Abstract
Objectives
Breast cancer screening guidelines could provide valuable tools for clinical decision making by reviewing the available evidence and providing recommendations. Little information is known about how many countries have issued breast cancer screening guidelines and the differences among existing guidelines. We systematically reviewed current guidelines and summarized corresponding recommendations, to provide references for good clinical practice in different countries.
Methods
Systematic searches of MEDLINE, EMBASE, Web of Science, and Scopus from inception to March 27th, 2021 were conducted and supplemented by reviewing the guideline development organizations. The quality of screening guidelines was assessed from six domains of the Appraisal of Guidelines for Research and Evaluation Ⅱ (AGREE Ⅱ) instrument by two appraisers. The basic information and recommendations of the issued guidelines were extracted and summarized.
Results
A total of 23 guidelines issued between 2010 and 2021 in 11 countries or regions were identified for further review. The content and quality varied across the guidelines. The average AGREE Ⅱ scores of the guidelines ranged from 33.3% to 87.5%. The highest domain score was "clarity of presentation" while the domain with the lowest score was "applicability". For average-risk women, most of the guidelines recommended mammographic screening for those aged 40–74 years, specifically, those aged 50–69 years were regarded as the optimal age group for screening. Nine of 23 guidelines recommended against an upper age limit for breast cancer screening. Mammography (MAM) was recommended as the primary screening modality for average-risk women by all included guidelines. Most guidelines suggested annual or biennial mammographic screening. Risk factors of breast cancer identified in the guidelines mainly fell within five categories which could be broadly summarized as the personal history of pre-cancerous lesions and/or breast cancer; the family history of breast cancer; the known genetic predisposition of breast cancer; the history of mantle or chest radiation therapy; and dense breasts. For women at higher risk, there was a consensus among most guidelines that annual MAM or annual magnetic resonance imaging (MRI) should be given, and the screening should begin earlier than the average-risk group.
Conclusions
The majority of 23 included international guidelines were issued by developed countries which contained roughly the same but not identical recommendations on breast cancer screening age, methods, and intervals. Most guidelines recommended annual or biennial mammographic screening between 40 and 74 years for average-risk populations and annual MAM or annual MRI starting from a younger age for high-risk populations. Current guidelines varied in quality and increased efforts are needed to improve the methodological quality of guidance documents. Due to lacking clinical practice guidelines tailored to different economic levels, low- and middle-income countries (LMICs) should apply and implement the evidence-based guidelines with higher AGREE Ⅱ scores considering local adaption.
Keywords: Breast neoplasms, Screening, Guideline, Systematic review
Highlights
-
•
This systematic review comprehensively maps the recommendations of the latest international breast screening guidelines, providing valuable tools for clinical decision making in different settings.
-
•
Most guidelines recommend annual or biennial mammographic screening between 40 and 74 years for the average-risk populations and annual MAM or annual MRI starting from a younger age for the high-risk populations. However, there are indeed discrepancies in screening age, methods, and intervals among countries.
-
•
High-quality evidence and rigorous methodology are the keys to guidance development, but current guidelines vary in methodological quality.
1. Introduction
In 2021, breast cancer has overtaken lung cancer to be the world's most commonly diagnosed cancer, accounting for the severe burden globally, especially among women [1]. Screening for breast cancer is an effective measure to detect early-stage disease and improve the survival rate of cancer patients [[2], [3]]. Population-based breast cancer screening programs have been implemented in many developed countries over the last decades, which contributed to reducing the mortality and the advanced cancer rate [[4], [5], [6]].
Screening guidelines could provide valuable tools for clinical decision making by reviewing the available evidence and providing recommendations. To date, several breast cancer screening guidelines have been issued in many developed countries [[7], [8], [9]]. However, the recommendations about screening age, methods, and intervals varied from different guidelines due to different institutions, based evidence, and development processes. This may confuse the clinical practice when they are applied to other countries. To our knowledge, it is currently unknown how many countries have issued breast cancer screening guidelines and the differences among these issued guidelines. Additionally, previous systematic reviews of international breast cancer screening guidelines were limited by publication date and screening population and did not systematically review screening recommendations for the population with different breast cancer risks [[10], [11], [12]].
Accordingly, our study reviewed existing breast cancer screening guidelines and summarized corresponding recommendations, in order to provide references for good clinical practice in different countries.
2. Material and methods
2.1. Data sources and searches
A search strategy was designed for MEDLINE, EMBASE, Web of Science, and Scopus from inception to March 27th, 2021 using variations on the search terms "breast cancer", "screening" and "guidelines/recommendations" (Appendix A). We also sought the additional guidelines by searching guideline development organizations, such as Guideline International Network (GIN), World Health Organization (WHO), Cancer Australia, Ministry of Health (MOH) Malaysia, and China Guideline Clearinghouse (CGC). Moreover, we meticulously examined the references of documents obtained above to further access potentially eligible articles.
2.2. Study selection and data extraction
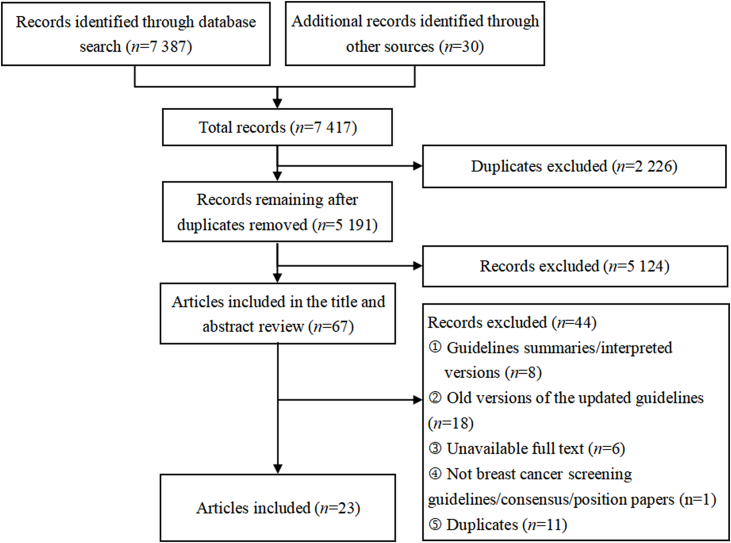
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is presented in Fig. 1. Two reviewers (MYC and WHR) independently reviewed the titles and abstracts of the included guidelines. Any discrepancies were resolved by discussion. Finally, both reviewers determined the included guidelines based on the full text. We included guidelines following inclusion criteria: (1) originally published guidelines, consensus, or position papers related to breast cancer screening; (2) the latest versions of the updated guidelines; (3) English or Chinese guidelines; and (4) full text was available. We excluded guidelines if they were: summaries or interpreted versions of guidelines.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Two independent reviewers (MYC and WHR) extracted information using a predesigned template. The information extracted included: (1) basic information (countries or regions, publication years, publication organizations, names of guidelines, number of updated versions, and publication years of old versions); (2) screening recommendations for the population at average risk and higher risk (screening age, screening methods, screening intervals, level of evidence, and strength of recommendation).
2.3. Quality assessment
The methodological quality of guidelines was evaluated using the Appraisal of Guidelines for Research and Evaluation Ⅱ (AGREE Ⅱ) instrument. This is a standardized tool for evaluating the methodological framework of guideline development which consists of 23 main items in six domains (scope and purpose, stakeholder involvement, rigour of development, clarity of presentation, applicability, and editorial independence) and two global rating items [13]. Each item is rated on a seven-point Likert-type scale from one (strongly disagree) to seven (strongly agree) according to the criteria and considerations articulated in the User's Manual. Scores are assigned depending on the completeness and quality of reporting. Scores increase as more criteria are met and considerations are addressed. Domain scores are calculated by summing up all the scores of the individual items in that domain and by scaling the total as a percentage of the maximum possible score for that domain. Two reviewers (MYC and WHR) independently scored each guideline. Evaluation results were compared and discrepancies of more than two points per item were discussed to reach a consensus. According to prior studies, the quality of guidelines was classified as high if the total score was 60% or higher and low if the score was less than 60% [14,15].
3. Results
A total of 7417 citations were included during the preliminary literature search process, but most were excluded after deleting duplicates and applying the inclusion and exclusion criteria. Of these, 23 guidelines were identified for further review (Fig. 1).
3.1. Guideline characteristics
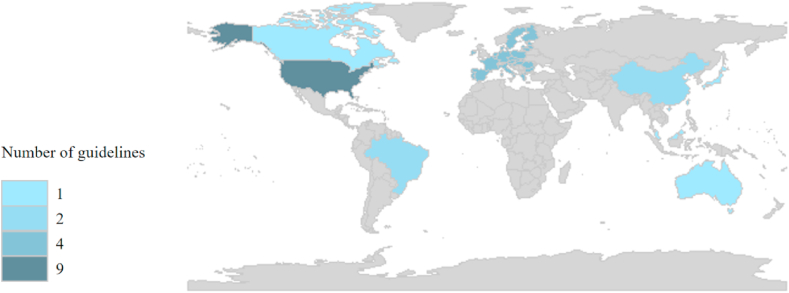
Table 1 displays the general characteristics of 23 included guidelines that were published between 2010 and 2021 [[7], [8], [9],[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]]. The majority of guidelines (17 of 23) were drawn from developed countries or regions. Guidelines from the United States accounted for the largest proportion, reaching 39.1%. One was developed by WHO, and four in Europe (Fig. 2). 12 of 23 guidelines have been updated.
Table 1.
Characteristics of 23 included guidelines on screening for breast cancer.
| Countries/Regions | Publication years | Publication organizations | Names of guidelines | Number of updated versions | Publication years of old versions |
|---|---|---|---|---|---|
| Global [16] | 2014 | WHO | WHO position paper on mammography screening | None | None |
| The United States [17] | 2019 | ACP | Screening for Breast Cancer in Average-Risk Women: A Guidance Statement From the American College of Physicians | 1 | 2007 |
| The United States [18] | 2019 | NCCN | Breast Cancer Screening and Diagnosis, Version 1.2019 | 9 | 1998 2003 2006 2010 2013 2015 2016 2017 2018 |
| The United States [19] | 2017 | ACR | ACR Appropriateness Criteria® Breast Cancer Screening | 2 | 1998 2013 |
| The United States [20] | 2018 | ACR | Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR | None | None |
| The United States [21] | 2017 | ACR | Breast Cancer Screening for Average-Risk Women: Recommendations From the ACR Commission on Breast Imaging | None | None |
| The United States [22] | 2010 | ACR and SBI | Breast Cancer Screening With Imaging: Recommendations From the Society of Breast Imaging and the ACR on the Use of Mammography, Breast MRI, Breast Ultrasound, and Other Technologies for the Detection of Clinically Occult Breast Cancer | None | None |
| The United States [7] | 2016 | USPSTF | Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement | 3 | 1996 2002 2009 |
| The United States [23] | 2015 | ACS | Breast Cancer Screening for Women at Average Risk 2015 Guideline Update From the American Cancer Society | 3 | 1992 1997 2003 |
| The United States [24] | 2019 | ACOG | Breast Cancer Risk Assessment and Screening in Average-Risk Women | 3 | 2003 2011 2017 |
| Europe [25] | 2020 | ECIBC | Breast Cancer Screening and Diagnosis: A Synopsis of the European Breast Guidelines | None | None |
| Europe [8] | 2019 | ESMO | Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up | 1 | 2015 |
| Europe [26] | 2010 | EUSOMA | Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group | None | None |
| Canada [27] | 2018 | CTFPHC | Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer | 3 | 1994 2001 2011 |
| Germany [28] | 2018 | AWMF, DKG, and DKH | The Screening, Diagnosis, Treatment, and Follow-Up of Breast Cancer | 1 | 2012 |
| Australia [29] | 2015 | Cancer Australia | Early detection of breast cancer | 2 | 2009 2004 |
| Singapore [30] | 2010 | MOH | Cancer screening | None | None |
| Malaysia [31] | 2019 | MOH | Management of Breast Cancer (3rd Edition) | 2 | 2002 2010 |
| Japan [9] | 2016 | NCC Japan | The Japanese Guidelines for Breast Cancer Screening | 2 | 2013 2015 |
| China [32] | 2021 | NCC China | China Guideline for the Screening and Early Detection of Female Breast Cancer (2021, Beijing) | None | None |
| Hong Kong, China [33] | 2018 | CEWG | Recommendations on prevention and screening for breast cancer in Hong Kong | None | None |
| Brazil [34] | 2018 | MOH | Guidelines for early detection of breast cancer in Brazil. II – New national recommendations, main evidence, and controversies | None | None |
| Brazil [35] | 2017 | CBR, SBM, and FEBRASGO | Breast Cancer Screening: Updated Recommendations of the Brazilian College of Radiology and Diagnostic Imaging, Brazilian Breast Disease Society, and Brazilian Federation of Gynecological and Obstetrical Associations | None | None |
Abbreviations: ACOG: American College of Obstetricians and Gynecologists; ACP: American College of Physicians; ACR: American College of Radiology; ACS: American Cancer Society; AWMF: German Association of Scientific Medical Societies; CBR: Brazilian College of Radiology and Diagnostic Imaging; CEWG: Cancer Expert Working Group; CTFPHC: Canadian Task Force on Preventive Health Care; DKG: German Cancer Society; DKH: German Cancer Aid; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; MOH: Ministry of Health; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; SBI: Society of Breast Imaging; SBM: Brazilian Society for Breast Disease; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization.
Fig. 2.
Geographical distribution of the included breast cancer screening guidelines.
3.2. Quality assessment
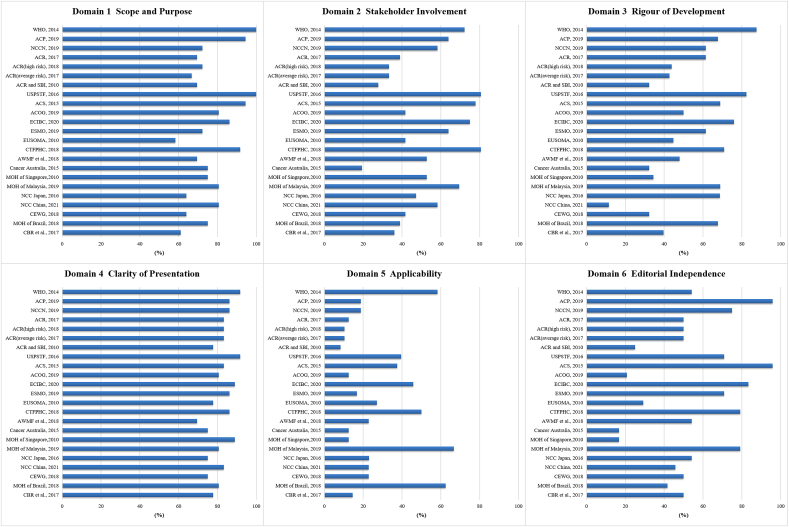
The included 23 guidelines were appraised using AGREE II Criteria (Fig. 3). The average AGREE II scores varied from 33.3% to 87.5%. 12 guidelines were scored over 60.0% [[7], [8], [16], [17], [23], [24], [25], [27], [30], [31], [32], [34]]. Among these, the guideline issued by Canadian Task Force on Preventive Health Care (CTFPHC) [27] was scored the highest (87.5%), followed by European Commission Initiative on Breast Cancer (ECIBC) [25], American Cancer Society (ACS) [23], United States Preventive Services Taskforce (USPSTF) [7], and WHO [16]. The highest domain score was "clarity of presentation" (domain 4), with an average score of 81.9%, followed by "scope and purpose" (domain 1). The domain with the lowest score was "applicability" (domain 5) with an average score of 21.3%, followed by "stakeholder involvement" (domain 2).
Fig. 3.
Quality of the included guidelines for the six domains of the AGREE Ⅱ instrument.
(Abbreviations: ACOG: American College of Obstetricians and Gynecologists; ACP: American College of Physicians; ACR: American College of Radiology; ACS: American Cancer Society; AWMF: German Association of Scientific Medical Societies; CBR: Brazilian College of Radiology and Diagnostic Imaging; CEWG: Cancer Expert Working Group; CTFPHC: Canadian Task Force on Preventive Health Care; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; MOH: Ministry of Health; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; SBI: Society of Breast Imaging; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization).
3.3. Strength of recommendations and quality of evidence
17 of 23 guidelines reported eight applied grading systems. Grading of Recommendations, Assessment, Development and Evaluations (GRADE) was the common system that was applied in six guidelines [16,23,25,27,32,34]. Four guidelines used the self-designated grading system [8,9,18,30]. The details about the strength of recommendations and the quality of evidence varied in different grading systems. The information of evidence and recommendation about the included guidelines is shown in Table 2.
Table 2.
Grading systems used in the included guidelines.
| Grading systems | Guideline organizations | Level of evidence | Strength of recommendations |
|---|---|---|---|
| GRADE | WHO, 2014 [16]; ACS, 2015 [23]; ECIBC, 2020 [25]; CTFPHC, 2018 [27]; MOH of Brazil, 2018 [34]; NCC China, 2021 [32] | High; Moderate; Low; Very low | Strong; Qualified/Conditional; Weak |
| GRADE + RAM | ACR, 2017 [19] | Strong; Moderate; Limited | Usually appropriate; May be appropriate; Usually not appropriate |
| USPSTF | USPSTF, 2010 [7]; ACOG, 2019 [24] | Ⅰ; Ⅱ-1; Ⅱ-2; Ⅱ-3; Ⅲ | A; B; C; D; I (insufficient) |
| USPSTF + GRADE | MOH of Malaysia, 2019 [31] | Ⅰ; Ⅱ-1; Ⅱ-2; Ⅱ-3; Ⅲ | Strong; Conditional |
| OCEBM | EUSOMA, 2010 [26]; AWMF, DKG, and DKH, 2018 [28] | 1 a/1 b/1 c; 2 a/2 b; 3 a/3 b; 4; 5 | A; B; C; D |
| OCEBM + GRADE | CBR, SBM, and FEBRASGO, 2018 [35] | None | A; B; C; D |
| NCCN | NCCN, 2019 [18] | 1; 2 A; 2 B; 3 | A; B; C; D |
| Adapted from the Infectious Disease Society of America-United States Public Health Service Grading System | ESMO, 2019 [8] | Ⅰ; Ⅱ; Ⅲ; Ⅳ; Ⅴ | A; B; C; D; E |
| JRGCSG | NCC Japan, 2016 [9] | None | A; B; C; D; I (insufficient) |
| MOH, Singapore | MOH of Singapore, 2010 [30] | 1++; 1+; 1-; 2++; 2+; 2-;3; 4 | A; B; C; D; GPP |
Abbreviations: ACOG: American College of Obstetricians and Gynecologists; ACS: American Cancer Society; ACR: American College of Radiology; AWMF: German Association of Scientific Medical Societies; CBR: Brazilian College of Radiology and Diagnostic Imaging; CTFPHC: Canadian Task Force on Preventive Health Care; DKG: German Cancer Society; DKH: German Cancer Aid; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; GPP: Good Practice Points; GRADE: Grading of Recommendations, Assessment, Development and Evaluations; JRGCSG: Japanese Research Group for the Development of Cancer Screening Guidelines; MOH: Ministry of Health; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; OCEBM: Oxford Centre for Evidence-based Medicine; RAM: RAND/UCLA Appropriateness Method; SBM: Brazilian Society for Breast Disease; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization.
3.4. The screening recommendations for women at average risk
The detailed information of recommendations for average-risk women is shown in Table 3, which summarized screening age, screening methods, screening intervals, and other recommended screening methods (Fig. 4).
Table 3.
The screening recommendations in average-risk women in eligible guidelines.
| Guidelines | Age range for screening | Age to end screening | Screening methods | Screening intervals | Recommendations for other screening methods |
|---|---|---|---|---|---|
| WHO, 2014 [16] | 40–49 years; 70-74 years | NR | MAM
|
NR | NR |
| 50–69 years |
|
|
CBE seems to be a promising approach in limited resource settings with weak health systems | ||
| ACP, 2019 [17] | 40–49 years | ≥ 75 years or in women with a life expectancy of 10 years or less | NR
|
NR | Not recommend CBE |
| 50–74 years | MAM | Biennial | |||
| NCCN, 2019 [18] | 25–39 years |
|
Clinical encounter
|
Every 1–3 years |
|
| ≥ 40 years | Clinical encounter
|
Annual | |||
MAM
|
Annual | ||||
| ACR, 2017 [19] | ≥ 40 years | NR | MAM or DBT | Annual | For women with dense breasts, US may be considered, but the increased cancer detection and the increased risk of a false-positive examination should be weighed |
| ACR (Average-risk), 2017 [21] | ≥ 40 years | The age to stop screening should be based on each woman's health status rather than an age-based determination | MAM | Annual | No sufficient data to support the use of breast MRI and MBI as a screening tool for average-risk women |
| ACR and SBI, 2010 [22] | ≥ 40 years |
|
MAM | Annual | NR |
| USPSTF, 2016 [7] | 40–49 years | 75 years
|
MAM
|
Biennial
|
|
| 50–74 years | MAM
|
Biennial
|
|||
| ACS, 2015 [23] | 40–44 years | Screening should continue as long as a woman is in good health and is expected to live at least 10 more years | MAM
|
Annual
|
Not recommend CBE |
| 45–54 years | MAM
|
Annual
|
|||
| ≥ 55 years | MAM
|
Annual or biennial
|
|||
| ACOG, 2019 [24] | 25–39 years |
|
CBE
|
Every 1–3 years
|
|
| ≥ 40 years | MAM
|
Annual or biennial
Biennial (after age 55)
|
|||
CBE
|
Annual
|
||||
| ECIBC, 2020 [25] | 45–49 years | NR | MAM
|
|
|
| 50–69 years | MAM
|
|
|||
| 70–74 years | MAM
|
|
|||
| ESMO, 2019 [8] | 40–49 years; 70–74years | NR | MAM [B] | NR | NR |
| 50–69 years | MAM [A] | Annual or biennial [A] | |||
| CTFPHC, 2018 [27] | 50–74 years | NR | MAM
|
Every 2–3 years
|
|
| AWMF, DKG, and DKH, 2020 [28] | 50–69 years | ≥ 70 years: taking into consideration their individual risk profile and health status, as well as a life expectancy of more than 10 years | MAM | Biennial | Insufficient evidence about other imaging examination (tomosynthesis, US, MRI, or other techniques) contributes to a reduction in breast cancer mortality, neither as a supplemental examination nor a substitute for MAM |
| Cancer Australia, 2015 [29] | 40–49 years | ≥ 75 years: be eligible to receive free MAM, but do not receive an invitation to attend | MAM (discuss, by SDM) | NR | No evidence to recommend for or against CBE |
| 50–74 years | MAM | Biennial | |||
| MOH of Singapore, 2010 [30] | 40–49 years | ≥70 years: be individualized by considering the potential benefits and risks of mammography in the context of current health status and estimated life expectancy | MAM
|
Annual
|
US and CBE are not routinely required |
| 50–69 years | MAM
|
Biennial
|
|||
| MOH of Malaysia, 2019 [31] | 50–74 years | NR | MAM | Biennial | NR |
| NCC Japan, 2016 [9] | 40–64 years | NR | MAM with CBE | NR | CBE and US are not recommended for population-based screening |
| 40–74 years | MAM without CBE | ||||
| NCC China, 2021 [32] | ≥ 45 years | NR |
|
Annual or biennial
|
|
| MOH of Brazil, 2018 [34] | 50–69 years |
|
MAM
|
Biennial
|
|
| CBR, SBM, and FEBRASGO, 2017 [35] | 40–74 years | ≥ 75 years
|
MAM (preferably digital MAM)
|
Annual
|
|
Abbreviations: ABUS: Automated Breast Ultrasonography; ACOG: American College of Obstetricians and Gynecologists; ACP: American College of Physicians; ACR: American College of Radiology; ACS: American Cancer Society; AWMF: German Association of Scientific Medical Societies; BSE: Breast Self-Examination; CBE: Clinical Breast Examination; CBR: The Brazilian College of Radiology and Diagnostic Imaging; CTFPHC: Canadian Task Force on Preventive Health Care; DKG: German Cancer Society; DKH: German Cancer Aid; DBT: Digital Breast Tomosynthesis; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; HHUS: Hand-Held Ultrasound; MAM: Mammography; MBI: Molecular Breast Imaging; MOH: Ministry of Health; MRI: Magnetic Resonance Imaging; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; NR: No Recommendation; SBM: The Brazilian Society for Breast Disease; SDM: Shared Decision Making; US: Ultrasound; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization.
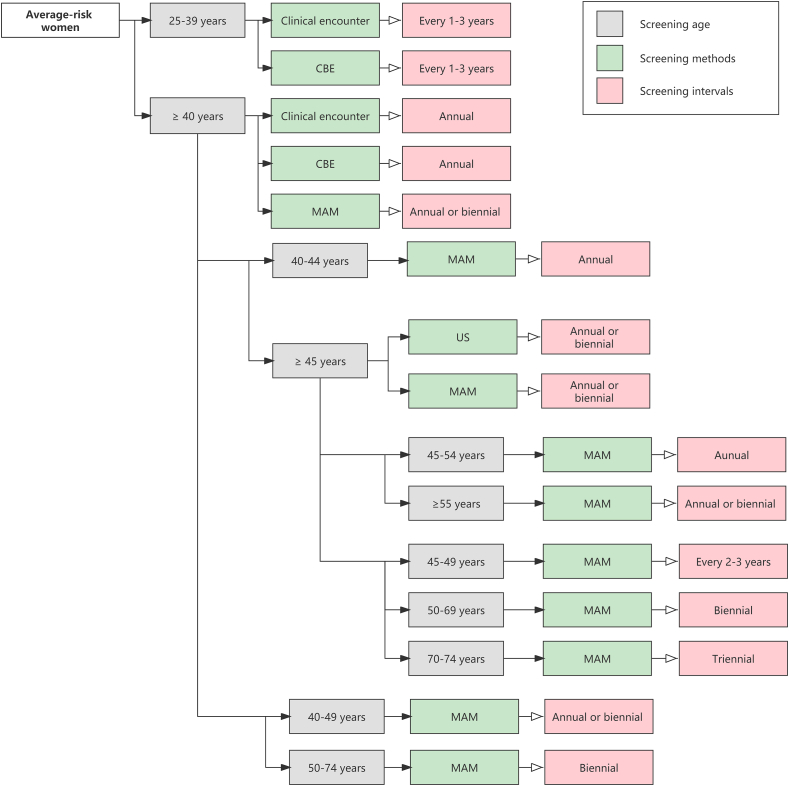
Fig. 4.
The main screening recommendations in average-risk women in the eligible guidelines.
(Abbreviations: CBE: Clinical Breast Examination; MAM: Mammography; US: Ultrasound)
3.4.1. Screening age
The majority of guidelines recommended mammographic screening for average-risk individuals aged 40–74 years [[7], [8], [9],16,17,29,35], and recommended women aged 50–69 years as the optimal age group for screening with strong recommendation [8,16,25,28,30,34]. National Comprehensive Cancer Network (NCCN) [18] and American College of Obstetricians and Gynecologists (ACOG) [24] suggested starting screening at age 25 by clinical encounter or clinical breast examination (CBE).
Nine of 23 guidelines did not recommend an upper age limit for breast cancer screening [8,9,16,18,20,25,27,31,32]. Some guidelines, including American College of Radiology (ACR) [21], ACR and Society of Breast Imaging (SBI) [22], and ACS [23] suggested that the age to end screening should be determined based on the women's health status, for example, stopping screening for women with life expectancy lower than 5–7 years or 10 years. Other guidelines, like USPSTF [7], American College of Physicians (ACP) [17], and Brazilian College of Radiology and Diagnostic Imaging (CBR)/Brazilian Society for Breast Disease (SBM)/Brazilian Federation of Gynecological and Obstetrical Associations (FEBRASGO) [35] did not recommend breast cancer screening for women aged over 75 years unless their life expectancy were higher than 7 years or 10 years. German Association German Cancer Society of Scientific Medical Societies (AWMF)/German Cancer Society (DKG)/German Cancer Aid (DKH) [28] and MOH of Singapore [30] recommended stopping screening at age 70.
3.4.2. Screening methods and intervals
Mammography (MAM) was recommended as the primary screening modality for average-risk women by all included guidelines [[7], [8], [9], [16], [17], [18], [19], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [34], [35]]. Most guidelines suggested annual or biennial mammographic screening [7,16,17,29,31]. Three guidelines recommended screening every 1–2 years [[8], [23], [32]]. Some guidelines agreed that screening intervals should be determined based on age [18,24]. ACS [23] recommended screening with MAM annually for women aged 40–54 years and every 1–2 years for women aged 55 years or older. ECIBC [25] recommended screening every 2–3 years for women aged 40–49 years and for women aged 70–74 years. For the priority screening groups (women aged 50–69 years), annual screening was not recommended, and biennial screening is better than triennial screening.
The recommendations of each guideline on CBE and ultrasound (US) were different in detail. NCCN [18] and ACOG [24] suggested that CBE should be given every 1–3 years for women aged 25–39 years and annually for women older than 40 years, but ACS [23] and CTFPHC [27] did not recommend CBE as a primary screening method. Among the included screening guidelines, only National Cancer Centre (NCC) of China [32] recommended screening every 1–2 years for women older than 45 years using US alone.
All guidelines did not recommend using breast self-examination (BSE), magnetic resonance imaging (MRI), and computed tomography (CT) to screen for average-risk women because of lacking evidence of benefit.
3.5. The screening recommendations for women at higher risk
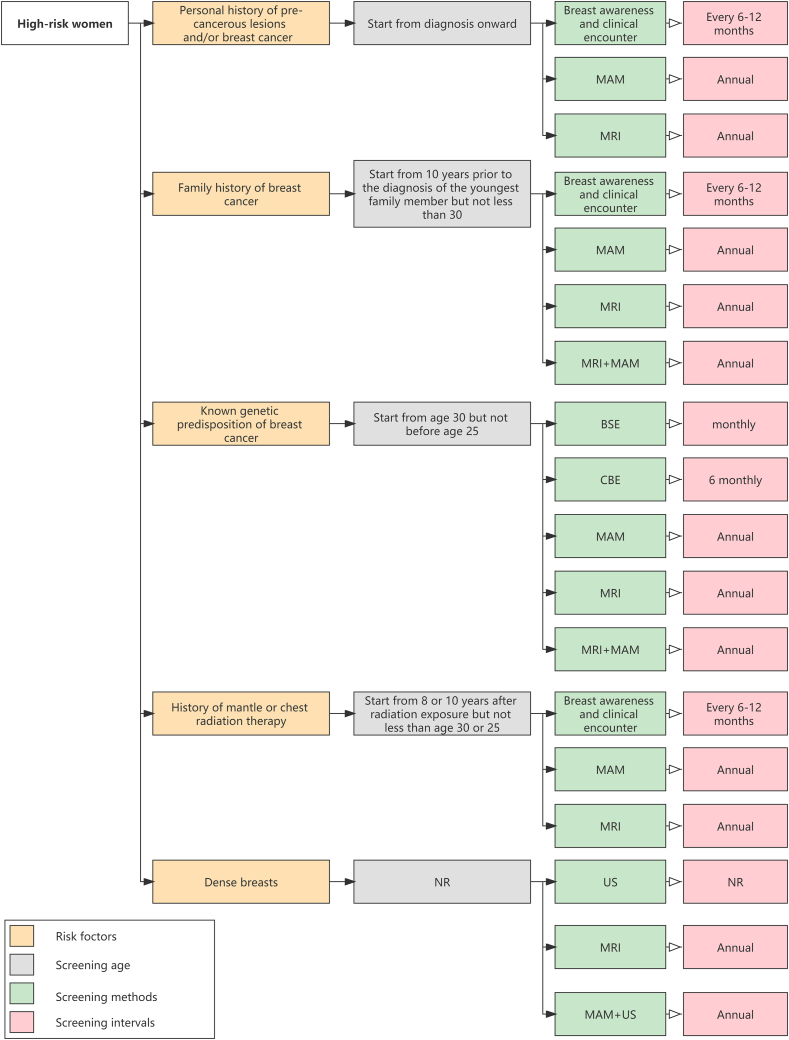
Risk factors of breast cancer identified in the guidelines mainly fell within five categories which could be broadly summarized as the personal history of pre-cancerous lesions and/or breast cancer; the family history of breast cancer; the known genetic predisposition of breast cancer; the history of mantle or chest radiation therapy; and dense breasts. For women at higher risk, there was a consensus among most guidelines that annual MAM screening or annual MRI screening should be given and the starting age should be earlier than the average-risk group (Table 4; Fig. 5).
Table 4.
The screening recommendations in high-risk women in eligible guidelines.
| Risk factors | Guidelines | Screening age | Screening methods and intervals |
|---|---|---|---|
|
|
|
|
| ACR (High-risk), 2017 [20] | From the time of diagnosis | MRI: annual | |
|
|
|
|
| MOH of Singapore, 2010 [30] | NR | MAM: annual [Grade D] | |
| MOH of Malaysia, 2019 [31] | 40–59 years, 30–39 years (may be considered) | MAM: annual | |
| ≥60 years | MAM: biennial | ||
| NCC China, 2021 [32] | NR | MAM and US: annual | |
| CEWG, 2018 [33] | From 35 years | MAM: annual | |
| CBR, SBM, and FEBRASGO, 2017 [35] |
|
|
|
| Family history of breast cancer |
|
|
|
|
|
||
|
|
||
| ESMO, 2019 [8] | NR | MAM and MRI: annual (concomitant or alternating) [Ⅲ, A] | |
| NCC China, 2020 [32] | NR | MAM and US: annual | |
| CEWG, 2018 [33] | Begin at age 35 or 10 years prior to the age at diagnosis of the youngest-affected relative (for those with family history), whichever is earlier, but not earlier than age 30. | MAM: annual | |
| Known genetic predisposition of breast cancer |
|
|
|
| ACR (High-risk), 2017 [20] | 30 years | DM+/DBT: annual | |
| 25–30 years | MRI: annual | ||
| ACR and SBI, 2010 [22] | Start by age 30 but not before age 25 | MAM: annual | |
| EUSOMA, 2010 [26] | Start from 30 years; Before 30 years [discuss, mutation carrier of BRCA1 or BRCA2 (start from 25 to 29) and TP53 (start from 20)] |
MRI: annual | |
| MOH of Singapore, 2010 [30] | Start at age 25–30 years for BRCA mutation carriers and their untested first-degree relatives, or as early as 5–10 years before the age of onset of breast cancer in the youngest family member in those with family history of breast cancer but no proven mutation |
|
|
| MOH of Malaysia, 2019 [31] | 30–49 years | MRI: annual | |
| 40–69 years | MAM: annual | ||
| ≥70 years | MAM: biennial | ||
| NCC China, 2020 [32] | NR | MRI: annual | |
| CBR, SBM, and FEBRASGO, 2017 [35] | From 30 years | MAM annual [category B recommendation] | |
| From 25 years | MRI annual [category A recommendation] | ||
| History of mantle or chest radiation therapy | NCCN, 2019 [18] | Start from 10 years after radiation exposure | Breast awareness and clinical encounter: every 6–12 months |
| Start from 10 years after radiation exposure but not less than age 30 | DM: annual, with consideration of tomosynthesis | ||
| Start from 10 years after radiation exposure but not less than age 25 | MRI: annual | ||
| Start from 10 years after radiation exposure for women younger than 25 years who have received prior thoracic irradiation | Breast awareness, counseling on risk and an annual clinical encounter | ||
| ACR, 2017 [19] | Start from age 25 or 8 years after radiation therapy, whichever is later | MAM | |
| ACR (High-risk), 2017 [20] | Start from age 25 or 8 years after radiation therapy, whichever is later | DM+/DBT: annual | |
| NCC China, 2020 [32] | NR | MRI: annual | |
|
|
|
|
| CBR, SBM, and FEBRASGO, 2017 [35] | Start from the 8th year after radiotherapy onward, but not begin before age 30 | MAM: annual [category C recommendation] | |
| Start from the 8th year after radiotherapy onward, but not begin before age 25 | MRI: annual [category C recommendation] | ||
| Dense breasts | ACR, 2017 [19] | NR | Consider: US |
| ACR (High-risk), 2017 [20] | NR |
|
|
| ACR and SBI, 2010 [22] | NR | Consider: US as an adjunct to MAM | |
| China NCC, 2020 [32] | NR | MAM and US: annual | |
| CBR, SBM, and FEBRASGO, 2017 [35] | NR | Consider: US as an adjunct to MAM |
Abbreviations: ACR: American College of Radiology; BRCA: Breast cancer gene; BSE: Breast Self-Examination; CBE: Clinical Breast Examination; CBR: Brazilian College of Radiology and Diagnostic Imaging; CEWG: Cancer Expert Working Group; DBT: Digital Breast Tomosynthesis; DM: Digital Mammography; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; MAM: mammography; MOH: Ministry of Health; MRI: Magnetic Resonance Imaging; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; NR: No Recommendation; SBI: Society of Breast Imaging; SBM: Brazilian Society for Breast Disease; US: Ultrasound.
Fig. 5.
The main screening recommendations in high-risk women in the eligible guidelines.
(Abbreviations: BSE: Breast Self Examination; CBE: Clinical Breast Examination; MAM: Mammography; MRI: Magnetic Resonance Imaging; NR: No Recommendation; US: Ultrasound)
3.5.1. Women with the personal history of pre-cancerous lesions and/or breast cancer
For women with biopsy-proven Lobular Carcinoma in Situ (LCIS), Atypical Ductal Hyperplasia (ADH), Ductal Carcinoma in Situ (DCIS), or invasive breast cancer or ovarian cancer, annual MAM or annual MRI was mainly recommended after diagnosis onward [18,20,22,31,33,35]. Especially for patients with unilateral invasive breast cancer, close monitoring of the contralateral breast was recommended. NCCN [18] also recommended breast awareness and clinical encounter every 6–12 months for this group of women. NCC of China [32] recommended MAM and US as screening methods for women at higher risk of breast cancer.
3.5.2. Women with the family history of breast cancer
For women with a family history suspicious of the inherited predisposition of breast cancer, two guidelines recommended that an annual MAM or annual MRI began 10 years before the age of diagnosis of the youngest-affected relative but not before the age of 30 [18,33]. NCCN [18] also recommended regular clinical visits every 6–12 months once the women were identified as begin at increased risk of breast cancer.
3.5.3. Women with the known genetic predisposition of breast cancer
Women with breast cancer susceptibility gene 1 (BRCA1) or breast cancer susceptibility gene 2 (BRCA2) mutations, or untested but have first-degree relatives (mothers, sisters, or daughters) who are proven to have BRCA mutations, have a higher risk for breast cancer. Two guidelines recommended that women with gene mutations should start to undertake annual MAM or annual MRI at 25–30 years [22,30]. European Society of Breast Cancer Specialists (EUSOMA) [26] recommended that annual MRI screening was performed for women carrying BRAC at 25–29 years, and those carrying TP53 at 20 years. MOH of Malaysia [31] provided age-specific recommendations for women carrying gene mutations, specifically, annual MRI for 30–49 years, annual MAM for 40–69 years, and biennial MAM for 70 years and above. For other recommended screening methods, ACR [19] recommended MRI as an adjunct to MAM or DBT and recommended US when the patient cannot tolerate MRI. MOH of Singapore [30] also recommended monthly BSE and 6 monthly CBE.
3.5.4. Women with the history of mantle or chest radiation therapy
For women with a history of mantle or chest radiation therapy that occurred before the age of 30 years or had a cumulative dose of 10 Gy radiation, most guidelines recommended starting regular screening 8 or 10 years after radiation therapy [[18], [19], [20],35]. Recommended screening strategies included annual MAM (not before age 30), annual MRI (not before age 25), or annual digital mammography (DM) (with or without digital breast tomosynthesis (DBT)). NCCN [18] also recommended increasing breast awareness or clinical encounters every 6–12 months.
3.5.5. Women with dense breasts
For women with dense breasts, ACR [20] recommended MRI should be performed annually. NCC of China [32] recommended screening with MAM and US annually. US (as adjunctive screening tools) was recommended for high-risk women who may be suitable for MRI but can not be accepted for any reason [20]. Two guidelines [[22], [35]] also recommended US as an adjunctive examination to MAM in asymptomatic women with dense breasts.
4. Discussion
To the best of our knowledge, this study is the largest and most comprehensive systematic review, which identified and compared the latest international breast screening guidelines and recommendations. A total of 23 guidelines issued between 2010 and 2021 in 11 countries or regions were included in this study. The content and quality varied between the guidelines. The average AGREE Ⅱ scores ranged from 33.3% to 87.5%, which is consistent with that reported by Li J et al. [12]. We found discrepancies between guidelines concerning screening age, methods, and intervals. In general, the majority of guidelines agreed upon annual or biennial MAM for average-risk women aged 40 to 74. Annual MAM or annual MRI should be given and start earlier for women at high risk for breast cancer.
Our study showed that many low- and middle-income countries (LMICs) lacked published clinical practice guidelines for breast cancer screening. Most included guidelines in our study were issued by developed countries, mainly in the United States (9/23) and Europe (4/23). One possible explanation is that high-income countries have accumulated more high-quality evidence for developing guidelines by implementing breast cancer screening programs and related research for a long time [[4], [5], [6]]. However, although LMICs have a severe breast cancer burden, few tailored guidelines have been issued due to lacking sufficient national evidence about breast cancer screening and the front-line impact of sparse resources to develop guidelines in these areas [36,37]. Additionally, some LMICs guidelines might be published in local languages and were not picked up in our search. We also found that some guidelines issued by LMICs are often based on evidence from high-income countries. The extent to which these guidelines can be applied to the clinical practice of routine screening in LMICs is unknown.
High-quality guidelines are vital to facilitate clinical decision making and to improve health outcomes and health service efficiency. Our findings showed nearly half of the included guidelines were rated as high quality. Most of the guidelines provided a clear description of "scope and purpose" as screening for populations with different breast cancer risks, and screening recommendations were described clearly. For these reasons, the domains "scope and purpose" and "clarity of presentation" received high scores. In contrast, the majority of the guidelines received low scores in the domains of "rigour of development" and "applicability". According to prior studies [[38], [39]], the domain "rigour of development" was the most relevant to the overall quality of the guideline. The main reason was that this domain reflects the evidence collection and synthesis process, as well as the formation and follow-up update of recommendations, which can provide enough information to evaluate whether the guidelines followed the best methodology and developed evidence-based recommendations. Meanwhile, the development process of guidelines is also one of the key reasons causing the variations between the recommendations from different guidance documents. In our study, 17 of 23 guidelines reported using eight different grading systems to evaluate the quality of evidence and strength of recommendations, which somewhat impeded the implementation of the guidelines and caused confusion in clinical practice. The most important purpose of guidelines is to promote their application to real-world medicine practice. Therefore, guideline developers should clearly describe the promotion conditions and hindrance factors in the implementation of recommendations and their improvement strategies, as well as consider the likely resource implications involved. At the same time, the quality of the "applicability" domain also plays a critical role in whether they can be extended to LMICs that might lack indigenous guidelines. Our study showed that the scores of different guidelines varied greatly in the domain "applicability". For example, the guideline issued by MOH of Malaysia [31] contained a separate section called "implementing the guidelines", which described the types of facilitators and barriers in detail, as well as put forward suggestions to ensure the implementation of the guideline. In contrast, the "ACR Appropriateness Criteria® Breast Cancer Screening" [19] did not mention facilitators and barriers to its application. Based on the above considerations, we considered the guideline developed by MOH of Malaysia with high "applicability" rather than ACR.
The majority of guidelines recommended mammographic screening for average-risk women aged 40–74 years. 50–69 years were regarded as the optimal age group for screening due to the steep increase of breast cancer beginning around age 50. In 2019, almost 82% of breast cancer was diagnosed among women aged ≥ 50 years in the United States [40]. Most randomized controlled trials (RCTs) from developed countries also showed that mammographic screening between 50 and 69 years had the greatest benefit in reducing mortality [[41], [42]]. However, due to the disease burden of breast cancer and the allocation of public health resources vary in different countries, a one-size-fits-all approach to screening is considered inapplicable. In several Asian countries, such as Japan and South Korea, the peak age of breast cancer incidence in women mainly ranges from 45 to 69 years old which is more than 10 years earlier than that in Europe and the United States [43,44]. Although some Asian guidelines agreed on beginning screening from the age of 40 or 45 years [9,30,32], high-quality evidence from large population-based RCTs is insufficient. In addition, based on several RCTs conducted in Canada, the UK, and Sweden, ECIBC and CTFPHC did not recommend regular screening begin at 40–44 years since the lower absolute benefit and higher overdiagnosis and false positives rate with related biopsies of this age group [2,25,27,[45], [46], [47]]. Furthermore, nine of 23 guidelines did not recommend an upper age limit. However, some guidelines recommended against regular screening for women older than age 70 or 75 years, as the harm potentially exceeds the benefits if screening is continued after these age groups [48]. The risk of breast cancer increases with age. Consequently, the decision to stop screening should be individually based on life expectancy or comorbid conditions.
Currently, MAM is widely accepted in developed countries with sufficient evidence to decrease breast cancer mortality among women aged 50–74 years and is recommended as a primary screening method in most screening guidelines [49]. Due to relatively high cost and the demand for high-quality radiologists, the application of MAM in low resource areas is limited [50]. Additionally, because higher mammographic density is associated with the masking of breast cancer on a mammogram, the sensitivity of MAM for women with dense breasts is lower than that for women with mainly fatty breasts [51]. Mammographic density among Asian women is higher than among Western women [52]. Several Asian studies have shown that US can improve the detection rate of breast cancer for women with dense breasts [53,54]. However, there is limited evidence for US in breast cancer screening to reduce mortality. Accordingly, the guidelines from European and American countries did not recommend US as the primary technique for breast cancer screening in average-risk population, but mainly as a supplemental method to MAM. Among the included guidelines of the present study, only Chinese guidelines recommended US as the primary screening tool. China has carried out a national breast cancer screening program since 2009. The screening tool of the program was changed from CBE to US in 2012, which provided preliminary evidence for the application of US in breast cancer screening in other Asian countries [55,56].
With greater emphasis on more accurate risk management based on patients and more personalized recommendations for diagnosis, treatment, and follow-up, age-oriented screening suggestions have been shifted to risk-based screening recommendations. By accurately identifying women who are above-average risk in the general population, we can provide timely and effective early diagnosis measures. High-risk women identified in the guidelines fell within many categories. The related recommendations for every category of high-risk women were different, which brought some difficulties to the implementation of breast cancer screening for high-risk women in the low resource areas. Thereby, identifying the risk factors of breast cancer by establishing a risk assessment model may be an effective way to prevent breast cancer. Currently, various risk prediction models were developed, such as the Gail model and BOADICEA model, whose application values in different countries are still under evaluation [57,58]. It is reported that China applies risk models as supplementary tools for screening in urban areas [59].
Few guidelines provided explicit recommendations for the management of women with positive findings except for NCCN [18] and NCC China [32]. Improper management of abnormal screening results may compromise the effectiveness of breast cancer screening programs. Doubeni et al. performed the PROSPR multi-model microsimulation study, which showed that the relative risk for the late-stage disease was higher when the time for diagnostic testing was delayed after an abnormal mammogram [60]. A previous study observed that low-income women and women of ethnic minority (African-American and Asian women) were less likely to have adequate follow-up abnormal breast cancer screening mammograms [61]. For these reasons, it is necessary to explore different referral and recall standards according to different initial screening results, to make a balance between the anxiety caused by false-positive breast cancer and the benefit of follow-up.
The strengths of this systematic review include its originality and the most comprehensive search strategy. This study was the largest and comprehensive systematic review to map the recommendations of the latest international breast screening guidelines. Furthermore, we systemically summarized the screening recommendations for both average-risk women and high-risk women.
Our study has some limitations. Even though we performed a comprehensive systematic search, we could not find all relevant guidelines. And we also did not include the breast screening program protocols in some countries. Another limitation was that non-English guidelines were not included in this review due to translation restrictions.
5. Conclusions
In summary, this study reviewed and compared the latest international breast screening guidelines for women both at average risk and at higher risk. The majority of guidelines were issued by developed countries, containing roughly the same but not identical recommendations for breast cancer on screening age, methods, and intervals. Most guidelines recommended annual or biennial mammographic screening for average-risk populations aged between 40 and 74 years and early annual MAM or annual MRI for high-risk populations. Current guidelines varied in methodological quality and increased efforts are needed to develop high-quality guidelines to provide more powerful supporting evidence for guidelines users. LMICs lacked published tailored clinical practice guideline. Therefore, we encourage policymakers and clinicians to use the evidence-based guidelines with higher AGREE Ⅱ scores considering local adaption.
Funding source
This work was supported by International Agency for Research on Cancer, France; World Health Organization, Switzerland [grant numbers CRA/SCR/2019/1].
Declaration of competing interest
All authors declare that they have no conflict of interest.
Acknowledgments
We gratefully acknowledge Ms. Huijiao Yan for her linguistic assistance during the revision of this manuscript.
Footnotes
Present address: Department of Cancer Epidemiology, National Cancer Centre/National Clinical Research Centre for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Pan Jia Yuan Lane, Beijing 100,021, China.
Contributor Information
Wenhui Ren, Email: rwh0617@outlook.com.
Mingyang Chen, Email: chenmy265@163.com.
Youlin Qiao, Email: qiaoy@cicams.ac.cn.
Fanghui Zhao, Email: zhaofangh@cicams.ac.cn.
Appendix A. Electronic search strategies
A). MEDLINE (via PubMed)
((Breast Neoplasms [MH] OR breast cancer* [tiab] OR breast neoplasm* [tiab] OR breast carcinoma* [tiab] OR breast tumor* [tiab] OR breast tumour* [tiab] OR mammary cancer* [tiab] OR mammary neoplasm* [tiab] OR mammary carcinoma* [tiab] OR mammary tumor* [tiab] OR mammary tumour* [tiab])
AND
("Mass Screening" [Mesh] OR "Early Detection of Cancer" [Mesh] OR screening [tiab] OR early detect*[tiab])
AND
(("Guideline" [Publication Type] OR "Practice Guideline" [Publication Type]) OR ("Guidelines as Topic" [Mesh] OR "Health Planning Guidelines" [Mesh] OR consensus [MeSH]) OR (guideline [Title] OR guidelines [Title] OR "practice guideline" [Title] OR "practice guidelines" [Title] OR "Health Planning Guidelines" [Title] OR Guidance [Title] OR consensus [Title] OR recommendations [Title] OR recommendation [Title] OR manual [Title] OR guidebook [Title] OR guidebooks [Title] OR guide [Title] OR guides [Title] OR handbook [Title] OR handbooks [Title])))
B). EMBASE via embase.com
('breast cancer'/exp OR 'breast tumor'/exp OR 'breast carcinoma*'/exp OR ('breast neoplasm*' OR 'breast tumor*' OR 'breast tumour*' OR 'mammary cancer*' OR 'mammary neoplasm*' OR 'mammary carcinoma*' OR 'mammary tumor*' OR 'mammary tumour*'):ab,ti
AND
('Mass Screening'/exp OR 'early cancer diagnosis'/exp OR "screening":ab, ti OR early detect:ab,ti)
AND
('Practice Guideline'/exp OR 'health care planning'/exp OR consensus/exp OR (guideline OR guidelines OR 'practice guideline' OR 'practice guidelines' OR 'Health Planning Guidelines' OR Guidance OR consensus OR recommendations OR recommendation OR manual OR guidebook OR guidebooks OR guide OR guides OR handbook OR handbooks):ti)
C). Web of Science
TI or AB=("breast cancer*" OR "breast neoplasm*" OR "breast carcinoma*" OR "breast tumor*" OR "breast tumour*" OR "mammary cancer*" OR "mammary neoplasm*" OR "mammary carcinoma*" OR "mammary tumor*" OR "mammary tumour*")
AND
TI or AB=("Mass Screening" OR "Early Detection of Cancer" OR screening OR "early detect*")
AND
TI=(guideline OR guidelines OR "Practice Guideline" OR "practice guidelines" OR consensus OR Guidance OR recommendation OR recommendations OR manual OR guide OR guides OR guidebook OR guidebooks OR handbook OR handbooks)
D). Scopus
TITLE-ABS("breast cancer*" OR "breast neoplasm*" OR "breast carcinoma*" OR "breast tumor*" OR "breast tumour*" OR "mammary cancer*" OR "mammary neoplasm*" OR "mammary carcinoma*" OR "mammary tumor*" OR "mammary tumour*")
AND
TITLE-ABS("Mass Screening" OR "Early Detection of Cancer" OR screening OR "early detect*")
AND
TITLE (guideline OR "Practice Guideline" OR consensus OR Guidance OR recommendation OR manual OR guide OR guidebook OR handbook)
References
- 1.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Available at: https://gco.iarc.fr/today/. [accessed 2021-05-01].
- 2.Moss S.M., Wale C., Smith R., Evans A., Cuckle H., Duffy S.W. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years' follow-up: a randomised controlled trial. Lancet Oncol. 2015;16:1123–1132. doi: 10.1016/S1470-2045(15)00128-X. [DOI] [PubMed] [Google Scholar]
- 3.Nelson H.D., Fu R., Cantor A., Pappas M., Daeges M., Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164(4):244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 4.Massat N.J., Dibden A., Parmar D., Cuzick J., Sasieni P.D., Duffy S.W. Impact of screening on breast cancer mortality: the UK program 20 Years on. Cancer Epidemiol Biomarkers Prev. 2016;25(3):455–462. doi: 10.1158/1055-9965. [DOI] [PubMed] [Google Scholar]
- 5.Plevritis S.K., Munoz D., Kurian A.W., Stout N.K., Alagoz O., Near A.M., et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA. 2018;319(2):154–164. doi: 10.1001/jama.2017.19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleyer A., Welch H.G. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 7.Siu A.L. U.S. Preventive services Task Force. Screening for breast cancer: U.S. Preventive services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 9.Hamashima C.C., Hattori M., Honjo S., Kasahara Y., Katayama T., Nakai M., et al. The Japanese guidelines for breast cancer screening. Jpn J Clin Oncol. 2016;46(5):482–492. doi: 10.1093/jjco/hyw008. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen K.J., Kalager M., Barratt A., Baines C., Zahl P.H., Brodersen J., et al. Overview of guidelines on breast screening: why recommendations differ and what to do about it. Breast. 2017;31:261–269. doi: 10.1016/j.breast.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Cai Y., Li J., Gao Y., Yang K., He J., Li N., et al. A systematic review of recommendations on screening strategies for breast cancer due to hereditary predisposition: who, When, and How? Cancer Med. 2021;10(10):3437–3448. doi: 10.1002/cam4.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Yang K.L., Cai Y.T., Tian J.H., Zheng Y.D., Wen Y., et al. Quality assessment of global breast cancer screening guidelines. Zhonghua Liuxingbingxue Zazhi. 2021;42(2):219–226. doi: 10.3760/cma.j.cn112338-20200806-01032. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers M.C., Kho M.E., Browman G.P., Burgers J.S., Cluzeau F., Feder G., et al. Agree II: advancing guideline development, reporting and evaluation in health care. CMAJ (Can Med Assoc J) 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann-Eßer W., Siering U., Neugebauer E.A.M., Lampert U., Eikermann M. Systematic review of current guideline appraisals performed with the Appraisal of Guidelines for Research & Evaluation II instrument-a third of AGREE II users apply a cut-off for guideline quality. J Clin Epidemiol. 2018;95:120–127. doi: 10.1016/j.jclinepi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Acuña-Izcaray A., Sánchez-Angarita E., Plaza V., Rodrigo G., de Oca M.M., Gich I., et al. Quality assessment of asthma clinical practice guidelines: a systematic appraisal. Chest. 2013;144(2):390–397. doi: 10.1378/chest.12-2005. [DOI] [PubMed] [Google Scholar]
- 16.World health Organization WHO position paper on mammography screening. https://www.who.int/publications/i/item/who-position-paper-on-mammography-screening accessed 2021-05-01] [PubMed]
- 17.Qaseem A., Lin J.S., Mustafa R.A., Horwitch C.A., Wilt T.J., et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170(8):547–560. doi: 10.7326/M18-2147. [DOI] [PubMed] [Google Scholar]
- 18.William J., Benjamin O.A., Jame A., et al. Breast cancer screening and Diagnosis,Version 1.2019, NCCN clinical practice guidelines in Oncology. J Natl Compr Cancer Netw. 2020;18(4):452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 19.Mainiero M.B., Moy L., Baron P., Didwania A.D., diFlorio R.M., Green E.D., et al. ACR appropriateness Criteria® breast cancer screening. J Am Coll Radiol. 2017;14(11S):S383–S390. doi: 10.1016/j.jacr.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Monticciolo D.L., Newell M.S., Moy L., Niell B., Monsees B., Sickles E.A. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408–414. doi: 10.1016/j.jacr.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Monticciolo D.L., Newell M.S., Hendrick R.E., Helvie M.A., Moy L., Monsees B., et al. Breast cancer screening for average-risk women: recommendations from the ACR commission on breast imaging. J Am Coll Radiol. 2017;14(9):1137–1143. doi: 10.1016/j.jacr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Lee C.H., Dershaw D.D., Kopans D., Evans P., Monsees B., Monticciolo D., et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7(1):18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Oeffinger K.C., Fontham E.T., Etzioni R., Herzig A., Michaelson J.S., Shih Y.C., et al. Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. JAMA. 2015;314(15):1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Practice bulletin number 179: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130(1):e1–e16. doi: 10.1097/AOG.0000000000002158. [DOI] [PubMed] [Google Scholar]
- 25.Schünemann H.J., Lerda D., Quinn C., Follmann M., Alonso-Coello P., Rossi P.G., et al. Breast cancer screening and diagnosis: a synopsis of the European breast guidelines. Ann Intern Med. 2020;172(1):46–56. doi: 10.7326/M19-2125. [DOI] [PubMed] [Google Scholar]
- 26.Sardanelli F., Boetes C., Borisch B., Decker T., Federico M., Gilbert F.J., et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46(8):1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Klarenbach S., Sims-Jones N., Lewin G., Singh H., Thériault G., Tonelli M., et al. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ (Can Med Assoc J) 2018;190(49):E1441–E1451. doi: 10.1503/cmaj.180463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wöckel A., Albert U.S., Janni W., Scharl A., Kreienberg R., Stüber T. The screening, diagnosis, treatment, and follow-up of breast cancer. Dtsch Arztebl Int. 2018;115(18):316–323. doi: 10.3238/arztebl.2018.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Australia. Early detection of breast cancer. Available at:https://www.canceraustralia.gov.au/resources/position-statements/early-detection-breast-cancer. [accessed 2021-03-01].
- 30.Ministry of Health Singapore. Cancer screening, MOH Clinical Practice Guidelines. Available at:https://www.moh.gov.sg/docs/librariesprovider4/guidelines/cpg_cancer-screening.pdf. [accessed 2021-03-01].
- 31.Ministry of Health of Malaysia. Management of Breast Cancer (3rd Edition). Available at: https://www2.moh.gov.my/moh/resources/Penerbitan/CPG/Kanser/Breast%20Cancer/CPG_Management_of_Breast_Cancer_(Third_Edition)_130720.pdf. [accessed 2021-03-01].
- 32.He J., Chen W.Q., Li N., Shen H.B., Li J., Wang Y., et al. China guideline for the screening and early detection of female breast cancer(2021, Beijing) Zhonghua Zhongliu Zazhi. 2021;43(4):357–382. doi: 10.3760/cma.j.cn112152-20210119-00061. [DOI] [PubMed] [Google Scholar]
- 33.Lam T.H., Wong K.H., Chan K.K., Chan M.C., Chao D.V., Cheung A.N., et al. Recommendations on prevention and screening for breast cancer in Hong Kong. Hong Kong Med J. 2018;24(3):298–306. doi: 10.12809/hkmj177037. [DOI] [PubMed] [Google Scholar]
- 34.Migowski A., Silva G.A.E., Dias M.B.K., Diz M.D.P.E., Sant'Ana D.R., Nadanovsky P. Guidelines for early detection of breast cancer in Brazil. II - new national recommendations, main evidence, and controversies. Cad Saúde Pública. 2018;34(6) doi: 10.1590/0102-311X00074817. [DOI] [PubMed] [Google Scholar]
- 35.Urban L.A.B.D., Chala L.F., Bauab S.D.P., Schaefer M.B., Dos Santos R.P., Maranhão N.M.A., et al. Breast cancer screening: updated recommendations of the Brazilian College of Radiology and diagnostic imaging, Brazilian breast disease society, and Brazilian federation of gynecological and obstetrical Associations. Radiol Bras. 2017;50(4):244–249. doi: 10.1590/0100-3984.2017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lodge M., Corbex M. Establishing an evidence-base for breast cancer control in developing countries. Breast. 2011;20(Suppl 2):S65–S69. doi: 10.1016/j.breast.2011.01.012. 10.1016/j.breast.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Pace L.E., Shulman L.N. Breast cancer in sub-saharan africa: challenges and opportunities to reduce mortality. Oncol. 2016;21(6):739–744. doi: 10.1634/theoncologist.2015-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dersch R., Toews I., Sommer H., Rauer S., Meerpohl J.J. Methodological quality of guidelines for management of Lyme neuroborreliosis. BMC Neurol. 2015;15:242. doi: 10.1186/s12883-015-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann-Eßer W., Siering U., Neugebauer E.A., Brockhaus A.C., Lampert U., Eikermann M. Guideline appraisal with AGREE II: systematic review of the current evidence on how users handle the 2 overall assessments. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D., Goding Sauer A., et al. Breast cancer statistics. CA Cancer J Clin 2019. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 41.Pace L.E., Keating N.L. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311(13):1327–1335. doi: 10.1001/jama.2014.1398. [DOI] [PubMed] [Google Scholar]
- 42.Tabar L., Fagerberg G., Chen H.H., Duffy S.W., Smart C.R., Gad A., et al. Efficacy of breast cancer screening by age new results from the Swedish two-county trial. Cancer. 1995;75:2507–2517. doi: 10.1002/1097-0142(19950515)75:10<2507::AID-CNCR2820751017>3.0.CO. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi N., Kumamaru H., Isozumi U., Aogi K., Asaga S., Iijima K., et al. Annual report of the Japanese breast cancer registry for 2017. Breast Cancer. 2020;27(5):803–809. doi: 10.1007/s12282-020-01139-3. [DOI] [PubMed] [Google Scholar]
- 44.Hong S., Won Y.J., Park Y.R., et al. Community of population-based regional cancer registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020;52(2):335–350. doi: 10.4143/crt.2020.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller A.B., Baines C.J., To T., Wall C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ (Can Med Assoc J) 1992 15;147(10):1459–1476. [PMC free article] [PubMed] [Google Scholar]
- 46.Bjurstam N.G., Bjorneld L.M., Duffy S.W. Updated results of the gothenburg trial of mammographic screening. Cancer. 2016;122:1832–1835. doi: 10.1002/cncr.29975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nyström L., Andersson I., Bjurstam N., Frisell J., Nordenskjöld B., Rutqvist L.E. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002 Mar 16;359(9310):909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 48.Lee C.S., Moy L., Joe B.N., Sickles E.A., Niell B.L. Screening for breast cancer in women age 75 Years and older. AJR Am J Roentgenol. 2018;210(2):256–263. doi: 10.2214/AJR.17.18705. [DOI] [PubMed] [Google Scholar]
- 49.Lauby-Secretan B., Scoccianti C., Loomis D., Benbrahim-Tallaa L., Bouvard V., Bianchini F., et al. International Agency for Research on Cancer Handbook Working Group. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353–2358. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 50.Yip C.H., Smith R.A., Anderson B.O., Miller A.B., Thomas D.B., Ang E.S., et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer. 2008;113(8 Suppl):2244–2256. doi: 10.1002/cncr.23842. [DOI] [PubMed] [Google Scholar]
- 51.Butler R.S. Invited commentary: the breast density dilemma–challenges, lessons, and future directions. Radiographics. 2015;35:324–326. doi: 10.1148/rg.352140276. 10.1148/rg.352140276. [DOI] [PubMed] [Google Scholar]
- 52.Bae J.M., Kim E.H. Breast density and risk of breast cancer in asian women: a meta-analysis of observational studies. J Prev Med Public Health. 2016;49(6):367–375. doi: 10.3961/jpmph.16.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohuchi N., Suzuki A., Sobue T., Kawai M., Yamamoto S., Zheng Y.F., et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 54.Yi A., Jang M.J., Yim D., Kwon B.R., Shin S.U., Chang J.M. Addition of screening breast US to digital mammography and digital breast tomosynthesis for breast cancer screening in women at average-risk. Radiology. 2021;298(3):568–575. doi: 10.1148/radiol.2021203134. [DOI] [PubMed] [Google Scholar]
- 55.Ma L., Ren W., Zhao Y., et al. Cost-effectiveness of optimized ultrasound-based breast cancer screening for Chinese rural women in 2015. China Cancer. 2019;28(12):891–895. doi: 10.11735/j.issn.1004-0242.2019.12.A002. [DOI] [Google Scholar]
- 56.Ma L., Lian Z., Zhao Y., et al. Breast ultrasound optimization process analysis based on breast cancer screening for 1 501 753 rural women in China. Zhonghua zhong liu za zhi [Chinese journal of oncology] 2021;43(4):497–503. doi: 10.3760/cma.j.cn112152-20190828-00549. [DOI] [PubMed] [Google Scholar]
- 57.Wang X., Huang Y., Li L., Dai H., Song F., Chen K. Assessment of performance of the Gail model for predicting breast cancer risk: a systematic review and meta-analysis with trial sequential analysis. Breast Cancer Res. 2018;20(1):18. doi: 10.1186/s13058-018-0947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ming C., Viassolo V., Probst-Hensch N., Dinov I.D., Chappuis P.O., Katapodi M.C. Machine learning-based lifetime breast cancer risk reclassification compared with the BOADICEA model: impact on screening recommendations. Br J Cancer. 2020;123(5):860–867. doi: 10.1038/s41416-020-0937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Chen H., Li N., Ren J., Zhang K., Dai M., et al. Ultrasound for breast cancer screening in high-risk women: results from a population-based cancer screening program in China. Front Oncol. 2019;9:286. doi: 10.3389/fonc.2019.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doubeni C.A., Gabler N.B., Wheeler C.M., McCarthy A.M., Castle P.E., Halm E.A., et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68(3):199–216. doi: 10.3322/caac.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reece J.C., Neal E.F.G., Nguyen P., McIntosh J.G., Emery J.D. Delayed or failure to follow-up abnormal breast cancer screening mammograms in primary care: a systematic review. BMC Cancer. 2021;21(1):373. doi: 10.1186/s12885-021-08100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]