Summary
Background
Few studies investigated the mechanisms of treatment-resistant depression (TRD) leading to the worsened survival outcome, and economic evidence was mostly restricted to short follow-ups. We aimed to examine the association and potential mediators between TRD and all-cause mortality, and estimate a longer-term associated health resource utilisation pattern.
Methods
This was a population-based cohort study using territory-wide electronic medical records in Hong Kong. Incident depression patients diagnosed in 2014 were followed up from the first diagnosis to death or December 2019 for TRD identification. We matched the TRD cohort 1:4 to the non-TRD cohort on propensity scores estimated by age, sex, history of physical disorders, and history of psychiatric conditions before depression diagnoses.
Findings
18% of incident patients developed TRD within six years of follow-up. Cox model showed that patients with TRD had 1⋅52-fold (95% CI: 1⋅14–2⋅02) greater risk of all-cause mortality, compared with non-TRD patients. Path analysis suggested that post-TRD psychiatric conditions significantly mediated 41⋅6% of mortality in patients with TRD (p=0.003). TRD was associated with 1⋅8-fold (95%CI: 1⋅63–2⋅00) higher healthcare costs compared to non-TRD patients over six years in negative binomial regression, with higher costs for both psychiatric and non-psychiatric services utilisation in all settings.
Interpretation
Identifying patients with TRD and subsequent monitoring for post-TRD psychiatric diagnoses could be a way to reduce premature mortality. Multidisciplinary care involving both psychiatric and general medical professionals is also warranted to relieve the multifaceted impacts on healthcare resources and overall cost.
Funding
Unconditional educational grant from Janssen.
Keywords: Treatment-resistant depression, Death-causing mechanism, Mediation analysis, Health Economics, Population-based electronic medical records, Restrospective cohort study
Research in context.
Evidence before this study
We searched PubMed and Google Scholar for research articles published up to 20th October, 2021 in English and Chinese with the terms “treatment-resistant depression” AND (“mortality” or “healthcare resource utilisation” or “economic burden”). Four studies from Denmark, Sweden and the United States all reported a positive association between treatment-resistant depression (TRD) and excess all-cause mortality or life years lost, but we did not identify studies that investigated mediating mechanisms between TRD and mortality. Several economic studies, with the majority of them based in the United States, found that TRD posed significant direct and indirect medical cost burden and heavier healthcare resource utilisation. The evidences were all generated from claims-based insurance data and mostly restricted to short follow-up within two years, and did not further stratify the utilization pattern into the service subtypes beyond outpatient, inpatient and emergency settings. Few studies evaluated both the clinical and economic consequences of TRD comprehensively in a single study.
Added value of this study
To the best of our knowledge, we present the first attempt to explore the potential mediators in the relationship between treatment resistance and the worsened survival outcome. Our path analysis of structural equation modelling suggested that the increased mortality risk associated with TRD was partially mediated by the post-TRD psychiatric conditions. Although the acquisition of post-TRD physical comorbidities was not a significantly mediator towards TRD-associated death, its burden may have manifested in terms of greater healthcare resource utilisation, given that TRD patients consumed significantly more resources in not only in psychiatric, but also non-psychiatric service subtypes in the settings of outpatient, inpatient and emergency. Our findings also validate the economic impacts of TRD in long-term and in the context of public taxation-based healthcare system.
Implications of all the available evidence
Our study highlights the difficult-to-treat nature of TRD and its subsequent burden to the health system, clinically and economically. Clinicians should be alert that identifying treatment resistance early and subsequent monitoring for post-TRD psychiatric comorbidities could be a way to prevent premature mortality. Healthcare providers and policy makers should expect that increased service demand arisen from TRD would manifest in both psychiatric and general medical services. A multidisciplinary disease management strategy, which involves communication and collaboration between psychiatric and non-psychiatric specialties, could be oriented to prevent disease progression; this would be beneficial not only to improve the multifaceted patient outcomes, but also save medical costs in a wide range of healthcare resources.
Alt-text: Unlabelled box
Introduction
More than 264 million people worldwide live with depression. Nearly half of all cases are from the Asia-pacific region.1,2 The World Health Organization ranks depression as the single greatest contributor to non-fatal health loss, contributing 7⋅5% of all years lived with disability. The number of incident cases continues to increase globally.1,3 Despite available treatments, up to half of patients fail to reach remission; as a result, there has been an increasing focus on the concept of treatment-resistant depression (TRD).4,5 Recent work has attempted to reconceptualise TRD as “difficult-to-treat” depression, based on which a new model of care was proposed but not wholly appropriate for patients who would normally be classified as TRD.6,7 Although there is no consensus on the definition of TRD to date,8 current literature generally defines TRD as treatment failure (i.e. failure to achieve remission and/or requiring switches in medication) following at least two trials of antidepressants at adequate doses, duration and adherence.9
Depressive disorder reduced life expectancy by seven to ten years, and mortality increases with depression severity.10,11 Studies from Denmark, Sweden and the United States (U.S.) consistently found that patients with TRD had 29–39% higher risk of all-cause mortality compared with those without TRD.12, 13, 14 Apart from elevated risk of self-harm,15 undertreated depression was associated with development of physical comorbidities, especially cardiovascular diseases and stroke, which further increase mortality risk.16,17 A cohort study of over 4,000 patients with depression in the U.S. suggested that the three-year mortality risk after myocardial infarction in patients with sub-optimally treated depression was three times higher than those with fully treated depression.18 Whilst for other mental health problems, previous studies suggested that 45–67% of patients with depression also had comorbid psychosis or anxiety disorder, which was associated with greater symptom severity, lower remission rates and increased treatment resistance.19, 20, 21 Comorbid psychosis was also more common in TRD patients than treatment-responsive patients.22 The complex interplay of genetic,23 physical and physiological factors, health-compromising behaviours, and poor medication adherence also contribute to the association between depression and physical and mental comorbidities.16
Treatment resistance also led to higher levels of healthcare resource use and economic impacts,24,25 which intensified the disease burden alongside premature mortality. TRD has been consistently linked with greater direct medical costs, productivity losses and employment changes.26,27 Studies from the U.S. and Japan indicated 1⋅4 to 2 times higher all-cause medical costs in outpatient, inpatient and emergency settings for TRD patients, and a study from Korea even reported a fivefold substantial difference.27, 28, 29, 30, 31 Total healthcare expenditure also increased in accordance with the degree of resistance.32
The chronicity and recurrent nature of TRD necessitates longer follow-up to obtain a clearer picture of the clinical and economic impacts. There was limited knowledge on how TRD subsequently led to death and the long-term care burden associated with TRD to the healthcare system. Moreover, it was unclear how patients used the sub-specialty services in the outpatient, inpatient and emergency settings, which however remained an unneglectable component to optimise resource allocation and evidence-based decision making. Using territory-wide longitudinal electronic medical records (EMR) in Hong Kong, we aimed to assess the disease burden in form of fatal and non-fatal health losses with the following objectives: 1) examine the impact of TRD on all-cause mortality and the potential mediators on worsening survivorship, and 2) evaluate the long-term healthcare resource utilisation pattern and the overall economic burden to the public taxation-based healthcare system. We hypothesise that 1) patients with TRD have higher risk of all-cause mortality, 2) TRD-associated physical and psychiatric conditions contribute to higher mortality, and 3) managing patients with TRD requires more healthcare resources compared to treatment-responsive patients.
Methods
Data source
We used the population-based, territory-wide EMR database in Hong Kong (Clinical Data Analysis and Reporting System), which covers all eligible citizens (population size: 7 million) who use public healthcare services.33,34 Patients’ records, including demographics, dates of death, dates of hospitalisation and service attendance, diagnoses and prescriptions, were captured across the outpatient, inpatient and emergency settings, and centralised for audit and research purposes, and anonymised to protect confidentiality. The database has been described in detail in previous epidemiology and economic impact studies.35, 36, 37
Study design and participants
This was an EMR-based matched cohort study. Incident patients with diagnosis codes for depression (ICD-9-CM codes: 296⋅2, 296⋅3, 300⋅4 and 311) in 2014 without history of depression since 1993, when the database first became available, were captured for analysis. We analysed prescription records from individual's first depression diagnosis date to death or December 2019, whichever came first, to identify whether they became TRD. Patients were considered treatment-resistant if they had taken at least two regimens of antidepressants for an adequate duration (same antidepressant or combination regimen of at least 28 days with gaps no longer than 14 days within regimens) and had a third antidepressant regimen to confirm failure in the previous two regimens. The 28-day duration was based on the minimum recommended duration needed to assess responsiveness to an intervention.38 Patients who did not meet the criteria for TRD were classified as non-TRD. Prior to the main analyses, we described the transition of incidence into resistance over the follow-up in a Sankey diagram39 to show the proportion of patients on different regimens at the three steps and the trajectory of regimen movement. The first regimen started with antidepressant monotherapy, classified by their pharmacological mechanisms, while the second and third regimens could be any antidepressant monotherapies or in augmentation/combination, second-generation antipsychotics, or mood stabilisers (Supplementary Tables 1-2). Behavioural therapies and psychotherapies were not included owing to data unavailability.
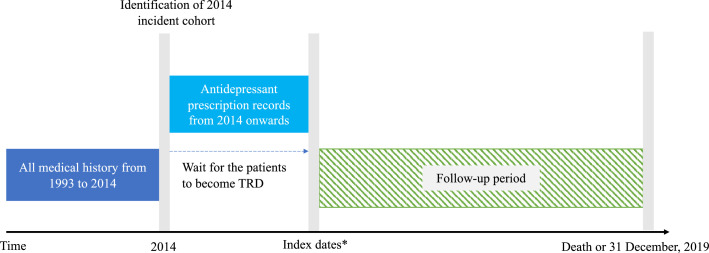
We matched the TRD cohort 1:4 to the non-TRD cohort on a propensity score to improve comparability between groups and address potential confounding, estimated using logistic regression with age, gender, history of physical disorders (all diseases in the calculation of the Charlson Comorbidity Index)40,41 and mental health conditions (attention-deficit-hyperactivity disorder, autism, psychosis, schizophrenia, epilepsy, anxiety disorder, personality disorder) from 1993 to the dates of depression diagnoses in 2014. Standardised mean difference values below 0⋅1 would indicate a meaningful balance of baseline variables between groups, which would not be included as model adjustment variables.42 In the survival, mediation and economic analyses, the follow-up started from the prescription date of the third regimen (index date for patients with TRD) until December 2019, and the same index date was used for the four non-TRD matches. Using the hazard ratio (HR: 1⋅35, 95% CI: 1⋅21-1⋅50) reported from a previous national-wide cohort study, the required sample size was 7047 to achieve a 80% statistical power.14,43 The schema for the study design is illustrated in Figure 1.
Figure 1.
Schematic presentation of study design for survival and mediation analyses
*For TRD patients, index dates were the dates on which they received the third prescription before the study end date. Same index dates were assigned to the matched non-TRD patients in the same matching stratum, who did not have a date of the third prescription by definition. The matching was performed using propensity score based on age, gender, history of physical comorbidities, psychiatric and suicidal attempts as of 2014. The follow-up of both groups started on the index dates until death or the end of study. Index dates, censoring and covariates adjustment used were the same throughout survival, mediation and healthcare resource utilisation analyses.
Survival and mediation analyses
We first fitted a multivariable Cox model to estimate the hazard ratio (HRs) of all-cause mortality. Since patients could become comorbid after matching before the index dates, we also adjusted for physical disorders and psychiatric conditions (same set of conditions measured in the baseline characteristics) recorded in the window between first diagnosis and index date as binary variables in the model. An interaction term between TRD status and time was added when Schoenfeld residual-based testing showed violation of proportional hazards assumption. To verify the robustness of results from the main survival analysis, we conducted two sensitivity analyses: 1) using the accelerated failure time (AFT) model in Weibull distribution to estimate the acceleration factor (AFs) of survival time;44 2) restricting TRD identification period to a two-year window and repeating the Cox regression as in the main analysis.
We then explored two potential mediators of the relationship between TRD status and all-cause mortality: 1) newly acquired physical disorders, and 2) newly acquired mental health conditions (same set of conditions measured in the baseline and post-matching characteristics), using structural equation modelling based on binary probit link (R ‘lavaan’ package), considering only the new conditions after the index date (Figure 1) and controlling for the significant post-matching confounders indicated from the survival analysis. We assessed model goodness-of-fit jointly using root-mean-square error of approximation, comparative fit index, and Tucker-Lewis index.
Healthcare resource utilisation and cost analysis
All-cause healthcare resource utilisation refers to all recorded episodes or hospitalisation days per patient-year from index date to death or December 2019, whilst the associated medical cost was estimated in a semi-macroeconomic manner. We first stratified the utilisation into 14 service subtypes in the outpatient (three specialist, one general, three day-hospital and three community services), inpatient (general/rehabilitation, psychiatric and high-dependency/intensive-care wards) and emergency settings. Medical costs were estimated by multiplying the obtained utilisation data by service-specific unit costs published in 2019 by the Hospital Authority (Supplementary Table 4). Fourteen service-specific costs were then aggregated into an overall cost. Despite a taxation-based subsidised healthcare financing system in Hong Kong, we used non-subsidised costs to reflect the overall economic burden from the decision-maker perspective. All monetary values are expressed in Hong Kong Dollars (2019). We applied negative binomial regression to compare the service-specific utilization and overall cost per patient-year over six years between groups, similarly adjusting for the post-matching characteristics as in the survival and mediation analyses.
Role of the funding source
This study was supported by an unconditional educational grant from Janssen and Internal Research Fund from the Department of Medicine, The University of Hong Kong. Funders did not participate throughout the study design, study conduct, interpretation, or manuscript writing.
RESULTS
Treatment trajectory and baseline characteristics
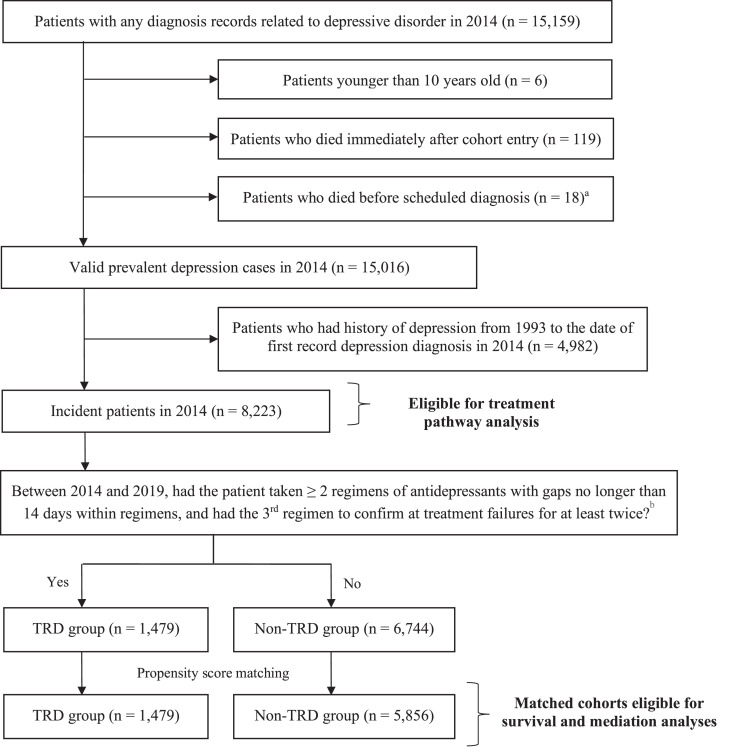
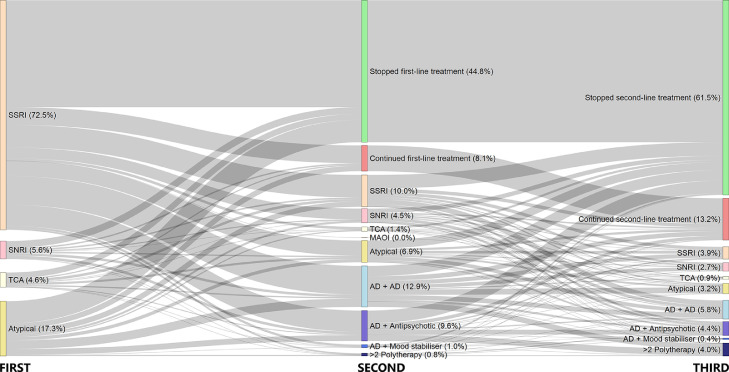
Figure 2 presents the identification process of the incident and matched cohorts. In 2014, we captured a total of 15,159 patients with depression diagnoses. Of the 8,223 incident patients, 5,834 had an adequate antidepressant monotherapy regimen as their first treatment. Of these, 47⋅1% (2745/5834) switched to a second regimen and a further 25⋅3% (1479/5834) switched to a third regimen; these were identified as TRD patients (Figure 3). 18⋅0% of the incident patients became treatment-resistant during our follow-up. The median treatment duration was 161 days (interquartile range [IQR]: 57–505 days) at the first-line, whilst durations were shorter at subsequent lines (The second-line: 130 days (IQR: 56–383); third-line: 84 days (IQR: 28–273), Supplementary Table 3).
Figure 2.
Flowchart of incident cohort and 1:4 matched cohorts identification
a Patients were excluded if their dates of death were earlier than their first dates in 2014 with depression-related diagnosis.
b Prescription records of antidepressant between 2014 and 2019 were extracted to define patients’ TRD status. Antidepressants treatment regimens could be monotherapy or combination treatments with antipsychotics or mood stabilisers.
Abbreviation: MDD – Major depressive disorder; TRD – Treatment-resistant depression.
Figure 3.
Treatment trajectory and resistance evolvement among incident patients with depression (N= 5,834)a
a 5,834 out of the 8,223 patients in the 2014 incident depression cohort were eligible for inclusion in this diagram as they had antidepressant monotherapy of adequate duration as their first treatment after incident diagnosis. The size of the colored nodes represents the number of patients taking different treatment regimens at each treatment step while the connecting grey bars represent the patient flow between the steps. ‘Stopped first/second-line treatment’ represents those with prescriptions from the previous step that ended before death or the study end date. ‘Continued first/second-line treatment’ represents those with prescription durations from the previous step that continued up until death or the study end date.
Abbreviation: SSRI – Selective serotonin reuptake inhibitor, SNRI – Selective norepinephrine reuptake inhibitor, TCA – Tricyclic antidepressant, MAOI – Monoamine oxidase inhibitor, AD - Antidepressant. The ‘Atypical’ group includes other antidepressants with mechanisms of action that are different from the major antidepressant classes.
Table 1 shows the baseline characteristics of patients with TRD and their matched controls. After matching, two groups had similar age, proportion of females, history of psychiatric conditions and history of physical disorders (all standardised mean difference<0⋅06). The mean time for patients to develop treatment resistance was 2⋅0 years (±1⋅5 years). The mean follow-up time was 3⋅4 years (±1⋅5 years) in the TRD group and 3⋅5 years (±1⋅5 years) in the non-TRD group (p=0⋅282).
Table 1.
Characteristics of patients with TRD and their matched controls at cohort entry in 2014
| Characteristics | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| TRD group(N = 1,479) | Non-TRD group(N = 6,744) | SMD | TRD group(N = 1,479) | Non-TRD group(N = 5,856)b | SMD | |
| Age (mean, SD) | 46.6 (17.0) | 49.1 (18.8) | 0.140 | 46.6 (17.0) | 47.1 (17.9) | 0.032 |
| Female (N, %) | 1093 (73.9) | 4789 (71.0) | 0.065 | 1093 (73.9) | 4306 (73.5) | 0.008 |
| History of psychiatric conditions (N, %) | - | - | - | - | - | - |
| Any psychiatric diseases or suicidal ideation | 218 (14.7) | 1,250 (18.5) | 0.102 | 218 (14.7) | 856 (14.6) | 0.003 |
| Attention-deficit Hyperactivity Disorder | 2 (0.1) | 10 (0.1) | 0.003 | 2 (0.1) | 8 (0.1) | <0.001 |
| Autism | 0 (0.0) | 4 (0.1) | 0.034 | 0 (0.0) | 3 (0.1) | 0.032 |
| Psychosis | 23 (1.6) | 201 (3.0) | 0.096 | 23 (1.6) | 128 (2.2) | 0.047 |
| Schizophrenia | 7 (0.5) | 69 (1.0) | 0.064 | 7 (0.5) | 46 (0.8) | 0.039 |
| Epilepsy | 11 (0.7) | 71 (1.1) | 0.033 | 11 (0.7) | 45 (0.8) | 0.003 |
| Anxiety disorder | 90 (6.1) | 491 (7.3) | 0.048 | 90 (6.1) | 323 (5.5) | 0.024 |
| Personality disorder | 19 (1.3) | 81 (1.2) | 0.008 | 19 (1.3) | 58 (1.0) | 0.028 |
| Suicidal behaviors (N, %) | 94 (6.4) | 540 (8.0) | 0.064 | 94 (6.4) | 388 (6.6) | 0.011 |
| History of physical disorders (N, %)a | - | - | - | - | - | - |
| Any physical comorbidities | 288 (19.5) | 1600 (23.7) | 0.103 | 288 (19.5) | 1158 (19.8) | 0.008 |
| Diabetes mellitus | 76 (5.1) | 499 (7.4) | 0.093 | 76 (5.1) | 351 (6.0) | 0.037 |
| Cerebrovascular vascular disease | 71 (4.8) | 440 (6.5) | 0.075 | 71 (4.8) | 288 (4.9) | 0.005 |
| Chronic pulmonary disease | 76 (5.1) | 322 (4.8) | 0.017 | 76 (5.1) | 244 (4.2) | 0.046 |
| Any tumors | 54 (3.7) | 348 (5.2) | 0.074 | 54 (3.7) | 252 (4.3) | 0.033 |
| Ulcer disease | 36 (2.4) | 169 (2.5) | 0.005 | 36 (2.4) | 120 (2.0) | 0.026 |
| Peripheral vascular disease | 22 (1.5) | 102 (1.5) | 0.002 | 22 (1.5) | 62 (1.1) | 0.038 |
| Congestive heart failure | 21 (1.4) | 147 (2.2) | 0.057 | 21 (1.4) | 95 (1.6) | 0.017 |
| Moderate/severe renal disease | 20 (1.4) | 124 (1.8) | 0.039 | 20 (1.4) | 90 (1.5) | 0.015 |
| Liver diseases | 14 (0.9) | 71 (1.1) | 0.011 | 14 (0.9) | 50 (0.9) | 0.010 |
| Dementia | 6 (0.4) | 71 (1.1) | 0.076 | 6 (0.4) | 48 (0.8) | 0.053 |
| Hemiplegia/paraplegia | 16 (1.1) | 110 (1.6) | 0.047 | 16 (1.1) | 86 (1.5) | 0.034 |
| Myocardial infarction | 7 (0.5) | 79 (1.2) | 0.077 | 7 (0.5) | 57 (1.0) | 0.059 |
| Connective tissue disease | 8 (0.5) | 63 (0.9) | 0.046 | 8 (0.5) | 50 (0.9) | 0.038 |
| Lymphoma | 0 (0.0) | 10 (0.1) | 0.054 | 0 (0.0) | 7 (0.1) | 0.049 |
| Leukaemia | 2 (0.1) | 12 (0.2) | 0.011 | 2 (0.1) | 11 (0.2) | 0.013 |
| Acquired Immunodeficiency Syndrome | 2 (0.1) | 10 (0.1) | 0.002 | 2 (0.1) | 7 (0.1) | 0.004 |
| Charlson Comorbidity Index (mean, SD) | 1.16 (1.81) | 1.52 (2.13) | 0.184 | 1.16 (1.81) | 1.27 (1.94) | 0.059 |
Categorization was based on the disease types included in the Charlson Comorbidity index.
257 patients did not proceed to survival and mediation analysis as deaths occurred earlier than their index dates. The SMD of matching variables remained below 0.1 in the absence of these patients.
Abbreviation: IQR – Interquartile range, SD – Standard deviation, SMD – Standardised mean difference, TRD – Treatment-resistant depression.
Survival and mediation analyses
There were 291 all-cause deaths and 11 deaths due to external causes (4⋅1% and 0⋅2% of the study population) during the follow-up (Table 2). Multivariable Cox model showed that TRD status significantly increased the risk of all-cause mortality (HR: 1⋅52, 95%CI: 1⋅14–2⋅02, p=0⋅004) (Supplementary Figure 1a). AFT model consistently showed that patients with TRD had a 30% reduction in survival time (AF: 0⋅70, 95%CI: 0⋅52–094, p=0⋅016) (Supplementary Figure 1b). Using two-year as the TRD identification window, Cox regression also returned similar mortality risk estimation as the main analysis (HR: 1⋅54, 95% CI: 1⋅00–2⋅36, p=0⋅049).
Table 2.
Number of recorded deaths, new-onset physical disorders and psychiatric conditions during follow-up period
| TRD groupN (%) | Non-TRD groupN (%) | p-value | |
|---|---|---|---|
|
Number of recorded deaths - All causes - External causes New onset of physical disorders New onset of psychiatric conditions |
78 (5.3) 6 (0.4) 130 (8.8) 85 (5.7) |
213 (3.6) 5 (0.1) 420 (7.2) 166 (2.8) |
0.014* 0.012* 0.112 <0.001* |
*Significant at 0.05 between TRD and non-TRD groups using chi-square or Fisher's exact tests.
Abbreviations: TRD – Treatment-resistant depression.
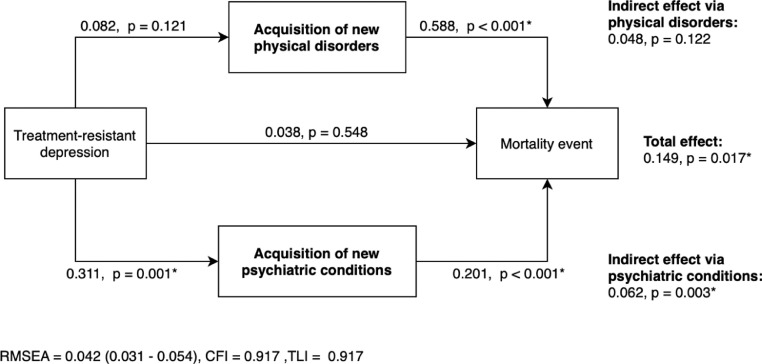
Table 2 describes the number of patients who died or who had new-onset physical disorders and psychiatric conditions in the two studied groups. During the post-TRD period, the most common new onset physical disorder was cardiovascular disease (2⋅0%), chronic obstructive pulmonary disease (1⋅4%) and diabetes mellitus (1⋅3%), whilst the most common new mental health conditions were self-harm behaviours (2⋅3%), psychosis (1⋅4%) and schizophrenia (1⋅0%). The path model illustrates the total effect of TRD status on all-cause mortality, and the mediated effects acting through the acquisition of new physical disorders and psychiatric conditions (Figure 4). The path model achieved acceptable goodness-of-fit and confirmed the significant total effect of TRD status on mortality (p=0⋅017) found in the survival analyses. The increased mortality associated with TRD was significantly mediated by the newly acquired psychiatric conditions (p=0⋅003), accounting for 41⋅6% of the total effect (i.e., the percentage of total effect attributable to indirect effect). There was a tendency towards a mediating effect via physical disorders (32⋅2% of the total effect), but the result did not reach statistically significant level (p=0⋅122).
Figure 4.
Mediating effect of TRD status on all-cause mortalitya-c
a The values are binary probit estimates illustrating the total, direct and mediated effects of TRD on mortality event through the post-index acquisition of new physical disorders and psychiatric conditions among matched incident patients in 2014. Total effect was the sum of direct effect and indirect effects via two mediators, whilst the indirect effect was the product of β values in the association between TRD and mediator and, between mediator and mortality.
b Path model adjusted for post-matching acquisition of new physical disorders before the index date as confounder control.
c Physical disorders include myocardial infarction, congestive heart failure, peripheral vascular siease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, liver disease, diabetes, hemiplegia or paraplegia, moderate-to-severe renal disease, tumours, leukaemia, lymphoma and acquired immunodeficiency syndrome. Mental health conditions include attention-deficit-hyperactivity disorder, autism, psychosis, schizophrenia, epilepsy, anxiety disorder and personality disorder.
Abbreviations = CFI – Comparative fit index, RMSEA – Root-mean-square error of approximation, TLI – Tucker-Lewis index.
Healthcare resource utilisation
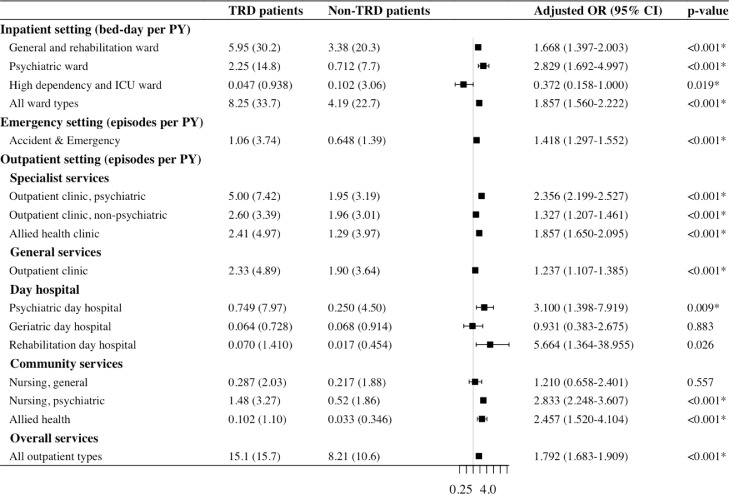
Over six years, patients with TRD had significantly greater mean healthcare resource utilisation per patient-year in outpatient visits (15⋅1 vs 8⋅2 episodes), emergency visits (1⋅1 vs 0⋅6 episodes) and inpatient days (8⋅3 vs 4⋅2 bed-days; all p<0⋅001), compared with non-TRD patients (Figure 5). When further stratifying the three settings into 14 psychiatric and non-psychiatric service subtypes, TRD patients had 2⋅4- to 3⋅1-fold greater utilisation of psychiatric-specific services, including psychiatric specialist outpatient clinic (5⋅0 vs 2⋅0 episodes), psychiatric day hospital (0⋅7 vs 0⋅3 episodes), psychiatric community nursing (1⋅5 vs 0⋅5 episodes) and psychiatric ward (2⋅3 vs 0⋅7 bed-days; all p<0.01). In most non-psychiatric service subtypes, TRD continued to exhibit greater utilisation including general and rehabilitation ward (6⋅0 vs 3⋅4 bed-days), specialist outpatient clinic of other specialties (2⋅6 vs 2⋅0 episodes) and allied health (2⋅4 vs 1⋅3 episodes), general outpatient clinic (2⋅3 vs 1⋅9 episodes), rehabilitation day hospital (0⋅07 vs 0⋅01 episode) and allied health community services (0⋅10 vs 0⋅03 episode; all p<0⋅05), with adjusted odds ratios between 1⋅2 and 5⋅7 compared with non-TRD patients.
Figure 5.
Healthcare resource utilisation comparison between treatment-resistant and treatment responsive patients
All-cause healthcare resource utilisation refers to all recorded episodes or hospitalisation days per patient-year from index date to death or December 2019.
*Significant at 0.05 between TRD and non-TRD patients using negative binomial regression with log link function, adjusting for post-matching acquisition of physical disorders and that of psychiatric conditions.
Abbreviation: OR – Odds ratio, PY – Patient-year, SD – Standard deviation, TRD – Treatment-resistant depression.
Aggregated from all settings, the unadjusted mean overall cost in TRD patients was $116,731±$213,743 (equivalent to US$14,966±27,403 in 2019) per patient-year — 54% higher than that in non-TRD patients (mean, $75,666±217,243). Results from the negative binominal regression showed that, after covariate adjustment, TRD continued to be positively associated with overall cost with an adjusted odds ratio of 1⋅80 (95%CI: 1⋅63-2⋅00, p<0⋅001).
Discussion
Summary of findings
Using an incident depression cohort with up to six years of follow-up, we found that patients with TRD had more than 50% increased risk of all-cause mortality compared with those remaining non-TRD. Our path analysis also identified that post-TRD psychiatric conditions partially mediated the mortality-causing mechanism. From the economic perspective, we observed greater healthcare resource utilisation by patients with TRD compared to non-TRD in both psychiatric and non-psychiatric service settings, leading to additional $41,000 annual healthcare costs per patient.
Treatment trajectory and prevalence of TRD
During the evolution towards TRD, overall median treatment durations were shortened following subsequent lines of treatment and regimen, and the application of combination therapies was increasingly common when the second and third regimens were attempted, reflecting the difficult-to-treat nature of TRD patients and the challenge of improving treatment strategies to prevent poor survival outcomes. We applied stringent criteria to define TRD based on previous literature.12,14,15 Despite this, the proportion of patients becoming treatment-resistant (18%) was higher than in previous studies using national registers or claims databases, which have reported prevalence rates of TRD between 10% and 15%. The higher prevalence may have stemmed from the use of a different study design that did not restrict the identification of TRD within a specific cut-off time window, as long as the patient fulfilled the definition of TRD. Another possible explanation could be that we included a different patient profile at cohort entry. Despite similar mean patient age compared with previous studies, scores on the Charlson Comorbidity Index and the proportion of patients with any physical health disorders were considerably higher in our study.12,14,15 A comorbid situation with depression could in turn complicate the care pathways and treatment responses, therefore increasing the chance of TRD identification.45,46
Mortality risk and mortality-causing mechanisms
We identified a consistent mortality risk (HR: 1⋅52, 95% CI: 1⋅14-2⋅02) as those reported in the U.S. (HR: 1⋅29, 95% CI: 1⋅22-1⋅38) and Sweden (HR: 1⋅35, 95% CI: 1⋅21-1⋅50).12,14 It is therefore clinically relevant to understand the reasons for the association between TRD and premature mortality, to inform strategies for secondary prevention. Previous research often portrays multiple mental health conditions as a risk factor for treatment resistance.47,48 whilst our results suggest that developing TRD could also be a precursor to diagnosis of other mental health conditions or self-harm, and that 42% of the causal relationship between TRD and all-cause mortality is mediated by the new onset of psychiatric conditions. In the TRD population, clinical attention should therefore focus on reducing the risk of further mental health problems and monitor for clinical signals that may lead to mortality, especially suicidal behaviours, psychosis, and schizophrenia as these were found to be the most common newly diagnosed post-TRD psychiatric conditions.
Our mediation analysis also highlights the complexity of depression management and reinforces the importance of ongoing monitoring for new psychiatric diagnoses after treatment resistance. TRD serves as a marker for mortality, and early identification of potential treatment-resistant patients is essential to prevent them enduring prolonged ineffective medication. Although the mediation effect of new physical disorders was non-significant, the effect coefficient suggested a direction and tendency towards a mediation effect that did not deviate from our initial hypothesis. As our data captured only one year of incident cohort, the study may be underpowered to detect underlying mediating effects of physical health on the relationship between TRD and mortality. Future studies with larger samples or multiple years of incident cohort will help to differentiate the reason.
Healthcare resource utilisation and economic burden
Consistent with previous research, our findings also suggest that TRD is associated with greater healthcare resource utilisation and higher cost in all service settings.27,28,30,31,49 Each TRD patient costs $116,731 per year to the system, which is equivalent to almost one-third of local gross domestic product (GDP) per capita in 2019.50 This has far exceeded the governmental annual healthcare budget at $23,753 per capita (6⋅2% of local GDP per capita in 2018/19),51 indicating an inadequate resource planning with respect to TRD. In terms of setting the distribution of healthcare resources, the burden found in our study appeared to incline towards inpatient setting compared with other country contexts. Claims-based studies, which similarly studied the impact of TRD on healthcare resource utilisation and healthcare cost in the U.S. and Japan, revealed that TRD patients had only 1⋅8 to 4⋅2 hospital days but 18⋅0 to 45⋅5 outpatient visits per patient year.27,28,30,31,49 Such discrepancies could have originated from the intrinsic difference between claims-based and routine-care based EMRs, for example, prescription renewal was often counted as one outpatient visit in a claims-based system.30 Alternatively, different health financing structures, particularly given that we exercise a taxation-based public system where eligible patients were heavily subsidised, may have potentiated different health-seeking behaviours. In the case of disproportionate reliance towards hospitalisation, nevertheless, re-consideration should be given to strengthening the role of outpatient and community resources, to which the costlier inpatient load could be reshuffled.
Clinical and health service implications
This comprehensive investigation on the clinical and economic consequences of TRD has brought about implications both clinically and in decision-making in a multifaceted manner. In contrast to post-TRD psychiatric diagnoses, post-TRD physical comorbidities did not seem to significantly sit in the pathway to TRD-associated death in the mediation analysis. However, the burden that arose from physical comorbidities might have instead manifested in healthcare resource utilisation as our results showed that TRD patients consumed significantly more resources in both psychiatric and general medical services in all three service settings. Physical complications are bidirectionally linked with the severity of depression, it is therefore reasonable that TRD patients had worse courses of both general and mental health, which exhibited and extended the healthcare burden to other medical services.16,18,45 Authorities should expect increased premature mortality and service demand associated with TRD. The findings also indicate that multidisciplinary disease management strategies to treat and prevent progression into TRD may not only be beneficial to the multifaceted patient outcomes, but also cost savings in both the aforementioned service subtypes.
Limitations
This study also has several limitations. First, as with other studies utilising routine care EMR, our definition of drug exposures was complicated when considering concurrent medications and the switching of drug regimens. In cases where patients switched or added medications before their previous prescription duration ended, it was difficult to determine whether the patient had stopped taking the previous medication or if it was added to their regimen and hence may be misclassified. To address this, we defined an adequate duration of regimen and gaps within the regimen based on clinical guidelines.38 Moreover, the lack of detailed clinical information, such as depression severity or reasons for switching medication and dosage adjustment were unavailable, may lead to misclassification of TRD. Given the lack of consensus on the TRD definition, pseudo-resistance (e.g. due to inadequate initial dosing, early discontinuation, atypical pharmacokinetics and medical compliance) could not be completely ruled out.52 Second, patients with TRD might likely have repeated visits with increased chance of detecting comorbidities early. Our current mediation analysis considered post-TRD diagnoses made from all service settings, including emergency and unplanned hospitalisation, which could minimise but not exclude the possibility of surveillance bias. Overestimation of mediation effect from post-TRD comorbidities was possible, which warrants future study with thoughtful study design. Third, the healthcare resource utilisation analyses did not account for costs of prescriptions and psychotherapies, which may represent an underestimation of overall medical cost. Last, sample size of the current study is relatively small comparing to previous national-wide cohort studies. With statistical power consideration, we limited our analyses to all-cause mortality but not depression-related death or healthcare resource utilisation. Future studies with larger sample size are encouraged, particularly for the reconfirmation of death-mediation effect from post-TRD psychiatric and physical comorbidities.
Conclusion
TRD increased mortality risk by 52% compared to treatment-responsive depression, partially mediated by post-TRD psychiatric conditions. Physical comorbidities, although not significantly mediating survival outcome, cast considerable burden to the healthcare system, given the increased service utilisation across multiple service types.
Contributors
X Li and ICK Wong conceived the study idea and study design. VKY Chan and ECL Cheung gathered the data and performed data analyses. X Li, M Fan, FTT Lai, RSM Wong and EYF Wan provided technical and statistical advice. All authors interpreted the results. VKY Chan, ECL Cheung and XL wrote and revised the drafts with all authors’ critical comments and revisions. All authors provided their final approval for manuscript submission. All authors agree to be accountable for all aspects of the work. X Li and ICK Wong obtained the funding and supervised the study conduct. The corresponding authors confirm that all co-authors meet authorship criteria.
Data sharing statement
We are unable to directly share the data used in this study since the data custodian, the Hong Kong Hospital Authority who manages the Clinical Data Analysis and Reporting System (CDARS), has not given permission. However, CDARS data can be accessed via the Hospital Authority Data Sharing Portal for research purpose. The relevant information can be found online (https://www3.ha.org.hk/data). The statistical procedures and R codes used in this study are available upon request.
Funding
This study was supported by the unconditional educational grant from Janssen and Internal Research Fund from Department of Medicine, The University of Hong Kong. All authors had no dependent relationship with the external funder.
Ethics approval
This study received ethics approval from the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong Western Cluster (UW 20-218).
Role of the funding source
This study was supported by an unconditional educational grant from Janssen and Internal Research Fund from the Department of Medicine, The University of Hong Kong. Funders did not participate throughout the study design, study conduct, interpretation, or manuscript writing.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
X Li received research grants from Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work; H Luo received research grants Research Grants Council Early Career Scheme (RGC/ECS, HKSAR) unrelated to this work. S Chan received research support from GRF, Research Grant Council; HMRF, Food and Health Bureau. FTT Lai was supported by the RGC Postdoctoral Fellowship 2020/21 (inaugural exercise) funded by the Research Grants Council, University Grants Committee, Hong Kong SAR. J F Hayes was supported by the Wellcome Trust (211085/Z/18/Z), University College London Hospitals NIHR Biomedical Research Centre and the NIHR ARC North Thames Academy. He received consultancy fees from the Wellcome Trust and Juli Health, unrelated to this work. P Ip received research grants from Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council (GRF and CRF, HKSAR) and project grant from the Hong Kong Jockey Club Charities Trust. EWY Chan received honorarium from the Hospital Authority, research grants from Innovation and Technology Commission of HKSAR, Narcotics Division of the Security Bureau of HKSAR, National Health and Medical Research Council (NHMRC, Australia), National Natural Science Foundation of China (NSFC), Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council (RGC, HKSAR), Wellcome Trust; Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, RGA, Takeda and Novartis, and consultancy fee from Novartis outside the submitted work. She is also the President of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Hong Kong Regional Chapter. ICK Wong received research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, Takeda, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and has also received speaker fees from Janssen and Medice over the past three years. He is also an independent non-executive director of Jacobson Medical in Hong Kong.
Acknowledgements
We thank Lisa Lam for proofreading this manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100426.
Contributor Information
Vivien KY Chan, Email: vchanky@connect.hku.hk.
Edmund CL Cheung, Email: edmundcc@connect.hku.hk.
Sandra SM Chan, Email: schan@cuhk.edu.hk.
Martin Knapp, Email: M.Knapp@lse.ac.uk.
Joseph F Hayes, Email: joseph.hayes@ucl.ac.uk.
Min Fan, Email: minfan@connect.hku.hk.
Francisco TT Lai, Email: fttlai@hku.hk.
Hao Luo, Email: haoluo@hku.hk.
Terry Lum, Email: tlum@hku.hk.
Rosa SM Wong, Email: rosawong@hku.hk.
Lauren KW Lau, Email: llkw127@hku.hk.
Eric YF Wan, Email: yfwan@hku.hk.
Gloria HY Wong, Email: ghywong@hku.hk.
Esther WY Chan, Email: ewchan@hku.hk.
Patrick Ip, Email: patricip@hku.hk.
Ian CK Wong, Email: wongick@hku.hk.
Xue Li, Email: sxueli@hku.hk.
Appendix. Supplementary materials
References
- 1.World Health Organisation . World Health Organisation; Geneva: 2017. Depression and Other Common Mental Disorders: Global Health Estimates. [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q., He H., Yang J., Feng X., Zhao F., Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatry Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Fava M., Davidson K.G. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 5.Trevino K., McClintock S.M., McDonald Fischer N., Vora A., Husain M.M. Defining treatment-resistant depression: a comprehensive review of the literature. Ann Clin Psychiatry. 2014;26(3):222–232. [PubMed] [Google Scholar]
- 6.McAllister-Williams R.H., Arango C., Blier P., et al. The identification, assessment and management of difficult-to-treat depression: An international consensus statement. J Affect Disord. 2020;267:264–282. doi: 10.1016/j.jad.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 7.McAllister-Williams R.H., Arango C., Blier P., et al. Reconceptualising treatment-resistant depression as difficult-to-treat depression. Lancet Psychiatry. 2021;8(1):14–15. doi: 10.1016/S2215-0366(20)30516-2. [DOI] [PubMed] [Google Scholar]
- 8.Brown S., Rittenbach K., Cheung S., McKean G., MacMaster F.P., Clement F. Current and Common Definitions of Treatment-Resistant Depression: Findings from a Systematic Review and Qualitative Interviews. Can J Psychiatry. 2019;64(6):380–387. doi: 10.1177/0706743719828965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guideline on clinical investigation of medicinal products in the treatment of depression 2013 [Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-depression_en.pdf.]
- 10.Chang C.K., Hayes R.D., Perera G., et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6(5):e19590. doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesney E., Goodwin G.M., Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153–160. doi: 10.1002/wps.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G., Fife D., Wang G., Sheehan J.J., Bodén R., Brandt L., et al. All-cause mortality in patients with treatment-resistant depression: a cohort study in the US population. Ann Gen Psychiatry. 2019;18:23. doi: 10.1186/s12991-019-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madsen K.B., Plana-Ripoll O., Musliner K.L., Debost J.P., Petersen L.V., Munk-Olsen T. Cause-specific life years lost in individuals with treatment-resistant depression: A Danish nationwide register-based cohort study. J Affect Disord. 2021;280(Pt A):250–257. doi: 10.1016/j.jad.2020.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Reutfors J., Andersson T.M., Brenner P., et al. Mortality in treatment-resistant unipolar depression: A register-based cohort study in Sweden. J Affect Disord. 2018;238:674–679. doi: 10.1016/j.jad.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Gronemann F.H., Jorgensen M.B., Nordentoft M., Andersen P.K., Osler M. Treatment-resistant depression and risk of all-cause mortality and suicidality in Danish patients with major depression. J Psychiatry Res. 2021;135:197–202. doi: 10.1016/j.jpsychires.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Hare D.L., Toukhsati S.R., Johansson P., Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 17.Lai F.T.T., Mercer S.W., Guthrie B., et al. Sociodemographic moderation of the association between depression and stroke incidence in a retrospective cohort of 0.4 million primary care recipients with hypertension. Psychol Med. 2020:1–9. doi: 10.1017/S0033291720001920. [DOI] [PubMed] [Google Scholar]
- 18.Scherrer J.F., Chrusciel T., Garfield L.D., Freedland K.E., Carney R.M., Hauptman P.J., et al. Treatment-resistant and insufficiently treated depression and all-cause mortality following myocardial infarction. Br J Psychiatry. 2012;200(2):137–142. doi: 10.1192/bjp.bp.111.096479. [DOI] [PubMed] [Google Scholar]
- 19.Fava M., Alpert J.E., Carmin C.N., et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34(7):1299–1308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- 20.Kessler R.C., Berglund P., Demler O., et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 21.Choi K.W., Kim Y.K., Jeon H.J. Comorbid Anxiety and Depression: Clinical and Conceptual Consideration and Transdiagnostic Treatment. Adv Exp Med Biol. 2020;1191:219–235. doi: 10.1007/978-981-32-9705-0_14. [DOI] [PubMed] [Google Scholar]
- 22.Dubovsky S.L., Thomas M. Psychotic depression: advances in conceptualization and treatment. Hosp Community Psychiatry. 1992;43(12):1189–1198. doi: 10.1176/ps.43.12.1189. [DOI] [PubMed] [Google Scholar]
- 23.Li G.H., Cheung C.L., Chung A.K., et al. Evaluation of bi-directional causal association between depression and cardiovascular diseases: a Mendelian randomization study. Psychol Med. 2020:1–12. doi: 10.1017/S0033291720003566. [DOI] [PubMed] [Google Scholar]
- 24.Mrazek D.A., Hornberger J.C., Altar C.A., Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatry Serv. 2014;65(8):977–987. doi: 10.1176/appi.ps.201300059. [DOI] [PubMed] [Google Scholar]
- 25.Brenner P., Nygren A., Hagg D., et al. Health care utilisation in treatment-resistant depression: a Swedish population-based cohort study. Int J Psychiatry Clin Pract. 2021:1–8. doi: 10.1080/13651501.2021.2003405. [DOI] [PubMed] [Google Scholar]
- 26.Greden J.F. The burden of disease for treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):26–31. [PubMed] [Google Scholar]
- 27.Amos T.B., Tandon N., Lefebvre P., et al. Direct and Indirect Cost Burden and Change of Employment Status in Treatment-Resistant Depression: A Matched-Cohort Study Using a US Commercial Claims Database. J Clin Psychiatry. 2018;79(2) doi: 10.4088/JCP.17m11725. [DOI] [PubMed] [Google Scholar]
- 28.Sussman M., O'Sullivan A.K., Shah A., Olfson M., Menzin J. Economic Burden of Treatment-Resistant Depression on the U.S. Health Care System. J Manag Care Spec Pharm. 2019;25(7):823–835. doi: 10.18553/jmcp.2019.25.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin D., Kim N.W., Kim M.J., et al. Cost analysis of depression using the national insurance system in South Korea: a comparison of depression and treatment-resistant depression. BMC Health Serv Res. 2020;20(1):286. doi: 10.1186/s12913-020-05153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahlich J., Tsukazawa S., Wiegand F. Estimating Prevalence and Healthcare Utilization for Treatment-Resistant Depression in Japan: A Retrospective Claims Database Study. Drugs Real World Outcomes. 2018;5(1):35–43. doi: 10.1007/s40801-017-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olfson M., Amos T.B., Benson C., McRae J., Marcus S.C. Prospective Service Use and Health Care Costs of Medicaid Beneficiaries with Treatment-Resistant Depression. J Manag Care Spec Pharm. 2018;24(3):226–236. doi: 10.18553/jmcp.2018.24.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell J.M., Hawkins K., Ozminkowski R.J., et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65(3):341–347. doi: 10.4088/jcp.v65n0309. [DOI] [PubMed] [Google Scholar]
- 33.Census and Statistics Department. Thematic Household Survey Report - Report No. 58 - Health status of Hong Kong residents 2015 [Available from: http://www.statistics.gov.hk/pub/B11302582015XXXXB0100.pdf.]
- 34.Hospital Authority. Hospital Authority Statistical Report 2014-2015 [Available from: http://www.ha.org.hk/haho/ho/stat/HASR1415_2.pdf.]
- 35.Chai Y., Luo H., Wong G.H.Y., et al. Risk of self-harm after the diagnosis of psychiatric disorders in Hong Kong, 2000-10: a nested case-control study. Lancet Psychiatry. 2020;7(2):135–147. doi: 10.1016/S2215-0366(20)30004-3. [DOI] [PubMed] [Google Scholar]
- 36.Man K.K.C., Chan E.W., Ip P., et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. Bmj. 2017;357:j2350. doi: 10.1136/bmj.j2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Man K.K.C., Coghill D., Chan E.W., et al. Association of Risk of Suicide Attempts With Methylphenidate Treatment. JAMA Psychiatry. 2017;74(10):1048–1055. doi: 10.1001/jamapsychiatry.2017.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder 2010 [Available from: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.] [PubMed]
- 39.Siegel C.A., Yang F., Eslava S., Cai Z. Treatment Pathways Leading to Biologic Therapies for Ulcerative Colitis and Crohn's Disease in the United States. Clin Transl Gastroenterol. 2020;11(2):e00128. doi: 10.14309/ctg.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Quan H., Li B., Couris C.M., et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T.L., Collins G.S., Spence J., et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17(1):78. doi: 10.1186/s12874-017-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lachin J.M. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2(2):93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 44.Royston P., Parmar M.K. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 45.Carney R.M., Freedland K.E. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry. 2009;166(4):410–417. doi: 10.1176/appi.ajp.2008.08081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sartorius N. Depression and diabetes. Dialogues Clin Neurosci. 2018;20(1):47–52. doi: 10.31887/DCNS.2018.20.1/nsartorius. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S.S., Chen H.H., Wang J., Chen W.J., Chen H.C., Kuo P.H. Investigation of early and lifetime clinical features and comorbidities for the risk of developing treatment-resistant depression in a 13-year nationwide cohort study. BMC Psychiatry. 2020;20(1):541. doi: 10.1186/s12888-020-02935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mogi T., Yoshino A. The multiple diagnoses of comorbid anxiety disorders and higher interpersonal sensitivity predict treatment-resistant depression. Asian J Psychiatry. 2017;26:131–135. doi: 10.1016/j.ajp.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Pilon D., Joshi K., Sheehan J.J., et al. Burden of treatment-resistant depression in Medicare: A retrospective claims database analysis. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The World Bank. GDP per capita (current US$) – Hong Kong SAR, China [Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=HK.]
- 51.Food and Health Bureau. Hong Kong's Domestic Health Accounts (HKDHA) [Available from: https://www.fhb.gov.hk/statistics/en/dha/dha_summary_report.htm ]
- 52.Souery D., Papakostas G.I., Trivedi M.H. Treatment-resistant depression. J Clin Psychiatry. 2006;67(Suppl 6):16–22. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.