Abstract
Introduction
Achalasia with megaesophagus is a pathology characterized by widespread and irregular dilation of the esophageal lumen. In most cases, this dilation is caused by contraction and subsequent failed relaxation of the lower esophageal sphincter (LES). It may be associated with a partial or complete slowing of the esophageal peristalsis.
Case overview
We present the case of a 58-year-old woman who developed dysphagia, regurgitation, and substantial weight loss (11 kg) over a span of 1 year. Symptomatic achalasia with megaesophagus was diagnosed following chest and abdominal computed tomography (CT) with contrast and transit RX with gastrografin and esophageal manometry. The patient refuse all minimally endoscopic treatments and opted straightly for the treatment with esophagectomy sec. Ivor–Lewis. At the 6-month follow-up, the patient appeared in excellent general clinical condition and oral gastrografin radiography (OGR) showed good channeling.
Discussion
Patients require medical attention when presenting with achalasia that has eroded the esophageal wall enough to form a megaesophagus. Early and minimally invasive treatments (i.e., medical therapy, endoscopic dilation, and myotomy) are insufficient at this stage, and thus esophageal surgery is required. Among the most common surgical approaches, we must mention esophagectomy sec. McKeown and esophagectomy with interposition of a colic loop sec. Wilkins; however, based on our experience, esophagectomy sec. Ivor–Lewis with intrathoracic anastomosis leads to excellent results and can therefore be considered a valid alternative for treating complex cases.
Conclusions
Subtotal esophagectomy sec. Ivor–Lewis with intrathoracic anastomosis is effective in treating achalasia with megaesophagus.
Keywords: Achalasia, Megaesophagus, LES, Dysphagia, Esophagectomy
Highlights
-
•
Achalasia with megaesophagus is a pathology characterized by widespread and irregular dilation of the esophageal lumen.
-
•
Dilation is caused by contraction and subsequent failed relaxation of the lower esophageal sphincter (LES).
-
•
Psychological disorders are well-known in achalasia patients.
-
•
Depression and frustration may influence patient's decision.
-
•
Esophageal resection sec. Ivor–Lewis may be considered for the treatment of megaesophagus.
-
•
Subtotal esophagectomy sec. Ivor–Lewis with intrathoracic anastomosis is effective in treating achalasia with megaesophagus.
1. Introduction
Megaesophagus is a rare disease with an annual incidence of 1 in 100,000 people and an equal distribution in both sexes. It consists of irregular dilation of the esophagus and excessive slowing of peristalsis. It can be present at birth or can result from certain diseases, most frequently endocrine, neuromuscular, and neoplastic. The most widely reported cause of megaesophagus is achalasia. Achalasia is a functional pathology of the esophagus in which the vagal bundles of the esophageal myenteric plexus cause spastic contraction of the lower esophageal sphincter (LES), first causing solid food dysphagia, coughing, vomiting, and weight loss, then exhaustion of the longitudinal muscles and formation of a megaesophagus.
Furthermore, esophageal dilation can cause retrosternal pain; in severe cases, the negative pressure in the chest cavity can cause reflux into the esophagus, causing an alimentary bolus to move from the stomach into the esophagus, which can lead to esophagitis and Barrett's esophagus with eventual neoplastic degeneration [1].
The diagnosis of megaesophagus is radiological and the gold standard is computed tomography (CT) with contrast. However, transit RX with gastrographin can also be useful, with examination conducted in orthostasis. An esophagogastroduodenoscopy (EGDS) is always recommended pre-surgery to provide morphological staging, finally is important to perform an esophageal manometry. For purposes of classification, a histological analysis should be performed to investigate possible degeneration of the esophageal myenteric plexus ganglion cells, which may evolve to fibrosis due to continuous inflammation. In recent years, prevention has become fundamental in avoiding the development of megaesophagus. The most common preventative methods include pharmacological or minimally invasive treatment of achalasia in its initial stages. Calcium antagonists and long-acting nitrates are the most used drugs. Botulinum toxin applied endoscopically on the LES has also provided excellent results. Minimally invasive procedures include pneumatic dilation and peroral endoscopic myotomy. Esophagectomy is indicated in cases of late diagnosis or recurrence following a minimally invasive treatment.
2. Case Overview
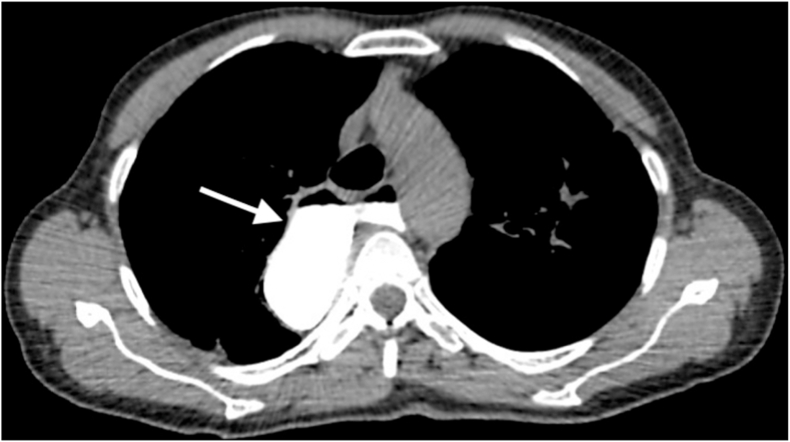
This case presents a 58-year-old woman who was a married housewife with asymptomatic history. Her genetic and family history was negative for major diseases. At the time of hospitalization, she did not declare the use of any drugs and declared that she had always been in apparent good health. For about a year, she complained of difficulty eating with dysphagia, coughing, vomiting, significant weight loss (11 kg), and halitosis. During her hospitalization, a chest CT with contrast enhancement was performed, which revealed widespread, irregular dilation of the esophagus and the presence of food material that lodged in the esophageal lumen (Fig. 1). Oral gastrografin radiography (OGR) highlighted slowed esophageal motility and an accumulation of contrast medium at the level of the LES with an obvious obstacle to transit (Fig. 2). The esophageal manometry showed a hypertone of LES and a peristalsis with synchronous waves of low amplitude and not propagated. The EGDS, conducted with a standard-sized Olympus radial gastroscope, showed irregular esophageal dilation with irritated mucosa; several random biopsies were taken. The discovery of an abundant alimentary bolus stagnating in the esophagus required interruption of the examination; the LES could not be crossed due to excessive contraction. After the procedure the endoscopic treatments was proposed as dilatations and infiltration of botulinum toxin [2]. The patient refused the treatments proposed probably for psychological reasons and long suffering [3].
Fig. 1.
Preoperative chest CT with gastrografin revealed the megaesophagus (arrow).
Fig. 2.
Preoperative oral gastrografin radiography highlighted a dilated esophagus (arrow).
Complete blood tests were also performed during the study phase and showed evidence of iron deficiency and chronic inflammation with high levels of white blood cells and polymerase-chain reaction (PCR).
Following completion of the study phase, the patient underwent esophagectomy sec. Ivor–Lewis. The abdominal phase was performed via xifo-subumbilical median laparotomy with mobilization of the hepatic flexure, Kocherization of the stomach, isolation and section of the left gastric artery. Cholecystectomy and tubulization of the stomach using a 60-mm GIA mechanical parenchymal stapler were also performed. The thoracic phase was performed via posterolateral thoracotomy with isolation of the esophagus, resection of the thoracic portion of the megaesophagus, and fixation of the gastroesophageal anastomosis using a mechanical stapler (EEA 25 mm) above the azygos vein.
The patient had a regular course of recovery; however, on the day 15 post-surgery, when the patient was switched to a semisolid diet, loss of food bolus from the pleural drainage tubes was observed. She then underwent a re-thoracotomy with detection of the fistula, closure of the leak, and affixation of a muscle flap from the intercostal muscle to protect the anastomosis. The patient then had a regular post-surgical course. A chest CT with contrast enhancement and gastrografin after 10 days from surgery (Fig. 3) showed no spreading of contrast.
Fig. 3.
Postoperative chest CT with gastrografin showed the gastric tubulization (arrow).
During the hospitalization, the patient was well-nourished and satisfied with a semisolid diet. At the 3-month follow-up, the patient was in good health and the esophageal lumen was patent with no sign of dysphagia and no specific symptoms. At the 6-month follow-up, RX transit with gastrografin revealed excellent channeling (Fig. 4).
Fig. 4.
Oral dynamic transit after 6 months from post-surgery showing adequate channeling of the esophagus (arrow).
3. Discussion
There are numerous preventive surgical, medical, or endoscopic treatments for achalasia, such as endoscopic pneumatic dilation of the LES. Surgical procedures are considered only after previous therapeutic attempts or when the disease has reached an advanced stage. The most described surgical procedure in the literature is laparoscopic Heller myotomy, which represents the referenced surgical treatment for achalasia [4,5]. Extra-mucous myotomy and fundoplication sec. Dohr have also shown excellent results in cases of megaesophagus; however, they do not prevent recurrence of the disease in all cases. In addition, subtotal esophagectomy sec. McKeown with transjatal approach and cervical anastomosis has shown excellent results. In a few, select cases, more invasive surgical techniques may be used, such as the subtotal esophagectomy sec. Ivor–Lewis with intrathoracic anastomosis [6,7]. This method, while retaining a small portion of dilated esophagus in the cervical area, represents an excellent alternative for treating the most complex cases of megaesophagus; because the obstacle of the LES is removed through the procedure, transit is perfectly restored. Finally, in highly complicated cases in which the megaesophagus appears widely dilated, total esophagectomy and reconstruction can be performed through the interposition of a colic loop sec. Wilkins. However, given the complexity of this procedure and the high degree of complications, this treatment is reserved for the small proportion of cases that are refractory to endoscopic and minimally invasive medical treatments [[8], [9], [10]]. Moreover, it is already known that patients affected by achalasia shown psychological disorders, as depression and frustration this aspect may represents a limitation in the relationship between doctor and patient [7,8]. In our case, the patient probably for psychological reasons refused all medical treatment and minimally invasive approaches and decided to undergo straightly for major surgical intervention. In this case, disturbances of the psychic sphere have led the patient to undergo a major surgical procedure at first glance, thus precluding minimally invasive treatments. At the six-month check, the patient was found to be in excellent physical and mental condition and in the absence of signs of recurrence. This shows that surgery in this type of patient can represent a valid alternative to medical or miinvasive therapy even in the first instance. This work has been reported in line with the SCARE criteria [9] and is compliant with the PROCESS Guidelines [11].
4. Conclusions
In select cases where previous treatments have failed or where proper prevention has not been carried out, esophagectomy is the only viable route. Subtotal esophagectomy sec. Ivor–Lewis, while leaving a piece of dilated esophagus in the cervical portion, is an excellent method because it removes the obstacle to transit at the level of the LES, resulting in perfect channeling.
Declarations
-
•
Patients signed an informed consent for the publication of the manuscript.
-
•
None of the Authors have any competing interests.
-
•
The idea for the manuscript was conceived by BA and LFR and was further developed by BA and LFZR. BA and LFZR wrote the first draft of the manuscript. BA, FS, DA, AC have been involved in surgery and tissue collection. FS and BA reviewed the manuscript and were involved in its critical revision before submission. All authors have read and approved the final manuscript.
-
•
Acknowledgements: We thank Prozetesis.org for the support.
Conflict of interest
No conflicts of interest to declare.
Funding
No funding.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. Please see consent section in instructions to authors for further information.
Guarantor
Prof. Franco Stella. Dr Beatrice Aramini.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103630.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Leeuwenburgh I., Haringsma J., Van Dekken H., Scholten P., Siersema P.D., Kuipers E.J. Long-term risk of esophagitis, Barrett's oesophagus and oesophageal cancer in achalasia patients. Scand. J. Gastroenterol. Suppl. 2006;(243):7–10. doi: 10.1080/00365520600664201. PMID: 16782616. [DOI] [PubMed] [Google Scholar]
- 2.Zaninotto G., Bennett C., Boeckxstaens G., Costantini M., Ferguson M.K., Pandolfino J.E., Patti M.G., Ribeiro U., Jr., Richter J., Swanstrom L., Tack J., Triadafilopoulos G., Markar S.R., Salvador R., Faccio L., Andreollo N.A., Cecconello I., Costamagna G., da Rocha J.R.M., Hungness E.S., Fisichella P.M., Fuchs K.H., Gockel I., Gurski R., Gyawali C.P., Herbella F.A.M., Holloway R.H., Hongo M., Jobe B.A., Kahrilas P.J., Katzka D.A., Dua K.S., Liu D., Moonen A., Nasi A., Pasricha P.J., Penagini R., Perretta S., Sallum R.A.A., Sarnelli G., Savarino E., Schlottmann F., Sifrim D., Soper N., Tatum R.P., Vaezi M.F., van Herwaarden-Lindeboom M., Vanuytsel T., Vela M.F., Watson D.I., Zerbib F., Gittens S., Pontillo C., Vermigli S., Inama D., Low D.E. The 2018 ISDE achalasia guidelines. Dis. Esophagus. 2018 Sep 1;31(9) doi: 10.1093/dote/doy071. PMID: 30169645. [DOI] [PubMed] [Google Scholar]
- 3.Loosen S.H., Kandler J., Luedde T., Kostev K., Roderburg C. Achalasia is associated with a higher incidence of depression in outpatients in Germany. PLoS One. 2021 Apr 30;16(4) doi: 10.1371/journal.pone.0250503. PMID: 33930060; PMCID: PMC8087033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweet M.P., Nipomnick I., Gasper W.J., Bagatelos K., Ostroff J.W., Fisichella P.M., Way L.W., Patti M.G. The outcome of laparoscopic Heller myotomy for achalasia is not influenced by the degree of esophageal dilatation. J. Gastrointest. Surg. 2008 Jan;12(1):159–165. doi: 10.1007/s11605-007-0275-z. Epub 2007 Aug 21. PMID: 17710504. [DOI] [PubMed] [Google Scholar]
- 5.El Hak N.G., Hamdy E., Abdalla T., Kandel T., El Raof A.A., El Hemaly M., Salah T., El Hanafy E. Laparoscopic Heller myotomy for achalasia: analysis of successes and failures. Hepato-Gastroenterology. 2012 Jul-Aug;59(117):1450–1454. doi: 10.5754/hge10060. PMID: 22683961. [DOI] [PubMed] [Google Scholar]
- 6.Gockel I., Eckardt V.F., Roth W., Junginger T. Dolichomegaösophagus bei Achalasie. Therapie durch Osophagusresektion bei einer alten Patientin [Dolichomegaesophagus in achalasia. Therapy by esophogectomy in an aged patient] Dtsch. Med. Wochenschr. 2004 Apr 2;129(14):735–738. doi: 10.1055/s-2004-821378. German, PMID: 15042488. [DOI] [PubMed] [Google Scholar]
- 7.Gockel I., Kneist W., Eckardt V.F., Oberholzer K., Junginger T. Subtotal esophageal resection in motility disorders of the esophagus. Dig. Dis. 2004;22(4):396–401. doi: 10.1159/000083605. PMID: 15812166. [DOI] [PubMed] [Google Scholar]
- 8.Peters J.H., Kauer W.K., Crookes P.F., Ireland A.P., Bremner C.G., DeMeester T.R. Esophageal resection with colon interposition for end-stage achalasia. Arch. Surg. 1995 Jun;130(6):632–636. doi: 10.1001/archsurg.1995.01430060070013. discussion 636-7, PMID: 7763172. [DOI] [PubMed] [Google Scholar]
- 9.Waters P.F., Pearson F.G., Todd T.R., Patterson G.A., Goldberg M., Ginsberg R.J., Cooper J.D., Ramirez J., Miller L. Esophagectomy for complex benign esophageal disease. J. Thorac. Cardiovasc. Surg. 1988 Mar;95(3):378–381. PMID: 3343848. [PubMed] [Google Scholar]
- 10.Hsu H.S., Wang C.Y., Hsieh C.C., Huang M.H. Short-segment colon interposition for end-stage achalasia. Ann. Thorac. Surg. 2003 Nov;76(5):1706–1710. doi: 10.1016/s0003-4975(03)01019-1. PMID: 14602317. [DOI] [PubMed] [Google Scholar]
- 11.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.