Abstract
Secretion of Immunoglobulin A (sIgA) and lactoferrin is a nutrient content in breast milk that can increase immunity in preventing infectious diseases such as Acute Respiratory Infection (ARI). This research aims to determine the role of exclusive breastfeeding (EBF) on sIgA and Lactoferrin levels in toddlers suffering from ARI. A cross-sectional study was conducted on 124 toddlers under five from January–July 2021. Toddlers were selected using a purposive technique sampling from three Community Health Centers in Central Jakarta. Data were analyzed using t-test and ANOVA. The history of immunization, EBF, and frequency of ARI was significantly associated with levels of sIgA and lactoferrin. Parity only correlated with sIgA but not with lactoferrin levels. Maternal and toddlers' age, gender, and nutritional status were not significantly related to sIgA and lactoferrin levels. There is a significant (p-value <0.001) difference in the mean protein sIgA and lactoferrin levels in toddlers who were given EBF with ARI frequency <2 times and toddlers who were given EBF with ARI frequency ≥2 times. Toddlers who were exclusively breastfed with ARI frequency <2 times had higher levels of sIgA and lactoferrin (188901.77 pg/ml and 262.32 ng/ml, respectively) compared to infants given EBF with ARI frequency ≥2 times (136683.47 pg/ml and 181.49 ng/ml, respectively). History of immunization was also significantly (p-value <0.05) associated with levels of sIgA and lactoferrin in infants with ARI. The content of sIgA and lactoferrin in breast milk and immunization can increase the body's immune system in toddlers suffering from ARI.
Keywords: Exclusive breastfeeding, Acute respiratory infection (ARI), Immunization, sIgA, Lactoferrin
Highlights
-
•
Breast milk contains sIg A and lactoferrin, which act as antibodies to increase immunity in toddlers suffering from ARI.
-
•
Exclusive breastfeeding significantly increased sIgA and lactoferrin levels in toddlers suffering from ARI.
-
•
Toddlers who are exclusively breastfed with ARI frequency < two times have higher levels of sIga A and lactoferrin than infants who are not exclusively breastfed and suffer from ARI ≥ two times.
1. Introduction
Breastfeeding can save the lives of more than 820.000 children under the age of five, every year and prevent breast and ovarian cancer and diabetes for mothers. Data collected from 129 countries, only 23 countries reached 60% coverage of EBF in 2017. The highest coverage is on the African continent and followed by the Asian continent in the second position [1]. The performance report of the Ministry of Health of the Republic of Indonesia in 2020 shows that the coverage of EBF in Indonesia is 66.1%. Increasing the coverage of exclusive breastfeeding is intended as an effort to overcome malnutrition in toddlers [2]. Exclusive breastfeeding is the best food needed by babies in the early period of a baby's life because it contains complete nutrients and various antibodies that can prevent various infectious diseases including ARI where this infectious disease is one of the leading causes of child morbidity and mortality the worldwide, including Indonesia [3,4]. The failure of exclusive breastfeeding causes the formation of immunity that is not optimal in toddlers so that they are vulnerable to the occurrence of ARI [5]. The prevalence of ARI among children under five in Indonesia is 12.8% [6].
The role of exclusive breastfeeding in preventing ARI has been widely studied [3,4]. Infants who are EBF are infants aged 0 months–5 months 29 days who are EBF without other food or liquids except for drugs, vitamins, and minerals. To improve nutrition, health, and child development, WHO recommends continued breastfeeding until the baby is 2 (two) years old with additional complementary foods (weaning food) [7]. However, there are still many mothers who fail to provide exclusive breastfeeding due to the lack of mother's intention, and low support from the family [4]. More than half of mothers who are successful in exclusive breastfeeding have an intention to breastfeed since the period of pregnancy and received strong support from their families [8]. For working mothers, the availability of breastfeeding rooms equipped with adequate facilities and policies that support breastfeeding in the workplace are the main factors in the success of exclusive breastfeeding [9]. In addition, mother's knowledge about the benefits of breastfeeding as an effort to prevent the occurrence of infectious diseases including ARI is also still lacking [10].
EBF is a protective factor against the incidence of ARI and various diseases because breast milk contains various substances that increase the baby's immunity [11,12]. Breast milk contains antibodies (immunoglobulins) and non-antibody antibacterial protection. Secretory IgA (sIgA) is an immunoglobulin that plays a critical role in the biological specificity of breast milk in infants. Lactoferrin (LF) is a non-antibody factor in breast milk that protects the infant against bacterial infection [13]. SIgA is an antibody that effectively coats the intestinal mucosa and prevents the entry of microorganisms into the tissue [14].
SIgA is the main immunoglobulin in all human secretions. This immunoglobulin gave initial bolus supplementing immunoglobulins transferred early across the placenta to the fetus. SIgA synthesis, during the lactation period, is stored in the breast, which reaches a higher and then decreases in mature milk. IgA levels are highest in colostrum (5 mg/ml), and gradually decrease to about 1 mg/ml during breastfeeding. The estimated mean infant IgA intake is 125 mg/kg/day at 1 month and 75 mg/kg/day at 4 months. Interleukin-6 in breast milk is partly responsible for the genesis of IgA and IgM-producing cells in the mammary gland. SIgA synthesis is intended to form the secretory immune system, which is carried out through gut-associated lymphoid tissue (GALT) or bronchus-associated lymphoid tissue (BALT). This pathway leads to the development of lymphoid cells in the mammary glands, which produce IgA. Antibodies after exposure to certain microbes or environments antigens in the gut or respiratory mucosa. When a mother earns more breast milk, the baby receives more sIgA so that the total level of sIgA that the baby receives during breastfeeding increases. Mothers of infants with systemic infection and poor suckling have higher IgA levels in their breastmilk [15,16]. A study by Rustam et al., in 2019 in Riau Province, Indonesia, reported that infants who did not receive exclusive breastfeeding had a risk of about 1.7 times increasing the incidence of ARI compared to infants who exclusively breastfed after being controlled by smokers at home and immunization variables [16]. Another study also showed that babies who were weaned early could increase the risk of ARI, where babies who were weaned early had a 2.7 times risk of suffering from ARI compared to babies who were weaned according to their age [17].
Lactoferrin found in breast milk is produced in the epithelial cells of the mammary gland and is secreted into milk. Once ingested by the infant, lactoferrin provides immune protection during early life. Lactoferrin (LF) is a non-antibody factor in breast milk that promotes intestinal epithelial growth and has a bactericidal effect by retaining iron from iron-requiring pathogens. LF contained in breast milk contains more than six hundred amino acids that have antibacterial, antifungal, antiviral, antiparasitic, anti-inflammatory, and immunomodulatory activity [18]. Availability of lactoferrin is abundant in breast milk, where levels are associated with the lactation stage. Colostrum contains 5–7 g/L lactoferrin, which gradually decreases over time. Infants aged 1 month consume about 260 mg/kg/day lactoferrin and at 4 months about 125 mg/kg/day. LF is distributed in saliva and all other secretions that wet mucous membranes such as the upper respiratory tract [19]. Lactoferrin synergistically with IgA readily absorbs enteric iron and thus prevents pathogenic organisms [14]. Lactoferrin, directly and indirectly, protects neonates against infections caused by bacteria and other microorganisms [19]. Breastfeeding can also confer long-term protection by stimulating an active immune response. A case-control study showed that a longer duration of breastfeeding may increase protective factors against respiratory tract infections [20]. Studies conducted by Alicia et al. on infants in Argentina, showed that the role of lactoferrin is synergistic with sIgA. The levels of these two compounds in breast milk were significantly associated with the experience of symptoms of the disease in the early period of life. Infants who suffer from ARI and are exclusively breastfed have higher levels of lactoferrin and sIgA than infants with ARI who are not exclusively breastfed [21].
Scientific evidence shows that exclusive breastfeeding can significantly increase the baby's immunity, thereby reducing the risk of infectious diseases including ARI. Breastfeeding is beneficial in infancy and early childhood concerning certain respiratory and gastrointestinal diseases, with a decreased incidence of otitis media from infancy to 4 years of age [22,23]. Oktaria et al. reported the results of an observational study that infants who were not EBF were at risk of developing respiratory tract infections compared to infants who were EBF [5]. Breastfeeding actively stimulates and directs the immune response of breastfed babies. Vaccine response to oral poliovirus vaccine and parenteral tetanus and diphtheria toxoid vaccine is enhanced by breastfeeding, with formula-fed infants sometimes exhibiting lower levels of antibodies to their immunizations [16]. Acute Respiratory Tract Infection (ARI) has a direct impact on toddlers' malnutrition [24]. The role of exclusive breastfeeding for toddlers who experience ARI requires strategic efforts based on scientific evidence to increase the coverage of exclusive breastfeeding to prevent other health impacts that can inhibit the growth and growth of toddlers. The burden of health costs due to the treatment of infectious diseases can be reduced. In addition, exclusive breastfeeding for toddlers is a strategic effort in preparing healthy and quality human resources for the future [25,26].
From the description above, this study was conducted to determine the role of EBF on sIgA and Lactoferrin Levels in Toddlers suffering from ARI.
2. Materials and methods
2.1. Study area and period
This research was carried out at the Integrated Management of Childhood Illness (IMCI) polyclinic of the Central Jakarta Regional Health Center Indonesia from January to July 2021. Central Jakarta is one of the administrative cities located in the capital of the State as the center of government, service, and trade, with development activities that are quite high. The estimated population of this municipality is 924.686, the lowest compared to the other 4 municipalities where most of the residents are highly educated. Central Jakarta consists of 8 sub-districts with lowland morphology [27].
2.2. Design and samples
Cross-sectional study with an independent test on children under five suffering from ARI. The population in this study were children under five who were treated for ARI at three Community Health Centers in Central Jakarta. Toddlers who meet the requirements included in the study were selected by the purposive sampling technique method.
2.3. Inclusion and exclusion criteria
The toddlers who were involved in this study were toddlers who were treated for ARI at the Community Health Center and aged 7–48 months, who were breastfed, and had a birth weight of more than 2500 g. Mothers are willing to have their toddlers participate in a series of research by signing informed consent. The exclusion criteria were toddlers who did not get breast milk and mothers who suffered from tuberculosis.
2.4. Sample size determination
The sample size was calculated using a two-sample proportion test formula with a two-tailed alternative hypothesis, using the following assumptions: 95% confidence level, 30,8 infants with ARI who received EBF (P1), and infants with ARI who did not receive EBF (P1) P2) in the previous study, 90% power, 10% contingency for loss to follow up. By using a formula calculated based on the proportion of the population of the previous study using the purposive sampling technique, the sample size was obtained as many as 124 (62 per group) children under five suffering from ARI in this study [28]:
2.5. Data collection and measurements
This study used a structured questionnaire instrument supported by medical record data to obtain data on the characteristics of mothers and toddlers including age, gender, parity, immunization history, frequency of ARI in the last 6 months, nutritional status (weight/age), and history of EBF. The questionnaire instrument used in this study was valid and reliable. The questionnaire tested on 10% of the minimum number of samples on respondents with appropriate characteristics with participants in this study. Measurement of sIgA and lactoferrin levels was obtained from the examination of blood samples taken as much as 100 μl, then centrifuged at 3000 rpm for 10 min to separate blood cells and serum. Blood serum was taken and put into an Eppendorf tube and stored in a freezer/refrigerator at −80 °C until all samples were filled. After the sample is fulfilled, it is then taken to the Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University for further examination. Examination of serum sIgA and lactoferrin levels using the Enzym linked Immunosorbent assay (ELISA) method according to the protocol of previous studies [12,[29], [30], [31], [32], [33], [34]].
2.6. Data processing and analysis
All data in the questionnaire were examined including the characteristics of mothers and children under five. Furthermore, the data was coded and input using SPSS version 22.0. Variable with continuous data, sIgA and lactoferrin levels were analyzed for normality using the Kolmogorov-Smirnov test. Based on the Kolmogorov test, sIgA and lactoferrin levels showed a normal distribution. Descriptive statistics consisting of mean, standard deviation, percentage, and categorical were analyzed by univariate analysis. 95% confidence level and P < 0,05 were used to assess statistical significance. Independent T-Test was used to see the difference in mean sIgA and lactoferrin for child's age, gender, immunization history, frequency of ARI, and EBF. ANOVA was used to see the difference in mean sIgA and lactoferrin for the mother's age, parity, and the history of breastfeeding with the frequency of ARI.
2.7. Ethical consideration
This research has passed the ethical test based on the research ethics notification letter from the Faculty of Medicine, Hasanuddin University on August 18, 2020, with Number 438/UN.4.6.4.5.31/PP36/2020. Data collection was carried out by the main researcher and assisted by enumerators (midwifery students). The mother filled out the questionnaire herself after receiving information about the study and signing the informed consent. In this study, the authors confirmed that all methods were carried out following the relevant guidelines and regulations (Helsinki Declaration) with Unique Identifying Number (UIN) or registration ID researchregistry7672 https://www.researchregistry.com/register-now#user-researchregistry/registerresearchdetails/6214706e7a29ed001f067ad4/. This manuscript has been reported in line with the STROCSS criteria [35]. This research is not experimental or intervention research.
3. Result
Interviews were conducted on 124 respondents, obtained data on the characteristics of mothers aged 20–35 years as much as 55.6%. Judging from maternal parity, multiparas were more than primiparas and grande, namely 57.3%. The characteristics of under-fives studied, aged 24 months were 55,6%, sex was almost comparable between 49.2% male and 50.8% female. Most of the children under five have received 75.0% complete immunization. Community Health Center is the place where the mother gives birth with the most 66.1%. 81.5% of children under five were born spontaneously. The frequency of ARI in children under five was more than 2 times in the last 6 months 45.2% and the frequency of ARI less than 2 times was 54.8%. In this study, the percentage of toddlers who received exclusive breastfeeding was comparable to that of toddlers who did not exclusively breastfeed, which was 50% for each category. Toddlers with normal weight status were 78.2%, 16.1% were underweight and 4.8% overweight. The mean lactoferrin level was 147.69 ng/ml ± 93.27 SD, with the lowest to highest lactoferrin levels, ie 0.80–304.98 ng/ml (see Table 1).
Table 1.
Univariate analysis.
| Variable | N | % |
|---|---|---|
| Mother Age | ||
| <20 Years | 7 | 5.6 |
| 20–35 Years | 69 | 55.6 |
| >35 Years | 48 | 38.7 |
| Parity | ||
| Primipara | 47 | 37.9 |
| Multipara | 71 | 57.3 |
| Grande | 6 | 4.8 |
| Gender | ||
| Boy | 61 | 49.2 |
| Girl | 63 | 50.8 |
| Toddler Age | ||
| ≤24 months | 69 | 55.6 |
| >24 months | 55 | 44.4 |
| Immunization history | ||
| Incomplete | 31 | 25.0 |
| Complete | 93 | 75.0 |
| EBF | ||
| No | 62 | 50.0 |
| Yes | 62 | 50.0 |
| Frequency ARI | ||
| ≥2 times | 56 | 45.2 |
| <2 times | 68 | 54.8 |
| Weight/Age Status | ||
| Underweight | 20 | 16.1 |
| Normal | 97 | 78.2 |
| Overweight | 6 | 4.8 |
| sIgA levels | ||
| Mean | 116918.50 pg/ml | |
| SD | 58451.99 | |
| Min-Max | 22426.77–212378.33 | |
| Lactoferrin levels | ||
| Mean | 147.69 ng/ml | |
| SD | 93.27 | |
| Min-Max | 0.80–304.98 | |
EBF: Exclusive Breastfeeding; ARI: Acute Respiratory Infection; SD: Standart Deviation.
Table 2 shows that the mean protein level of sIgA at maternal age <20 years is 132038.97 pg/ml, age 20–35 years is 122852.25 pg/ml, >35 years is 106183.66 pg/ml. The mean level of sIgA protein was not much different based on maternal age, so this indicates that there is no significant relationship between maternal age and sIgA protein content (p-value 0.248).
Table 2.
Factors related to sIgA and Lactoferrin levels in Independent T-Test and ANOVA analysis.
| Characteristics | sIgA (pg/ml) |
Lactoferrin (ng/ml) |
||
|---|---|---|---|---|
| Mean ± SD (95% CI) | p-value | Mean ± SD (95% CI) | p-value | |
| Mother Age | ||||
| <20 Years | 132038.97 ± 45367.75 | 0.248 | 160.61 ± 78.80 | 0.347 |
| (90080.80–173997.14) | (29.79–87.72) | |||
| 20–35 Years | 122852.25 ± 62788.02 | 157.07 ± 100.35 | ||
| (107768.92–137935.58) | (12.08–132.96) | |||
| >35 Years | 106183.66 ± 52644.30 | 132.34 ± 83.71 | ||
| (90897.35–121469.97) | (12.08–108.03) | |||
| Toddler Age | ||||
| ≤24 months | 119887.95 ± 61069.68 | 0.529 | 151.13 ± 91.63 | 0.648 |
| (-14272.62-27662.16) | (-25.75-41.21) | |||
| >24 months | 113193.19 ± 55321.98 | 143.39 ± 95.95 | ||
| (-14042.32-27431.86) | (-25.95-41.42) | |||
| Parity | ||||
| Primipara | 103289.32 ± 56095.16 | 0.033* | 128.37 ± 90.13 | 0.072 |
| (86819.17–119759.48) | (0.80–302.09)\ | |||
| Multipara | 128381.30 ± 58002.56 | 163.86 ± 92.94 | ||
| (114652.31–142110.28) | (1.65–304.78) | |||
| Grande | 88037.20 ± 58196.90 | 107.90 ± 95.95 | ||
| (26963.30–149111.10) | (7.13–270.57) | |||
| Gender | ||||
| Boy | 114706.10 ± 59968.73 | 0.680 | 144.23 ± 95.20 | 0.685 |
| (-25210.08-16500.97) | (-40.11-26.45) | |||
| Girl | 119060.66 ± 57344.97 | 151.06 ± 91.99 | ||
| (-25226.50-16517.39) | (-40.13-26.47) | |||
| Immunization history | ||||
| Incomplete | 90691.58 ± 53047.63 | 0.004** | 103.01 ± 87.50 | 0.002** |
| (-58235.48 to −11702.96) | (-96.52 to −22.65) | |||
| Complete | 125660.80 ± 57803.41 | 162.59 ± 90.75 | ||
| (-57521.72 to −12416.72) | (-96.32 to −22.84) | |||
| Frequency ARI | ||||
| ≥2 times | 84888.22 ± 49128.64 | 0.000*** | 99.01 ± 78.16 | 0.000*** |
| (-76573.59 to −40242.73) | (-118.21 to −59.35) | |||
| <2 times | 143296.38 ± 52223.75 | 187.79 ± 85.69 | ||
| (-76469.06 to −40347.26) | (-117.95 to −59.61) | |||
| Weight/Age Status | ||||
| Underweight | 112070.61 ± 57292.19 | 0.142 | 149.62 ± 91.59 | 0.239 |
| (85991.52–138149.70) | (107.93–191.31) | |||
| Normal | 120674.88 ± 59128.15 | 151.17 ± 95.32 | ||
| (108757.92–132591.84) | (131.96–170.38) | |||
| Overweight | 73157.88 ± 33516.88 | 84.77 ± 30.96 | ||
| (37984.06–108331.69) | (52.28–117.27) | |||
| EBF | ||||
| No | 65990.99 ± 26565.23 | 0.000*** | 65.67 ± 39.28 | 0.000*** |
| (-111964.75 to −91745.27) | (-179.68 to −148.44) | |||
| Yes | 167845.01 ± 30187.96 | 229.72 ± 48.15 | ||
| (-111966.39 to −91743.64) | (-179.69 to −148.43) | |||
*p < 0.05 (good significant), **p < 0.01 (strong significant), ***p < 0.001 (very strong significant).
Based on the age of toddlers, the mean protein level of sIgA at the age of ≤24 months was 119887.95 pg/ml, and >24 months 113193.19 pg/ml. The mean protein level of sIgA was not much different between the age groups of toddlers. This shows that there is no relationship between the age of toddlers and the mean protein level of sIgA (p-value 0.529).
Based on parity, the mean protein content of sIgA in primiparas was 103289.32 pg/ml, multiparas 128381.30 pg/ml, and grande 88037.20 pg/ml. The mean protein level of sIgA in the multiparous group was higher than that of the primipara and grande groups. So that the results obtained that parity is related to protein levels of sIgA (p-value 0.033).
The mean protein content of sIgA for boys is 114706.10 pg/ml, while the mean protein level for sIgA for girls is 119060.66 pg/ml. The mean value of sIgA protein levels between boys and girls sexes is not much different. So that the results obtained that the gender of children under five was not associated with protein levels of sIgA (p-value 0.680).
The mean sIgA protein level with a history of incomplete immunization was 119060.66 pg/ml, while the mean sIgA protein level with a history of complete immunization was 125660.80 pg/ml. The mean sIgA protein level in the history of incomplete immunization was lower than the mean sIgA protein level in the history of complete immunization. It was found that the history of immunization was associated with protein levels of sIgA (p-value 0.004).
The mean sIgA protein level of respondents who did not receive exclusive breastfeeding was 65990.99 pg/ml, while the mean sIgA protein level of respondents who received exclusive breastfeeding was 167845.01 pg/ml. The mean IgA protein levels of respondents who did not receive exclusive breastfeeding were lower than those who received exclusive breastfeeding. It was found that exclusive breastfeeding was associated with protein levels of sIgA (p-value 0.000).
The mean sIgA protein level with an ARI frequency of more than 2 times in the last 6 months was 84888.22 pg/ml, while the mean sIgA protein level with an ARI frequency of fewer than 2 times in the last 6 months was 143296.38 pg/ml. The mean sIgA protein level with an ARI frequency of more than 2 times in the last 6 months is lower than the mean sIgA protein level with an ARI frequency of fewer than 2 times in the last 6 months. From the results of the study, it was found that the frequency of ARI was associated with protein levels of sIgA (p-value 0.000).
The mean protein level of sIgA under five with underweight children was 112070.61 pg/ml, normal weight 117906.90 pg/ml, and body weight over 73157.88 pg/ml. The mean sIgA protein level of infants with excess body weight was lower than that of infants with normal weight. The results showed that weight/age status was not related to sIgA protein levels (p-value 0.678).
Table 2 shows that the mean lactoferrin levels at the age of the mother <20 years old are 160.61 ng/ml, the age from 20 to 35 years old is 157.07 ng/ml, >35 years old is 132.34 ng/ml. The mean lactoferrin levels are not much different based on maternal age, so this shows that there is no significant relationship between maternal age and lactoferrin levels (p-value 0.347).
Based on the age of toddlers, the mean lactoferrin levels at the age of ≤24 months were 151.13 ng/ml and >24 months 143.39 ng/ml. The mean lactoferrin levels were not much different between the toddler age groups. This shows that there is no relationship between the age of toddlers and the mean lactoferrin level (p-value 0.648).
Based on parity, the mean lactoferrin levels in primiparas were 128.37 ng/ml, multiparas 163.86 ng/ml, and grande 107.90 ng/ml. The mean lactoferrin levels in the multiparous group were higher than in the primipara and grande groups. However, the results showed that parity was not related to lactoferrin levels (p-value 0.072).
The mean lactoferrin level in male children under five was 144.23 ng/ml, while the mean female lactoferrin level was 151.06 ng/ml. The mean value of lactoferrin levels between the male and female sexes is not much different. So that the results obtained that the gender of children under five was not associated with lactoferrin levels (p-value 0.685).
The mean lactoferrin level with a history of incomplete immunization was 103.01 ng/ml, while the mean lactoferrin level with a history of complete immunization was 162.59 ng/ml. The mean lactoferrin level with a history of incomplete immunization is lower than the mean lactoferrin level with a history of complete immunization. The results showed that immunization history was associated with lactoferrin levels (p-value 0.002).
The mean lactoferrin levels of respondents who did not receive EBF were 65.67 ng/ml, while the mean lactoferrin levels of respondents who received EBF were 229.72 ng/ml. The mean lactoferrin levels of respondents who did not receive EBF were lower than in those who received EBF. The results showed that EBF was associated with lactoferrin levels (p-value 0.000).
The mean lactoferrin level with an ARI frequency of ≥ two times in the last 6 months was 99.01 ng/ml, while the mean lactoferrin level with a frequency <2 times the ARI in the last 6 months was 187.79 ng/ml. The mean lactoferrin level with an ARI frequency of ≥2 times in the last 6 months is lower than the mean lactoferrin level with a frequency <2 times the ARI in the last 6 months. From the results of the study, it was found that the frequency of ARI was related to lactoferrin levels (p-value 0.000).
The mean lactoferrin level of toddlers with underweight status is 149.62 ng/ml, while the mean lactoferrin levels of toddlers with weight normal status are 151.17 ng/ml. The mean lactoferrin levels between under-fives with underweight, normal and overweight status were not significantly different. The results showed that weight/age status was not related to lactoferrin levels (p-value 0.918).
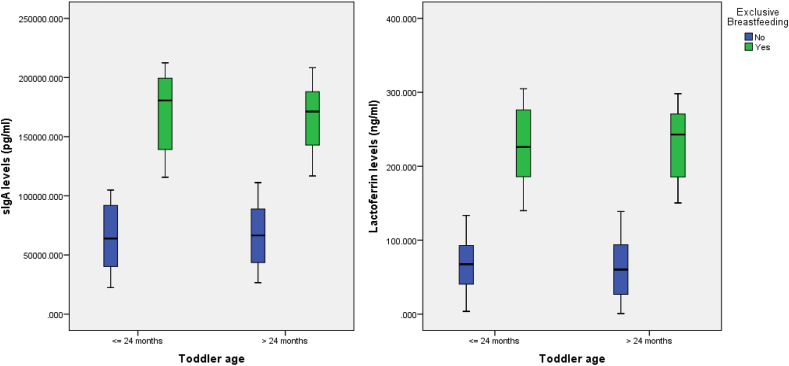
Differences in lactoferrin levels in toddlers who are not EBF and those who are EBF are presented in Fig. 1. Lactoferrin levels in toddlers who are not EBF are lower than in those who are EBF.
Fig. 1.
Boxplot of sIgA and Lactoferrin levels based on age and EBF for toddlers with ARI.
Table 3 also shows a significant difference in the mean protein sIgA between the group non-EBF with ARI frequency ≥2 times (43117.85 ± 13375.61), non-EBF with ARI frequency <2 times (88864.14 ± 13222.18). EBF with ARI frequency ≥2 times (136683.47 ± 16106.02), and EBF with ARI frequency <2 times (188901.77 ± 15530.46) with a significance value of p < 0.001.
Table 3.
Differences in sIgA protein levels and lactoferrin levels based on the history of breastfeeding with the frequency of ARI in the last 6 months.
| Biomarker | Breastfeeding with ARI Frequency | N | Mean | SD | 95% CI | p-value |
|---|---|---|---|---|---|---|
| sIgA | non-EBF with ARI FQ ≥ 2 times | 31 | 43117.85 | 13375.61 | 37932.02–48303.68 | 0.000a |
| non-EBF with ARI FQ < 2 times | 31 | 88864.14 | 13222.18 | 83678.31–94049.96 | ||
| EBF with ARI FQ ≥ 2 times | 25 | 136683.47 | 16106.02 | 130908.78–142458.17 | ||
| EBF with ARI FQ < 2 times | 37 | 188901.77 | 15530.46 | 184155.00–193648.54 | ||
| Lactoferrin | non-EBF with ARI FQ ≥ 2 times | 31 | 32.50 | 19.33 | 23.91–41.08 | 0.000a |
| non-EBF with ARI FQ < 2 times | 31 | 98.84 | 22.12 | 90.26–107.42 | ||
| EBF with ARI FQ ≥ 2 times | 25 | 181.49 | 27.02 | 171.94–191.05 | ||
| EBF with ARI FQ < 2 times | 37 | 262.32 | 27.11 | 254.46–270.18 |
Oneway ANOVA, p < 0.001 (very strong significant).
A significant difference in the mean lactoferrin levels between the group non-EBF with ARI frequency ≥2 times (32.50 ± 19.33), non-EBF with ARI frequency <2 times (98.84 ± 22.12), EBF with ARI frequency ≥2 times (181.49 ± 27.02), and EBF with ARI frequency <2 times (262.32 ± 27.11) with a significance value of p < 0.001.
4. Discussion
The findings of this study indicate that exclusive breastfeeding significantly increased sIgA and lactoferrin levels in toddlers suffering from ARI. The mean protein sIgA and lactoferrin levels were higher in toddlers who were given EBF with ARI frequency <2 times and lower in toddlers who were not given EBF with ARI frequency ≥2 times. Inline previous scientific evidence, breast milk has a main role in preventing the occurrence of infectious diseases in infants. Breast milk contains many bioactive factors such as immunoglobulins especially sigA, lactoferrin, and other important components (hormones, cytokines, leukocytes, lysozyme, stem cells, human milk oligosaccharides, microbiota, and microRNA) [36]. There are two general concepts regarding the role of bioactive factors in breast milk on infant health. The first concept is related to the concept of bioactive factors as 'protectors', where high levels of these two immune compounds can prevent infectious diseases in infants. The second concept, the ‘responsive’ concept, suggests that increased levels of sIgA and lactoferrin are a response to infection [21]. Secretory IgA in breast milk is the main source of passively acquired immunity for several weeks before endogenous production of sIgA, the concentration is highest in the first few days postpartum. During the postpartum period, infants are susceptible to pathogenic infections that enter, therefore sIgA is a protective factor important against infection. SIgA is the main source of immunity transferred from breast milk that protects mucous membranes of the digestive and respiratory tracts, IgG and IgM antibodies, hormones, antioxidants, vitamins, cytokines, growth factors, components, prostaglandins, granulocytes, macrophages, and B and T lymphocytes that can bind specific antigens in the maternal gastrointestinal and respiratory tract via the lymphatic system. sIgA has advantages in performing its function that is easier releases fluid from the adrenal glands oral mucosa because it has secretory components that contain receptors, especially in the excretory ducts, and is more resistant to proteolytic degradation bacteria and hydrolases (digestion) compared to other immunoglobulins. Several studies clearly show clinical benefits showing a reduced risk of gastrointestinal and respiratory infections, especially during the first year of life. The increased incidence of bioactive and immune factors may explain the reduced risk of gastrointestinal and respiratory allergies and autoimmune diseases in breastfed infants [21,37]. The study conducted by Fujita et al. showed that the content of sIgA in breast milk can significantly reduce the baby's susceptibility to infection [15].
Lactoferrin is the second most abundant protein in breastmilk. Lactoferrin can limit infectious proliferation in several ways. The main role of lactoferrin is to bind iron in the digestive tract so that it can weaken the activity of pathogenic bacteria that require iron to multiply [38]. The role of lactoferrin in protecting infants in the first 6 months of life, when the baby's immune system is developing immunological competence. The role of the two substances has been strongly proven to play a role in preventing respiratory diseases and various other infectious diseases such as gastrointestinal diseases in infants [21,37]. The study conducted by Turin et al. showed that lactoferrin levels in breast milk increased up to 2 months postpartum, including in breast milk for mothers who gave birth to low birth weight babies. Lactoferrin (LF) is a glycoprotein that has a role to protect against neonatal infections. It attaches to any of the extra iron that the baby doesn't absorb and prevents it from allowing harmful bacteria to grow in the baby's respiratory and digestive tracts [39].
In this study, it was shown that levels of sIgA and lactoferrin decreased with the increasing age of mothers and toddlers. Although there was a decrease, it did not show a significant relationship between sIgA and lactoferrin levels with the age of mothers and toddlers. The results of previous studies also found that IgA and lactoferrin in breast milk were not associated with maternal age, but there was a significant relationship between lactoferrin levels and infant age [40,41]. Meanwhile, according to parity, lactoferrin levels were higher in multiparas compared to primiparas and grandes. This shows a significant relationship between parity and sIgA levels, but no significant relationship was found with lactoferrin levels. Research by Rahfiludin and Pangestuti found that there was no significant relationship between lactoferrin concentration and parity [41].
In this study, the results showed that the levels of sIgA and lactoferrin between the male and female sexes were not much different. So that the results obtained that the sex of toddlers is not related to lactoferrin levels. This is in line with the study of Mercil et al. who found that gender was not positively correlated with plasma lactoferrin levels [42].
The levels of sIgA and lactoferrin in toddlers with a history of incomplete immunization were lower than the mean levels of sIgA and lactoferrin with a history of complete immunization. The results showed that the history of immunization was associated with levels of sIgA and lactoferrin. Research conducted on pregnant women who received tetanus toxoid (TT) immunization, showed that TT immunization can increase the body's response to form serum IgG and colostrum IgA [43]. Immunization along with breastfeeding can increase lactoferrin levels in infants [44]. Therefore, breastfeeding accompanied by vaccination can increase the immunity of toddlers to prevent the occurrence of various infectious diseases such as ARI and tuberculosis [45].
The sIgA and lactoferrin levels of ARI frequency of more than 2 times in the last 6 months are lower than the mean sIgA and lactoferrin levels of ARI frequency less than 2 times in the last 6 months. From the results of the study, it was found that the frequency of ARI was related to the levels of sIgA and lactoferrin. The results of Lars' research show that EBF protects sIgA antibodies that can protect infants from Haemophilus influenza microbes in the mouth and nose and reduce the risk of respiratory tract infections [46].
Lactoferrin is another major milk protein (1–3 g I−1) capable of killing certain bacteria, viruses, and candida. At the same time, it enters the leukocyte nucleus and binds to the transcription factor nuclear factor-kB (NF-kB), blocking the capacity of leukocytes to produce proinflammatory cytokines. In experimental animals, lactoferrin and some of its fragments, administered orally, resulted in a reduction in inflammatory colitis. It also protects against urinary tract infections, because lactoferrin and its fragments are present in the intestine and appear in the urine [47].
In this study, the results showed that nutritional status was not related to levels of sIgA and lactoferrin. The results of this study are in line with research conducted by Smidowicz and Reguta in 2018, which found that there was no significant correlation between IgA levels and nutritional status [48]. In contrast to the results of studies conducted in Africa, the level of sIgA secretion was significantly lower in obese and overweight children compared to normal-weight children. Another study found age and BMI predict the level of sIgA secretion, with BMI as an independent predictor of the level of sIgA secretion [49]. Metabolic disorders (obesity and metabolic syndrome) in adults are positively associated with IgA levels. Abdominal obesity and hypertriglyceridemia are components of the metabolic syndrome that are most closely associated with IgA [50]. Pollaro et al. also observed lower total sIgA levels in obese compared with lean children [51].
The relationship between nutritional status and lactoferrin was based on the results of a cohort study conducted on children of French Canadians aged 6, 13, and 16 years. Where it was found that plasma lactoferrin levels were positively correlated with Body Mass Index (BMI) and body weight [42]. These results are different from the findings in this study.
The levels of sIgA and lactoferrin of respondents who did not receive EBF were lower than those who received EBF. Following the results of previous studies, it was found that EBF was associated with levels of sIgA and lactoferrin. The concentrations of sIgA and lactoferrin decreased progressively with the lactation stage [14,19,41,52]. Lactation duration was found to be positively associated with IgA content up to two years postpartum [40]. A study in Sweden stated that levels of IgA and IgM antibodies were significantly higher in breastfeeding infants than in non-breastfeeding infants [53]. Immunoglobulin A contained in maternal antibodies obtained from the gastrointestinal and respiratory immune systems is carried through the blood and lymphatic circulation to the breast glands, finally being excreted in breast milk as sIgA [37].
From the results of this study, it was found that there was a significant difference in the mean protein sIgA and lactoferrin levels between groups the non-EBF with ARI frequency ≥2 times, non-EBF with ARI frequency <2 times, EBF with ARI frequency ≥2 times, and EBF with ARI frequency 2 times. The mean protein sIgA and lactoferrin levels were highest in the EBF group with an ARI frequency <2 times and the lowest in the non-EBF group with an ARI frequency ≥2 times. This shows that the protein sIgA and lactoferrin levels present in breast milk protect against the recurrence of ARI in toddlers.
Scientifically other studies have also argued that the relationship between diarrhea and EBF is due to the protein lactoferrin, which is found in breast milk in all age groups. Thus in lymphocyte growth factors, lactoferrin can destroy disease-causing pathogens to reduce the inflammatory response and can increase the activity of the immune system [54].
5. Conclusion
The mean protein sIgA and lactoferrin levels were higher in toddlers who were given EBF with ARI frequency <2 times and lower in toddlers who were not given EBF with ARI frequency ≥2 times. sIgA and lactoferrin in breast milk can increase the body's immune system in toddlers suffering from ARI.
Limitations of the study
sIgA and lactoferrin levels which play a role in increasing the body's immunity of infants with ARI are influenced by multifactor. However, this study only assessed the relationship between EBF and several variables from all the variables associated with the two biomarkers. In addition, this study only assessed two biomarkers associated with infant immunity, among many other biomarkers. Thus, a more comprehensive explanation can be obtained regarding the role of EBF on the immunity of infants suffering from ARI.
Ethical approval
This research has passed the ethical test based on the research ethics notification letter from the Faculty of Medicine Hasanuddin University on August 18, 2020, with Number 438/UN.4.6.4.5.31/PP36/2020.
Source of funding
This research was funded by the Faculty of Medicine and Health, Universitas Muhammadiyah Jakarta.
Authors’ contributions
Fatimah: Conceptualization funding acquisition role/writing-original draft; MNM, ASBF, and MH: Conceptualization, role/writing- original draft; AK, SF, SW, and FH: Resources, project administration; TAEP: Data curation, methodology; MH: Supervision; Farsida: Formal analysis, validation.
Trail registry number
-
1.
Name of the registry: The Role of Exclusive Breastfeeding on sIgA and Lactoferrin Levels in Toddlers suffering from Acute Respiratory Infection: A Cross-Sectional Study
-
2.
Unique Identifying number or registration ID: researchregistry7672
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/register-now#user-researchregistry/registerresearchdetails/6214706e7a29ed001f067ad4/
Guarantor
Fatimah, SST., MKM.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We extend our highest appreciation and gratitude to all research respondents. We also thank the data collectors and supervisors for their outstanding contributions. We thank the Faculty of Medicine and Health, Universitas Muhammadiyah Jakarta for facilitating this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103644.
Contributor Information
Fatimah, Email: fatimahagus2013@gmail.com.
Muhammad Nasrum Massi, Email: nasrum2000@yahoo.com.
Andi Dwi Bahagia Febriani, Email: bahagiadwi@yahoo.com.
Mochammad Hatta, Email: hattaram@yahoo.com.
Anis Karuniawati, Email: anis.karuniawatimk@ui.ac.id.
Syahrul Rauf, Email: syahrulrauf@yahoo.com.
Sitti Wahyuni, Email: sittiwahyuni@gmail.com.
Firdaus Hamid, Email: firdaus.hamid@gmail.com.
Ema Alasiry, Email: alasiryema@yahoo.com.
Ilham Patellongi, Email: ilham_pt@yahoo.com.
Tria Astika Endah Permatasari, Email: tria.astika@umj.ac.id.
Farsida, Email: farsidazaenudin@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.WHO and UNICEF, “Global Breastfeeding Scorecard . 2017. Tracking Progress for Breastfeeding Policies and Programmes. 2017. [Google Scholar]
- 2.Ministry of Health R.I. Laporan Kinerja Kementrian Kesehatan Tahun 2020); 2021. Ministry of Health Performance Report 2020. [Google Scholar]
- 3.Wibawa P.G.S.S., Indrarto F.W., Samodra Y.L. Protective effect of exclusive breastfeeding on acute respiratory infections (Ari) among children in Tabanan, Bali. JHE (Journal Heal. Educ. 2019;4(2):65–71. [Google Scholar]
- 4.Melati I., Susan, Nawang G. Breastfeeding with recurrent acute respiratory tract infections (Ari) in toddler at Gotong Royong Hospital Surabaya. J. Widya Med. Jr. 2019;1(4) [Google Scholar]
- 5.Oktaria V., et al. Nutritional status, exclusive breastfeeding and management of acute respiratory illness and diarrhea in the first 6 months of life in infants from two regions of Indonesia. BMC Pediatr. Dec. 2017;17(1):211. doi: 10.1186/s12887-017-0966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health R.I. Badan Penelitian dan Pengembangan Kesehatan; Jakarta: 2019. Basic Health Research National Report 2018 (Laporan Nasional Riset Kesehatan Dasar 2018) [Google Scholar]
- 7.WHO and UNICEF . 2017. Nurturing the Health and Wealth of Nations: the Investment Case for Breastfeeding.http://www.who.int/nutrition/publications/infantfeeding/global-bf-collective-investmentcase.pdf?ua=1 [Online]. Available: [Google Scholar]
- 8.Robert E., Coppieters Y., Swennen B., Dramaix M. Breastfeeding duration: a Survival analysis—data from a Regional immunization survey. BioMed Res. Int. 2014 doi: 10.1155/2014/529790. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Permatasari T.A.E., Sudiartini N.W. Do health workers play a role in exclusive breastfeeding among working mothers in industrial Area? J. Nutr. Sci. Vitaminol. 2020;66(Supplement):S94–S98. doi: 10.3177/jnsv.66.S94. [DOI] [PubMed] [Google Scholar]
- 10.Permatasari T.A.E., Rizqiya F., Kusumaningati W., Suryaalamsah I.I., Hermiwahyoeni Z. The effect of nutrition and reproductive health education of pregnant women in Indonesia using quasi experimental study. BMC Pregnancy Childbirth. 2021;21(1):1–15. doi: 10.1186/s12884-021-03676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan J., Vesel L., Bahl R., Martines J.C. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity--a systematic review and meta-analysis. Matern. Child Health J. 2015;19(3):468–479. doi: 10.1007/s10995-014-1526-8. Mar. [DOI] [PubMed] [Google Scholar]
- 12.Fatimah, et al. Effect of breastfeeding on children's health and its relationship to NRAMP1 expression: a cross-sectional study. Ann. Med. Surg. 2021;71(November) doi: 10.1016/j.amsu.2021.103017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajeebhoy N., Nguyen P.H., Mannava P., Nguyen T.T., Mai L.T. Suboptimal breastfeeding practices are associated with infant illness in Vietnam. Int. Breastfeed. J. 2014;9(1):1–7. doi: 10.1186/1746-4358-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmeira P., Carneiro-Sampaio M. Immunology of breast milk. Rev. Assoc. Med. Bras. 2016;62(6):584–593. doi: 10.1590/1806-9282.62.06.584. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M., Wander K., Paredes Ruvalcaba N., Brindle E. Human milk sIgA antibody in relation to maternal nutrition and infant vulnerability in northern Kenya. Evol. Med. Public Heal. 2019;(1):201–211. doi: 10.1093/emph/eoz030. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rustam M., Mahkota R., Kodim N. Exclusive breastfeeding and upper respiratory infection in infants aged 6-12 months in Kampar district, Riau Province. Kesmas. 2019;13(3):117–123. doi: 10.21109/kesmas.v13i3.1892. [DOI] [Google Scholar]
- 17.Rosa E.F., Pome G., Harsanto D. Early weaning risk factors for acute respiratory infections. Int. J. Publ. Health Sci. 2017;6(2):118. doi: 10.11591/.v6i2.6641. [DOI] [Google Scholar]
- 18.Kell D.B., Heyden E.L., Pretorius E. The Biology of lactoferrin, an iron-Binding protein that can Help defend against viruses and bacteria. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czosnykowska-Łukacka M., Orczyk-Pawiłowicz M., Broers B., Królak-Olejnik B. Lactoferrin in human milk of prolonged lactation. Nutrients. Oct. 2019;11(10) doi: 10.3390/nu11102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandolfi E., et al. Breastfeeding and respiratory infections in the first 6 Months of life: a case control study. Front. Pediatr. 2019;7:152. doi: 10.3389/fped.2019.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breakey A.A., Hinde K., Valeggia C.R., Sinofsky A., Ellison P.T. Illness in breastfeeding infants relates to concentration of lactoferrin and secretory Immunoglobulin A in mother's milk. Evol. Med. Public Heal. 2015;(1):21–31. doi: 10.1093/emph/eov002. Jan. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank N.M., et al. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019;19(1):339. doi: 10.1186/s12887-019-1693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tromp I., et al. Breastfeeding and the risk of respiratory tract infections after infancy: the Generation R Study. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nurbaiti L., Taslim N., Hatta M., Bukhari A. Evaluation of feeding practices for infants and children (PMBA) for stunting children in Lombok. Ann. Rom. Soc. Cell Biol. 2021;25(1):2019–2027. [Google Scholar]
- 25.Iskandar I., et al. Gene prolactine receptor (PRLR) and signal transducer and activator of transcription 5 (STAT5) on milk production. Med. Clínica Práctica. 2021;4 doi: 10.1016/j.mcpsp.2021.100223. [DOI] [Google Scholar]
- 26.Siregar A.Y.M., Pitriyan P., Walters D. The annual cost of not breastfeeding in Indonesia: the economic burden of treating diarrhea and respiratory disease among children (< 24mo) due to not breastfeeding according to recommendation. Int. Breastfeed. J. 2018;13:10. doi: 10.1186/s13006-018-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakarta Dinkes DKI. Profil Kesehatan Provinsi DKI Jakarta Tahun 2018); 2018. DKI Jakarta Province Health Profile 2018. [Google Scholar]
- 28.Lemeshow S., Hosmer D.W., Klar J., Lwanga S.K., Who . World Health Organization; Chichester: 1990. Adequacy of Sample Size in Health Studies. [Google Scholar]
- 29.Tommy T., et al. Effect of folinic acid on serum homocysteine, TNFα, IL-10, and HMGB1 gene expression in head injury model. Ann. Med. Surg. May 2021;65 doi: 10.1016/j.amsu.2021.102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oley M.C., et al. Hyperbaric oxygen therapy ameliorates the symptoms of post-concussion syndrome by inhibiting MMP-9 activity: a randomized controlled trial in Indonesia. F1000Research. 2021;10(501) doi: 10.12688/f1000research.53289.1. [DOI] [Google Scholar]
- 31.Karnina R., et al. Systemic lidocaine administration influences NF-kβ gene expression, NF-kβ and TNF- α protein levels on BALB/c mice with musculoskeletal injury. Ann. Med. Surg. Sep. 2021;69 doi: 10.1016/j.amsu.2021.102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agustin H., et al. Analysis of CD4 and CD8 expression in multidrug-resistant tuberculosis infection with diabetes mellitus: an experimental study in mice. Ann. Med. Surg. Aug. 2021;68 doi: 10.1016/j.amsu.2021.102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marlina R., Hatta M., Sridiana E., Djaharuddin I., Patellongi I., Murtiani F. The effect of miana (coleus scutellariodes [L]) on vascular endothelial growth factor expression in Balb/C mice infected with mycobacterium tuberculosis. Biomed. Pharmacol. J. 2021;14(2):525–532. doi: 10.13005/bpj/2154. [DOI] [Google Scholar]
- 34.Wardani I.S., et al. Serum vitamin D receptor and High Mobility Group Box-1 (HMGB1) levels in HIV-infected patients with different immunodeficiency status: a cross-sectional study. Ann. Med. Surg. Mar. 2021;63 doi: 10.1016/j.amsu.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew G., Agha R. Strocss 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. Dec. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 36.Carr L.E., et al. Role of human milk bioactives on infants' gut and immune health. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.604080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aldy O.S., Lubis B.M., Sianturi P., Azlin E., Tjipta G.D. Impact of breast milk protection against infection (dampak proteksi Air Susu ibu Terhadap infeksi) Sari Pediatr. 2009;11(3):167–173. doi: 10.14238/sp11.3.2009.167-73. [DOI] [Google Scholar]
- 38.Peroni D.G., Fanos V. Lactoferrin is an important factor when breastfeeding and COVID-19 are considered. Acta Paediatr. Int. J. Paediatr. 2020;109(10):2139–2140. doi: 10.1111/apa.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turin C.G., et al. Lactoferrin concentration in breast milk of mothers of low-birth-weight newborns. J. Perinatol. May 2017;37(5):507–512. doi: 10.1038/jp.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ongprasert K., et al. Macronutrient, immunoglobulin a and total antioxidant capacity profiles of human milk from 1 to 24 months: a cross-sectional study in Thailand. Int. Breastfeed. J. 2020;15(1):1–10. doi: 10.1186/s13006-020-00333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zen Rahfiludin M., Pangestuti D.R. Lactoferrin association with maternal nutritional status and lactation stages. Curr. Res. Nutr. Food Sci. 2020;8(1):174–181. doi: 10.12944/CRNFSJ.8.1.16. [DOI] [Google Scholar]
- 42.Marcil V., et al. Cardiometabolic risk factors and lactoferrin: polymorphisms and plasma levels in French-Canadian children. Pediatr. Res. 2017;82(5):741–748. doi: 10.1038/pr.2017.72. [DOI] [PubMed] [Google Scholar]
- 43.Saidin M. Sukarti, Mucherdiyantiningsih, and Muhilal, “immunoglobulin levels in colostrum and maternal blood relation with maternal nutrition status (Kadar immunoglobulin kolostrum dan darah ibu dalam hubungannya dengan status gizi ibu) J. Nutr. Food Res. 2012;17:22–32. [Google Scholar]
- 44.Mwila-Kazimbaya K., et al. Effect of innate antiviral glycoproteins in breast milk on seroconversion to rotavirus vaccine (Rotarix) in children in Lusaka, Zambia. PLoS One. Dec. 2017;12(12) doi: 10.1371/journal.pone.0189351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shabariah R., et al. Comparison TLR2 and TLR4 serum levels in children with pulmonary and extrapulmonary tuberculosis with and without a Bacillus Calmette-Guérin (BCG) scar. J. Clin. Tuberc. Other Mycobact. Dis. Dec. 2021;25 doi: 10.1016/j.jctube.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson L.Å. Breast-feeding and protection against infection. Scand. J. Food Nutr. 2006;50(1):32–34. doi: 10.1080/11026480600601083. [DOI] [Google Scholar]
- 47.Håversen L.A., Baltzer L., Dolphin G., Hanson L.A., Mattsby-Baltzer I. Anti-inflammatory activities of human lactoferrin in acute dextran sulphate-induced colitis in mice. Scand. J. Immunol. Jan. 2003;57(1):2–10. doi: 10.1046/j.1365-3083.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 48.Śmidowicz A., Reguła J. Assessment of the relationship between nutritional status, inflammatory marker CRP and serum immunoglobulin G, M, A in adults. Prog. Nutr. 2018;20(2):236–240. doi: 10.23751/pn.v20i2.6198. [DOI] [Google Scholar]
- 49.Starzak D.E., Konkol K.F., McKune A.J. Effects of cardiorespiratory fitness and obesity on salivary secretory IgA and Alpha-Amylase in South African children. Child. (Basel, Switzerland) Jul. 2016;3(3) doi: 10.3390/children3030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Quintela A., et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. Jan. 2008;151(1):42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pallaro A., et al. Total salivary IgA, serum C3c and IgA in obese school children. J. Nutr. Biochem. Sep. 2002;13(9):539. doi: 10.1016/s0955-2863(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 52.Haschke F., Haiden N., Thakkar S.K. Nutritive and bioactive proteins in breastmilk. Ann. Nutr. Metab. 2016;69(suppl 2):16–26. doi: 10.1159/000452820. Suppl. 2. [DOI] [PubMed] [Google Scholar]
- 53.Butte N.F., Garza C., Lopez M.G. 2002. Nutrient Adequacy of Exclusive for the Term Infant during the First Six Months of Life. no. 9241562110. Geneva. [Google Scholar]
- 54.Gebremedhin T., Geberu D.M., Atnafu A. Less than one-fifth of the mothers practised exclusive breastfeeding in the emerging regions of Ethiopia: a multilevel analysis of the 2016 Ethiopian demographic and health survey. BMC Publ. Health. 2021;21(1):18. doi: 10.1186/s12889-020-10071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.