Abstract
Introduction
This multicenter review evaluated the efficacy and safety of osimertinib dose escalation for central nervous system (CNS) progression developing on osimertinib 80 mg in EGFR-mutant NSCLC.
Methods
Retrospective review identified 105 patients from eight institutions with advanced EGFR-mutant NSCLC treated with osimertinib 160 mg daily between October 2013 and January 2020. Radiographic responses were clinically assessed, and Kaplan-Meier analyses were used. We defined CNS disease control as the interval from osimertinib 160 mg initiation to CNS progression or discontinuation of osimertinib 160 mg.
Results
Among 105 patients treated with osimertinib 160 mg, 69 were escalated for CNS progression, including 24 treated with dose escalation alone (cohort A), 34 who received dose-escalated osimertinib plus concurrent chemotherapy and/or radiation (cohort B), and 11 who received osimertinib 160 mg without any prior 80 mg exposure. The median duration of CNS control was 3.8 months (95% confidence interval [CI], 1.7–5.8) in cohort A, 5.1 months (95% CI, 3.1–6.5) in cohort B, and 4.2 months (95% CI 1.6–not reached) in cohort C. Across all cohorts, the median duration of CNS control was 6.0 months (95% CI, 5.1–9.0) in isolated leptomeningeal progression (n = 27) and 3.3 months (95% CI, 1.0–3.1) among those with parenchymal-only metastases (n = 23). Patients on osimertinib 160 mg experienced no severe or unexpected side effects.
Conclusion
Among patients with EGFR-mutant NSCLC experiencing CNS progression on osimertinib 80 mg daily, dose escalation to 160 mg provided modest benefit with CNS control lasting approximately 3 to 6 months and seemed more effective in patients with isolated leptomeningeal CNS progression.
Keywords: EGFR, Osimertinib, 160 mg, CNS progression, Leptomeningeal progression
Introduction
For patients with advanced EGFR-mutant NSCLC, osimertinib is the preferred first-line EGFR inhibitor based on the FLAURA study, which revealed improved progression-free survival (PFS) and overall survival (OS) for first-line osimertinib compared with erlotinib or gefitinib.1 Although osimertinib has improved central nervous system (CNS) penetrance over older EGFR tyrosine kinase inhibitors (TKIs),2,3 progression within the CNS remains an important clinical challenge in patients with EGFR-mutant NSCLC. Among patients with baseline CNS disease treated with osimertinib on the FLAURA study, the CNS response rate was 91% (95% confidence interval [CI], 71%–99%).4 However, 30% of patients with baseline CNS disease developed CNS progression or death due to CNS progression during a median follow-up of 12.4 months. CNS progression on osimertinib is particularly challenging given the limited CNS efficacy of alternative systemic therapies.
When patients experience CNS progression on osimertinib, treatment options include local therapies, such as surgery and radiation (stereotactic radiosurgery [SRS] or whole brain radiation [WBRT]), or systemic therapies, among which pemetrexed is often considered the most CNS penetrant.5,6 Although the evaluation of resistance mechanisms through tissue or plasma is used to guide post-osimertinib therapy among patients with systemic disease progression, it is more challenging to evaluate mechanisms of resistance in patients with CNS-only progression.7 Finally, clinical trials often exclude those with active CNS disease, further limiting next-line treatment options.8,9
Although the standard dose of osimertinib is 80 mg daily, the 160 mg dose has been evaluated within the context of CNS disease. In the BLOOM trial, osimertinib was given at 160 mg and had an efficacy in patients progressing on early generation EGFR TKIs with leptomeningeal disease (with or without concurrent parenchymal metastases).10 On the basis of these data and case reports of leptomeningeal response with osimertinib dose escalation,11,12 many oncologists use dose escalation from 80 mg to 160 mg to treat CNS progression. The effectiveness of this approach, however, remains unclear given only limited prospective data.13,14 We conducted a multi-institutional, retrospective study of osimertinib 160 mg at the time of CNS progression and herein report the largest series, to our knowledge, of patients who underwent osimertinib dose escalation for CNS progression.
Methods
Study Design and Patients
We retrospectively collected data from eight institutions on patients with advanced EGFR-mutant NSCLC treated with osimertinib at a dose of 160 mg daily. Data were collected by investigators at each participating institution according to approved institutional review board protocols, including sex, smoking status, date of diagnosis, EGFR mutation type, reason for osimertinib 160 mg therapy, pattern of CNS progression (parenchymal metastases, leptomeningeal disease, or both), and clinical outcomes. For this analysis, we focused on patients treated with osimertinib 160 mg for CNS progression, regardless of having simultaneous systemic progression or not. Patients treated with osimertinib 160 mg for any reason other than CNS progression were excluded. All included patients had provided informed consent to participate in retrospective clinical research at their respective institutions or data were collected by approved institutional review board protocols that allow use of a waiver of consent for retrospective data collection. Analyses were performed in accordance with an approved local institutional review board protocol and data use policies at the lead site.
Treatments, Assessments, and Statistical Considerations
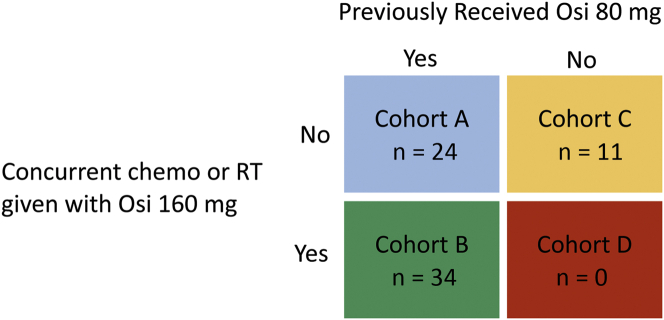
Patients treated with osimertinib 160 mg were categorized into four cohorts on the basis of whether they had previously received osimertinib 80 mg before 160 mg and whether they received osimertinib 160 mg alone or with any other therapies, such as chemotherapy or radiation (Fig. 1).
Figure 1.
Treatment-based cohort assignments. Patients were assigned to cohorts A, B, or C according to treatment with osimertinib 80 mg before CNS PD and concurrent chemotherapy or RT at the time of CNS PD. Chemo, chemotherapy; CNS, central nervous system; Osi, osimertinib; PD, disease progression; RT, radiation therapy.
CNS progression was defined as detection of new or increasing parenchymal brain metastases on routine surveillance imaging or clinical evidence of leptomeningeal progression indicated by imaging, symptoms, or malignant cells on cerebrospinal fluid (CSF) cytology. Radiographic responses and progression were clinically evaluated by investigators at each participating site. Response of leptomeningeal disease was also determined clinically by improvement in clinician-assessed symptoms attributed to leptomeningeal disease or clearance of CSF cytology when available. Treatment-related toxicities were clinically assessed by investigators at each site.
Kaplan-Meier analyses were used for time-to-event end points including duration of CNS control on osimertinib 160 mg and OS. The duration of CNS disease control on osimertinib 160 mg was defined as the interval from initiation of osimertinib 160 mg to CNS progression or discontinuation of osimertinib 160 mg.
Results
Patients
A total of 105 patients from eight institutions treated with osimertinib 160 mg daily for advanced EGFR-mutant NSCLC between October 2013 and January 2020 were identified. Of these, the 69 who received osimertinib 160 mg for CNS progression are included in this analysis (Table 1). Median age was 57 years (range 36–79) and 43 (62%) were female. EGFR mutations included L858R (n = 31), exon 19 deletions (n = 29), and other activating mutations (n = 9). Patients had been treated with a median of 1 (range 0–8) line of therapy before treatment with osimertinib. The type of CNS progression experienced included leptomeningeal progression only in 27 (39%), brain parenchymal progression only in 23 (33%), or both in 11 (16%). In addition, among the 69 patients, 58 (84%) had CNS-only progression, without concurrent systemic disease progression. The proportion of patients with systemic progression concurrent with CNS progression was similar across cohorts (Table 1; overall, 11.4%; cohort A, 12.5%, cohort B, 12%; cohort C, 9%).
Table 1.
Characteristics of Patients Treated With Osimertinib 160 mg for Central Nervous System Progression
| Characteristic | Total, N = 69 | Cohort A, n = 24 | Cohort B, n = 34 | Cohort C, n = 11 |
|---|---|---|---|---|
| Age, median (range) | 57 (36–79) | 55 (36–71) | 58 (41–73) | 63 (40–79) |
| Female, n (%) | 43 (62%) | 16 (67%) | 21 (62%) | 6 (55%) |
| Median prior lines of systemic therapy (range): | 1 (0–8) | 1 (0–8) | 1 (0–6) | 3 (0–6) |
| Prior TKI exposure:a | ||||
| Osimertinib 80 mg | 58 (84%) | 24 (100%) | 34 (100%) | 0 |
| Erlotinib | 36 (52%) | 12 (50%) | 17 (50%) | 7 (64%) |
| Afatinib | 13 (19%) | 3 (13%) | 7 (21%) | 3 (27%) |
| Rociletinib | 5 (7%) | 1 (4%) | 0 | 4 (36%) |
| Gefitinib | 2 (3%) | 1 (4%) | 1 (3%) | 0 |
| None | 15 (22%) | 5 (21%) | 9 (26%) | 1 (9%) |
| CNS progression pattern: | ||||
| Leptomeningeal only | 27 (39%) | 11 (46%) | 11 (32%) | 5 (45%) |
| Parenchymal only | 23 (33%) | 8 (33%) | 12 (35%) | 3 (27%) |
| Both | 19 (27%) | 5 (21%) | 11 (33%) | 3 (27%) |
| Concurrent CNS and systemic progression | 12 (11.4%) | 3 (12.5%) | 4 (12%) | 1 (9%) |
| Therapies given concurrently with osimertinib 160 mg: | ||||
| Chemotherapy | Cohort B only | - | 14 (41%) | - |
| Stereotactic radiosurgery | - | 14 (41%) | - | |
| Whole brain radiotherapy | - | 8 (24%) | - | |
| EGFR mutation subtype: | ||||
| L858R | 31 (45%) | 13 (54%) | 15 (44%) | 3 (27%) |
| Deletion 19 | 29 (42%) | 8 (33%) | 14 (41%) | 7 (64%) |
| Other | 9 (13%) | 3 (13%) | 5 (15%) | 1 (9%) |
Cohort A: osimertinib dose-escalated from 80 mg to 160 mg; cohort B: osimertinib dose-escalated from 80 mg to 160 mg and concurrent chemotherapy and/or radiotherapy; cohort C: initial osimertinib dose of 160 mg.
CNS, central nervous system; TKI, tyrosine kinase inhibitor.
TKIs preceding osimertinib 160 mg.
Patients were categorized into cohorts (Fig. 1) such that cohort A (osimertinib dose escalation without chemotherapy or radiation) consisted of 24 patients, cohort B (osimertinib dose escalation with concurrent chemotherapy and/or radiation) consisted of 34 patients, and cohort C (osimertinib 160 mg as initial osimertinib treatment) consisted of 11 patients. There were no patients who fell into cohort D (osimertinib 160 mg as initial osimertinib treatment with concurrent chemotherapy and/or radiation). The breakdown of concurrent therapies received in cohort B included chemotherapy in 41% (n = 14), SRS in 41% (n = 14), and WBRT in 24% (n = 8). Patients in cohort B accounted for more than half of those (six of 11) who were treated with osimertinib dose escalation for concurrent systemic and CNS progression.
Efficacy
Patient outcomes were analyzed by cohort and type of CNS progression prompting osimertinib 160 mg QD. In cohort A (n = 24), dose escalation to osimertinib 160 mg monotherapy was associated with a median duration of CNS disease control of 3.8 months (95% CI, 1.7–5.8) and a median OS of 14.8 months (95% CI, 7.0–not reached [NR]; Table 2). In cohort B (n = 34), dose escalation to osimertinib 160 mg in combination with radiotherapy (n = 22) and/or chemotherapy (n = 14) yielded a median duration of CNS control of 5.1 months (95% CI, 3.1–6.5) and a median OS of 10.5 months (95% CI, 7.0–25.3). Among cohort B patients treated only with osimertinib dose escalation and chemotherapy (n = 10; nine with leptomeningeal disease), the median duration of CNS control was 8.2 months (95% CI, 1.5–NR) and OS was 10 months (95% CI, 1.5–NR).
Table 2.
Duration of CNS Control and Overall Survival on Osimertinib 160 mg QD
| Cohort | Median Duration of CNS Control, mo (95% CI) | Median OS, mo (95% CI) | |
|---|---|---|---|
| Full cohorts treated with osi 160 for any type of CNS progression (N = 69) | |||
| Cohort A (n = 24) | 3.8 (1.7–5.8) | 14.8 (7.0–NR) | |
| Cohort B (n = 34) | 5.1 (3.1–6.5) | 10.5 (7.0–25.3) | |
| Cohort C (n = 11) | 4.2 (1.6–NR) | NA | |
| Subsets treated with osi 160 for CNS leptomeningeal-only progression (n = 27) | |||
| Cohort A (n = 11) | 5.8 (1.7–9.1) | 14.8 (4.1–NR) | |
| Cohort B (n = 11) | 7.1 (5.0–NR) | a | |
| Cohort C (n = 5) | a | a | |
| Combined A/B/C (n = 27) | 6.0 (5.1–9.0) | a | |
| Subsets treated with osi 160 for CNS parenchymal-only progression (n = 23) | |||
| Cohort A (n = 8) | 2.0 (1–4.9) | 13.0 (2.2–NR) | |
| Cohort B (n = 12) | 3.1 (0.8–NR) | a | |
| Cohort C (n = 3) | a | a | |
| Combined A/B/C (n = 23) | 3.3 (1–3.1) | a | |
CNS, central nervous system; CI, confidence interval; NA, insufficient number of events to calculate median; NR, not reached; OS, overall survival; osi, osimertinib.
Outcome data not evaluated. Time-to-event outcomes calculated using Kaplan-Meier analyses.
Within all groups of patients, whether divided into cohorts or considered together, patients with a leptomeningeal-only pattern of progression achieved prolonged duration of CNS disease control compared with parenchymal-only progression. Combining all cohorts, the leptomeningeal group (n = 27) had a median duration of CNS disease control of 6.0 months (95% CI, 5.1–9.0), whereas the parenchymal-only pattern of progression had 3.3 months (95% CI 1.0–3.1). Notably, patients in cohort B with CNS parenchymal-only versus leptomeningeal-only progression were more most often treated with CNS radiation [9 of 12, 75% (7 SRS, 2 WBRT) versus 5 of 11, 45% (2 SRS, 3 WBRT)].
Neurologic Function
Improvement in neurologic symptoms on osimertinib 160 mg was clinically documented by providers in 14 (20%) patients, including 5 of 24 (21%) patients in cohort A, 5 of 34 (15%) patients in cohort B, and 4 of 11 (36%) of patients in cohort C. Conversely, documentation of clinical worsening of neurologic symptoms despite osimertinib 160 mg was present in 10 (15%) patients.
Safety
Though adverse events were not prospectively graded according to the Common Terminology Criteria for Adverse Events for most patients included in this study, retrospective review revealed no reported grade 3 or higher toxicities with osimertinib 160 mg. All reported treatment-related toxicities were grade 1 or grade 2 and those occurring in more than one patient included diarrhea (grade 1, n = 12; grade 2, n = 2), xerosis (grade 1, n = 11), fatigue (grade 1, n = 8, grade 2, n = 1), rash (grade 1, n = 6), paronychia (grade 1, n = 5, grade 2, n = 2), cytopenia (grade 1, n = 4), anorexia (grade 1, n = 4), dysgeusia (grade 1, n = 1, grade 2, n = 2), and mucositis (grade 2, n = 2). There were no reports of radiation necrosis on osimertinib 160 mg.
Discussion
In this retrospective multicenter study, osimertinib dose escalation revealed modest clinical benefit among patients with EGFR-mutant NSCLC who developed CNS progression on osimertinib 80 mg daily with an acceptable safety profile.
Among 58 patients treated with an increase in osimertinib dose from 80 to 160 mg daily at the time of CNS progression, the median duration of CNS control was 3.8 months (95% CI, 1.7–5.8) in those without overlapping chemotherapy or radiation and 5.1 months (95% CI, 3.1–6.5) in those who were treated with chemotherapy and/or radiation in addition to osimertinib dose escalation. The 11 patients who had not been treated with any prior osimertinib until their 160 mg initiation also derived similar benefit, with a median duration of CNS control of 4.2 months (95% CI, 1.6–NR). Statistical power was limited by sample size and heterogeneous therapies, and direct comparisons of one cohort to another revealed no statistical differences. Nevertheless, trends suggested that the benefit of osimertinib dose escalation may be greatest for those with leptomeningeal-only CNS progression (ranging 6–7 mo) compared with those with parenchymal-only CNS progression (ranging 2–3 mo), in both cohorts A and B individually and in all cohorts combined. Notably, the combination of osimertinib dose escalation and chemotherapy conferred a duration of CNS control of 8.2 months (95% CI, 1.5–NR) among 10 patients in cohort B, nine of whom had leptomeningeal disease at the time of escalation. The median OS with osimertinib dose escalation ranged from 10.5 to 14.8 months, which is encouraging in this high-risk population. Osimertinib dose escalation was overall well-tolerated in this study with no grade 3 or greater adverse events reported.
In EGFR-mutant lung cancer, there have previously been retrospective studies and one prospective nonrandomized study evaluating the strategy of continuing TKI beyond progression with the addition of locally ablative therapy targeting sites of oligoprogression. These approaches have revealed an additional duration of disease control of approximately 6 to 16 months,15, 16, 17 are most often used in practice, and are included in the National Comprehensive Cancer Network lung cancer treatment guidelines.18 The strategy studied here, osimertinib dose escalation to 160 mg for CNS progression, is an analogous approach with a comparable magnitude of benefit. Our study contributes to the growing literature supporting consideration of dose escalation of osimertinib to 160 mg as a viable clinical strategy, especially for patients with leptomeningeal progression. Although treatment with platinum-pemetrexed regimens can provide some CNS benefit for chemotherapy-naive patients,5,6 osimertinib dose escalation remains a relevant addition to currently available therapies given that CNS progression on osimertinib is a challenging clinical scenario with limited active options.
Pulse-dose treatment strategies with earlier generation EGFR TKIs have been explored to improve CNS activity. The postulated mechanism of this approach is increasing the peripheral blood levels of drug, therefore increasing CNS penetration. Side effects of first- and second-generation EGFR TKIs, primarily mediated by EGFR wild-type inhibition, prohibited the possibility of simply raising the daily dose to achieve this effect. Pulsatile dosing was initially attempted with gefitinib19 and erlotinib,20, 21, 22 but suggested only transient clinical benefit. For example, the largest series reporting efficacy for pulse-dose erlotinib (1500 mg once weekly) was a retrospective study that included nine patients with either new CNS disease (three patients) or CNS progression on standard-dose EGFR TKI (six patients).20 Although the radiographic response rate to pulse-dose erlotinib was high at 67%, the median time to CNS progression was only 2.7 months. Pulse-dose afatinib was also tested in a phase I study at a variety of doses, but the study was not confined to EGFR-mutant cancers and CNS progression was an exclusion criterion for enrollment.23
Pulsatile dosing strategies have not been explored with osimertinib, but with decreased EGFR wild-type inhibition, osimertinib seems to be safe and generally tolerable at 160 mg daily.24 The phase I BLOOM study was the first prospective trial to assess osimertinib 160 mg in those with leptomeningeal progression after first- and second-generation EGFR TKIs.10 This study was performed before regulatory approval of osimertinib, and all patients were receiving osimertinib for the first time at 160 mg. The study had an encouraging median PFS of 8.6 months and median OS of 11.0 months; however, it remains unclear whether this benefit was due to the high-dose strategy or simply the first exposure to an active drug for the T790M resistance mechanism and with excellent CNS penetration. A recent retrospective study found no difference in outcomes between patients with leptomeningeal progression on earlier EGFR TKIs treated with osimertinib 80 mg or 160 mg,25 though patients in both groups received additional therapies (such as intrathecal chemotherapy and WBRT) and none of these patients underwent dose escalation. To our knowledge, only two prospective studies have assessed the efficacy of osimertinib dose escalation after CNS progression on a third-generation EGFR TKI.13,14 Importantly, in the study by Park et al.,14 although a subset of patients (33 of 80) were treated with osimertinib 160 mg after progression on a third-generation EGFR TKI, only 13 of 80 specifically underwent osimertinib dose escalation. In a subgroup analysis of a phase II study by Goldstein et al.,13 11 patients underwent dose escalation though only two had leptomeningeal disease at the time of CNS disease progression. The median PFS with dose escalation in these two prospective studies was 7.6 months (95% CI, 5–16.6 mo) and 4.3 (0.7–25.5 mo), respectively. Our multi-institutional experience describes a similar magnitude of benefit for 58 patients who underwent osimertinib dose escalation for CNS progression, contributing to the growing evidence for a clinical strategy not likely feasible for study with larger prospective trials. Of note, most patients treated with osimertinib in our study received it in the second-line or later setting for T790M-mediated acquired resistance (only 15 of 69 patients were treated with first-line osimertinib).
Historical studies suggested that T790M was rarely detected in the CSF of patients with CNS progression on first-generation EGFR TKIs, suggesting treatment failures were more likely related to pharmacokinetic limitations rather than genomic-acquired resistance, and therefore may be more responsive to dose increases.26, 27, 28 Whether the same trend holds with osimertinib 80 mg is not known, though growing evidence suggests that this may be the case given the substantial disparity between plasma and CSF osimertinib concentrations (CSF-to-free plasma ratio of 16%).10 The recent OCEAN study assessed blood concentrations of osimertinib and its metabolite, AZ5014, among 37 patients treated with osimertinib 80 mg and did not find a significant difference in CNS PFS among patients with “high” versus “low” plasma concentrations of AZ5014, though there was a trend toward longer CNS PFS in the “high” cohort (34.5 versus 19.8 mo, p = 0.197).29 CSF drug levels were tested in only seven patients in the OCEAN study; thus, it was unclear whether the CSF concentration of osimertinib correlated with CNS response. Previous studies have reported CSF penetration rates ranging from 2.5% to 31.7% with osimertinib 80 mg30,31 and 16% with osimertinib 160 mg10 with differences potentially attributable to calculation methods. In the APOLLO study of standard-dose osimertinib for patients with CNS progression on earlier generation EGFR TKIs, patients with intracranial responses had an overall higher median CSF “penetration rate” (36.5%) than those without a response (25.8%) (“penetration rate” was defined as the ratio of osimertinib CSF-to-plasma concentration, each measured simultaneously 6 ± 2 h after the last dose).31 Importantly, it is also unknown, and our study did not evaluate, whether dose escalation of osimertinib from 80 to 160 mg leads to a meaningful increase in drug levels in the CNS in individual patients.
In addition to drug levels, the CSF can provide insight into resistance mechanisms underlying CNS progression on osimertinib. Our retrospective study did not include CSF molecular analyses, but emerging data suggest that there may be increased frequency of MET amplification and on-target resistance mutations, such as C797S, when the CSF is sampled.32,33 Unlike those with pharmacokinetic failure, we hypothesize that patients who develop genomic resistance mechanisms within the CNS would be less likely to benefit from TKI dose escalation but may be candidates for combination TKI strategies targeting identified mechanisms of resistance, highlighting the potential role of molecular analysis of cerebrospinal fluid, when available, in the clinic.
In this context, the results of our study were encouraging but should be interpreted with caution. Our retrospective study design yielded a heterogeneous group of patients from eight academic medical centers, making direct comparisons across the entire cohort challenging. In addition, responses were clinically assessed by investigators at each site rather than using a formal system such as the Response Evaluation Criteria In Solid Tumors. Assessing for radiographic response is particularly challenging in CNS disease, and criteria developed for this purpose—such as RANO-LM34—warrant further prospective validation.
In conclusion, we have revealed that osimertinib dose escalation from 80 mg daily to 160 mg daily had modest clinical prolongation of CNS control in patients with CNS progression on osimertinib. The benefit is approximately 3 to 6 months, on par with locally ablative therapy to oligoprogressing sites in EGFR mutation-positive patients. Given the limited number of effective therapeutic options for patients who develop CNS progression on osimertinib, this may be a reasonable clinical option especially for those with leptomeningeal progression, though further prospective study of osimertinib dose escalation should be pursued.
CRediT Authorship Contribution Statement
AJ Piper-Vallillo: Writing—Original draft, Conceptualization, Methodology, Data curation, Formal analysis.
Julia K. Rotow: Investigation, Writing—Review and editing.
Jacqueline V. Aredo: Investigation, Writing—Review and editing.
Khvaramze Shaverdashvilli: Investigation, Writing—Review and editing.
J. Luo: Investigation, Writing—Review and editing.
J. Carlisle: Investigation, Writing—Review and editing.
H. Husain: Investigation, Writing—Review and editing.
A. Muzikansky: Formal analysis.
R. Heist: Investigation, Writing—Review and editing.
D. Rangachari: Investigation, Writing—Review and editing.
Suresh S. Ramalingam: Investigation, Writing—Review and editing.
Heather A. Wakelee: Investigation, Writing—Review and editing.
Helena A. Yu: Investigation, Writing—Review and editing.
Lecia Sequist: Writing—Review and editing, Conceptualization, Methodology, Supervision.
Joshua M. Bauml: Investigation, Writing—Review and editing.
Joel W. Neal: Investigation, Writing—Review and editing.
Zofia Piotrowska: Writing—Review and editing, Conceptualization, Methodology, Supervision.
Footnotes
Dr. Bauml is now employed by Janssen R&D.
Disclosure: Dr. Piper-Vallillo has received honoraria from Sanofi-Genzyme. Dr. Rotow has served in a consulting or advisory role for AstraZeneca, AbbVie, Gritstone, Lilly, Takeda, and Sanofi-Genzyme and has received honoraria from AstraZeneca, Pfizer, Merck, Janssen, Sanofi-Genzyme, Jazz Pharmaceuticals, and Lilly. Dr. Aredo has received honoraria from Targeted Oncology and Physicians’ Education Resource. Dr. Luo has received honoraria from Targeted Oncology and Physicians’ Education Resource. Dr. Husain has served as a compensated consultant or received honoraria from Foundation Medicine, Blueprint Medicines, Takeda, AstraZeneca, Phillips, Boehringer Ingelheim, Mirati, and Turning Point Therapeutics and received institutional research support from Roche Sequencing Solutions, Eli Lilly Oncology, Pfizer, and Bristol Myers Squibb. Dr. Heist has served as a compensated consultant or received honoraria from AbbVie, Novartis, EMD Serono, and Daichii Sankyo and received institutional research support from Novartis, Genentech/Roche, Corvus, Incyte, Exelixis, AbbVie, Daichii Sankyo, Agios, Mirati, Turning Point, and Eli Lilly. Dr. Rangachari reported receiving personal fees from Advance Medical Expert, DynaMed, and AstraZeneca and institutional research support from AbbVie/Stemcentrx, Novocure, and Bristol Myers Squibb outside the submitted work. Dr. Ramalingam received honoraria for consulting from Amgen, Bristol Myers Squibb, Genentech/Roche, Merck, AstraZeneca, Takeda, Eisai, Daiichi Sankyo, Sanofi, GlaxoSmithKline, and Eli Lilly; and grants from Merck, AstraZeneca, Advaxis, Bristol Myers Squibb, Amgen, Takeda, Genmab, and GlaxoSmithKline. Dr. Wakelee receives institutional research support from ACEA Biosciences, Arrys Therapeutics, AstraZeneca/Medimmune, Bristol Myers Squibb, Celgene, Clovis Oncology, Exelixis, Genentech/Roche, Gilead, Merck, Novartis, Pharmacyclics, Sea Gen, and Xcovery; serves on data safety monitoring or advisory boards for AstraZeneca, Xcovery, Janssen, Daiichi Sankyo, Blueprint, Mirati, Helsinn, Merck (not compensated), and Genentech/Roche (not compensated); and serves in a leadership role for IASLC and ECOG-ACRIN. Dr. Yu has consulted for AstraZeneca, Blueprint Medicine, Black Diamond, Janssen Oncology, Cullinan Oncology, C4 Therapeutics, and Daiichi; and received research funding through her institution for clinical trials from AstraZeneca, Daiichi, Pfizer, Novartis, Cullinan Oncology, Lilly, Janssen Oncology, ERASCA, and Blueprint Medicine. Dr. Sequist has served as a compensated consultant or received honoraria from AstraZeneca, Janssen, Pfizer, Takeda, and Genentech; and received institutional research support from Novartis, AstraZeneca, Boehringer Ingelheim, Genentech, Blueprint Medicines, and LOXO. Dr. Bauml has received consulting honoraria from Clovis, Bristol Myers Squibb, AstraZeneca, Celgene, Boehringer Ingelheim, Janssen, Merck, Guardant Health, Genentech, Takeda, Ayala, Regeneron, Inivata, Novartis; and has received institutional research support from Merck, Clovis, Carevive Systems, Novartis, Bayer, Janssen, Astra Zeneca, Takeda, and Carisma Therapeutics. Dr. Neal received grants and personal fees from Takeda during the conduct of the study; personal fees from AstraZeneca, Jounce Therapeutics, Eli Lilly and Company, Calithera Biosciences, Amgen, Iovance Biotherapeutics, Blueprint Pharmaceuticals, Regeneron Pharmaceuticals, and Natera; grants, personal fees, and nonfinancial support from Genentech/Roche and Exelixis; and grants and nonfinancial support from Merck, Novartis, Boehringer Ingelheim, Nektar Therapeutics, Adaptimmune, GlaxoSmithKline, Janssen, and AbbVie, outside of the submitted work. Dr. Piotrowska has served as a compensated consultant or received honoraria from AstraZeneca, Takeda, AbbVie, Novartis, Guardant Health, Spectrum, Genentech, C4 Therapeutics, Eli Lilly, InCyte, Blueprint Medicines, Jazz Pharmaceutics, Janssen, Daiichi Sankyo, and Cullinan; receiving research support (to institution) from Novartis, ARIAD/Takeda, Spectrum, AstraZeneca, Tesaro, Cullinan, Daiichi Sankyo, AbbVie, Janssen, and Blueprint Medicines; and received travel reimbursement from AstraZeneca and ARIAD/Takeda. The remaining authors declare no conflicts of interest.
This study was previously presented as a poster at ASCO 2020 and during the General Session of the EGFR Resisters Conference 2020.
Cite this article as: Piper-Vallillo AJ, Rotow JK, Aredo JV, et al. High-dose osimertinib for CNS progression in EGFR+ NSCLC: a multi-institutional experience. JTO Clin Res Rep. 2022;3:100328.
References
- 1.Ramalingam S.S., Vansteenkiste J., Planchard D., et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2019;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 2.YJWYZ B.P., Yates J.W., Yang Z., et al. Pre-clinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 3.Soria J.C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 4.Reungwetwattana T., Nakagawa K., Cho B.C., et al. CNS response to osimertinib versus Standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol. 2018;36:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 5.Yu X., Fan Y. Effect of pemetrexed on brain metastases from nonsmall cell lung cancer with wild-type and unknown EGFR status. Medicine. 2019;98 doi: 10.1097/MD.0000000000014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bearz A., Garassino I., Tiseo M., et al. Activity of pemetrexed on brain metastases from non-small cell lung cancer. Lung Cancer. 2010;68:264–268. doi: 10.1016/j.lungcan.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 7.De Mattos-Arruda L., Mayor R., Ng C.K.Y., et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. doi: 10.1038/ncomms9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlisle J.W., Ramalingam S.S. Improving outcomes for brain metastases in EGFR mutated NSCLC. Transl Lung Cancer Res. 2019;8:S355–S359. doi: 10.21037/tlcr.2019.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper-Vallillo A.J., Sequist L.V., Piotrowska Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: a review. J Clin Oncol. 2020 doi: 10.1200/JCO.19.03123. 0:JCO.19.03123. [DOI] [PubMed] [Google Scholar]
- 10.Yang J.C.H., Kim S.-W., Kim D.-W., et al. Osimertinib in patients with epidermal growth factor receptor mutation–positive non–small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38:538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang M.W.-K. Osimertinib 160 mg daily for advanced non-small cell lung cancer with leptomeningeal metastasis: a case report. Asia Pac J Clin Oncol. 2019;15(suppl 6):5–7. doi: 10.1111/ajco.13246. [DOI] [PubMed] [Google Scholar]
- 12.Cordova C., Chi A.S., Chachoua A., et al. Osimertinib dose escalation induces regression of progressive EGFR T790M-mutant leptomeningeal lung adenocarcinoma. J Thorac Oncol. 2017;12:e188–e190. doi: 10.1016/j.jtho.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein I.M., Roisman L.C., Keren-Rosenberg S., et al. Dose escalation of osimertinib for intracranial progression in EGFR mutated non-small-cell lung cancer with brain metastases. Neurooncol Adv. 2020;2:vdaa125. doi: 10.1093/noajnl/vdaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S., Lee M.H., Seong M., et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol. 2020;31:1397–1404. doi: 10.1016/j.annonc.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Weickhardt A.J., Scheier B., Burke J.M., et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park K., Yu C.J., Kim S.W., et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol. 2016;2:305–312. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 17.Le X., Puri S., Negrao M.V., et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018;24:6195–6203. doi: 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network Non-Small Cell Lung Cancer. 4.2021 version, Principles of Radiation Therapy. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 19.Jackman D.M., Cioffredi L.A., Jacobs L., et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget. 2015;6:4527–4536. doi: 10.18632/oncotarget.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grommes C., Oxnard G.R., Kris M.G., et al. Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant nonsmall cell lung cancer. Neurooncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura T., Hata A., Takeshita J., et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol. 2015;75:1261–1266. doi: 10.1007/s00280-015-2759-y. [DOI] [PubMed] [Google Scholar]
- 22.How J., Mann J., Laczniak A.N., Baggstrom M.Q. Pulsatile erlotinib in EGFR-positive non–small-cell lung cancer patients with leptomeningeal and brain metastases: review of the literature. Clin Lung Cancer. 2017;18:354–363. doi: 10.1016/j.cllc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Camidge D.R., Sequist L.V., Jänne P.A., et al. Phase Ib study of high-dose intermittent afatinib in patients with advanced solid tumors. Clin Lung Cancer. 2018;19:e655–e665. doi: 10.1016/j.cllc.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Jänne P.A., Yang J.C.-H., Kim D.-W., et al. AZD9291 in EGFR inhibitor–resistant non–small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 25.Lee J., Choi Y.L., Han J., et al. Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol. 2020;15:1758–1766. doi: 10.1016/j.jtho.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Hata A., Katakami N., Yoshioka H., et al. Spatiotemporal T790M heterogeneity in individual patients with EGFR-mutant non-small-cell lung cancer after acquired resistance to EGFR-TKI. J Thorac Oncol. 2015;10:1553–1559. doi: 10.1097/JTO.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki S., Yoshioka Y., Ko R., et al. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefitinib therapy failure. Respir Investig. 2016;54:14–19. doi: 10.1016/j.resinv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J., Ye X., Xu Y., et al. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol. 2016;78:1305–1310. doi: 10.1007/s00280-016-3155-y. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi H., Wakuda K., Fukuda M., et al. A phase II study of osimertinib for radiotherapy-naive central nervous system metastasis from NSCLC: results for the T790M cohort of the OCEAN study (LOGIK1603/WJOG9116L) J Thorac Oncol. 2021;16:2121–2132. doi: 10.1016/j.jtho.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Nanjo S., Hata A., Okuda C., et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118:32–37. doi: 10.1038/bjc.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing L., Pan Y., Shi Y., et al. Biomarkers of osimertinib response in patients with refractory, EGFR-T790M-positive non-small cell lung cancer and central nervous system metastases: the Apollo study. Clin Cancer Res. 2020;26:6168–6175. doi: 10.1158/1078-0432.CCR-20-2081. [DOI] [PubMed] [Google Scholar]
- 32.Li Y.S., Jiang B.Y., Yang J.J., et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol. 2018;29:945–952. doi: 10.1093/annonc/mdy009. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Zhang R., Zhou Y., et al. Combined use of crizotinib and gefitinib in advanced lung adenocarcinoma with leptomeningeal metastases harboring MET amplification after the development of gefitinib resistance: a case report and literature review. Clin Lung Cancer. 2019;20:e251–e255. doi: 10.1016/j.cllc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlain M., Junck L., Brandsma D., et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neurooncol. 2017;19:484–492. doi: 10.1093/neuonc/now183. [DOI] [PMC free article] [PubMed] [Google Scholar]