PDB were collected, placed on the sterile filter paper (Whatman No. 1), and left to dry for 10 min. Using a sterile mortar and pestle, the dried mycelia were ground to a fine powder in liquid nitrogen. Then, 0.05 g of the fine powdered mycelia was placed in a 1.5 ml microcentrifuge tube for DNA extraction. The InnuPREP Plant DNA kit (Analytik Jena, Germany) was used to extract DNA by referring to the manufacturer's protocols. For amplification of internal transcribed spacer (ITS), elongation translation factor 1-alpha (tef1-α), β-tubulin (tub2), and RNA polymerase subunit II (rpb2), primer pairs of ITS1 (TCCGTAGGTGAACCTGCGG)/ITS4 (TCCTCCGCTTATTGATATGC)69, EF1-688F (CGGTCACTTGATCTACAAGTGC)/EF1-1251R (CCTCGAACTCACCAGTACCG)30, Bt2a (GGTAACCAAATCGGTGCTGCTTTC)/Bt2b (ACCCTCAGTGTAGTGACCCTTGGC)70, and rpb2-LasF (GGTAGCGACGTCACTCCT)/rpb2-LasR (GCGCAAATACCCAGAATCAT)52 were adopted, respectively. A reaction mixture of 50 µl was prepared by adding 8 µl of green buffer (Promega, USA), 8 µl of MgCl2 (Promega, USA), 1 µl of deoxynucleotide triphosphate polymerase (dNTP) (Promega, USA), 8 µl of each primer (Promega, USA), 0.3 µl of Taq polymerase (Promega, USA), 1 µl of genomic DNA, and sterile distilled water to obtain a total volume of 50 µl. The following conditions were used in the polymerase chain reaction (PCR) with the MyCycler™ Thermal Cycler (Bio-rad, Hercules, USA): Initial denaturation at 95 °C for 7 min (ITS)/5 min (tef1-α and tub2)/2 min (rpb2), then 25 cycles (ITS)/30 cycles (tef1-α and tub2)/35 cycles (rpb2) of denaturation at 94 °C for 1 min (ITS)/30 s (tef1-α, tub2, and rpb2), annealing at 50 °C for 1 min (ITS)/55 °C for 45 s (tef1-α and tub2)/54 °C for 30 s (rpb2), extension at 72 °C for 1 min (ITS and rpb2)/90 s (tef1-α and tub2), and final extension at 72 °C for 10 min (ITS, tef1-α, and tub2)/8 min (rpb2). The PCR products were electrophoresed for 90 min at 80 V and 400 mA in a 1.0% agarose gel (Promega, USA) containing FloroSafe DNA stain (First Base) in a 1.0× Tris–borate EDTA buffer. The Bio-Rad Molecular Imager® Gel Doc™ XR System and Bio-Rad Quantity One® Software were used to view and photograph the gel. The size of the amplified PCR products was determined using a 100 bp GeneRulers™ DNA ladder (Thermo Scientific, USA). The PCR products were sent to the First BASE Laboratories Sdn Bhd in Seri Kembangan, Malaysia, for DNA purification and sequencing.
The sequences obtained were compared, and phylogenetic analysis was performed using the Molecular Evolutionary Genetic Analysis (MEGA7) software71. The nucleotide homogeneity of the resulting consensus sequences was assessed by comparing with other sequence data in the GenBank database using Basic Local Alignment Search Tools (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). All sequences obtained were submitted to the GenBank database. Table 2 lists the sequences from the present study and the reference isolates used for phylogenetic analysis. The phylogenetic classification of the isolates from the present study was performed by analyzing the combination of multi-sequence alignments of fungal isolates and reference isolates in MEGA7 using the maximum likelihood (ML) method. The ML tree of combined genes was constructed using the Tamura 3-parameter model72 and 1000 bootstrap replicates73.
Table 2.
List of GenBank accession numbers of Lasiodiplodia species and the outgroup (Botryosphearia dothidea) used in the phylogenetic analysis.
| Species | Isolate | Host | Location | GenBank accession number | References | |||
|---|---|---|---|---|---|---|---|---|
| ITS | tef1-α | tub2 | rpb2 | |||||
| Lasiodiplodia brasiliensis | CBS123095 | Theobroma cacao | Cameroon | MT587423 | MT592135 | MT592615 | MT592309 | Zhang et al.75 |
| L. brasiliensis | CBS115447 | Psychotria tutcheri | Hong Kong | MT587422 | MT592134 | MT592614 | MT592308 | Zhang et al. 75 |
| L. brasiliensis | CMM4015a | Mangifera indica | Brazil | JX464063 | JX464049 | MT592614 | MT592308 | Marques et al.76 |
| L. brasiliensis | CSM11 | Theobroma cacao | Venezuela | MF436018 | MF436006 | MF435998 | MT592308 | Mohali-Castillo and Stewart19 |
| Lasiodiplodia citricola | CBS124707a | Citrus sp. | Iran | GU945354 | GU945340 | KU887505 | KU696351 | Cruywagen et al.52; Abdollahzadeh et al.55 |
| L. citricola | CBS124706 | Citrus sp. | Iran | GU945353 | GU945339 | KU887504 | KU696350 | Cruywagen et al.52; Abdollahzadeh et al.55 |
| Lasiodiplodia crassispora | CBS118741a | Santalum album | Australia | DQ103550 | DQ103557 | KU887506 | KU696353 | Cruywagen et al.52 |
| L. crassispora | CBS125626 | Vitis vinifera | South Africa | MT587424 | DQ103557 | MT592617 | MT592312 | Zhang et al.75 |
| L. crassispora | CMW33262 | Adansonia sp. | Unknown | KU887068 | DQ103557 | KU887426 | KU887364 | Cruywagen et al.52 |
| Lasiodiplodia euphorbiicola | CMM3609a | Jatropha curcas | Brazil | KF234543 | KF226689 | KF254926 | KU887367 | Machado et al.77 |
| L. euphorbiicola | CMM3651 | Jatropha curcas | Brazil | KF234553 | KF226711 | KF254937 | KU887367 | Machado et al.77 |
| L. euphorbiicola | CMW33268 | Adansonia sp. | Unknown | KU887131 | KU887008 | KU887430 | KU887367 | Cruywagen et al.52 |
| Lasiodiplodia hormozganensis | CBS124709a | Olea sp. | Iran | GU945355 | GU945343 | KU887515 | KU696361 | Cruywagen et al.52; Abdollahzadeh et al.55 |
| L. hormozganensis | CBS124708 | Mangifera indica | Iran | GU945356 | GU945344 | KU887514 | KU696360 | Cruywagen et al.52; Abdollahzadeh et al.55 |
| Lasiodiplodia iraniensis | CBS124710a | Salvadora persica | Iran | GU945348 | GU945336 | KU887516 | KU696363 | Cruywagen et al.52; Abdollahzadeh et al.55 |
| L. iraniensis | CBS124711 | Juglans sp. | Iran | GU945347 | GU945335 | KU887517 | KU696362 | Cruywagen et al.52; Abdollahzadeh et al.55 |
| L. iraniensis | CMW35881 | Adansonia sp. | Unknown | KU887092 | KU886970 | KU887464 | KU887388 | Cruywagen et al.52 |
| Lasiodiplodia lignicola | CBS134112a | Dead wood | Thailand | JX646797 | KU887003 | JX646845 | KU696364 | Cruywagen et al.52; Liu et al.78 |
| L. lignicola | MFLUCC110656 | Dead wood | Thailand | JX646798 | KU887003 | JX646846 | KU696364 | Cruywagen et al.52; Liu et al.78 |
| Lasiodiplodia mahajangana | CBS124925a | Terminalia catappa | Madagascar | FJ900595 | FJ900641 | KU887518 | KU696365 | Cruywagen et al.52; Begoude et al.79 |
| L. mahajangana | CBS124926 | Terminalia catappa | Madagascar | FJ900596 | FJ900642 | KU887519 | KU696366 | Cruywagen et al.52; Begoude et al.79 |
| Lasiodiplodia margaritacea | CBS122519a | Adansonia gibbosa | Australia | EU144050 | EU144065 | KU887520 | KU696367 | Cruywagen et al.52 |
| L. margaritacea | CBS138289 | Combretum elaeagnoides | Namibia | KP872320 | KP872349 | KP872379 | KP872429 | Zhang et al.75 |
| L. margaritacea | CBS138290 | Combretum collinum | Zambia | KP872321 | KP872350 | KP872380 | KP872430 | Zhang et al.75 |
| Lasiodiplodia mediterranea | CBS137783a | Quercus ilex | Italy | KJ638312 | KJ638331 | KU887521 | KU696368 | Cruywagen et al.52; Linaldeddu et al.80 |
| L. mediterranea | CBS137784 | Vitis vinifera | Italy | KJ638311 | KJ638330 | KU887522 | KU696369 | Cruywagen et al.52; Linaldeddu et al.80 |
| Lasiodiplodia pseudotheobromae | CBS116459a | Gmelina arborea | Costa Rica | EF622077 | EF622057 | EU673111 | KU696376 | Alves et al.30; Phillips et al.81 |
| L. pseudotheobromae | CBS116460 | Acacia mangium | Costa Rica | MT587433 | MT592145 | KU198428 | MT592322 | Zhang et al.75 |
| L. pseudotheobromae | CBS130991 | Mangifera indica | Egypt | MT587433 | MT592145 | MT592629 | MT592325 | Zhang et al.75 |
| L. pseudotheobromae | I46 | Theobroma cacao | Puerto Rico | MK693211 | MK693707 | MK693702 | KU696376 | Serrato-Diaz et al.17 |
| Lasiodiplodia theobromae | CBS164.69a | Fruit on coral reef coast | Indonesia: New Guinea | AY640255 | AY640258 | EU673110 | KU696383 | Cruywagen et al.52 |
| L. theobromae | CBS214.50 | Cajanus cajan | India | MT587440 | MT592152 | MT592637 | MT592333 | Zhang et al.75 |
| L. theobromae | CMW13490 | Eucalyptus urophylla | Venezuela: Acarigua | KY473071 | KY473019 | KY472962 | KY472888 | Mehl et al.82 |
| L. theobromae | CMM4019 | Mangifera indica | Brazil | JX464096 | JX464026 | EU673110 | KU696383 | Marques et al.76 |
| L. theobromae | CSM57 | Theobroma cacao | Venezuela | MF436029 | MF436017 | MF435999 | KU696383 | Mohali-Castillo and Stewart19 |
| L. theobromae | M400 | Theobroma cacao | USA: Puerto Rico | MN446021 | MN536705 | MN536694 | KU696383 | Puig et al.25 |
| L. theobromae | NS2F | Theobroma cacao | Malaysia: Negeri Sembilan | OL831055 | OL863319 | OL863262 | OL863376 | This study |
| L. theobromae | M3F | Theobroma cacao | Malaysia: Melaka | OL831056 | OL863320 | OL863263 | OL863377 | This study |
| L. theobromae | M4F | Theobroma cacao | Malaysia: Melaka | OL831057 | OL863321 | OL863264 | OL863378 | This study |
| L. theobromae | NS7F | Theobroma cacao | Malaysia: Negeri Sembilan | OL831058 | OL863322 | OL863265 | OL863379 | This study |
| L. theobromae | NS8F | Theobroma cacao | Malaysia: Negeri Sembilan | OL831059 | OL863323 | OL863266 | OL863380 | This study |
| L. theobromae | PP9F | Theobroma cacao | Malaysia: Pulau Pinang | OL831060 | OL863324 | OL863267 | OL863381 | This study |
| L. theobromae | PP11F | Theobroma cacao | Malaysia: Pulau Pinang | OL831061 | OL863325 | OL863268 | OL863382 | This study |
| L. theobromae | J13F | Theobroma cacao | Malaysia: Johor | OL831062 | OL863326 | OL863269 | OL863383 | This study |
| L. theobromae | J15F | Theobroma cacao | Malaysia: Johor | OL831063 | OL863327 | OL863270 | OL863384 | This study |
| L. theobromae | J16F | Theobroma cacao | Malaysia: Johor | OL831064 | OL863328 | OL863271 | OL863385 | This study |
| L. theobromae | M19F | Theobroma cacao | Malaysia: Melaka | OL831065 | OL863329 | OL863272 | OL863386 | This study |
| L. theobromae | PE20F | Theobroma cacao | Malaysia: Perak | OL831066 | OL863330 | OL863273 | OL863387 | This study |
| L. theobromae | PE22F | Theobroma cacao | Malaysia: Perak | OL831067 | OL863331 | OL863274 | OL863388 | This study |
| L. theobromae | PP23F | Theobroma cacao | Malaysia: Pulau Pinang | OL831068 | OL863332 | OL863275 | OL863389 | This study |
| L. theobromae | K25F | Theobroma cacao | Malaysia: Kedah | OL831069 | OL863333 | OL863276 | OL863390 | This study |
| L. theobromae | K27F | Theobroma cacao | Malaysia: Kedah | OL831070 | OL863334 | OL863277 | OL863391 | This study |
| L. theobromae | S30F | Theobroma cacao | Malaysia: Selangor | OL831071 | OL863335 | OL863278 | OL863392 | This study |
| L. theobromae | PE31F | Theobroma cacao | Malaysia: Perak | OL831072 | OL863336 | OL863279 | OL863393 | This study |
| L. theobromae | PE32F | Theobroma cacao | Malaysia: Perak | OL831073 | OL863337 | OL863280 | OL863394 | This study |
| L. theobromae | S34F | Theobroma cacao | Malaysia: Selangor | OL831074 | OL863338 | OL863281 | OL863395 | This study |
| L. theobromae | S35F | Theobroma cacao | Malaysia: Selangor | OL831075 | OL863339 | OL863282 | OL863396 | This study |
| L. theobromae | PR36F | Theobroma cacao | Malaysia: Perlis | OL831076 | OL863340 | OL863283 | OL863397 | This study |
| L. theobromae | PR37F | Theobroma cacao | Malaysia: Perlis | OL831077 | OL863341 | OL863284 | OL863398 | This study |
| L. theobromae | PE39F | Theobroma cacao | Malaysia: Perak | OL831078 | OL863342 | OL863285 | OL863399 | This study |
| L. theobromae | K41L | Theobroma cacao | Malaysia: Kedah | OL831081 | OL863343 | OL863286 | OL863400 | This study |
| L. theobromae | K42L | Theobroma cacao | Malaysia: Kedah | OL831082 | OL863344 | OL863287 | OL863401 | This study |
| L. theobromae | PR43L | Theobroma cacao | Malaysia: Perlis | OL831083 | OL863345 | OL863288 | OL863402 | This study |
| L. theobromae | PR44L | Theobroma cacao | Malaysia: Perlis | OL831084 | OL863346 | OL863289 | OL863403 | This study |
| L. theobromae | PE45L | Theobroma cacao | Malaysia: Perak | OL831085 | OL863347 | OL863290 | OL863404 | This study |
| L. theobromae | PE46L | Theobroma cacao | Malaysia: Perak | OL831086 | OL863348 | OL863291 | OL863405 | This study |
| L. theobromae | S47L | Theobroma cacao | Malaysia: Selangor | OL831087 | OL863349 | OL863292 | OL863406 | This study |
| L. theobromae | S48L | Theobroma cacao | Malaysia: Selangor | OL831088 | OL863350 | OL863293 | OL863407 | This study |
| L. theobromae | S49L | Theobroma cacao | Malaysia: Selangor | OL831089 | OL863351 | OL863294 | OL863408 | This study |
| L. theobromae | M50L | Theobroma cacao | Malaysia: Melaka | OL831090 | OL863352 | OL863295 | OL863409 | This study |
| L. theobromae | M51L | Theobroma cacao | Malaysia: Melaka | OL831091 | OL863353 | OL863296 | OL863410 | This study |
| L. theobromae | NS52L | Theobroma cacao | Malaysia: Negeri Sembilan | OL831080 | OL863354 | OL863297 | OL863411 | This study |
| L. theobromae | NS53L | Theobroma cacao | Malaysia: Negeri Sembilan | OL831079 | OL863355 | OL863298 | OL863412 | This study |
| L. theobromae | J54S | Theobroma cacao | Malaysia: Johor | OL831092 | OL863356 | OL863299 | OL863413 | This study |
| L. theobromae | J55S | Theobroma cacao | Malaysia: Johor | OL831093 | OL863357 | OL863300 | OL863414 | This study |
| L. theobromae | J56S | Theobroma cacao | Malaysia: Johor | OL831094 | OL863358 | OL863301 | OL863415 | This study |
| L. theobromae | J57S | Theobroma cacao | Malaysia: Johor | OL831095 | OL863359 | OL863302 | OL863416 | This study |
| L. theobromae | J58S | Theobroma cacao | Malaysia: Johor | OL831096 | OL863360 | OL863303 | OL863417 | This study |
| L. theobromae | J59S | Theobroma cacao | Malaysia: Johor | OL831097 | OL863361 | OL863304 | OL863418 | This study |
| L. theobromae | NS60S | Theobroma cacao | Malaysia: Negeri Sembilan | OL831098 | OL863362 | OL863305 | OL863419 | This study |
| L. theobromae | NS61S | Theobroma cacao | Malaysia: Negeri Sembilan | OL831099 | OL863363 | OL863306 | OL863420 | This study |
| L. theobromae | NS62S | Theobroma cacao | Malaysia: Negeri Sembilan | OL831100 | OL863364 | OL863307 | OL863421 | This study |
| L. theobromae | M63S | Theobroma cacao | Malaysia: Melaka | OL831101 | OL863365 | OL863308 | OL863422 | This study |
| L. theobromae | M64S | Theobroma cacao | Malaysia: Melaka | OL831102 | OL863366 | OL863309 | OL863423 | This study |
| L. theobromae | S65S | Theobroma cacao | Malaysia: Selangor | OL831103 | OL863367 | OL863310 | OL863424 | This study |
| L. theobromae | S66S | Theobroma cacao | Malaysia: Selangor | OL831104 | OL863368 | OL863311 | OL863425 | This study |
| L. theobromae | PE67S | Theobroma cacao | Malaysia: Perak | OL831105 | OL863369 | OL863312 | OL863426 | This study |
| L. theobromae | PE68S | Theobroma cacao | Malaysia: Perak | OL831106 | OL863370 | OL863313 | OL863427 | This study |
| L. theobromae | PP69S | Theobroma cacao | Malaysia: Pulau Pinang | OL831107 | OL863371 | OL863314 | OL863428 | This study |
| L. theobromae | PP70S | Theobroma cacao | Malaysia: Pulau Pinang | OL831108 | OL863372 | OL863315 | OL863429 | This study |
| L. theobromae | PP71S | Theobroma cacao | Malaysia: Pulau Pinang | OL831109 | OL863373 | OL863316 | OL863430 | This study |
| L. theobromae | J72S | Theobroma cacao | Malaysia: Johor | OL831110 | OL863374 | OL863317 | OL863431 | This study |
| L. theobromae | J73S | Theobroma cacao | Malaysia: Johor | OL831111 | OL863375 | OL863318 | OL863432 | This study |
| Lasiodiplodia viticola | CBS128313a | hybrid grape Vignoles | USA | HQ288227 | HQ288269 | HQ288306 | KU696385 | Cruywagen et al.52 |
| L. viticola | CBS128314 | Chardonel | USA | HQ288228 | HQ288270 | HQ288307 | KU696386 | Cruywagen et al.52 |
| Botryosphearia dothidea | CBS115476 | Prunus sp. | Switzerland | KF766151 | AY236898 | MT592470 | DQ677944 | Slippers et al.83 |
aEx-type isolates.
Pathogenicity tests
A total of 57 fungal isolates were assessed for pathogenicity on leaves (13 isolates), stems (20 isolates), and pods (24 isolates) of T. cacao using KM clone. The 1-year-old healthy seedlings of T. cacao grown using clay loam soil with a pH of 6.5–7 in polythene bags; and healthy mature pods (5 months old and 17 cm in size) taken from 3-year-old trees were purchased from the MCB. The seedlings were placed in the plant house of the School of Biological Sciences, Universiti Sains Malaysia (USM) at a temperature of 26 °C to 32 °C.
A fungal mycelial plug used as an inoculum was prepared from a 7-day-old PDA culture using a sterile cork borer (5 mm diameter). For control, the PDA plugs without fungal mycelia were prepared from the blank PDA using the same methods. Pathogenicity tests for all fungal isolates were performed twice. The tests were carried out on 84 healthy attached young leaves (84 seedlings), 126 stems (126 seedlings), and 150 detached pods of T. cacao. The targeted plant parts were surface-sterilized with 70% ethanol prior to inoculation.
To inoculate 13 fungal isolates on leaves of T. cacao, a total of 84 healthy leaves (78 for the fungal treatment and six for the control) from 84 seedlings of T. cacao were used for two pathogenicity tests. Each surface-sterilized leaf was aseptically pricked at one point with a sterile toothpick represented a replicate. For each pathogenicity test, three replicates were performed for each fungal isolate, using three different leaves from three different seedlings. Controls were performed in the same ways but treated with the blank PDA plugs. A sterile scalpel was used to inoculate control and mycelial plugs onto the control and treatment points, respectively. The plugs were wrapped in sterile cotton wool and fixed to the leaf with cellophane tape to avoid dryness. Each inoculated leaf was covered in a sterile zip lock bag. The inoculated seedlings were kept in the plant house of the School of Biological Sciences, USM for 9 days at temperatures ranging from 26 to 32 °C.
A total of 126 healthy stems of T. cacao (126 seedlings) were used to inoculate 20 fungal isolates for twice pathogenicity tests. A small wound (0.5 cm) was created on the sterilized surface of each stem by removing the bark with a sterile scalpel. For each pathogenicity test, three wounded stems from three different seedlings were used to inoculate each fungal isolate, representing triplicates. Control was treated similarly using the blank PDA plugs. Using a sterile scalpel, the mycelial and control plugs were placed on the wounded points, with the mycelium positioned towards the cambium. The moisture of the plugs was maintained by wrapping in sterilized cotton and sealing with parafilm. All the inoculated seedlings were incubated in the plant house of the School of Biological Sciences, USM at temperatures ranging from 26 to 32 °C.
Twice pathogenicity tests conducted on healthy detached cocoa pods involved 150 pods (144 for the fungal treatment and six pods for the control). Control and fungal treatments were inoculated on different pods to avoid symptoms overlapping if both were performed on the same pods. For each pathogenicity test, a wound point was created on the three different pods for each fungal isolate by piercing the pod surface with a sterile cork borer. Then, 5 mm mycelial plugs with the mycelium facing the surface of the pods were placed on the wounded points. The three control pods were treated in the same way but using the blank PDA plugs. To retain moisture, all the plugs were wrapped with sterilized cotton wool and the cotton was fixed with cellophane tape. The inoculated cocoa pods were incubated for 12 days at 25 °C ± 2 °C in sterilized trays and covered with transparent plastic to maintain humidity.
The area of the lesion developed on the inoculated leaves, stems, and pods of T. cacao was measured using grid paper adopted by Parker et al.74 with slight modifications. The area of diseased lesion was calculated by multiplying the number of small squares covering the lesion with the value calculated for one small square. Differences in lesion area were evaluated using the one-way method ANOVA and means were compared with the Tukey’s test (p < 0.05) using the software IBM SPSS Statistics version 26. To confirm Koch's postulates, fungi from symptomatic inoculated leaves, stems, and pods of T. cacao were reisolated and reidentified using morphological characteristics.
Supplementary Information
Acknowledgements
The authors thank the Malaysian Cocoa Board (MCB) for permission to collect samples and provide healthy seedlings for pathogenicity tests. Special thanks to the MCB staff who have assisted in the fieldwork activities.
Author contributions
A.R.H-S.: conceptualization, methodology, formal analysis, investigation, writing-original draft preparation. N.M.I.M.N., L.Z., Y.-H.L.: writing-review and editing. M.H.M.: conceptualization, methodology, investigation, writing-review and editing, supervision.
Funding
This research was funded by Fundamental Research Grant Scheme (FRGS/1/2019/WAB01/USM/02/1) from Ministry of Higher Education, Malaysia.
Data availability
All sequence data are available in NCBI GenBank [https://www.ncbi.nlm.nih.gov/genbank/] following the accession numbers [OL831055–OL831111 (ITS); OL863319–OL863375 (tef1-α); OL863262–OL863318 (tub2); OL863376–OL863432 (rpb2)] in the manuscript. All data analyzed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13057-9.
References
- 1.Azhar I, Kelvin L, Denamany G, Azmi CA, Saripah B, Nuraziawati MY. Cocoa Planting Manual. Malaysian Cocoa Board; 2009. [Google Scholar]
- 2.Wood GAR, Lass RA. Cocoa (Tropical Agriculture) Wiley Blackwell; 2001. [Google Scholar]
- 3.Lachenaud PH, Paulin D, Ducamp M, Thevenin JM. Twenty years of agronomic evaluation of wild cocoa trees (Theobroma cacao L.) from French Guiana—review. Sci. Hortic. 2007;113:313–321. doi: 10.1016/j.scienta.2007.05.016. [DOI] [Google Scholar]
- 4.Bailey BA, Meinhardt LW. Cacao Diseases: A History of Old Enemies and New Encounters. Springer International Publishing; 2016. [Google Scholar]
- 5.Dillinger TL, et al. Food of the gods: Cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J. Nutr. 2000;130:2057–2072. doi: 10.1093/jn/130.8.2057S. [DOI] [PubMed] [Google Scholar]
- 6.Nair KP. The Agronomy and Economy of Important Tree Crops of the Developing World. Springer; 2021. [Google Scholar]
- 7.Beckett ST, Fowler MS, Ziegler GR. Beckett’s Industrial Chocolate Manufacture and Use. Wiley; 2017. [Google Scholar]
- 8.Malaysian Cocoa Board (MCB). https://www.koko.gov.my/lkm/loader.cfm?page=1 (2021).
- 9.Ishaq S, Jafri L. Biomedical importance of cocoa (Theobroma cacao): Significance and potential for the maintenance of human health. Matrix Sci. Pharm. 2017;1:1–5. doi: 10.26480/msp.01.2017.01.05. [DOI] [Google Scholar]
- 10.Steinberg FM, Bearden MM, Keen CL. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- 11.Dryden GW, Song M, McClain C. Polyphenols and gastrointestinal diseases. Curr. Opin. Gastroenterol. 2006;22:165. doi: 10.1097/01.mog.0000208463.69266.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selmi C, Mao TK, Keen CL, Schmitz HH, Gershwin ME. The anti-inflammatory properties of cocoa flavanols. J. Cardiovasc. Pharmacol. 2006;47:163–171. doi: 10.1097/00005344-200606001-00010. [DOI] [PubMed] [Google Scholar]
- 13.Taubert D, Roesen R, Schömig E. Effect of cocoa and tea intake on blood pressure: A meta-analysis. Arch. Intern. Med. 2007;167:626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 14.Latif R. Health benefits of cocoa. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:669–674. doi: 10.1097/MCO.0b013e328365a235. [DOI] [PubMed] [Google Scholar]
- 15.Guest D, Keane P. Vascular-streak dieback: A new encounter disease of cacao in Papua New Guinea and Southeast Asia caused by the obligate basidiomycete Oncobasidium theobromae. Phytopathology. 2007;97:1654–1657. doi: 10.1094/PHYTO-97-12-1654. [DOI] [PubMed] [Google Scholar]
- 16.James RS, Ray J, Tan YP, Shivas RG. Colletotrichum siamense, C. theobromicola and C. queenslandicum from several plant species and the identification of C. asianum in the northern territory, Australia. Australas. Plant Dis. Notes. 2014;9:1–6. doi: 10.1007/s13314-014-0138-x. [DOI] [Google Scholar]
- 17.Serrato-Diaz LM, Mariño YA, Guadalupe I, Bayman P, Goenaga R. First report of Lasiodiplodia pseudotheobromae and Colletotrichum siamense causing cacao pod rot, and first report of C. tropicale causing cacao pod rot in Puerto Rico. Plant Dis. 2020;104:592. doi: 10.1094/PDIS-06-19-1333-PDN. [DOI] [Google Scholar]
- 18.Rojas EI, et al. Colletotrichum gloeosporioides sl associated with Theobroma cacao and other plants in Panama: Multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia. 2010;102:1318–1338. doi: 10.3852/09-244. [DOI] [PubMed] [Google Scholar]
- 19.Mohali-Castillo, S. & Stewart, J.E. Microfungi associated with diseases on Theobroma cacao L. in Merida State, Venezuela. In Proceedings of the APS Annual Meeting (2017).
- 20.Mbenoun M, Momo Zeutsa EH, Samuels G, NsougaAmougou F, Nyasse S. Dieback due to Lasiodiplodia theobromae, a new constraint to cocoa production in Cameroon. Plant Pathol. 2008;57:381. doi: 10.1111/j.1365-3059.2007.01755.x. [DOI] [Google Scholar]
- 21.Kannan C, Karthik M, Priya K. Lasiodiplodia theobromae causes a damaging dieback of cocoa in India. Plant Pathol. 2010;59:410. doi: 10.1111/j.1365-3059.2009.02192.x. [DOI] [Google Scholar]
- 22.Twumasi P, Ohene-Mensa G, Moses E. The rot fungus Botryodiplodia theobromae strains cross infect cocoa, mango, banana and yam with significant tissue damage and economic losses. Afr. J. Agric. Res. 2014;9:613–619. doi: 10.5897/AJAR2013.7528. [DOI] [Google Scholar]
- 23.Alvindia DG, Gallema FLM. Lasiodiplodia theobromae causes vascular streak dieback (VSD)-like symptoms of cacao in Davao Region, Philippines. Australas. Plant Dis. Notes. 2017;12:1–4. doi: 10.1007/s13314-017-0279-9. [DOI] [Google Scholar]
- 24.Asman, A. et al. Lasiodiplodia theobromae: an emerging threat to cocoa causes dieback and canker disease in Sulawesi. In Proceedings of the Asia-Pacific Regional Cocoa IPM Symposium (2019).
- 25.Puig AS, Keith LM, Matsumoto TK, Gutierrez OA, Marelli JP. Virulence tests of Neofusicoccum parvum, Lasiodiplodia theobromae, and Phytophthora palmivora on Theobroma cacao. Eur. J. Plant Pathol. 2021;159:851–862. doi: 10.1007/s10658-021-02210-1. [DOI] [Google Scholar]
- 26.Meinhardt LW, et al. Moniliophthora perniciosa, the causal agent of witches’ broom disease of cacao: What's new from this old foe? Mol. Plant Pathol. 2008;9:577–588. doi: 10.1111/j.1364-3703.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips-Mora W, Wilkinson MJ. Frosty pod of cacao: A disease with a limited geographic range but unlimited potential for damage. Phytopathology. 2007;97:1644–1647. doi: 10.1094/PHYTO-97-12-1644. [DOI] [PubMed] [Google Scholar]
- 28.Puig AS, Marelli JP, Matsumoto TK, Keith LM, Gutierrez OA. First report of Neofusicoccum parvum causing pod rot on cacao in Hawaii. Plant Dis. 2019;103:1416. doi: 10.1094/PDIS-10-18-1719-PDN. [DOI] [Google Scholar]
- 29.Akrofi AY, Amoako-Atta I, Assuah M, Asare EK. Black pod disease on cacao (Theobroma cacao L.) in Ghana: Spread of Phytophthora megakarya and role of economic plants in the disease epidemiology. Crop Prot. 2015;72:66–75. doi: 10.1016/j.cropro.2015.01.015. [DOI] [Google Scholar]
- 30.Alves A, Crous PW, Correia A, Phillips AJL. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008;28:1–13. [Google Scholar]
- 31.Phillips AJL, et al. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013;76:51–167. doi: 10.3114/sim0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Punithalingam E. Plant diseases attributed to Botryodiplodia theobromae. Bibl. Mycol. 1980;71:1–123. [Google Scholar]
- 33.Burgess TI, Sakalidis ML, Hardy GES. Gene flow of the canker pathogen Botryosphaeria australis between Eucalyptus globulus plantations and native eucalypt forests in Western Australia. Austral. Ecol. 2006;31:559–566. doi: 10.1111/j.1442-9993.2006.01596.x. [DOI] [Google Scholar]
- 34.Slippers B, Wingfield MJ. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology, and impact. Fungal Biol. Rev. 2007;21:90–106. doi: 10.1016/j.fbr.2007.06.002. [DOI] [Google Scholar]
- 35.Latha P, Prakasam V, Kamalakakannan A, Gopalakrishnan C, Thiruvengadam R, Paramathma M. First report of Lasiodiplodia theobromae (Pat.) Griff. and Maubl causing root rot and collar rot of physic nut (Jatropha curcas L.) in India. Australas. Plant Dis. Notes. 2009;4:19–20. [Google Scholar]
- 36.Sakalidis ML, Ray JD, Lanoiselet V, Hardy GES, Burgess TI. Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley region of Western Australia. Eur. J. Plant Pathol. 2011;130:379–391. doi: 10.1007/s10658-011-9760-z. [DOI] [Google Scholar]
- 37.Ismail AM, Cirvilleri G, Polizzi G, Crous PW, Groenewald JZ, Lombard L. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas. Plant Pathol. 2012;41:649–660. doi: 10.1007/s13313-012-0163-1. [DOI] [Google Scholar]
- 38.Urbez-Torres JR, et al. Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers. 2012;52:169–189. doi: 10.1007/s13225-011-0110-4. [DOI] [Google Scholar]
- 39.Netto MSB, et al. Species of Lasiodiplodia associated with papaya stem-end rot in Brazil. Fungal Divers. 2014;67:127–141. doi: 10.1007/s13225-014-0279-4. [DOI] [Google Scholar]
- 40.Slippers B, et al. Confronting the constraints of morphological taxonomy in the Botryosphaeriales. Persoonia. 2014;33:155–168. doi: 10.3767/003158514X684780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yildiz A, Benlioglu K, Benlioglu HS. First report of strawberry dieback caused by Lasiodiplodia theobromae. Plant Dis. 2014;98:1579. doi: 10.1094/PDIS-11-13-1192-PDN. [DOI] [PubMed] [Google Scholar]
- 42.Li HL, et al. Lasiodiplodia theobromae and L. pseudotheobromae causing leaf necrosis on Camellia sinensis in Fujian Province, China. Can. J. Plant Pathol. 2019;41:277–284. doi: 10.1080/07060661.2019.1569559. [DOI] [Google Scholar]
- 43.Berraf-Tebbal A, et al. Lasiodiplodia mitidjana sp. nov. and other Botryosphaeriaceae species causing branch canker and dieback of Citrus sinensis in Algeria. PLoS ONE. 2020;15:1–18. doi: 10.1371/journal.pone.0232448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norhayati M, Erneeza MH, Kamaruzaman S. Morphological, pathogenic and molecular characterization of Lasiodiplodia theobromae: A causal pathogen of black rot disease on kenaf seeds in Malaysia. Int. J. Agric. Biol. 2016;18:80–85. doi: 10.17957/IJAB/15.0065. [DOI] [Google Scholar]
- 45.Kee YJ, Zakaria L, Mohd MH. Lasiodiplodia species associated with Sansevieria trifasciata leaf blight in Malaysia. J. Gen. Plant Pathol. 2019;85:66–71. doi: 10.1007/s10327-018-0814-3. [DOI] [Google Scholar]
- 46.Li L, Mohd MH, Mohamed Nor NMI, Subramaniam S, Latiffah Z. Identification of Botryosphaeriaceae associated with stem-end rot of mango (Mangifera indica L.) in Malaysia. J. Appl. Microbiol. 2020;130:1273–1284. doi: 10.1111/jam.14828. [DOI] [PubMed] [Google Scholar]
- 47.Maid M, et al. First report of stem canker disease on Acacia mangium induced by Lasiodiplodia theobromae and Lasiodiplodia pseudotheobromae species in Sabah, Malaysia. Malays. Appl. Biol. 2018;47:147–151. [Google Scholar]
- 48.Sulaiman R, Thanarajoo SS, Kadir J, Vadamalai G. First report of Lasiodiplodia theobromae causing stem canker of Jatropha curcas in Malaysia. Plant Dis. 2012;96:767. doi: 10.1094/PDIS-06-11-0482-PDN. [DOI] [PubMed] [Google Scholar]
- 49.Munirah MS, Azmi AR, Yong SYC, Nur Ain Izzati MZ. Characterization of Lasiodiplodia theobromae and L. pseudotheobromae causing fruit rot on pre-harvest mango in Malaysia. Plant Pathol. Quar. 2017;7:202–213. doi: 10.5943/ppq/7/2/14. [DOI] [Google Scholar]
- 50.Zee KY, Asib N, Ismail SI. First report of Lasiodiplodia theobromae causing postharvest fruit rot on guava (Psidium guajava) in Malaysia. Plant Dis. 2021;105:2716. doi: 10.1094/PDIS-12-20-2732-PDN. [DOI] [Google Scholar]
- 51.Hyde KD, Abd-Elsalam K, Cai L. Morphology: Still essential in a molecular world. Mycotaxon. 2010;114:439–451. doi: 10.5248/114.439. [DOI] [Google Scholar]
- 52.Cruywagen EM, Slippers B, Roux J, Wingfield MJ. Phylogenetic species recognition and hybridisation in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017;121:420–436. doi: 10.1016/j.funbio.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Schoch CL, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kashyap PL, Rai P, Kumar S, Chakdar H, Srivastava AK. DNA barcoding for diagnosis and monitoring of fungal plant pathogens. In: Singh BP, Gupta VK, editors. Molecular Markers in Mycology: Diagnostics and Marker Development. Springer; 2017. pp. 87–122. [Google Scholar]
- 55.Abdollahzadeh J, Javadi A, Goltapeh EM, Zare R, Philip AJL. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia. 2010;25:1–10. doi: 10.3767/003158510X524150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geiser DM, et al. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004;110:473–479. doi: 10.1023/B:EJPP.0000032386.75915.a0. [DOI] [Google Scholar]
- 57.Udayanga D, Liu X, Crous PW, McKenzie EH, Chukeatirote E, Hyde KDA. Multi-locus phylogenetic evaluation of Diaporthe (Phomopsis) Fungal Divers. 2012;56:157–171. doi: 10.1007/s13225-012-0190-9. [DOI] [Google Scholar]
- 58.Lopes UP, Zambolim L, Pereira OL. First report of Lasiodiplodia theobromae causing leaf blight on the orchid Catasetum fimbriatum in Brazil. Australas. Plant Dis. Notes. 2009;4:64–65. [Google Scholar]
- 59.Ramjegathesh R, Johnson I, Hubballi M, Maheswarappa HP. Characterization of Lasiodiplodia theobromae causing leaf blight disease of coconut. J. Plant Crops. 2019;47:62–71. [Google Scholar]
- 60.Santos PH, et al. Is Lasiodiplodia theobromae the only species that causes leaf blight disease in Brazilian coconut palms? Trop. Plant Pathol. 2020;45:434–442. doi: 10.1007/s40858-020-00344-x. [DOI] [Google Scholar]
- 61.Fan R, et al. First report of Lasiodiplodia theobromae causing leaf blight of Kadsura longipedunculata in China. Plant Dis. 2020;104:3063. doi: 10.1094/PDIS-02-20-0330-PDN. [DOI] [Google Scholar]
- 62.Chen F, Zheng X, Zhao X, Chen F. First report of Lasiodiplodia theobromae causing stem canker of Fraxinus americana. Plant Dis. 2019;103:3276. doi: 10.1094/PDIS-04-19-0892-PDN. [DOI] [Google Scholar]
- 63.Borrero C, Pérez S, Avilés M. First report of canker disease caused by Lasiodiplodia theobromae on blueberry bushes in Spain. Plant Dis. 2019;103:2684. doi: 10.1094/PDIS-03-19-0473-PDN. [DOI] [PubMed] [Google Scholar]
- 64.Khanzada MA, Lodhi AM, Shahzad S. Mango dieback and gummosis in Sindh, Pakistan caused by Lasiodiplodia theobromae. Plant Health Prog. 2004;15:13. doi: 10.1094/PHP-2004-0302-01-DG. [DOI] [Google Scholar]
- 65.Cardoso JE, Vidal JC, dos Santos AA, Freir FCO, Viana FMP. First report of black branch dieback of cashew caused by Lasiodiplodia theobromae in Brazil. Plant Dis. 2002;86:558. doi: 10.1094/PDIS.2002.86.5.558B. [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Song X. First report of Lasiodiplodia theobromae and L. pseudotheobromae causing canker disease of sacha inchi in Hainan, China. Plant Dis. 2021;105:3757. doi: 10.1094/PDIS-11-20-2507-PDN. [DOI] [Google Scholar]
- 67.Bautista-Cruz MA, et al. Phylogeny, distribution, and pathogenicity of Lasiodiplodia species associated with cankers and dieback symptoms of persian lime in Mexico. Plant Dis. 2019;103:1156–1165. doi: 10.1094/PDIS-06-18-1036-RE. [DOI] [PubMed] [Google Scholar]
- 68.Úrbez-Torres JR, Gubler WD. Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis. 2009;93:584–592. doi: 10.1094/PDIS-93-6-0584. [DOI] [PubMed] [Google Scholar]
- 69.White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; 1990. pp. 315–322. [Google Scholar]
- 70.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- 73.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 74.Parker SR, Shaw MW, Royle DJ. The reliability of visual estimates of disease severity on cereal leaves. Plant Pathol. 1995;44:856–864. doi: 10.1111/j.1365-3059.1995.tb02745.x. [DOI] [Google Scholar]
- 75.Zhang W, Groenewald JZ, Lombard L, Schumacher RK, Phillips AJL, Crous PW. Evaluating species in Botryosphaeriales. Persoonia. 2021;46:63–115. doi: 10.3767/persoonia.2021.46.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marques MW, et al. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013;61:181–193. doi: 10.1007/s13225-013-0231-z. [DOI] [Google Scholar]
- 77.Machado AR, Pinho DB, Pereira OL. Phylogeny, identification and pathogenicity of the Botryosphaeriaceae associated with collar and root rot of the biofuel plant Jatropha curcas in Brazil, with a description of new species of Lasiodiplodia. Fungal Divers. 2014;67:231–247. doi: 10.1007/s13225-013-0274-1. [DOI] [Google Scholar]
- 78.Liu JK, et al. Towards a natural classification of Botryosphaeriales. Fungal Divers. 2012;57:149–210. doi: 10.1007/s13225-012-0207-4. [DOI] [Google Scholar]
- 79.Begoude BD, Slippers B, Wingfield MJ, Roux J. Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycol. Prog. 2010;9:101–123. doi: 10.1007/s11557-009-0622-4. [DOI] [Google Scholar]
- 80.Linaldeddu BT, et al. Diversity of Botryosphaeriaceae species associated with grapevine and other woody hosts in Italy, Algeria and Tunisia, with descriptions of Lasiodiplodia exigua and Lasiodiplodia mediterranea sp. nov. Fungal Divers. 2015;71:201–214. doi: 10.1007/s13225-014-0301-x. [DOI] [Google Scholar]
- 81.Phillips AJL, et al. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia. 2008;21:29–55. doi: 10.3767/003158508X340742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehl J, Wingfield MJ, Roux J, Slippers B. Invasive everywhere? Phylogeographic analysis of the globally distributed tree pathogen Lasiodiplodia theobromae. Forests. 2017;8:145. doi: 10.3390/f8050145. [DOI] [Google Scholar]
- 83.Slippers B, et al. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2006;55:35–52. doi: 10.3114/sim.55.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequence data are available in NCBI GenBank [https://www.ncbi.nlm.nih.gov/genbank/] following the accession numbers [OL831055–OL831111 (ITS); OL863319–OL863375 (tef1-α); OL863262–OL863318 (tub2); OL863376–OL863432 (rpb2)] in the manuscript. All data analyzed during this study are included in this published article and its supplementary information files.