Abstract
Intervertebral disc degeneration is a natural process of aging. It can cause physical, psychological, and socioeconomic impact due to the decreasing function of the spine and pain manifestation. Conservative and surgical treatment to correct symptoms and structural anomalies does not fully recover the degenerated disc. Several therapeutic approaches have been developed to improve the clinical result and patient's quality of life. This paper aims to review previous studies that discussed potential novel approach in order to make effective degenerated disc restoration. We tried to briefly describe IVD, IDD, also review several promising current therapeutic approaches for degenerated disc treatment, including its relevance to the degeneration process and limitation to be applied in a clinical setting. There are generally four current therapeutic approaches that we reviewed; growth factors, small molecules, gene therapy, and stem cells. These new approaches aim to not only correct the symptoms but also restore and delay the degeneration process.
Keywords: Intervertebral disc degeneration, Current therapeutic approach, Cell therapy, Gene therapy, Growth factors, Stem cells
Highlights
-
•
Intervertebral Disc Degeneration.
-
•
Current Therapeutic Approach.
-
•
Stem Cell Therapy.
1. Introduction
The intervertebral disc (IVD) is a structure consisting of fibrocartilage that lies between the vertebral bodies to provide flexibility and hold compression. Intervertebral disc degeneration (IDD) is a natural process of aging [[1], [2], [3]] [[1], [2], [3]] [[1], [2], [3]]. Degenerative disc disease has been associated with structural failure and lowering the patient's quality of life, manifesting as chronic back pain and limiting activities [[2], [3], [4]] [[2], [3], [4]] [[2], [3], [4]]. The start of the IDD process has been observed as early as age eleven, and blood supply to the vertebral endplates started to diminish since the age of twenties [3].
The incidence of degenerative disc disease of the whole spine in a population aged less than 50 years was 71% for men and 77% for women, and in population aged, more than 50 years was >90% in both genders [5,6]. Back pain is a major health issue with prevalence between 12% and 30%, with around 10% developing into chronic back pain [5]. It affects not only physical well-being, but also psychological as it increases stress, depression, an.83d anxiety. The socioeconomic impact has also been affected due to the increasing cost by the year [[3], [4], [5]] [[3], [4], [5]] [[3], [4], [5]].
There are several treatment options currently available for IDD, but the widely used method are still either conservative treatment with medications or invasive treatment with surgery [4,5]. These methods are only correcting symptoms and not reversing the degeneration process, thus requiring follow-up treatments and building up further medical bills [1,5]. Several novel treatment options are currently under research and orthopedic doctors are required to have an understanding of current therapy development to give the best medical advice for their patients. We tried to briefly describe IVD, IDD, also review several promising current therapeutic approaches for degenerated disc treatment, including its relevance to the degeneration process and limitation to be applied in a clinical setting. This study followed the PRISMA criteria [7] and.
2. Anatomy and physiology of the normal intervertebral disc
The IVD is an important structure of the spine to support the normal functioning of the spine, providing connection to the vertebral bodies [8,9]. It is a pad of fibrocartilage and the primary joint between two vertebrae in the spinal column [9,10]. It depends on oxygen and nutrients diffused via the endplate since it is avascular [9]. Major compilers of the IVD are glycosaminoglycans, collagen, and aggrecans. There are 25 discs in the human spine: seven in the cervical region (neck), twelve in the thoracic region (middle back), five in the lumbar region (lower back), and one in the sacrum region [1,2,9].
The IVD consists of mainly three parts: the central nucleus pulposus, surrounding annulus fibrosus, and two cartilaginous endplates [10,11]. The gelatinous nucleus pulposus is composed of small chondrocyte-like cell and notochordal cells clusters embedded in the extracellular matrix made up of type II collagen fibers and elastin that contain negative-charged side chains of proteoglycans that contributes to the high hydration and osmolarity of the nucleus pulposus, making the IVD able to resist burden from pressure and to reverse its form [1,11]. Since the beginning of nucleus pulposus formation, it has been trapped in the IVD by the surrounding annulus fibrosus and vertebral cartilaginous endplate, thus isolating the nucleus pulposus from the host immune system [12,13].
The annulus fibrosus consists of a sequence of 15–25 stacked lamellae of collagen fibers, therefore providing a tensible structure and effective resistance from multidirectional movement. It is also composed of with interspersed proteoglycans, glycoproteins, elastic fibers, and the connective tissue cells that secrete these extracellular matrix products [2,9]. Annulus fibrosus consists of outer and inner portions, which differ primarily in their collagen composition. The inner annulus fibrosus is composed of several layers of fibrocartilage with predominantly type II collagen and more proteoglycans, whereas the outer annulus fibrosus is a fibrous tissue comprised of type I collagen fibers, making the structure able to stretch and hold compressive loads. Approaching the outer annulus fibrosis, type I collagen fibers content increases, while type II collagen fibers and proteoglycans are reduced [1,2,14].
The cartilaginous vertebral endplate is a layer of hyaline cartilage that covers the inferior and superior ends of the disc (each about 0.6–1.1 mm thick) which plays a key role in transporting fluids and solutes from going inside or outside of the disc, thus providing nutrition for the disc. Similar to the articular cartilage, the extracellular matrix of the cartilaginous vertebral endplate consists of type II collagen inveterate with chondrocytes [14].
3. Intervertebral disc degeneration
The IDD can result from the physiologic aging process or pathological process, both may present as symptomatic or asymptomatic cases. The etiology of IDD is complicated [15,16]. Almost immediately after birth, the biochemical and cellular structure of the IVD begins to change and the process progresses through life. Most degeneration begins in early adulthood and progresses with age [16].
The disappearance of large vacuolated notochordal cells in the nucleus pulposus at around 10 years of age and calcification of the cartilaginous vertebral endplates are considered to initiate and exacerbate the degeneration process [17]. With the loss of notochordal cells, the dominant cells that make proteoglycans and maintain the gelatinous consistency, proteoglycans synthesis is decreased [18]. Accompanied by the conversion of collagen synthesis, reducing type II and increasing type III, the increase of matrix metalloproteinase (MMPs) synthesis/activity and apoptotic nucleus pulposus cells, contribute to the degeneration process. Degradation of the extracellular matrix and loss of proteoglycans reduced the height and loading capacity of the IVD [1,18]. Calcification of the vertebral endplates decreased the supply of nutrients to the IVD, further exacerbating the process [18].
The possible environmental risk factors for IDD are unsanitary lifestyle, smoking, lack of physical activity, trauma, and exposure to mechanical load. The aging process results in dehydrated nucleus pulposus which is unable to balance the loads properly within the adjacent vertebrae. Thus, the loads eventually outspread the annulus pulposus region causing mechanical and gradual structural destruction, resulting in radial annular tear and herniation of the nucleus pulposus [4,19].
Discogenic pain is mostly present in IDD patients as a result of inflammatory responses [20,21]. It is exacerbated by pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). In degenerated disc, the number of senescent cells are increased, and these senescent cells secrete a senescence-associated secretory phenotype (SASP), which increases the secretion of pro-inflammatory cytokines, chemokines, and tissue-damaging proteases [21].
In the normal spine, only the outer annulus fibrosus is innervated. In the degenerated discs, the distorted tissue contains nerves, therefore causing pain [22]. In a mouse model with spontaneous degeneration, an increase of sensory innervation corresponding with age was found in the IVD. The vertebral endplates also experience an increase of innervation, found in discogenic low back pain patients. The identified inducers of nerve growth are TNF-α, IL-1, IL-37, IL-38, IL-39, and nerve growth factor (NGF) [18,23]. The discogenic pain signal passes through the IVD and adjacent structures, transmitted through peripheral afferent nerve fibers. Nerve fibers interact with inflammatory mediators in the nucleus pulposus and cause discogenic low back pain [23].
4. Treatment for intervertebral disc degeneration
There are two common strategies for IDD management, conservative and surgical treatment [4,5]. In the early stages of the disease, treatment options such as analgesics, physical therapy, and anti-inflammatories are given to reduce pain. In advanced cases, corticosteroid injections can be given to treat radicular symptoms [24]. Surgical treatment may be undertaken to help relieve symptoms as definitive therapy for patients unresponsive to symptomatic medications [4,24].
Treatment options for a pain reliever in conservative therapy consist of physical strengthening, physiotherapy, injection, and oral medication. The role of conservative therapy is primarily to improve the patient's physical well-being and quality of life by providing a platform for the body to adapt [1,25]. Physical exercise, especially back muscle strengthening, is clinically recommended to help reduce pain. In animal model studies, physical exercise helps in IVD cell proliferation, specifically exercises with moderate to a high volume of low repetition and frequency. It improves the paraspinal muscle strength, therefore reducing pain and disability [25].
Pain reliever oral medication choices non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, opioids, and muscle relaxants. Medication is given to patients with symptomatic degenerative disc disease without contraindication to the drugs [4,26]. Education for self-management back positions and reassurance is given alongside the medication. Patients are encouraged to have an early return to activity [20,26]. In patients with chronic lower back pain, multimodal rehabilitation is needed on top of the treatment for acute back pain. These medications only work in relieving pain for the short term but did not interfere in delaying the development of disc degeneration [26].
Injection pain reliever aims to decrease inflammation, providing temporary anesthesia in the area where the irritated nerve is involved. Giving a volume of fluid to the region gives hydrostatic pressure effect to correct adhesiolysis between the neural elements and the degenerated disc. Epidural corticosteroid injections and peri radicular corticosteroid injection are the choices in IDD pain reliever injection [27].
Epidural steroid injection (ESI) is given within the spinal cord's epidural space through several routes such as interlaminar, transforaminal, and caudal injection [28]. The epidural steroid injection effect is limited in areas of the spine where adhesions are formed from previous spinal surgery, ruptured disc, cyst, and chronic degeneration of disc and flavum. It is preferred to have adhesion lysis done before starting ESI treatment to increase responsiveness. The effect of ESI is temporary, and the rare of long-term success is variable [27,28].
It is important to determine the area of pain in treating IDD, because patients are often present with several levels of disc degeneration with associated spinal canal central, lateral recess, and foraminal stenosis as a result of the facet hypertrophy due to arthritis, decreased of disc height, and thickening of the ligamentum flavum. The patient may present with mixed pain associated with multidirectional movement (Wu et al., 2020).
Peri radicular injection is indicated for nerve root irritation symptoms and plays a role as selective nerve root block. However, the efficacy is limited in cases in which pain originated from mechanical compressions, such as spinal stenosis and foraminal disc herniation [29]. Epidural and peri radicular corticosteroid injection can lead to immediate temporary improvements in pain, function, and quality of life in radiculopathy. But it is less effective for spinal stenosis, non-radicular back pain, and facet joint pathology. There is no effect to develop long term risk of surgery. There is no evidence that shows dose-dependent effectiveness in amount, frequency, and administration technique of corticosteroid [30].
The definitive treatment for IDD is surgical treatment [5]. This method is indicated for patients with acute neurology disruption or cauda equina syndrome. Chronic lower back pain with the degenerative disc is a strong candidate for early surgical management [31]. Patients with clinical symptoms who failed conservative management and were motivated to have surgery treatment should be evaluated by diagnostic injection or provocative discogram to assess if symptoms can be relieved temporarily before being chosen as a surgical candidate [5,31]. Previous study showed evidence that patients with back pain and disability who underwent fusion show significant clinical improvement compared to patients who undergo conservative management with physiotherapy [31]. It is noted that in the surgery group, there is a higher short-term cost in 2 years, but the effect of long-term treatment effects calculated with its economic benefits suggests that surgery is superior to conservative treatment. After fusion was done, there are twice the number of patients returning to work compared with conservative treatment [5,27].
5. Current therapeutic approaches for Intervertebral Disc Degeneration
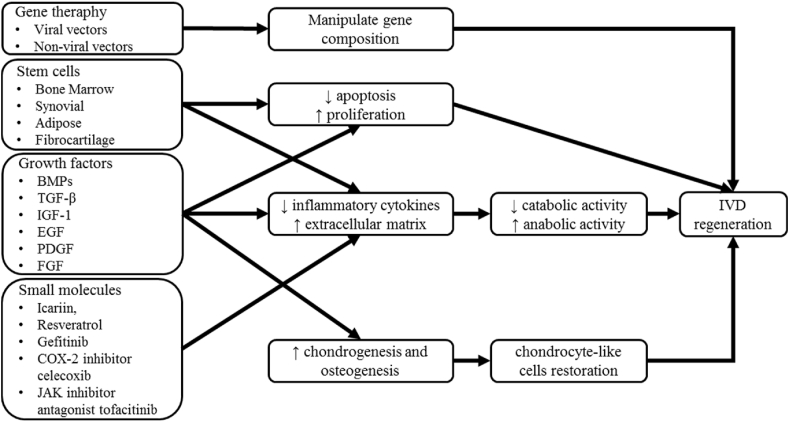
There are several areas of research growing in search of novel approaches for IDD therapy, aiming to restore the degeneration process with the less invasive method. From cellular and genetic modification, biochemical approach, tissue engineering, and biomaterials application have been showing beneficial effects on IVD regeneration through in vivo and in vitro research [10]. We try to summarize the use of growth factors, small molecules, gene therapy, and stem cells as a current therapeutic approach for IDD (Fig. 1.).
Fig. 1.
Current approaches to the treatment of IDD and mechanisms.
5.1. Growth factors
The injection of growth factors into the IVD can stimulate the extracellular matrix synthesis, relieve inflammation and delay the degeneration process [3]. Growth factors are peptides that cause cellular actions in the target receptors, such as synthesis of proteins, proliferation, differentiation, and apoptosis of cells. Growth factors commonly used in treating spine areas are bone morphogenic proteins (BMPs), and members of transforming growth factor-beta (TGF-β), which stimulates chondrogenesis and osteogenesis [3,32]. Growth factors increase the proteins of the extracellular matrix, such as TGF- β, insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), BMPs, and platelet-derived growth factor (PDGF), promoting anabolic function. There are other growth factors that downregulate catabolic activity by reducing inflammatory cytokines such as IL-1, IL-6, TNF-α, MMPs, nitric oxide, and prostaglandin E2 (PGE2). Both growth factors with these activities are applied to help the restoration of IVD. The limitation of this method is the short biological half-lives, only in hours to days, thus limiting the application to restore degenerative disc where the growth factors stability is needed [27].
The multiple growth factors contained in platelet-rich plasma (PRP) have been a promising alternative strategy for IVD regeneration [24,33]. Platelet-rich plasma can stimulate cell proliferation through its numerous bioactive compounds, such as TGF-β3, BMPs, and FGF. Injection of a single growth factor has limitations because not a single growth factor is potent enough in reversing the degenerative process. In the rabbit disc model, the administration of PRP via hydrogel with gelatin-based microspheres to the degenerated nucleus pulposus suppressed the degenerative disc progression significantly [24]. Furthermore, the therapeutic effects analysis of this PRP model indicated that type II collagen mRNA expression levels were higher, and is associated with greater disc height and preservation of water content. Magnetic resonance imaging (MRI) findings of reduced inflammatory cells, increased water content and detainment of morphological features in a rat model with degenerated IVD, show that administration of PRP has protective effects. PRP injection in a similar rabbit model of degenerated IVD also shows significant improvement of disc height and restoration of chondrocyte-like cells. In human IDD, experimental protocols still need further research [24,33].
5.2. Small molecules
In IDD, pro-inflammatory cytokines play an important role in developing the degeneration process. Cytokines such as interleukins and tumor necrosis factors activate the signaling pathway (nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and mitogen-activated protein kinase (MAPK)) and trigger extracellular matrix degeneration and attenuate the synthesis of prostaglandin. Cyclooxygenase-2 (COX-2) also plays a role as inflammatory cytokines in IDD [34]. Thus, studies among small molecules that can inhibit the NFκB and MAPK signaling pathway are under research to inhibit the decrease of extracellular matrix and prostaglandin synthesis [34,35]. Icariin, resveratrol, gefitinib, COX-2 inhibitor celecoxib, and janus kinase inhibitor (JAK inhibitor) antagonist tofacitinib are currently under research and show promising result in inflammation control, pain reduction, and delay of the degeneration process prostaglandin [[34], [35], [36]] [[34], [35], [36]] [[34], [35], [36]]. Small molecule therapy is under in vitro study and shows promising and beneficial effects in the IDD model. Further research in relevant animal models and organ culture are still needed to confirm the benefits of delaying degeneration, reservation of weight-bearing physiological function, duration of treatment, and safety effects. In the future, the selection of small molecules used should be customized based on individual approaches and adjust the medical and hormonal balance of the patient to achieve the optimal result, such as menopause or any underlying diseases [34].
5.3. Gene therapy
Gene therapy studies are mostly still conducted in the laboratory. This method introduces genes to the target cells via viral or non-viral vectors in vivo. The target cells are then taken out into a culture medium. After the target genes were altered, the cells are re-implanted to the target organs by ex vivo. This method has a potentially long-term effect once the gene is transferred successfully to the original target cells. These cells with manipulated gene composition will produce proteins that support IVD regeneration [37]. The challenges in this method are the involvement of a viral vector for genetic material transfer contains a risk of complications related to the viral component. Rather than degenerative condition, the clinical applications of this method are still limited to medical-emergency threatening conditions, such as sickle cell disease and cystic fibrosis [37,38].
5.4. Stem cells
Stem cells from synovial tissue, bone marrow, and adipose tissue mediate changes in vascular and fibrocartilage-like tissue. De novo cells made in the laboratory are used in cell therapy [39,40]. Through cell transplant, these cells induce paracrine signaling to stimulate endemic cells producing substances for disc regeneration. De novo cells can also directly participate in homeostasis and production of extracellular matrix production [40]. These cells are generally obtained from embryonic human nucleus pulposus; chondrocytes, and mesenchymal stem cells (MSCs) through autologous matured bone marrow and adipose tissue or allogeneic juvenile and embryonic cells [41,42]. The advantage of this method is the target injection is the nucleus pulposus which has an immune-privileged milieu, thus preventing cell migration because it is contained by the annulus [39,42].
Adipose mesenchymal stem cells (ASCs) used on nucleus pulposus cells can reduce apoptosis, inhibit the expression of proinflammatory cytokines (IL-1b and TNF-a), and catabolic factors (MMPs and disintegrin). The of ASCs effects on annulus fibrosus cells will increase proliferation, increase anabolic action, and decrease apoptosis and expression of catabolic factors and proinflammatory cytokines [43]. ASCs are one of the MSCs and can differentiate into mesoderm tissue, such as adipocytes, osteocytes, and chondrocytes, allowing them to be used as material for disc regeneration. There are various sources and techniques for obtaining ASCs, but the largest source comes from abdominal fat which can be obtained by abdominoplasty or endoscopically [43,44].
In order for MSCs can be effectively used in regenerative medicine is to have a sufficient number of cells to reach the location of damage and be able to survive for a long time. To maximize its reparative capacity and regeneration, ASCs requires a preconditioning strategy [45]. Environmentally modified precondition strategy of lowering oxygen levels multiplies the multiplication and migration of ASCs by the generation of reactive oxygen species (ROS) and downstream phosphorylation of platelet-derived growth factor receptor-beta, extracellular signal-regulated kinase (ERK) 1/2, and protein kinase [46,47]. Hypoxia environment and thermal preconditioning can reduce the number of cells undergoing apoptosis so that it can increase the potency of MSC [41].
Apart from environmental modification, the use of a conditioned medium derived from growth factors, cytokines, chemokines, and hormones can increase the motility and migration capabilities of MSCs without affecting their multipotential ability [39,48]. The use of EGF in preconditioning MSCs increases the expression of EGF receptor proteins, which play a role in accelerating wound healing [43]. Pharmacological or chemical agents preconditioning is another way to maximize the yield of ASCs. Agents such as atorvastatin, curcumin, diazoxide, deferoxamine, valproate, and lithium chloride will improve the survival of the transplanted MSCs by increasing growth factors, immunomodulatory capacity, regenerative efficacy, cell migration, and preventing damage from oxidative stress [45,49].
6. Conclusion
IDD is one of the major health problems that cause chronic pain and affect a person's quality of life. The IVD is composed of the nucleus pulposus, annulus fibrosus, and cartilaginous endplates, which play an important role in maintaining back structure and mobility. Degeneration of the IVD occurs physiologically or can be a result of a pathologic cascade and is influenced by several risk factors. Changes in biochemical and cellular structure in IDD cause pain and IVD destruction. IDD management with conservative therapy (physical therapy, NSAIDs, and steroid injection) and surgery did not effective in restoring the structure and function of the degenerated disc but focused on pain relief.
The aim of current therapeutic approaches is to delay the degeneration process and stimulate IVD regeneration to give more resilient outcomes with various mechanisms. There are methods developed for the current approach in IDD, which are using growth factors, small molecules, gene therapy, and stem cells. These methods have their own challenges and limitations, such as survival of the factors in IVD environment to create a regeneration effect; infection and mutation due to the viral vector effects in delivering the gene target; and costs. Clinical studies in humans are still in progress by taking into account safety, duration of treatment, individual approach. These are a promising therapeutic approach but still needs further research to be applied in clinical.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Please state any conflicts of interest
No potential conflict of interest relevant to this article was reported.
Please state any sources of funding for your research
The authors declare that this study had no funding resource.
Ethical approval
No need ehical approval for this review article.
Consent
This is review article, no need consent.
Author contribution
Romaniyanto, Ferdiansyah Mahyudin, Cita Rosita Sigit Prakoeswa, Hari Basuki Notobroto, Damayanti Tinduh, Ryan Ausrin, Fedik Abdul Rantam, Heri Suroto, Dwikora Novembri Utomo, Sholahuddin Rhatomy conceived the study. Romaniyanto, Ferdiansyah Mahyudin, Cita Rosita Sigit Prakoeswa, Hari Basuki Notobroto, and Damayanti Tinduh prepared and drafted the manuscript. Romaniyanto, Fedik Abdul Rantam, Heri Suroto, Dwikora Novembri Utomo, Sholahuddin Rhatomy edited manuscript. Romaniyanto, Ryan Ausrin, Ferdiansyah Mahyudin, Cita Rosita Sigit Prakoeswa, Hari Basuki Notobroto, and Fedik Abdul Rantam reviewed the manuscript. Romaniyanto, Ryan Ausrin, Ferdiansyah Mahyudin, Hari Basuki Notobroto, and Damayanti Tinduh, Sholahuddin Rhatomy prepared, reviewed, edited and improved the final version manuscript.
Registration of research studies
-
1.
Name of the registry:research registry
-
2.
Unique Identifying number or registration ID: reviewregistry1337
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/register-now/register-your-systematic-review#registryofsystematicreviewsmeta-analyses/registryofsystematicreviewsmeta-analysesdetails/62500312e7d4df001eb63368/.
Guarantor
Sholahuddin Rhatomy, MD, Adult Reconstruction Surgeon.
Acknowledgements
Nil.
Footnotes
Department of Orthopaedics and Traumatology, dr. Soeradji Tirtonegoro General Hospital, Klaten, Indonesia/Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Abbreviations
- ASCs
adipose mesenchymal stem cells
- BMPs
bone morphogenic proteins
- COX
cyclooxygenase
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- ESI
epidural steroid injection
- IDD
Intervertebral disc degeneration
- IGF
insulin-like growth factor
- IL
interleukin
- IVD
intervertebral disc
- JAK
janus kinase
- MAPK
mitogen-activated protein kinase
- MMPs
matrix metalloproteinase
- MRI
magnetic resonance imaging
- MSCs
mesenchymal stem cells
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NGF
nerve growth factor
- NSAIDs
non-steroidal anti-inflammatory drugs
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- PRP
platelet-rich plasma
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- TGF
transforming growth factor
- TNF
tumor necrosis factor
References
- 1.Rustenburg C.M.E., Emanuel K.S., Peeters M., Lems W.F., Vergroesen P.-P.A., Smit T.H. Osteoarthritis and intervertebral disc degeneration: quite different, quite similar. JOR Spine. 2018;1 doi: 10.1002/jsp2.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogendoorn R.J.W., Lu Z.F., Kroeze R.J., Bank R.A., Wuisman P.I., Helder M.N. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future: tissue Engineering Review Series. J. Cell Mol. Med. 2008;12:2205–2216. doi: 10.1111/j.1582-4934.2008.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowdell J., Erwin M., Choma T., Vaccaro A., Iatridis J., Cho S.K. Intervertebral disk degeneration and repair. Neurosurgery. 2017;80:S46–S54. doi: 10.1093/NEUROS/NYW078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casiano V.E., Dydyk A.M., Varacallo M. StatPearls Publishing; 2020. Back Pain. [PubMed] [Google Scholar]
- 5.Ishiguro H., Kaito T., Yarimitsu S., Hashimoto K., Okada R., Kushioka J., Chijimatsu R., Takenaka S., Makino T., Sakai Y., Moriguchi Y., Otsuru S., Hart D.A., Fujie H., Nakamura N., Yoshikawa H. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019;87:118–129. doi: 10.1016/j.actbio.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 6.Teraguchi M., Yoshimura N., Hashizume H., Muraki S., Yamada H., Minamide A., Oka H., Ishimoto Y., Nagata K., Kagotani R., Takiguchi N., Akune T., Kawaguchi H., Nakamura K., Yoshida M. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthr. Cartil. 2014;22:104–110. doi: 10.1016/J.JOCA.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro I.M., Vresilovic E.J., Risbud M.V. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone. 2012;50:771–776. doi: 10.1016/j.bone.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waxenbaum J.A., Futterman B. StatPearls; 2018. Anatomy, Back, Cervical Vertebrae. [PubMed] [Google Scholar]
- 10.Iatridis J.C., Nicoll S.B., Michalek A.J., Walter B.A., Gupta M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pattappa G., Li Z., Peroglio M., Wismer N., Alini M., Grad S. Diversity of intervertebral disc cells: phenotype and function. J. Anat. 2012;221:480–496. doi: 10.1111/J.1469-7580.2012.01521.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capossela S., Schläfli P., Bertolo A., Janner T., Stadler B.M., Pötzel T., Baur M., Stoyanov J.V. Degenerated human intervertebral discs contain autoantibodies against extracellular matrix proteins. Eur. Cell. Mater. 2014;27:251–263. doi: 10.22203/ECM.V027A18. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z., Liu B., Luo Z.J. The immune privilege of the intervertebral disc: implications for intervertebral disc degeneration treatment. Int. J. Med. Sci. 2020;17:685. doi: 10.7150/IJMS.42238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oichi T., Taniguchi Y., Oshima Y., Tanaka S., Saito T. Pathomechanism of intervertebral disc degeneration. JOR Spine. 2020;3 doi: 10.1002/JSP2.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y., Feng C., Liu H., Yang Y., Huang B. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell. Physiol. Biochem. 2015;35:1–16. doi: 10.1159/000369670. [DOI] [PubMed] [Google Scholar]
- 16.Kos N., Gradisnik L., Velnar T. A brief review of the degenerative intervertebral disc disease. Med. Arch. 2019;73:421–424. doi: 10.5455/medarh.2019.73.421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues-Pinto R., Richardson S.M., Hoyland J.A. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur. Spine J. 2014;23:1803–1814. doi: 10.1007/S00586-014-3305-Z. [DOI] [PubMed] [Google Scholar]
- 18.Wuertz K., Haglund L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob. Spine J. 2013;3:175–184. doi: 10.1055/S-0033-1347299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molinos M., Almeida C.R., Caldeira J., Cunha C., Gonçalves R.M., Barbosa M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface. 2015;12 doi: 10.1098/RSIF.2014.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urits I., Burshtein A., Sharma M., Testa L., Gold P.A., Orhurhu V., Viswanath O., Jones M.R., Sidransky M.A., Spektor B., Kaye A.D. Low back pain, a comprehensive review: pathophysiology, diagnosis, and treatment. Curr. Pain Headache Rep. 2019;23:23. doi: 10.1007/s11916-019-0757-1. [DOI] [PubMed] [Google Scholar]
- 21.Papavassiliou A.G., Pneumaticos S.G., Evangelopoulos D.S. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol. Med. 2014;20:400–409. doi: 10.2119/MOLMED.2014.00145/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtori S., Inoue G., Miyagi M., Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15:1347–1355. doi: 10.1016/J.SPINEE.2013.07.490. [DOI] [PubMed] [Google Scholar]
- 23.Lyu F.J., Cui H., Pan H., Mc Cheung K., Cao X., Iatridis J.C., Zheng Z. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;91(9):1–14. doi: 10.1038/s41413-020-00125-x. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Moure J., Moore C.A., Kim K., Karim A., Smith K., Barbosa Z., Van Eps J., Rameshwar P., Weiner B. Novel therapeutic strategies for degenerative disc disease: review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018;6 doi: 10.1177/2050312118761674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S., Kim H., Chung J. Effects of spinal stabilization exercise on the cross-sectional areas of the lumbar multifidus and psoas major muscles, pain intensity, and lumbar muscle strength of patients with degenerative disc disease. J. Phys. Ther. Sci. 2014;26:579. doi: 10.1589/JPTS.26.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong J.J., Côté P., Sutton D.A., Randhawa K., Yu H., Varatharajan S., Goldgrub R., Nordin M., Gross D.P., Shearer H.M., Carroll L.J., Stern P.J., Ameis A., Southerst D., Mior S., Stupar M., Varatharajan T., Taylor-Vaisey A. Clinical practice guidelines for the noninvasive management of low back pain: a systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Eur. J. Pain. 2017;21:201–216. doi: 10.1002/EJP.931. [DOI] [PubMed] [Google Scholar]
- 27.Wu P.H., Kim H.S., Jang I.T. Intervertebral disc diseases part 2: a review of the current diagnostic and treatment strategies for intervertebral disc disease. Int. J. Mol. Sci. 2020;21 doi: 10.3390/IJMS21062135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J., Zhou H., Lu L., Li X., Jia J., Shi Z., Yao X., Wu Q., Feng S. The effectiveness of transforaminal versus caudal routes for epidural steroid injections in managing lumbosacral radicular pain: a systematic review and meta-analysis. Medicine (Baltim.) 2016;95 doi: 10.1097/MD.0000000000003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanayama M., Oha F., Hashimoto T. What types of degenerative lumbar pathologies respond to nerve root injection? A retrospective review of six hundred and forty one cases. Int. Orthop. 2015;39:1379–1382. doi: 10.1007/S00264-015-2761-3. [DOI] [PubMed] [Google Scholar]
- 30.Chou R., Hashimoto R., Friedly J., Fu R., Bougatsos C., Dana T., BScPharm S.D.S., Jarvik J. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Ann. Intern. Med. 2015;163:373–381. doi: 10.7326/M15-0934. [DOI] [PubMed] [Google Scholar]
- 31.Försth P., Ólafsson G., Carlsson T., Frost A., Borgström F., Fritzell P., Öhagen P., Michaëlsson K., Sandén B. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N. Engl. J. Med. 2016;374:1413–1423. doi: 10.1056/NEJMOA1513721. [DOI] [PubMed] [Google Scholar]
- 32.Tamama K., Kawasaki H., Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/795385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obata S., Akeda K., Imanishi T., Masuda K., Bae W., Morimoto R., Asanuma Y., Kasai Y., Uchida A., Sudo A. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res. Ther. 2012;14 doi: 10.1186/AR4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamali A., Ziadlou R., Lang G., Pfannkuche J., Cui S., Li Z., Richards R.G., Alini M., Grad S. Small molecule-based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics. 2021;11:27. doi: 10.7150/THNO.48987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuertz K., Vo N., Kletsas D., Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-κB and MAP kinases. Eur. Cell. Mater. 2012;23:103–120. doi: 10.22203/ECM.V023A08. [DOI] [PubMed] [Google Scholar]
- 36.Hua W., Zhang Y., Wu X., Kang L., Tu J., Zhao K., Li S., Wang K., Song Y., Luo R., Shao Z., Yang S., Yang C. Icariin attenuates interleukin-1β-induced inflammatory response in human nucleus pulposus cells. Curr. Pharmaceut. Des. 2018;23:6071–6078. doi: 10.2174/1381612823666170615112158. [DOI] [PubMed] [Google Scholar]
- 37.Sampara P., Banala R.R., Vemuri S.K., Av G.R., Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018;25:67–82. doi: 10.1038/S41434-018-0004-0. [DOI] [PubMed] [Google Scholar]
- 38.Gonçalves G.A.R., Paiva R. de M.A. Gene therapy: advances, challenges and perspectives. Einstein. 2017;15:369. doi: 10.1590/S1679-45082017RB4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schäfer R., Spohn G., Baer P.C. Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy? Transfus. Med. Hemotherapy. 2016;43:256–267. doi: 10.1159/000447458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai D., Schol J. Cell therapy for intervertebral disc repair: clinical perspective. J. Orthop. Transl. 2017;9:8–18. doi: 10.1016/J.JOT.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z., Chen L., Zeng C., Wang W.E. Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells Int. 2018 doi: 10.1155/2018/7045245. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong W., Lu Z., Qin L., Mauck R.L., Smith H.E., Smith L.J., Malhotra N.R., Heyworth M.F., Caldera F., Enomoto-Iwamoto M., Zhang Y. Cell therapy for the degenerating intervertebral disc. Transl. Res. 2017;181:49–58. doi: 10.1016/J.TRSL.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma A. The role of adipokines in intervertebral disc degeneration. Med. Sci. 2018;6(6):34. doi: 10.3390/MEDSCI6020034. (2018) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francis S.L., Duchi S., Onofrillo C., Di Bella C., Choong P.F.M. Adipose-derived mesenchymal stem cells in the use of cartilage tissue engineering: the need for a rapid isolation procedure. Stem Cells Int. 2018;2018 doi: 10.1155/2018/8947548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baer P.C., Overath J.M., Urbschat A., Schubert R., Koch B., Bohn A.A., Geiger H. Effect of different preconditioning regimens on the expression profile of murine adipose-derived stromal/stem cells. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kakudo N., Morimoto N., Ogawa T., Taketani S., Kusumoto K. Hypoxia enhances proliferation of human adipose-derived stem cells via HIF-1α activation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang S., Kim S.M., Sung J.H. Cellular and molecular stimulation of adipose-derived stem cells under hypoxia. Cell Biol. Int. 2014;38:553–562. doi: 10.1002/cbin.10246. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira J.R., Teixeira G.Q., Santos S.G., Barbosa M.A., Almeida-Porada G., Gonçalves R.M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinjikji W., Luetmer P.H., Comstock B., Bresnahan B.W., Chen L.E., Deyo R.A., Halabi S., Turner J.A., Avins A.L., James K., Wald J.T., Kallmes D.F., Jarvik J.G. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am. J. Neuroradiol. 2015;36:811–816. doi: 10.3174/AJNR.A4173. [DOI] [PMC free article] [PubMed] [Google Scholar]